Abstract
The mitochondrial chaperonin Hsp60 (Hspd1) plays important roles in sustaining cellular viability, regulate cellular functions and maintain homeostasis. Mutations in the Hsp60 gene or erratic expression has been frequently observed in wide-ranging human diseases. Targeting Hsp60 to ameliorate the prognosis of mitochondrial dysfunction-related diseases were proposed in the past. Genetically engineered mice provide a compelling tool to investigate the aetiology and pathogenesis of these diseases. Eventually, this will benefit the development of therapeutics towards these physiological complications. Conventional Hsp60 transgenic mice are often neonatally lethal. We’ve generated a unique conditional Hsp60 transgenic (Tg) mouse model to investigate the mitochondrial activities and demonstrated that ubiquitous expression of human Hsp60 protein in mice leads to neonatal death due to septum defect (ASD) and cardiac myopathy. This chapter concisely reviews recent advances regarding manipulating Hsp60 levels in cells and mouse models along with depicts our quest to develop transgenic mice to study Hsp60-related human diseases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cardiac myopathy
- Chaperonopathies
- Conditional transgenic mice
- Heart failure
- Hsp60
- Mitochondrial molecular chaperone
1 Introduction
In all organisms, a distinct group of proteins termed as the heat shock proteins (HSP) are specially synthesized under heat stress and by a diverse range of external impetus including increased temperature (Currie et al. 1988), pressure overload (Katayose et al. 1993), ischemia (Richard et al. 1996), hypoxia (Heads et al. 1995), or changes in chemical environment. Commonly, HSP are molecular chaperones important for physiological and protective roles in cells, as they facilitate crucial activities, such as protein folding, material transport, and cellular signalling. Triggered by stress or protein denaturation, HSP facilitate to preserve the natural metabolic, structural and functional stability of the cell, as a protective feedback responses by preserving in their native forms and refolding denatured proteins (Benjamin and McMillan 1998; Liu et al. 2012; Macario and Conway de Macario 2007). This brisk induction of HSP in response to stress, attributed to the heat shock response (HSR) (Shamovsky and Nudler 2008).
1.1 The Curious Case of Hsp60
In general, HSP have been categorized based on their molecular weights. Mitochondrial chaperonin Hsp60 (Hspd1) together with Hsp10 (Hspe1) are constitutively expressed in normal condition as a folding machine for refolding imported proteins into the mitochondrial matrix (Ciocca and Calderwood 2005). Thus, Hsp60 is crucial in cell survival and to maintain mitochondrial functions, including the TCA cycle, respiration, and synthesis of ATP (Hartl 1996; Horwich et al. 2007). Hsp60 is concurrently induced under stress conditions by various stressors like heat shock, DNA damage, oxidative stress, and the unfolded protein response in mitochondria (Gupta and Knowlton 2002; Habich and Burkart 2007; Ohashi et al. 2000; Wick 2000). Hsp60 induction can result in pro-survival or pro-death consequences depending on the tissue type and stressors.
Upregulation of Hsp60 is an indicator of mitochondrial stress. This is well demonstrated in mitochondrial unfolded protein response (UPRmt), where under stress condition nucleus-encoded mitochondrial chaperones (Hsp60, Hsp10 and mtHsp70) are induced by still poorly defined mitochondria-to-nucleus communication (Juwono and Martinus 2016). UPRmt has been identified in worm, flies, and mouse, but the signalling pathways responsible for sensing the mitochondrial stress and activating nuclear gene transcription are only identified in flies and worms mediating transcriptional activation of Hsp60, including ATFS-1, DVE-1, UBL-5, and chromatin remodelling factor. Other forms of mitochondrial stress including oxidative stress (low concertation of hydrogen peroxide), hyperglycemic condition (100 mM glucose), and respiration stress (sodium azide 50 mM), were shown to result in ROS production, inhibition of mitochondrial dehydrogenase, and the induction of Hsp60 and mtHsp70 (Hall and Martinus 2013; Pellegrino et al. 2013). UPRmt was shown to influence longevity, innate immunity, and diseases affecting the central nervous system (CNS) (Jovaisaite et al. 2014).
In human, Hsp60 gene is situated on chromosome 2 and it shares a bidirectional promoter with Hsp10 gene (Wu et al. 2017). Three major domains of Hsp60 are: the apical, intermediate, and equatorial domains (Sigler et al. 1998). The mechanistic integral biology of Hsp60, regarding substrate folding, has been investigated extensively. However, in the past few years, there has been an upsurge of interest about Hsp60, as roles of mitochondrial, cytosolic, and extracellular Hsp60 have been widely documented in numerous diseases. Although most of the Hsp60 proteins are transported and stayed in the mitochondrial matrix, they also appear in the cytoplasm. The elevated level of extracellular Hsp60, at least in part due to enhanced Hsp60 secretion, have been associated with type 2 diabetes, cancer, cardiovascular, and immunity-related diseases (Cappello et al. 2014; Caruso Bavisotto et al. 2017; Deocaris et al. 2006; Hohfeld and Hartl 1994).
