Abstract
Autoantibodies against human heat shock protein 60 (Hsp60) have been implicated in the pathogenesis of autoimmune and inflammatory rheumatic diseases (AIRDs), including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), systemic sclerosis, Sjogren’s syndrome and various idiopathic vasculitides. Anti-human Hsp60 autoantibodies can be detected in the sera of patients with AIRDs in various frequencies and levels; therefore, their diagnostic, clinical and pathogenic importance remains enigmatic. Regarding their clinical significance, several studies have implicated them in AIRDs with vascular manifestations, including SLE and primary vasculitides. For other diseases, however, clinical associations have not been comprehensively investigated. Their pathogenetic role is also questionable, although several studies have suggested an apoptotic capacity of anti-Hsp60 autoantibodies on osteoblasts and endothelial cells, linking them with joint inflammation and bone erosion in RA and vascular inflammation in SLE and vasculitides, respectively. In addition, molecular mimicry based on amino acid similarities between human Hsp60 and bacterial Hsp60 or other bacterial antigens has been implicated in the production of cross-reactive autoantibodies in AIRDS, connecting infection with autoimmunity and autoimmune disease. In search of the connection between anti-human Hsp60 autoantibodies and AIRDS, the current chapter reviews research advances in the field and discusses prospective investigations.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Autoimmune and inflammatory rheumatic diseases (AIRDs) , such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), Sjogren’s syndrome (SjS), systemic sclerosis (SSc), vasculitides and others are characterized by the breakdown of immunological self–tolerance, aberrant activation of immune cells and production of autoantibodies (autoAbs). AutoAbs may result from insufficient clearance of apoptotic or necrotic cells, modification of self-antigens that generates neoepitopes that are perceived as foreign by B cells or from cross-reactivity between foreign and self-antigens (Anders et al. 2005; Bogdanos et al. 2001; Ehser et al. 2013; Getts et al. 2013; Kivity et al. 2009; Polymeros et al. 2006; Saeki and Ishihara 2014; Suurmond and Diamond 2015). The cumulative evidence in support of infectious-triggered autoimmunity has led investigators to introduce the concept of the infectome and autoinfectome, which describes the totality of specific pathogens that are responsible for the development and maintenance of autoreactive immune responses in susceptible individuals (Bogdanos and Sakkas 2017; Bogdanos et al. 2013a, b, 2015). Among the autoAbs with a potential diagnostic, clinical and pathogenic importance are those against human heat shock protein 60 (Hsp60), particularly in atherosclerosis, but equally importantly in AIRDs (Alard et al. 2007, 2008; Mandal et al. 2005). In a similar vein, although unrelated to the topic of the present chapter, several studies have investigated the role of bacterial antibodies against other Hsp antigens as triggers of autoimmunity and organ-specific autoimmune diseases (Bogdanos et al. 2013a, 2015; Dubaniewicz 2010; Kaufmann et al. 1991; Res et al. 1991; Schultz and Arnold 1993; van der Zee et al. 1998).
Hsp60 is a phylogenetically and functionally conserved molecular chaperone that assists in the folding of nascent and denatured proteins (Horvath et al. 2002). The bacterial homologues are GroEL in Escherichia coli, Hsp60 in Chlamydia pneumoniae and Hsp65 in Mycobacterium tuberculosis, all sharing a high sequence similarity with the human Hsp60 (Gupta 1990; Jones et al. 1993). Bacterial Hsp60 is very immunogenic and acknowledged as the “common antigen” of gram-negative bacteria (van Eden et al. 2017). Hence, anti-bacterial Hsp60 antibodies are detected in many infectious diseases (Alard et al. 2007). As we are going to discuss later, infections are commonly found in patients with AIRDS, secondary to immune-suppressive treatment, which weakens immune system and makes the affected individuals prone to infections. Thus, anti-bacterial Hsp60 antibodies may be merely epiphenomena rather than potentially relevant to disease pathogenesis in AIRDs. On the other hand, bacterial anti-Hsp60 antibodies can be found in treatment-naive patients, raising concerns as to whether they bear a pathogenic potential (Kaufmann et al. 1991; Leung and Gershwin 1991; Res et al. 1991; Schultz and Arnold 1993).
