Abstract
The accumulation of intra- and extracellular misfolded proteins is found to play an imperative role in the progression of several neurological disorders including epilepsy, Alzheimer’s disease, brain tumors, etc. which ultimately causes death worldwide. Heat shock protein (Hsp60) was found to be an important biomolecule that plays an essential role in the removal or degradation of these misfolded proteins and also act as a biomarker in disease prognosis. In neurological diseases, these systems are compromised due to deregulation or mutation of Hsp60 resulting in a large amount of aggregated proteins accumulation. Therefore, the development of novel and more efficient Hsp60 modulators is essential that can modulate the Hsp60 and involved pathways for the treatment of neurological diseases. For this propose, several In silico computational tools are developed, and many tools are under development. In this chapter, we discuss the role of Hsp60 in some more prominent neurological diseases and modulators developed in this direction. Further, we also highlight advanced computational tools that could be used for designing more Hsp60 modulators.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Heat shock proteins (Hsp) are a special kind of protein generally found in all three kingdom prokaryotes, archaea and eukaryotes including human beings (Kim et al. 2013). The primary roles of Hsp are to help in the folding of nascent polypeptides to their native and accurate confirmation, prevent the aggregation of other proteins, degrade severely damaged proteins, and to regulate the apoptosis (Akerfelt et al. 2010). The failure of this cellular process leads to several neurological disorders. Although, different kinds of Hsp, like Hsp40, Hsp60, Hsp70, Hsp90, and Hsp100, among them, Hsp60 has been reported a critical molecule involved in several neurological disorders (Bross et al. 2012).
Hsp60 involved in translocation, folding and assembly of different proteins. It acts as a molecular chaperone which cooperates with other chaperones such as Hsp10/GroES, Hsp70 to fulfill their specific functions (Hartl 1996). Hsp60 (HspD1) is most commonly present in mitochondria, help in the transportation of nascent polypeptide from the cytoplasm to the mitochondrial matrix. It mainly works in combination with Hsp10 (Cpn10), resides in the mitochondria (Veereshwarayya et al. 2006). In addition to its classical chaperone function, Hsp60 is also involved in the replication and transmission of mitochondrial DNA molecule. It has been reported that mutations in Hsp60 molecules, unregulated the mtDNA levels that cause transmission defects (Samluk et al. 2018).
There are mainly two functions perform by Hsp60 concerning mitochondrial protein transport. First is to catalyze the folding of proteins destined for the matrix and then maintains protein in an unfolded state for transportation across the inner membrane of the mitochondria. Hsp60 binds to incoming proteins and induces conformational and structural changes, the hydrophobic portion of Hsp60 is responsible for maintaining the protein in unfolded conformation during transmembrane transport (Hartl and Hayer-Hartl 2002; Natalello et al. 2013). Further, consequent changes in the concentration of ATP hydrolyze the bonds between the protein and Hsp60 which signals the protein to exit from the mitochondria. Hsp60 is also capable of distinguishing between proteins designated for export and proteins destined to remain in the mitochondrial matrix by looking for an amphiphilic alpha-helix of 15–20 residues (Priya et al. 2013).
Additionally, some evidence indicates that Hsp60 also represents a regulator of an enormous and diverse range of cellular activities as far beyond to identify roles in protein folding and chaperoning (M van Noort et al. 2017). Now, it is well understood that Hsp60 to be involved in several processes such as synaptic transmission, autophagy, ER stress response, protein kinase and cell death signaling (Bie et al. 2016). Further, dysfunction of Hsp60 has robust effects on the fate of cells in neurological injury and disease states that leads to a variety of severe neurodegenerative disorders including Alzheimer’ and Parkinson’s disease, familial amyloidotic polyneuropathy and bovine spongiform encephalopathy as well as Jacob-Creutzfeldt disease (Cardinale et al. 2014). Deficiency in Hsp60 has recently been identified as being associated with atypical mitochondrial diseases in combination with multisystem failure (Amor et al. 2014). Further, proteomic analysis revealed that Hsp60 acts as an interactor of Parkin, PINK1, α-synuclein, and DJ-1. Among all these proteins PINK1 has different mitochondrial localization, but all these are involved in Parkinson’s disease (Campanella et al. 2012). Hsp60 is among the most potent suppressors of neurodegeneration in animal models. Thus, Hsp60 provides a potential target for protective pharmacotherapy in many neurologic disorders (Benarroch 2011). This chapter presents the information about the role of Hsp60 in different neurological disorder with their multiple functions and dynamics of Hsp modulators with different computational tools designed to predict the conformational changes occurs during binding of Hsp60 with different proteins.