The over-expression of Hsp60 has been reported to be linked with various cancers including colorectal cancer (Hamelin et al. 2011), hepatocellular carcinoma (Abdalla and Haj-Ahmad 2012), gastric cancer (Giaginis et al. 2009; Li et al. 2014), large bowel cancer (Campanella et al. 2015), prostate cancer (Skvortsov et al. 2011), head and neck cancer (Tsai et al. 2009), breast cancer (Desmetz et al. 2008), ovarian cancer (Hjerpe et al. 2013) and cervical cancer (Hwang et al. 2009). There are several reports that Hsp60 promotes cancer cell survival, such as in neuroblastoma cells by binding and inhibiting the intracellular CLU (clusterin) (Chaiwatanasirikul and Sala 2011). In another report, cytosolic Hsp60 interacts and regulates the inhibitor of κB kinase (IκB kinase or IKK) in human cervical cancer HeLa cells, which lead to the survival of cancer cells via nuclear factor-κB (NF-κB) (Chun et al. 2010). Inhibition of Hsp60 leads to caspase-dependent apoptosis and suppress tumour growth (Ghosh et al. 2010). By interacting with β-catenin, over-expression of Hsp60 promotes metastatic phenotypes in cancer cells (Tsai et al. 2009). In a murine model of ovarian cancer, treatment of tumour cells with a proteasome inhibitor, bortezomib, ensues into the upregulation of Hsp60 and Hsp90 on the surface of cancer cells and promotes phagocytosis by dendritic cells (Chang et al. 2012). Moreover, an anti-leukemic agent, azacytidine, has been reported to induce over-expression of Hsp60 in tumour cells (Tian et al. 2013). The pro-apoptotic role of Hsp60 in HeLa and Jurkat cells was also reported two decades ago (Samali et al. 1999; Xanthoudakis et al. 1999). Loss of Hsp60 expression has been documented, in the case of esophageal squamous cell carcinoma (Faried et al. 2004), ovarian cancer (Schneider et al. 1999) and bladder carcinoma (Lebret et al. 2003).
The role of Hsp60 in metabolic diseases has not been explored enough. Increased level of Hsp60 was observed in metabolic diseases, such as type 2 diabetes mellitus patients (Juwono and Martinus 2016; Yuan et al. 2011). Hsp60 has been identified as a mediator of adipose tissue inflammation and circulating Hsp60 levels were found elevated in obese individuals compared to lean controls (Märker et al. 2012). Moreover, it has been reported that obese mice develop an autoimmune response to Hsp60, which partially responsible for metabolic anomalies (Şelli et al. 2017). A recent study showed that elevated Hsp60 secretion as the response to IL-1β increases the phosphorylation of ERK, JNK, and p38 MAPK, and further augment the inflammation primarily via TLR4-p38 MAPK axis (Swaroop et al. 2016). Endurance exercise training increases Hsp60 expression in skeletal muscle, particularly in the type I muscle fibres and in the blood (Barone et al. 2016). This is the first report demonstrating differential responses to exercise, in various muscle types, by varying Hsp60 induction. Also, exercise increases Hsp60 expression level in the subcutaneous adipose tissue of diabetic and obese individuals, concomitantly alleviates inflammation (Khadir et al. 2018).
Interestingly, with age and in the case of metabolic diseases, Hsp60 expression has been reduced in the heart. According to the study, caloric restriction increases lifespan, improve cardiovascular activities and restore ageing-related abatement of Hsp60 expression in the heart (Colotti et al. 2005). Hsp60 is involved in protecting cardiac myopathy by preserving mitochondrial function, ATP synthesis and by suppressing cardiac myocyte apoptosis (Rizzo et al. 2011). In vitro studies have shown Hsp60 over-expression can result in cell survival or in cell death, depending on the cell type and models of the study. In neonatal rat cardiomyocytes (CMC), cells infected with an adenoviral construct by concomitantly overexpressing Hsp60 and Hsp10 were reported to be protected against simulated ischemia, whereas, cells infected with adenoviral constructs by overexpression of only Hsp60 or Hsp10 was less effective to ischemic injury (Lau et al. 1997). A follow-up study showed that combined or individual overexpression of Hsp60 and Hsp10 protect myocytes against apoptosis, preserve mitochondrial integrity and capability for ATP generation after simulated ischaemia and reperfusion (Lin et al. 2001). In the case of heart failure, cardiomyocytes secrete Hsp60 and its presence in the serum related to the severity of heart failure and cardiovascular risk (Bonanad et al. 2013; Nahas et al. 2014). Hsp60 is released via exosomes by adult cardiomyocytes and ectopic trafficking of Hsp60 to the cell surface may lead to the loss of myocyte and heart failure progression (Gupta and Knowlton 2007; Lin et al. 2007). Moreover, in cardiac myocytes, cytosolic Hsp60 interacts with apoptotic molecules Bax and Bak (Gupta and Knowlton 2005; Kirchhoff et al. 2002). Also, extracellular Hsp60 (exHsp60) binds to cardiac myocytes and involves in apoptosis (Kim et al. 2009). Even though, the involvement of Hsp60 in apoptosis of CMC was demonstrated in various in-vitro studies, its role and underlying molecular mechanisms for resulting in mitochondrial dysfunction and apoptosis in CMC remain elusive in vivo. Thus, genetically engineered mice models are needed to examine the underlying mechanisms of Hsp60 on the pathogenesis of cardiovascular risk and its possibilities in prognosis. Amid the inconsistency and complexity of in-vitro study reports, the role of Hsp60 as a potential biomarker and therapeutic target for the diagnosis and prognosis will remain clouded without the development of in vivo Hsp60 expressing transgenic mouse models.