In human, under physiological conditions Hsp60 has a housekeeping role and is located in the mitochondrial matrix (Alard et al. 2007). However, under stress conditions Hsp60 can be over-expressed and translocated on the cell membrane (Jamin et al. 2005; Pfister et al. 2005), or can be detected in serum (Davies et al. 2006) and in cell culture supernatant (Basu et al. 2000). In addition, elevated levels of Hsp60 are detected in sera of patients of inflammatory diseases, including Behçet’s disease (BD) (Shaker et al. 2007). However, in some autoimmune diseases, such as immune thrombocytopenia, serum Hsp60 levels are decreased (Dolasik et al. 2015), but whether this is due to its decreased expression or its binding and concomitant precipitation by anti-Hsp antibodies remains elusive (Alard et al. 2011; Dolasik et al. 2015; Rai et al. 2015).
Cellular immune responses to human Hsp60 have been detected in almost all chronic, inflammatory diseases. There is evidence that cellular responses to heat shock proteins, including Hsp60, are associated with anti-inflammatory regulation and theses are extensively reported in well-informative reviews (van Eden et al. 2005, 2017).
Humoral immune responses to Hsp60 have been detected in autoimmune and non-autoimmune diseases, including multiple sclerosis (Chiba et al. 2006; Efthymiou et al. 2016), type 1 diabetes mellitus (Horvath et al. 2002), atherosclerosis (Kilic and Mandal 2012; Wick et al. 2004) and cystic fibrosis (de Graeff-Meeder et al. 1993). Antibodies against both bacterial (Efthymiou et al. 2016) and human Hsp60 (Alard et al. 2007) can also be detected in the sera of healthy individuals. In this case, they are mainly considered as part of physiological, pathogen-induced immune responses (Zugel and Kaufmann 1999), rather than triggers of disease induction.
Several investigators have suggested that the production of anti-human Hsp60 antibodies may be triggered by bacterial Hsp60 via antibody cross-reaction (Fig. 11.1). Not all of them have been able to demonstrate the presence of cross-reactive humoral (and/or cellular) responses (Alard et al. 2008; Efthymiou et al. 2016; Richter et al. 1994). In addition, endogenous Hsp60 can also act as a target of autoAbs, possibly through mechanisms induced by changes in the structure of the protein, post-translational modifications or the formation of immunogenic complexes with other foreign or self-antigens. Several regions of the human Hsp60 molecule have been recognized as antigenic epitopes (Fig. 11.2), such as epitopes located within Hsp60383–447 (Boog et al. 1992), three overlapping epitopes spanning Hsp60394–460 (Horvath et al. 2002) and an epitope spanning Hsp60286–315 corresponding to apical I helix of human Hsp60 (Elfaitouri et al. 2013).
Molecular mimicry and antibody cross-reaction as potential mechanism of infection-induced anti-human Hsp60 antibodies production. Bacterial infection (1) stimulates the production of antigen-specific anti-bacterial antigen antibodies, such as those against bacterial Hsp60 (2). Molecular mimicry (3) between a bacterial Hsp60 epitope and a human Hsp60 epitope leads to the induction of cross-reactive antibodies (4) that recognize both the bacterial and human mimics. To this end, Boog et al. and Horvath et al. have reported on a human Hsp60 highly sequence-homologous epitope who is recognized by autoimmune disease-specific antibodies that cross-react with mycobacterial Hsp60 as noted in the insert (5). Eventually, cross-reactive antibodies (and in a similar vein cross-reactive T-cell responses) mediate self-tissue inflammation and subsequent tissue destruction (6)
Alignment using BLASTp2 of human Hsp60 and mycobacterial Hsp60 shows a significant degree of amino acid similarity at various sequences. Double underlined: the sequence of the human Hsp60383–419 epitope recognized by antibodies in patients with juvenile chronic arthritis (Boog et al. 1992), overlaps with the epitope recognized by antibodies in patients with type 1 diabetes mellitus (Horvath et al. 2002). Single underlined is the sequence of the human Hsp60286–315 epitope recognized by IgG and IgM antibodies in patients with myalgic encephalomyelitis (Elfaitouri et al. 2013)
1.1 Rheumatoid Arthritis
Rheumatoid arthritis (RA) is an autoimmune disease characterized mainly by chronic destructive polyarthritis, but may also affect extra-articular organs/tissues (Choy and Panayi 2001; McInnes and Schett 2011). The presence of anti-citrullinated peptide antibodies (ACPAs) is a highly specific biomarker for this disease (Sakkas et al. 2014). ACPAs are directed against various citrullinated antigens, such as fibrinogen, vimentin, α-enolase, collagen type II, histone and others (Sakkas et al. 2014).