1.1 HSP60 in Neurological Diseases
Neuron dysfunction is caused by abnormal aggregation of misfolded or mutant proteins which is a vague and significant medical challenging condition (Cardinale et al. 2014). The plagued brain region and disruption of daily activities including sensory and motor functions are major clinical manifestations. These include a problem in moving, speaking, swallowing, breathing and cognitive dysfunctions. In different neurodegenerative diseases, misfolding and accumulation of proteins are known to be a significant cause of neuronal death and loss of synapses (Bross et al. 2012). There are various cellular processes which exacerbate or attenuate the diseases process (Wyttenbach et al. 2000). There are several mechanisms involved to clear off these protein aggregates which include molecular chaperones, the autophagy pathway, and the ubiquitin-proteasome system. Among all these mechanisms chaperone-mediated autophagy is known to be the most effective and promising approach to remove misfolded protein aggregation in the cells (Maiti et al. 2014).
Protein quality control mechanism of the mitochondrial matrix has been taken up by two protein system mitochondrial m-AAA protease system paraplegin and Afg312 (Neuspiel 2008). Defects of genes encoding in these systems lead to neurological disorders like hereditary spastic paraplegia SPG7, spinocerebellar ataxia SCA and early onset spatic ataxia-neuropathy syndrome (Rugarli and Langer 2012). Along with these findings mutation in the gene encoding, Hsp60 is associated with another form of hereditary spastic paraplegia SPG13 (Hansen et al. 2002). This disease is a subgroup of heterogeneous neurological disorders characterized by primary motor neuron degeneration, and the disease affects motor and sensory neurons with the longest axons. This degeneration is known as dying back degeneration (Laser et al. 2003).
A further role of Hsp60 was supported by findings that homozygosity for a missense mutation in the HSPD1 gene which is found to be associated with fatal hypomyelinatingleukodystrophy (MitCHAP60) (Magen et al. 2008). MRI revealed severe hypomyelination in the central nervous system and characterized by roatary nystagmus, early onset and progressive spastic paraplegia which is followed by neurological decline and worsening situations with severe mental retardation (Hansen et al. 2007). MitCHAP60 mutations for heterozygous siblings are entirely asymptomatic which recommends that a single mutant allele does not considerably impair the overall activity of Hsp60 in mitochondria (Bross et al. 2012).
1.1.1 Epilepsy
It is a central nervous system disorder affecting approximately 1% of the world population (Sendrowski and Sobaniec 2013). It is characterized by spontaneous recurrent seizures (SRSs) with a high frequency of neuronal discharges, unusual behavior, sensations and sometimes loss of consciousness (Gu and Daltone 2017). Temporal lobe epilepsy (TLE) is the most common one in adult humans which is characterized by the advanced development of SRSs from temporal lobe foci and morphological fluctuations in the hippocampus (Liu et al. 2008). Generally, TLE is initiated by head injury or stroke, infection in brain or delirious seizures which induces a status epilepticus (SE) and epileptogenesis is the period between initial injury and seizure. This period duration is 5–10 years in which many neurobiological events occur. At the site of injury, inflammation develops which involves glial and endothelial cells (Van Liefferinge et al. 2013). Clinically for diagnostic and prognosis purposes, biochemical measurements of inflammatory mediators in blood serum serve as a powerful tool. Hsp serves as promising candidates can be used as biomarkers among neuroinflammatory mediators including TLE (Chang et al. 2012). Hsp60, a constitutively expressed mitochondrial protein is expressed endogenously in astrocytes, neurons, oligodendrocytes, and microglia, ependymal cells of the brain. Thus, Hsp60 distribution in many of the cells of the brain suggests its active participation in normal and pathological conditions (Gu and Daltone 2017).