1.2 Transgenic Hsp60 Mouse Models: A Brief History and New Possibilities
Though HSP can be induced by a variety of stimulants, yet in cell culture studies HSR is primarily induced by increasing temperature. Protocols used to induce the synthesis of HSP by exposing the cells to 40–45 °C heating for 15–20 min. Apparently, this temperature is standard in terms of thermotolerance. However, for cells, this temperature is extreme and may lead to disturbance to the cell’s cytoskeleton and cytotoxicity. The heat-associated impairment includes the disintegration of the organization of keratin filaments (Shyy et al. 1989), actin filaments (Glass et al. 1985; van Bergen en Henegouwen and Linnemans 1987) and other undesired alterations in cellular metabolism. Because of the significance of Hsp60 in numerous diseases, transgenic animal models with inducible and tissue-specific Hsp60 expression will be beneficial to understand the pathogenesis and prognosis of these diseases. Moreover, by the development and introduction of genetically engineered animals which overexpress Hsp60 at any desired level, most of the problems related to thermal/stress induction of Hsp60 can be avoided. The significant benefit of using transgenic mouse models is, it’s achievable to induce and intensify the level of Hsp60 in a tissue-specific manner, without the introduction of other metabolic alterations. The genetically engineered Hsp60 mouse models in human diseases are summarized in Table 14.1.
The story begins with the significance of Hsp60 in autoimmune diabetes. Hsp60 and Hsp70 of both prokaryotic and eukaryotic origins were identified as antigens of human diseases involving innate immunity (Dieude et al. 2004; Quintana and Cohen 2011; Quintana et al. 2004; Tanaka et al. 1999; van Eden et al. 2005; Zugel and Kaufmann 1999). By using nonobese diabetic (NOD) mice as a spontaneous mouse model of type I diabetes, murine Hsp60 transgene induced by the major histocompatibility complex class II-Eα (HIIEα) promoter was generated in NOD strain. The researchers achieved to express Hsp60 distinctly in the thymus and bone marrow and also have shown a significantly restrained propensity to autoimmunity induced diabetes mellitus in this nonobese diabetic (NOD) HIIEα-HSP60 Tg mice (Birk et al. 1996).
The existence of cytosolic Hsp60 involved in cellular signalling has been shown in certain cell types, such as cardiac myocytes and hepatocytes (Gupta and Knowlton 2002; Lai et al. 2007; Park et al. 2003). The researchers have expressed human Hsp60, lacking mitochondrial targeting sequence (MTS; amino acids 1–26 according to human sequence) into CAGGS transgenic vector in C57BL/6j mice. This transgenic mouse study, expressing truncated Hsp60 instead of the complete Hsp60, demonstrated that the resultant cytosolic Hsp60 Tg mice were impervious to hepatic stress with increased cell survival (Chun et al. 2010). As this study reported, Hsp60 directly interacts and influence the activation of the inhibitor of κB kinase (IκB kinase or IKK) and regulate mitochondrial-derived reactive oxygen species (ROS) via nuclear factor-κB (NF-κB) target gene expression, and this mechanism consequently leads to cell survival. A previous study also showed that Hsp60 interacts with the IKK (Cappello et al. 2008). As mitochondrial ROS has been related with human diseases like cancer, degenerative diseases, therefore, further research on the pro-survival role of cytosolic Hsp60 which fails to enter mitochondria, but regulates the ROS production through cytosolic pathways can shed a light on new therapeutics for these maladies (Coelho and Faria 2012).
In human, Hsp60 is encoded by Hspd1 gene located within Chromosome 2. Its dysfunction is associated with some hereditary diseases such as autosomal dominantly inherited hereditary spastic paraplegia 13 (SPG13) and autosomal recessively inherited hypomyelinating leukodystrophy termed MitCHAP-60, caused by mutations in the Hspd1 gene at equatorial domain of Hsp60 protein (Bross et al. 2008; Hansen et al. 2007; Hansen et al. 2003; Hansen et al. 2002; Magen et al. 2008), with a functional consequence affecting only the central nervous system. Hsp60 knockout (KO) mice were not successfully produced until recently. It was shown that Hsp60 homozygous KO mice which lack both functional Hspd1 alleles, are lethal at early embryonical stage (at 7.5 dpc); by contrast, the heterozygous Hspd1+/− mouse, in which Hsp60 expression had been reduced by 50% in most organs was postnatally viable up to a few weeks (Christensen et al. 2010). The Hspd1+/− mice developed a late-onset, gradual dysfunction in motor functions due to Hspd1 haploinsufficiency ensues in the hereditary spastic paraplegia-like features in mice, suggests a role for Hsp60 in late-onset motor neuron disorder (Magnoni et al. 2013). This heterozygous Hspd1+/− mice in combination with tissue-specific cre mouse have the possibility to serve as valuable mouse models and shed light on mechanistic details for diseases related to mitochondrial functional deficiencies and neurodegenerative disorders such as, Parkinson’s disease, Alzheimer’s disease, Huntington’s disease, and multiple sclerosis (Dutta et al. 2006; Kwong et al. 2006). Hspd1+/− mice present swollen mitochondria and deficient complex III activity in spinal cord and brain cortex with an increase of protein carbonylation (oxidation of protein side chains), indicative of increased ROS generation in these tissues. In the affected tissue, the decreased level of complex III subunit ubiquinone cytochrome c core protein1 (Uqcrc1), and the increase of ROS levels may be due to increased turnover of matrix superoxide dismutase (SOD2) as a result of impaired protein folding (Magnoni et al. 2014).
A prevalent characteristic of human obesity is leptin resistance and it’s linked with insulin resistance and mitochondrial dysfunction (Myers et al. 2008). A recent study reported obesity is linked to mitochondrial dysfunction in the hypothalamus due to the reduction of Hsp60 and demonstrated Hsp60 as a leptin-induced mitochondrial chaperone. This study investigated a new perspective of Hsp60 in obesity and type 2 diabetes by documenting decreased Hsp60 in the brain of diabetic mice and humans. Mitochondrial dysfunction and lowered Hsp60 expression lead to weakened hypothalamic insulin signalling in mice. Heterozygous obliteration of Hsp60 in the hypothalamus results in mitochondrial dysfunction, elevated ROS, and insulin resistance. Hsp60 downregulation in the hypothalamus was also achieved by bilaterally injecting lentiviral vector enclosing shRNA against Hsp60 into the ventral hypothalamus, and resulted in insulin resistance in the mice. Thus, by using knockdown mouse model, Hsp60 has been found as a novel mediator correlates leptin/insulin crosstalk in the brain (Kleinridders et al. 2013).
Control of intestinal epithelial stemness is important for tissue homeostasis and disturbances in epithelial function can lead to gastrointestinal tract diseases (Sartor 2006). To understand how Hsp60 regulates the epithelial cell homeostasis in the intestine, the researchers have established the epithelial-specific knockout mice. In intestinal epithelial cell (IEC)-specific mouse model, intestinal epithelial-specific Hsp60 deletion resulted in defected UPRmt and leads to mitochondrial dysfunction, impedes epithelial stem cell homeostasis (Berger et al. 2016). This finding may suggest that Hsp60 induction can potentially be beneficial by aggravating or simulating local UPRmt in targeted lesions.
A newly reported study confirmed the pathological role of Hsp60 in osteoarthritis. Transgenic mice that overexpress human Hsp60 driven by phosphoglycerate kinase promoter were generated, which had higher chondrocyte proliferation along with thicker articular cartilage compared to wild-type mice. These findings suggest a therapeutic potential of targeting Hsp60 for osteoarthritis (Ko et al. 2016).
1.3 Conditional Hsp60 Transgenic Mouse Models to Study Cardiovascular Disorders
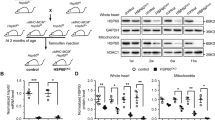
Protective roles of Hsp60, together with Hsp10, in the cardiovascular system by maintaining mitochondrial function and protecting from ischemia/reperfusion injury has been shown previously through a mechanism involving collaborative folding by Hsp60 and Hsp10 (Lau et al. 1997; Lin et al. 2001, 2004). Reduction in Hsp60 expression and subsequent decline of insulin-like growth factor-1 receptor (IGF-1R) signalling in cardiac muscle cells have been implicated in the development of diabetic cardiomyopathy (Shan et al. 2003). We’ve generated a unique Hsp60 Tg mouse model (G-lox-HSP60) in FVB strain driven by a ubiquitous CMV early enhancer/chicken β-actin promoter (CAGGS) to investigate the mitochondrial function and generation of ROS in tissues isolated from our Hsp60 Tg mouse (Chen et al. 2015). This Hsp60 Tg vector allows tissue-specific induction of human Hsp60 in Tg mice. We reported that ubiquitous expression of human Hsp60 protein in mice results in neonatal death due to septum defect (ASD) and cardiac myopathy (Fig. 14.1). This conditional Hsp60 transgenic mice model surmounted the early lethality of the conventional transgenic method.
(a) G-Lox-HSP60 and EGFP-Cre Tg vectors. The CAGGS promoter was used to drive both Tg vectors. After the LoxP sites were rejoined using the Cre DNA recombinase, the Hsp60 transcript was expressed. (b) Analysis of possible littermate genotypes using PCR on tailDNA. PCR amplification using the primer pair complementary to human Hsp60 (top row), amplification of EGFP-Cre (middle row), and the abridged sequence in the recombined vector from CAGGS promoter to human Hsp60 (bottom row). (c) Western blotting for Hsp60 and EGFP proteins in samples of B. (d) Pictures of neonatal Tg litters. The white arrow indicates cyanosis and abdominal bleeding in H+/C+ neonates; middle, fluorescent images of the same mice; right, the lungs and heart of neonatal mice. Scale bar = 3 mm. (e) Atrial septal defect in H+/C+ neonatal mice. (f) Transmission electron microscopy showing ultrastructure of myofibril defect in H+/C+ neonatal heart. This research was originally published in Biomed Res Int. (Chen et al. 2015)
In our another project, we’ve generated conditional Hsp60 transgenic mice for heart-specific Hsp60 expression involving the G-Lox-HSP60 Tg vector and Myh6-creERT2 Tg vector driven by the Myh6 promoter. A strong induction of human Hsp60 expression in the heart of double-Tg mice by 2-week tamoxifen feeding, was validated by western blotting. In the double Tg mice, we’ve observed the rapid induction of cardiac hypertrophy and dilated heart failure within 6–8 weeks after the tamoxifen treatment, which supports the hypothesis that perturbation of the Hsp60 level in cardiac myocytes can result in mitochondrial and calcium dysregulation, and in certain circumstances, precipitate cardiomyopathy. By using Langendorff isolated heart perfusion model and simulated ischemia/reperfusion protocol, the left ventricular developed pressure (LVDP) of hearts isolated from double-Tg mice were recorded. Strikingly, 4 weeks after Hsp60 induction, both LVDP at baseline and after reperfusion were significantly higher than uninduced double Tg mice or wild-type mice. Baseline LVDP as well as after reperfusion LVDP have been diminished at 6–7 weeks since the induction of Hsp60 (Fig. 14.2). The results suggest an opportunity of employing Hsp60 induction for treating diseases involving a reduced Hsp60 level, such as in brains and muscles during ageing and diabetes. This hypothesis has been further supported by a recent report showing benefit from enhanced Hsp60 expression during endurance training (Barone et al. 2016). Optimal Hsp60 induction in cardiac myocytes can be beneficial for cardiac function and ameliorate cardiac ischemic injuries. Whereas, prolonged Hsp60 induction may result in pathological consequences in the heart such as pathological remodelling and hypertrophy. Thus, this will be a potential conditional transgenic mice model to study cardiac myopathy.