Gammazza et al. 2015 studied the expression and distribution of Hsp60 in the hippocampus of rats. They induce partial seizures in anesthetized rats which were based on the phenomenon of maximal dentate activation (MDA) which was recorded in the dentate gyrus (DG). This was induced by repetitive electrical stimulation of the perforant path in these rats and conducted analysis using western blot and immunohistochemistry of hippocampal tissues. Epileptic rats were assessed for Hsp60 by using ELISA. A similar technique was used to assay Hsp60 levels in the bloodstream of patients suffering from temporal lobe epilepsy. It was found that immunoreactivity of Hsp60 in rat models of TLE increased. The epileptic rats and patients with seizures showed a high level of circulating Hsp60 in the bloodstream. Their results demonstrate that Hsp60 can be potentially used as a biomarker and diagnostic for patients with these types of seizures.
1.1.2 Alzheimer’s Disease
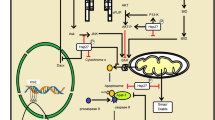
Alzheimer’s disease (AD) is a type of dementia which is irreversible progressive and slowly destroys memory and thinking skills (Sonkusare et al. 2005). It is currently sent as the 6th leading cause of death in the US after heart disease and cancer (Rodgers 2002). In the AD, the expression of an Hsp60 is associated with the deposition of Aβ tangles of neurofibrils (Venegas and Heneka 2017). Current studies on Hsp showed that they prevent the accumulation of Aβ tangles. Papuc et al. 2015 studied the humoral response against Hsp60 in the AD and found out its potential as biomarkers for early diagnosis of disease. It has been known that anti-60 KD Hsp60 antibodies are present in the serum of healthy humans and the serum of patients with inflammatory and autoimmune disorders. A cellular signaling pathway involved in Hsp60-mediated NLRP3 inflammasome activation and subsequent IL-1β production is described in Fig. 15.1. So, it might be hypothesized that AD patients may be accompanied by the presence of anti-HSP antibodies due to the inflammatory process present in the brains of these patients. They didn’t find any significant difference in antibody titer of the healthy individual as compared to AD patients. So, they concluded that anti Hsp60 antibodies that are present in these patients might belongs to a natural human immune system and does not significantly induce any effect on immunoreactivity against Hsp60.
Scheme of the signaling pathway involved in Hsp60-mediated NLRP3 inflammasome activation and subsequent IL-1β production. IL-1β induces its production by the activated microglia in an Hsp60-dependent manner. Hsp60, after being up-regulated by IL-1β, gets secreted outside and binds with TLR4 of the microglia to activate p38 MAPK. Binding of Hsp60 with TLR4 facilitates NF-κB phosphorylation, mitochondrial damage, and ROS generation and finally activates NLRP3 inflammasome leading to IL-1β production. JEV also augments Hsp60 production and thus influences inflammasome complex to induce a consecutive expression of IL-1β and, in turn, induces an exaggerated immune response
Hsp60 is a mitochondrial chaperone which is required for its homeostasis and there are many human diseases associated with mutation in Hsp60 (also known as HSPD1 gene) (Meng et al. 2018).V98I mutation in Hsp60 was reported to be associated with hereditary spastic paraplegia SPG 13 which is a rare neurodegenerative disorder characterized by weakness of the lower Limbs (Bross et al. 2008). Another mutation in Hsp60 (D27G) was identified from patients suffering in an autosomal recessive neurodegenerative disorder also called MitCHAP-60 disease (Magen et al. 2008). It is characterized by hypomyelination and leukodystrophy in the brain, and it was found that D27G mutant was less stable in forming hepta and decameric oligomers as compared to wild-type (Meng et al. 2018).
1.1.3 Brain Tumors
The frequency of development of brain tumors accounts for 2% of human neoplasms in adults and 20% in pediatric patients (McKeever 1999). These tumors comprise a different group of neoplasms which arises in the brain and its surrounding structures as shown in Fig. 15.2. Hsp had a promising role for cell survival functions and believed to be involved in the development of some human diseases known as chaperonopathies (Macario and Macario 2005). According to some studies, chaperones favors the tumor by inhibiting tumor cell apoptosis and presence of high levels of various Hsp in human brain tumors might be useful in early detection of disease (Campanella et al. 2012). Rappa et al. 2013 studied the level and cellular distribution of Hsp60 and Hsp70 in a series of brain tumors. They found the significantly higher level of Hsp60 in neuroepithelial tumors as compared to meningeal neoplasms, and Hsp60 was mainly present in the cytoplasmic level. They hypothesized that increment of level of Hsp60 is not by passive phenomenon but might be implicated in tumor progression. In another study by Kato et al. 2001, demonstrated expression and immunopositivity of Hsp60 in a series of 158 human brain tumors as compared to normal brain tissues.