(a) G-Lox-HSP60 and Myh6-creERT2 Tg vectors. (b) Western blotting for Hsp60 and GFP proteins in hearts at 4th week or 8th week after the period of tamoxifen feeding. (c) Bright-field images of hearts from double-Tg mice, which haven’t received tamoxifen and sacrificed at 4 or 7 weeks after receiving tamoxifen. (d) Heart weight of double-Tg mice received control chow and mice sacrificed at 0–2, 4, 6–8 weeks after receiving tamoxifen. (e) Left ventricular developed pressure (LVDP) in wild-type and double-Tg mice hearts, that were uninduced or induced for 0–2, 4, or 6–7 weeks and subjected to Langendorff preparation. Simulated ischemia/reperfusion was induced by stopping the flow for 45 mins followed by reperfusion. Both LVDP at baseline and after reperfusion in hearts of double Tg mice at 4 weeks after Hsp60 induction, were significantly higher than other groups. Baseline LVDP as well as after reperfusion LVDP have been diminished at 6–7 weeks after the induction of Hsp60 (∗p < 0.05)
2 Conclusions
Lately, the involvement of Hsp60 with a wide array of human diseases has gained increasing interests and focus on Hsp60. Though, Hsp60 has been explored extensively for more than three decades from the molecular, genetic, or protein aspects, its involvement in complex biological pathways are not yet completely explored. In vitro studies on Hsp60 have come a long way to discover the enormous amount of information on fundamental mechanisms and biological roles. As we’ve seen in this chapter that there are many contrasting results regarding the impact of Hsp60 in cells, in vivo, or in diseases. With the advances of molecular biology and cell analysis techniques such as cellular imaging, cryo EM, and due to the rapid developments in metabolic research, the unsolved puzzles of mitochondrial Hsp60 can be revisited, particularly about the molecular mechanisms of Hsp60 in the context of myopathy and protection against noxious stress. Moreover, the presence of extramitochondrial Hsp60 adds a new dimension to the research of Hsp60. The development of genetically engineered mouse models will enable to decode this highly complex mitochondrial chaperonin in in vivo settings. Knowledge to be gained will be beneficial to the better diagnosis and prognosis of diseases such as Hsp60 chaperonopathies, cancer, cardiovascular disorders, type 2 diabetes mellitus and other metabolic diseases.
Abbreviations
- ASD:
-
Atrial septum defect
- ATP:
-
Adenosine triphosphate
- CAGGS:
-
CMV enhancer/chicken β-actin
- CLU:
-
Clusterin
- CMC:
-
Cardiomyocytes
- CNS:
-
Central nervous system
- H&E:
-
Hematoxylin and eosin
- HIIEα:
-
Histocompatibility complex class II-Eα
- HSP:
-
Heat shock proteins
- HSR:
-
Heat shock response
- IKK:
-
Inhibitor of κB kinase
- KO:
-
Knockout
- LVDP:
-
Left ventricular developed pressure
- NF-κB:
-
Nuclear factor-κB
- NOD:
-
Nonobese diabetic
- ROS:
-
Reactive oxygen species
- SPG13:
-
Spastic paraplegia 13
- Tg:
-
Transgenic
- UPRmt:
-
Mitochondrial unfolded protein response
References
Abdalla MA, Haj-Ahmad Y (2012) Promising urinary protein biomarkers for the early detection of hepatocellular carcinoma among high-risk hepatitis C virus Egyptian patients. J Cancer 3:390–403
Barone R, Macaluso F, Sangiorgi C et al (2016) Skeletal muscle heat shock protein 60 increases after endurance training and induces peroxisome proliferator-activated receptor gamma coactivator 1 alpha1 expression. Sci Rep 6:19781
Benjamin IJ, McMillan DR (1998) Stress (heat shock) proteins: molecular chaperones in cardiovascular biology and disease. Circ Res 83:117–132
Berger E, Rath E, Yuan D et al (2016) Mitochondrial function controls intestinal epithelial stemness and proliferation. Nat Commun 7:13171
Birk OS, Douek DC, Elias D et al (1996) A role of Hsp60 in autoimmune diabetes: analysis in a transgenic model. Proc Natl Acad Sci 93:1032
Bonanad C, Nunez J, Sanchis J et al (2013) Serum heat shock protein 60 in acute heart failure: a new biomarker? Congest Heart Fail 19:6–10
Bross P, Naundrup S, Hansen J et al (2008) The Hsp60-(p.V98I) mutation associated with hereditary spastic paraplegia SPG13 compromises chaperonin function both in vitro and in vivo. J Biol Chem 283:15694–15700
Campanella C, Rappa F, Sciume C et al (2015) Heat shock protein 60 levels in tissue and circulating exosomes in human large bowel cancer before and after ablative surgery. Cancer 121:3230–3239
Cappello F, Conway de Macario E, Marasa L, Zummo G, Macario AJ (2008) Hsp60 expression, new locations, functions and perspectives for cancer diagnosis and therapy. Cancer Biol Ther 7:801–809
Cappello F, Marino Gammazza A, Palumbo Piccionello A et al (2014) Hsp60 chaperonopathies and chaperonotherapy: targets and agents. Expert Opin Ther Targets 18:185–208