1.2 HSP60 Modulators
There is a need to develop Hsp60 modulators that can target Hsp60 and useful in therapeutics of these disease conditions (Nakamura and Minegishi 2013). Already numerous compounds have been designed to focus on Hsp90, but few have been developed up till now for Hsp60 (Capello et al. 2014). Most of the Hsp60 modulators that are developed to date are bioactive compounds from studies of chemo proteomics. These modulators are classified into two types Type I and Type II (Pace et al. 2013). Type 1 inhibitors are work through inhibiting ATP binding and its hydrolysis. These inhibitors inhibit Hsp60 refolding activity as ATP-dependent conformation changes are affected (Meng et al. 2018). Type II inhibitors covalently react with specific cysteine residues in Hsp60. There is some natural product based Hsp-inhibitors and other from synthetic source (Radons 2017). Mizoribine is the first smallest organic molecule to be known as an Hsp60 inhibitor (Mizuno et al. 1974). This compound act as imidazole nucleoside antibiotic isolated from Eupenicillium brefeldianum. It does not contain any antimicrobial activity, but it is a potential immunosuppressor (Itoh et al. 1999).
Another natural compound which inhibits Hsp60 is epolactaene isolated from fungus Penicillium sp. Recently, MyrtucommulaneA (MC) is a non prenylatedacylphloroglucinol which contains antibacterial, antioxidant, anti-inflammatory, antitumor properties and isolated from human leukemia cells (Nagumo et al. 2004). Experiments have shown that Hsp60 is a direct target of MC. Indeed there is an excellent opportunity to develop potential therapeutic by targeting Hsp60 because the expression level of Hsp60 is increased in almost all neurodegenerative disorders (Nagumo et al. 2005). Few small modulators of Hsp60 have been identified which includes both natural products and synthetic molecules but these all molecules does not share any common structural motif or pharmacophore, so it will be crucial to understanding how these inhibitors can interact with Hsp60.
1.3 Computational Tools Used in Drug Discovery
Hsp60 is a complex molecule having a large number of therapeutic application in drug discovery, by stimulation of different genes and protein effectively (Abramovitch 2018). The pro-tumoral strategies in Hsp60 include stimulation of pro-apoptotic pathways, pro-survival pathways, surface expression, and antitumor immune responses. The target of Hsp60 inhibitors is ATP binding sites and hydrolysis sites whose change in conformation can lead to the tumor (Nakamoto et al. 2018). A variety of docking databases can help to identify these conformational changes such as PDB bind database, some commercial programs such as LigandFit, Glide, GOLD, MOE Dock, and Surflex-Dock and some academic programs such as AutoDock, AutoDock Vina, LeDock, rDock, and UCSF DOCK also helpful in this direction (Padmadas et al. 2018). These programs can help in finding the accuracies of binding pose prediction (sampling power) and binding affinity (scoring power). Docking can assist not only in identifying these ligands with their specific sites also they can contribute to predicting the interactions between them (de Ruyck et al. 2016). Another place for Hsp60 attack is oxidation sites and binding sites with specific ligands. Improvement in computational tools such as target/ligand databases, homology modeling, ligand fingerprint methods, etc. can sort out the difficulties of predicting ligand activity, based on only similarities or dissimilarities. Various computer-aided drug designing can act as a companion for efficient drugs development (Hauser et al. 2017). Tumor-derived Hsp60 act as a loyal candidate for anti-tumor vaccines; derived peptides shows high immunomodulatory effect such as in arthritis. In addition to this, levels of Hsp60 influence by the levels of flavonoids and other proteins (Carlson 2002). Certain web tools such as SwissADME can detect these physicochemical properties, pharmacokinetics, drug-likeness. Some methods in cheminformatics such as BOILED-Egg and iLOGP are used to support drug discovery endeavors (Daina et al. 2017). They function collaboratory and interacts with others also; some proteins inhibit the expression of other Hsp such as Hsp60, Hsp70, Hsp27, and Hsp47. Photodynamic therapy (PDT) can help in identifying treatments for different oncologic and nononcologic lesions; it can act as a powerful tool for modulating HSP60/HSPD1 gene expression tumor cell resistance to anti-tumor agents induced by PDT might be related to Hsp60 overexpression. For example, E. coli Hsp90 and the DnaK system work synergistically to remodel of the client protein. E. coli Hsp90 and DnaK interact both in vivo and in vitro, additional evidence justify that E. coli Hsp90 and the DnaK system function together (Genest et al. 2011).
Some DNA vaccines encoded with Hsp60 directly targets different disease such as arthritis, diabetes, obesity, etc. so, they can be modified and predicted by using various computational strategies. Moreover, various computational studies can be used to predict the epitopes design for particular diseases especially mycobacterium Hsp60which is responsible for T-cell activations. Here, Docking of peptides to the binding groove of MHC I proteins can be used to study the binding interactions of 15 antigenic CTL epitopes with three class I major histocompatibility complex (MHC I). The self-reactive T-cell population can be protected by the cross-reactivity between non-self- and self-Hsp60. Exosome-based tumor vaccines represent an exciting approach in inducing strong anti-tumor immune responses CD8(+) T cells have been recognized as the significant T-cell subset responsible for the anti-tumor effect of Hsp60-containing exosomes interpretation of anti-tumor vaccines based on Hsp60-containing exosomes act as novel anti-cancer therapies. In addition to this, chemotherapy possibly impacts Hsp60 expression has been the subject of numerous investigation (Leelananda and Lindert 2016).
In neurological disorders especially that targets the genes and protein expression, Hsp60 plays a significant role. Hsp60 plays a crucial role in protecting the brain, as it consists of proteins consisting of microglial triggering receptor expressed in myeloid (TREM). They bind on the surface of neuroblastoma cells and astrocytes when exposed to the surface of Hsp60. Under nonpathological conditions Hsp60-complexed TREM-2 synchronize functions of brain cells, these types of correlations can be studied by using various computational strategies such as Brain-coX which provide a wide range of transcriptomics studies based on brain and gene expressions. In microglia, it was found that different interactions of extracellular Hsp60 with microglial LOX-1 boost the production of pro-inflammatory factors (IL-1β, NO and ROS) and propagate neuronal damage. For a better analysis of gene networks for hsp60 in different neurological disorders, NeuroDNet is a web server using Mysql - 5.0.18 - Win32 and PHP - 5.2.0 (Bendl et al. 2016; Jorgensen 2004). For the comparative analysis, an Integrated Neurodegenerative Disease Database (INDD) has been made of frontotemporal lobar degeneration (FLD) (Sliwoski et al. 2014). Extracellular Hsp60 induces an inflammatory response and soluble neuronal injury signal having a direct link with neuroinflammation and neurotoxicity. More informative studies can be done by Alzforum, by which diagnostics and treatment related to Alzheimer disease and related disorders can be retrieved quickly. Alzforum has developed other ten open-access databases- AlzBiomarker, AlzGene, AlzPedia, AlzRisk, Antibodies, Brain Banks, Mutations, Research Models, Protocols, Therapeutics which directly links it to all aspects of AD and PD (Weinberg et al. 2017). In addition to this other database such as CIDeR and CombiROC can be used for integrative studies of metabolic and neurological disorders, it can help in determining best possible biomarkers for specific diseases (Scott et al. 2016).
2 Conclusions
In recent years, there has been a spectacular augment in cases where individual suffering from neurological disorders worldwide. It was observed from different studies that Hsp60 deregulation or malfunction play a significant role in the progression of these diseases. In a healthy individual, Hsp60 involves in refolding, or degradation of misfolded proteins thus prevents the development of the disease. So, the development of Hsp60 modulators could be a better and effective treatment option for several neurological diseases. In this direction, several modulators such as Mizoribine and Myrtucommulane A were discovered from natural sources that can inhibit or modulate the Hsp60 activity and pathways where Hsp60 takes part. Further, several computer-based advanced docking and simulation models have been developed that can be used for designing and screening of more potent modulators for disease treatment.