Caruso Bavisotto C, Cappello F, Macario AJL et al (2017) Exosomal HSP60: a potentially useful biomarker for diagnosis, assessing prognosis, and monitoring response to treatment. Expert Rev Mol Diagn 17:815–822
Chaiwatanasirikul KA, Sala A (2011) The tumour-suppressive function of CLU is explained by its localisation and interaction with HSP60. Cell Death Dis 2:e219
Chang C-L, Hsu Y-T, Wu C-C et al (2012) Immune mechanism of the antitumor effects generated by bortezomib. J Immunol 189:3209–3220
Chen TH, Liu SW, Chen MR et al (2015) Neonatal death and heart failure in mouse with transgenic HSP60 expression. Biomed Res Int 2015:539805
Christensen JH, Nielsen MN, Hansen J et al (2010) Inactivation of the hereditary spastic paraplegia-associated Hspd1 gene encoding the Hsp60 chaperone results in early embryonic lethality in mice. Cell Stress Chaperones 15:851–863
Chun JN, Choi B, Lee KW et al (2010) Cytosolic Hsp60 is involved in the NF-kappaB-dependent survival of cancer cells via IKK regulation. PLoS One 5:e9422
Ciocca DR, Calderwood SK (2005) Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones 10:86–103
Coelho V, Faria AM (2012) HSP60: issues and insights on its therapeutic use as an immunoregulatory agent. Front Immunol 2:97
Colotti C, Cavallini G, Vitale RL et al (2005) Effects of aging and anti-aging caloric restrictions on carbonyl and heat shock protein levels and expression. Biogerontology 6:397–406
Currie RW, Karmazyn M, Kloc M, Mailer K (1988) Heat-shock response is associated with enhanced postischemic ventricular recovery. Circ Res 63:543–549
Deocaris CC, Kaul SC, Wadhwa R (2006) On the brotherhood of the mitochondrial chaperones mortalin and heat shock protein 60. Cell Stress Chaperones 11:116–128
Desmetz C, Bibeau F, Boissiere F et al (2008) Proteomics-based identification of HSP60 as a tumor-associated antigen in early stage breast cancer and ductal carcinoma in situ. J Proteome Res 7:3830–3837
Dieude M, Senecal JL, Raymond Y (2004) Induction of endothelial cell apoptosis by heat-shock protein 60-reactive antibodies from anti-endothelial cell autoantibody-positive systemic lupus erythematosus patients. Arthritis Rheum 50:3221–3231
Dutta R, McDonough J, Yin X et al (2006) Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann Neurol 59:478–489
Faried A, Sohda M, Nakajima M, Miyazaki T, Kato H, Kuwano H (2004) Expression of heat-shock protein Hsp60 correlated with the apoptotic index and patient prognosis in human oesophageal squamous cell carcinoma. Eur J Cancer 40:2804–2811
Ghosh JC, Siegelin MD, Dohi T, Altieri DC (2010) Heat shock protein 60 regulation of the mitochondrial permeability transition pore in tumor cells. Cancer Res 70:8988–8993
Giaginis C, Daskalopoulou SS, Vgenopoulou S, Sfiniadakis I, Kouraklis G, Theocharis SE (2009) Heat shock Protein-27, −60 and −90 expression in gastric cancer: association with clinicopathological variables and patient survival. BMC Gastroenterol 9:14
Glass JR, DeWitt RG, Cress AE (1985) Rapid loss of stress fibers in Chinese hamster ovary cells after hyperthermia. Cancer Res 45:258–262
Gupta S, Knowlton AA (2002) Cytosolic heat shock protein 60, hypoxia, and apoptosis. Circulation 106:2727–2733
Gupta S, Knowlton AA (2005) HSP60, Bax, apoptosis and the heart. J Cell Mol Med 9:51–58
Gupta S, Knowlton AA (2007) HSP60 trafficking in adult cardiac myocytes: role of the exosomal pathway. Am J Phys Heart Circ Phys 292:H3052–H3056
Habich C, Burkart V (2007) Heat shock protein 60: regulatory role on innate immune cells. Cell Mol Life Sci 64:742–751
Hall L, Martinus RD (2013) Hyperglycaemia and oxidative stress upregulate HSP60 & HSP70 expression in HeLa cells. Springerplus 2:431
Hamelin C, Cornut E, Poirier F et al (2011) Identification and verification of heat shock protein 60 as a potential serum marker for colorectal cancer. FEBS J 278:4845–4859
Hansen JJ, Durr A, Cournu-Rebeix I et al (2002) Hereditary spastic paraplegia SPG13 is associated with a mutation in the gene encoding the mitochondrial chaperonin Hsp60. Am J Hum Genet 70:1328–1332
Hansen JJ, Bross P, Westergaard M et al (2003) Genomic structure of the human mitochondrial chaperonin genes: HSP60 and HSP10 are localised head to head on chromosome 2 separated by a bidirectional promoter. Hum Genet 112:71–77
Hansen J, Svenstrup K, Ang D et al (2007) A novel mutation in the HSPD1 gene in a patient with hereditary spastic paraplegia. J Neurol 254:897–900
Hartl FU (1996) Molecular chaperones in cellular protein folding. Nature 381:571–579
Heads RJ, Yellon DM, Latchman DS (1995) Differential cytoprotection against heat stress or hypoxia following expression of specific stress protein genes in myogenic cells. J Mol Cell Cardiol 27:1669–1678
Hjerpe E, Egyhazi S, Carlson J et al (2013) HSP60 predicts survival in advanced serous ovarian cancer. Int J Gynecol Cancer 23:448–455
Hohfeld J, Hartl FU (1994) Role of the chaperonin cofactor Hsp10 in protein folding and sorting in yeast mitochondria. J Cell Biol 126:305–315