Abbreviations
- AD:
-
Alzheimer’s disease
- FLD:
-
Frontotemporal lobar degeneration
- Hsp:
-
Heat shock protein
- INDD:
-
Integrated neurodegenerative disease database
- PDT:
-
Photodynamic therapy
- SRSs:
-
Spontaneous recurrent seizures
- TLE:
-
Temporal lobe epilepsy
References
Abramovitch RB (2018) Mycobacterium tuberculosis reporter strains as tools for drug discovery and development. IUBMB Life 70:818–825
Åkerfelt M, Morimoto RI, Sistonen L (2010) Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol 11:545
Amor S, Peferoen LA, Vogel DY, Breur M, van der Valk P, Baker D, van Noort JM (2014) Inflammation in neurodegenerative diseases–an update. Immunology 142:151–166
Benarroch EE (2011) Heat shock proteins multiple neuroprotective functions and implications for neurologic disease. Neurology 76:660–667
Bendl J, Stourac J, Sebestova E, Vavra O, Musil M, Brezovsky J et al (2016) HotSpot Wizard 2.0: automated design of site-specific mutations and smart libraries in protein engineering. Nucleic Acids Res 44:W479–W487
Bie AS, Fernandez-Guerra P, Birkler RI, Nisemblat S, Pelnena D, Lu X, Deignan JL, Lee H, Dorrani N, Corydon TJ, Palmfeldt J (2016) Effects of a mutation in the HSPE1 gene encoding the mitochondrial co-chaperonin HSP10 and its potential association with a neurological and developmental disorder. Front Mol Biosci 3:65
Bross P, Naundrup S, Hansen J, Nielsen MN, Christensen JH, Kruhøffer M, Palmfeldt J, Corydon TJ, Gregersen N, Ang D, Georgopoulos C (2008) The Hsp60-(p. V98I) mutation associated with hereditary spastic paraplegia SPG13 compromises chaperonin function both in vitro and in vivo. J Biol Chem 283(23):15694–15700
Bross P, Magnoni R, SigaardBie A (2012) Molecular chaperone disorders: defective Hsp60 in neurodegeneration. Curr Top Med Chem 12(22):2491–2503
Campanella C, Bucchieri F, Merendino AM, Fucarino A, Burgio G, Corona DF, de Macario EC (2012) The odyssey of Hsp60 from tumor cells to other destinations includes plasma membrane-associated stages and Golgi and exosomal protein-trafficking modalities. PLoS One 7(7):e42008
Cappello F, Marino Gammazza A, Palumbo Piccionello A, Campanella C, Pace A, Conway de Macario E, Macario AJ (2014) Hsp60 chaperonopathies and chaperonotherapy: targets and agents. Expert Opin Ther Targets 18(2):185–208
Cardinale A, Chiesa R, Sierks M (2014) Protein misfolding and neurodegenerative diseases. Int J Biochem Cell Biol 2014:2014
Carlson HA (2002) Protein flexibility and drug design: how to hit a moving target. Curr Opin Chem Biol 6:447–452
Chang CC, Lui CC, Lee CC, Chen SD, Chang WN, Lu CH, Chuang YC (2012) Clinical significance of serological biomarkers and neuropsychological performances in patients with temporal lobe epilepsy. BMC Neurol 12:15
Daina A, Michielin O, Zoete V (2017) SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep 7:42717
de Ruyck J, Brysbaert G, Blossey R, Lensink MF (2016) Molecular docking as a popular tool in drug design, an in silico travel. Adv Appl Bioinforma Chem: AABC 9:1
Gammazza AM, Colangeli R, Orban G, Pierucci M, Di Gennaro G, Bello ML, Muscat R (2015) Hsp60 response in experimental and human temporal lobe epilepsy. Sci Rep 5:9434
Genest O, Hoskins JR, Camberg JL, Doyle SM, Wickner S (2011) Heat shock protein 90 from Escherichia coli collaborates with the DnaK chaperone system in client protein remodeling. Proc Natl Acad Sci 108:8206–8211
Gu B, Daltone KA (2017) Models and detection of spontaneous recurrent seizures in laboratory rodents. Zool Res 38(4):171–179
Hansen JJ, Dürr A, Cournu-Rebeix I, Georgopoulos C, Ang D, Nielsen MN, Davoine CS, Brice A, Fontaine B, Gregersen N, Bross P (2002) Hereditary spastic paraplegia SPG13 is associated with a mutation in the gene encoding the mitochondrial chaperonin Hsp60. Am J Hum Genet 70(5):1328–1332