Horwich AL, Fenton WA, Chapman E, Farr GW (2007) Two families of chaperonin: physiology and mechanism. Annu Rev Cell Dev Biol 23:115–145
Hwang YJ, Lee SP, Kim SY et al (2009) Expression of heat shock protein 60 kDa is upregulated in cervical cancer. Yonsei Med J 50:399–406
Jovaisaite V, Mouchiroud L, Auwerx J (2014) The mitochondrial unfolded protein response, a conserved stress response pathway with implications in health and disease. J Exp Biol 217:137–143
Juwono J, Martinus RD (2016) Does Hsp60 provide a link between mitochondrial stress and inflammation in diabetes mellitus? J Diabetes Res 2016:6
Katayose D, Isoyama S, Fujita H, Shibahara S (1993) Separate regulation of heme oxygenase and heat shock protein 70 mRNA expression in the rat heart by hemodynamic stress. Biochem Biophys Res Commun 191:587–594
Khadir A, Kavalakatt S, Cherian P et al (2018) Physical exercise enhanced heat shock protein 60 expression and attenuated inflammation in the adipose tissue of human diabetic obese. Front Endocrinol (Lausanne) 9:16
Kim SC, Stice JP, Chen L et al (2009) Extracellular heat shock protein 60, cardiac myocytes, and apoptosis. Circ Res 105:1186–1195
Kirchhoff SR, Gupta S, Knowlton AA (2002) Cytosolic heat shock protein 60, apoptosis, and myocardial injury. Circulation 105:2899–2904
Kleinridders A, Lauritzen HP, Ussar S et al (2013) Leptin regulation of Hsp60 impacts hypothalamic insulin signaling. J Clin Invest 123:4667–4680
Ko JY, Sun YC, Li WC, Wang FS (2016) Chaperonin 60 regulation of SOX9 ubiquitination mitigates the development of knee osteoarthritis. J Mol Med (Berl) 94:755–769
Kwong JQ, Beal MF, Manfredi G (2006) The role of mitochondria in inherited neurodegenerative diseases. J Neurochem 97:1659–1675
Lai HC, Liu TJ, Ting CT et al (2007) Regulation of IGF-I receptor signaling in diabetic cardiac muscle: dysregulation of cytosolic and mitochondria HSP60. Am J Phys Endocrinol Metab 292:E292–E297
Lau S, Patnaik N, Sayen MR, Mestril R (1997) Simultaneous overexpression of two stress proteins in rat cardiomyocytes and myogenic cells confers protection against ischemia-induced injury. Circulation 96:2287–2294
Lebret T, Watson RW, Molinie V et al (2003) Heat shock proteins HSP27, HSP60, HSP70, and HSP90: expression in bladder carcinoma. Cancer 98:970–977
Li X-s, Xu Q, Fu X-y, Luo W-s (2014) Heat shock protein 60 overexpression is associated with the progression and prognosis in gastric cancer. PLoS One 9:e107507
Lin KM, Lin B, Lian IY, Mestril R, Scheffler IE, Dillmann WH (2001) Combined and individual mitochondrial HSP60 and HSP10 expression in cardiac myocytes protects mitochondrial function and prevents apoptotic cell deaths induced by simulated ischemia-reoxygenation. Circulation 103:1787–1792
Lin KM, Hollander JM, Kao VY, Lin B, Macpherson L, Dillmann WH (2004) Myocyte protection by 10 kD heat shock protein (Hsp10) involves the mobile loop and attenuation of the Ras GTP-ase pathway. FASEB J 18:1004–1006
Lin L, Kim SC, Wang Y et al (2007) HSP60 in heart failure: abnormal distribution and role in cardiac myocyte apoptosis. Am J Phys Heart Circ Phys 293:H2238–H2247
Liu T, Daniels CK, Cao S (2012) Comprehensive review on the HSC70 functions, interactions with related molecules and involvement in clinical diseases and therapeutic potential. Pharmacol Ther 136:354–374
Macario AJ, Conway de Macario E (2007) Molecular chaperones: multiple functions, pathologies, and potential applications. Front Biosci 12:2588–2600
Magen D, Georgopoulos C, Bross P et al (2008) Mitochondrial hsp60 chaperonopathy causes an autosomal-recessive neurodegenerative disorder linked to brain hypomyelination and leukodystrophy. Am J Hum Genet 83:30–42
Magnoni R, Palmfeldt J, Christensen JH et al (2013) Late onset motoneuron disorder caused by mitochondrial Hsp60 chaperone deficiency in mice. Neurobiol Dis 54:12–23
Magnoni R, Palmfeldt J, Hansen J, Christensen JH, Corydon TJ, Bross P (2014) The Hsp60 folding machinery is crucial for manganese superoxide dismutase folding and function. Free Radic Res 48:168–179
Märker T, Sell H, Zilleßen P et al (2012) Heat shock protein 60 as a mediator of adipose tissue inflammation and insulin resistance. Diabetes 61:615–625
Myers MG, Cowley MA, Munzberg H (2008) Mechanisms of leptin action and leptin resistance. Annu Rev Physiol 70:537–556
Nahas EA, Nahas-Neto J, Orsatti CL et al (2014) The 60- and 70-kDa heat-shock proteins and their correlation with cardiovascular risk factors in postmenopausal women with metabolic syndrome. Cell Stress Chaperones 19:559–568
Ohashi K, Burkart V, Flohe S, Kolb H (2000) Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol 164:558–561
Park SG, Lee SM, Jung G (2003) Antisense oligodeoxynucleotides targeted against molecular chaperonin Hsp60 block human hepatitis B virus replication. J Biol Chem 278:39851–39857
Pellegrino MW, Nargund AM, Haynes CM (2013) Signaling the mitochondrial unfolded protein response. Biochim Biophys Acta 1833:410–416
Quintana FJ, Cohen IR (2011) The HSP60 immune system network. Trends Immunol 32:89–95
Quintana FJ, Hagedorn PH, Elizur G, Merbl Y, Domany E, Cohen IR (2004) Functional immunomics: microarray analysis of IgG autoantibody repertoires predicts the future response of mice to induced diabetes. Proc Natl Acad Sci U S A 101(Suppl 2):14615–14621
Richard V, Kaeffer N, Thuillez C (1996) Delayed protection of the ischemic heart – from pathophysiology to therapeutic applications. Fundam Clin Pharmacol 10:409–415
Rizzo M, Macario AJ, de Macario EC et al (2011) Heat shock protein-60 and risk for cardiovascular disease. Curr Pharm Des 17:3662–3668
Samali A, Cai J, Zhivotovsky B, Jones DP, Orrenius S (1999) Presence of a pre-apoptotic complex of pro-caspase-3, Hsp60 and Hsp10 in the mitochondrial fraction of jurkat cells. EMBO J 18:2040–2048
Sartor RB (2006) Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol 3:390–407
Schneider J, Jimenez E, Marenbach K, Romero H, Marx D, Meden H (1999) Immunohistochemical detection of HSP60-expression in human ovarian cancer. Correlation with survival in a series of 247 patients. Anticancer Res 19:2141–2146
Şelli ME, Wick G, Wraith DC, Newby AC (2017) Autoimmunity to HSP60 during diet induced obesity in mice. Int J Obes (2005) 41:348–351
Shamovsky I, Nudler E (2008) New insights into the mechanism of heat shock response activation. Cell Mol Life Sci 65:855–861
Shan YX, Yang TL, Mestril R, Wang PH (2003) Hsp10 and Hsp60 suppress ubiquitination of insulin-like growth factor-1 receptor and augment insulin-like growth factor-1 receptor signaling in cardiac muscle: implications on decreased myocardial protection in diabetic cardiomyopathy. J Biol Chem 278:45492–45498
Shyy TT, Asch BB, Asch HL (1989) Concurrent collapse of keratin filaments, aggregation of organelles, and inhibition of protein synthesis during the heat shock response in mammary epithelial cells. J Cell Biol 108:997–1008
Sigler PB, Xu Z, Rye HS, Burston SG, Fenton WA, Horwich AL (1998) Structure and function in GroEL-mediated protein folding. Annu Rev Biochem 67:581–608
Skvortsov S, Schafer G, Stasyk T et al (2011) Proteomics profiling of microdissected low- and high-grade prostate tumors identifies Lamin A as a discriminatory biomarker. J Proteome Res 10:259–268
Swaroop S, Sengupta N, Suryawanshi AR, Adlakha YK, Basu A (2016) HSP60 plays a regulatory role in IL-1β-induced microglial inflammation via TLR4-p38 MAPK axis. J Neuroinflammation 13:27
Tanaka T, Yamakawa N, Koike N, Suzuki J, Mizuno F, Usui M (1999) Behcet’s disease and antibody titers to various heat-shock protein 60s. Ocul Immunol Inflamm 7:69–74
Tian E, Tang H, Xu R, Liu C, Deng H, Wang Q (2013) Azacytidine induces necrosis of multiple myeloma cells through oxidative stress. Proteome Sci 11:24
Tsai YP, Yang MH, Huang CH et al (2009) Interaction between HSP60 and beta-catenin promotes metastasis. Carcinogenesis 30:1049–1057
van Bergen en Henegouwen PM, Linnemans AM (1987) Heat shock gene expression and cytoskeletal alterations in mouse neuroblastoma cells. Exp Cell Res 171:367–375
van Eden W, van der Zee R, Prakken B (2005) Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat Rev Immunol 5:318
Wick G (2000) Atherosclerosis – an autoimmune disease due to an immune reaction against heat-shock protein 60. Herz 25:87–90
Wu J, Liu T, Rios Z, Mei Q, Lin X, Cao S (2017) Heat shock proteins and cancer. Trends Pharmacol Sci 38:226–256
Xanthoudakis S, Roy S, Rasper D et al (1999) Hsp60 accelerates the maturation of pro-caspase-3 by upstream activator proteases during apoptosis. EMBO J 18:2049–2056
Yuan J, Dunn P, Martinus RD (2011) Detection of Hsp60 in saliva and serum from type 2 diabetic and non-diabetic control subjects. Cell Stress Chaperones 16:689–693
Zugel U, Kaufmann SH (1999) Role of heat shock proteins in protection from and pathogenesis of infectious diseases. Clin Microbiol Rev 12:19–39
Acknowledgements
The research works were supported by intramural grants from the National Health Research Institutes of Taiwan [BNPP02-014]. We would like to thank Dr. Vivia Kao of Chia Nan University of Pharmacy and Science, Tainan, Taiwan for generating conditional constructs and Dr. Chun-Hong Chen of NHRI for valuable discussions.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Chen, TH. et al. (2019). Cardiac Myopathy in Conditional Hsp60 Transgenic Mice. In: Asea, A., Kaur, P. (eds) Heat Shock Protein 60 in Human Diseases and Disorders. Heat Shock Proteins, vol 18. Springer, Cham. https://doi.org/10.1007/978-3-030-23154-5_14
Download citation
DOI: https://doi.org/10.1007/978-3-030-23154-5_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-23153-8
Online ISBN: 978-3-030-23154-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)