Hansen J, Svenstrup K, Ang D, Nielsen MN, Christensen JH, Gregersen N, Bross P (2007) A novel mutation in the HSPD1 gene in a patient with hereditary spastic paraplegia. J Neurol 254(7):897–900
Hartl FU (1996) Molecular chaperones in cellular protein folding. Nature 381:571–579
Hartl FU, Hayer-Hartl M (2002) Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295:1852–1858
Hauser AS, Attwood MM, Rask-Andersen M, Schiöth HB, Gloriam DE (2017) Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov 16:829
Itoh H, Komatsuda A, Wakui H, Miura AB, Tashima Y (1999) Mammalian HSP60 is a major target for an immunosuppressant mizoribine. J Biol Chem 274(49):35147–35151
Jorgensen WL (2004) The many roles of computation in drug discovery. Science (80- ) 303:1813–1818
Kato S, Kato M, Hirano A, Takikawa M, Ohama E (2001) The immunohistochemical expression of stress-response protein (srp) 60 in human brain tumours: relationship of srp 60 to the other five srps, proliferating cell nuclear antigen and p53 protein. Histol Histopathol 16(3):809–820
Kim YE, Hipp MS, Bracher A, Hayer-Hartl M, Ulrich Hartl F (2013) Molecular chaperone functions in protein folding and proteostasis. Annu Rev Biochem 82:323–355
Laser H, Mack TG, Wagner D, Coleman MP (2003) Proteasome inhibition arrests neurite outgrowth and causes “dying-back” degeneration in primary culture. J Neurosci Res 74(6):906–916
Leelananda SP, Lindert S (2016) Computational methods in drug discovery. Beilstein J Org Chem 12:2694
Liu XY, Yang JL, Chen LJ, Zhang Y, Yang ML, Wu YY, Wei YQ (2008) Comparative proteomics and correlated signaling network of rat hippocampus in the pilocarpine model of temporal lobe epilepsy. Proteomics 8(3):582–603
M van Noort J, Bugiani M, Amor S (2017) Heat shock proteins: old and novel roles in neurodegenerative diseases in the central nervous system. CNS Neurol Disord-Drug Targets 16:244–256
Macario AJ, de Macario EC (2005) Sick chaperones, cellular stress, and disease. N Engl J Med 353(14):1489–1501
Magen D, Georgopoulos C, Bross P, Ang D, Segev Y, Goldsher D, Heno B (2008) Mitochondrial hsp60 chaperonopathy causes an autosomal-recessive neurodegenerative disorder linked to brain hypomyelination and leukodystrophy. Am J Hum Genet 83(1):30–42
Maiti P, Manna J, Veleri S, Frautschy S (2014) Molecular chaperone dysfunction in neurodegenerative diseases and effects of curcumin. Biomed Res Int 2014:2014
McKeever PE (1999) The brain, spinal cord and meninges. Diagn Surg Pathol 1:408–409
Meng Q, Li BX, Xiao X (2018) Toward developing chemical modulators of Hsp60 as potential therapeutics. Front Mol Biosci 5:35
Mizuno K, Tsujino M, Takada M, Hayashi M, Atsumi K, Asano K, Matsuda T (1974) Studies on bredinin. J Antibiot 27(10):775–782
Nagumo Y, Kakeya H, Yamaguchi J, Uno T, Shoji M, Hayashi Y, Osada H (2004) Structure–activity relationships of epolactaene derivatives: structural requirements for inhibition of Hsp60 chaperone activity. Bioorg Med Chem Lett 14(17):4425–4429
Nagumo Y, Kakeya H, Shoji M, Hayashi Y, Dohmae N, Osada H (2005) Epolactaene binds human Hsp60 Cys442 resulting in the inhibition of chaperone activity. Biochem J 387(3):835–840
Nakamoto H, Amaya Y, Komatsu T, Suzuki T, Dohmae N, Nakamura Y et al (2018) Stimulation of the ATPase activity of Hsp90 by zerumbone modification of its cysteine residues destabilizes its clients and causes cytotoxicity. Biochem J 475:2559–2576. BCJ20180230
Nakamura H, Minegishi H (2013) HSP60 as a drug target. Curr Pharm Des 19(3):441–451
Natalello A et al (2013) Biophysical characterization of two different stable misfolded monomeric polypeptides that are chaperone-amenable substrates. J Mol Biol 425:1158–1171
Neuspiel M (2008) Mitochondrial dynamics, mechanisms and meaning: Investigations into fusion and fission proteins Mitofusin 2 and MAPL (Doctoral dissertation, University of Ottawa (Canada)
Pace A, Barone G, Lauria A, Martorana A, Palumbo Piccionello A, Pierro P, Angileri F (2013) Hsp60, a novel target for antitumor therapy: structure-function features and prospective drugs design. Curr Pharm Des 19(15):2757–2764
Padmadas N, Panda PK, Durairaj S (2018) Binding patterns associated Aß-HSP60 p458 conjugate to HLA-DR-DRB allele of human in Alzheimer’s disease: an in silico approach. Interdiscip Sci Comput Life Sci 10:93–104
Papuć E, Kurys-Denis E, Krupski W, Tatara M, Rejdak K (2015) Can antibodies against glial derived antigens be early biomarkers of hippocampal demyelination and memory loss in Alzheimer’s disease? J Alzheimers Dis 48:115–121
Priya S, Sharma SK, Goloubinoff P (2013) Molecular chaperones as enzymes that catalytically unfold misfolded polypeptides. FEBS Lett 587:1981–1987
Radons J (2017) The ATP-driven Hsp60 machinery: biological and clinical implications. Curr Immunol Rev 13(1):19–43
Rappa F, Unti E, Baiamonte P, Cappello F, Scibetta N (2013) Different immunohistochemical levels of Hsp60 and Hsp70 in a subset of brain tumors and putative role of Hsp60 in neuroepithelial tumorigenesis. Eur J Histochem 57(2):e20
Rodgers AB (2002) Alzheimer’s disease: unraveling the mystery, vol 1. National Institutes of Health
Rugarli EI, Langer T (2012) Mitochondrial quality control: a matter of life and death for neurons. EMBO J 31(6):1336–1349
Samluk L, Chroscicki P, Chacinska A (2018) Mitochondrial protein import stress and signaling. Curr Opin Physiol 3:41–48
Scott DE, Bayly AR, Abell C, Skidmore J (2016) Small molecules, big targets: drug discovery faces the protein–protein interaction challenge. Nat Rev Drug Discov 15:533
Sendrowski K, Sobaniec W (2013) Hippocampus, hippocampal sclerosis and epilepsy. Pharmacol Rep 65(3):555–565
Sliwoski G, Kothiwale S, Meiler J, Lowe EW (2014) Computational methods in drug discovery. Pharmacol Rev 66:334–395
Sonkusare SK, Kaul CL, Ramarao P (2005) Dementia of Alzheimer’s disease and other neurodegenerative disorders—memantine, a new hope. Pharmacol Res 51(1):1–7
Van Liefferinge J, Massie A, Portelli J, Di Giovanni G, Smolders I (2013) Are vesicular neurotransmitter transporters potential treatment targets for temporal lobe epilepsy? Front Cell Neurosci 7:139
Veereshwarayya V, Kumar P, Rosen KM, Mestril R, Querfurth HW (2006) Differential effects of mitochondrial heat shock protein 60 and related molecular chaperones to prevent intracellular β-amyloid-induced inhibition of complex IV and limit apoptosis. J Biol Chem 281:29468–29478
Venegas C, Heneka MT (2017) Danger-associated molecular patterns in Alzheimer’s disease. J Leukoc Biol 101(1):87–98
Weinberg BA, Yu H, Rosen R, Staudt J, Ross MB, Marschke G (2017) Identifying potentially transformative research: a case study of Alzheimer’s disease research. Alzheimers Dement 13(7):860
Wyttenbach A, Carmichael J, Swartz J, Furlong RA, Narain Y, Rankin J, Rubinsztein DC (2000) Effects of heat shock, heat shock protein 40 (HDJ-2), and proteasome inhibition on protein aggregation in cellular models of Huntington’s disease. Proc Natl Acad Sci 97(6):2898–2903
Acknowledgements
The author acknowledges Maharshi Dayanand University, Rohtak for providing infrastructure and lab facility for the compilation of this interesting and informative book chapter.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sharma, B., Smita, M., Khangwal, I., Maheshwari, R., Dangi, A.K. (2019). Heat Shock Protein 60: An Effective Target Candidate in Neurological Diseases Treatment. In: Asea, A., Kaur, P. (eds) Heat Shock Protein 60 in Human Diseases and Disorders. Heat Shock Proteins, vol 18. Springer, Cham. https://doi.org/10.1007/978-3-030-23154-5_15
Download citation
DOI: https://doi.org/10.1007/978-3-030-23154-5_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-23153-8
Online ISBN: 978-3-030-23154-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)