Anti-human Hsp60 antibodies are commonly found at low frequency in RA (Table 11.1). The prevalence of anti-human Hsp60 autoAbs was found to be 10% by immunoblotting and 20% by ELISA (Jamin et al. 2005). Jarjour et al. using immunoblotting found that IgM and IgG autoAbs were uncommon in RA and not different from healthy controls (Jarjour et al. 1991). Similarly, several other groups did not detect increased levels of anti-human Hsp60 autoAbs in RA (Dieude et al. 2004; Horvath et al. 2001; Lu et al. 2016). However, elevated titres of IgG autoAbs against two members of the Hsp60 chaperonin family were reported, namely Hsp60 itself and chaperonin containing t-complex polypeptide 1 (CCT), in RA (Yokota et al. 2000). The elevated levels of anti-Hsp60 autoAbs were also reported in two other studies (Hirata et al. 1997; van Halm et al. 2006). Anti-human Hsp60 autoAbs might be of clinical significance for cardiovascular disease in RA (van Halm et al. 2006). Humoral immune responses against Hsp60 have been linked with cardiovascular disease (Zhu et al. 2001) and implicated in the development of atherosclerosis (Kilic and Mandal 2012; Wick et al. 2004). However, anti-Hsp60 autoAbs levels were comparable between RA patients with or without cardiovascular disease (van Halm et al. 2006). Therefore, anti-human Hsp60 autoAbs has not been considered as a marker for cardiovascular risk in RA.
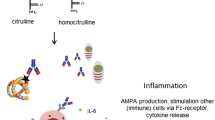
The pathogenetic potential of anti-citrullinated Hsp60 antibodies was elegantly demonstrated by Lu et al. (2016). ACPAs isolated from RA patients can bind to citrullinated Hsp60 on the surface of human mature osteoblasts in vitro, a finding that had been previously reported by mass spectrometry (Goeb et al. 2009). In addition, ACPAs can mediate osteoblast apoptosis by binding to cell surface-expressed citrullinated Hsp60 through Toll-like receptor (TLR) 4 signaling and stimulate IL-6 and IL-8 gene expression (Lu et al. 2016). Both interleukins promote osteoclast proliferation and could contribute to bone erosion in RA (Pathak et al. 2015). Serum levels of anti-citrullinated Hsp60 autoAbs were elevated in RA and correlated with joint damage, but there was no difference in the levels of anti-Hsp60 autoAbs between RA patients and healthy controls (Lu et al. 2016). It would be interesting to determine whether citrullinated Hsp60 is localized on the cell membrane in arthritic joints in RA. The homologue mycobacterial Hsp65 has been detected in the synovial membrane of arthritic joints both in RA and animal models of arthritis (de Graeff-Meeder et al. 1990; Karlsson-Parra et al. 1990). However, anti-Hsp60 antibody levels were found to be lower in synovial fluids than in sera, which argues against local production of antibodies in arthritic joints (Hirata et al. 1997).
The mechanism of molecular mimicry between bacterial and endogenous Hsp60 has been explored in the study of Yokota et al. who demonstrated by inhibition studies that antibodies recognizing CCT, human Hsp60, E. coli GroEL and mycobacterial Hsp65 were cross-reactive (Yokota et al. 2000), indicating that these antibodies recognize common epitopes on all four proteins. The observed anti-Hsp60 and anti-CCT antibody titres correlated strongly in sera of patients with rheumatic autoimmune diseases, a correlation that was not affected by age, disease duration or treatment, suggesting a common mechanism of production. Immunoblotting experiments showed that the epitopes recognized by the anti-Hsp60 and anti-CCT autoAbs were conformational and not sequence-specific. Of relevance, when sera of RA patients were absorbed with E. coli GroEL the reactivity to human Hsp60 was lost, but when sera were absorbed with human Hsp60 reactivity to E. coli GroEL remained (Hirata et al. 1997). Furthermore, in RA the levels of anti-human Hsp60 autoAbs and anti-E. coli GroEL abs were elevated in sera but lower in synovial fluid. These results suggest that molecular mimicry is the mechanism of anti-human Hsp60 antibodies in RA, rather than a synovium-localized, RA-specific generation (Hirata et al. 1997). Similar cross-reactivity was detected between mycobacterial Hsp70287–306 and human binding immunoglobulin protein (BiP)336–355. These data strengthen the hypothesis that molecular mimicry and immunological cross-reactivity, involving bacterial and human Hsp, is a mechanism for anti-human Hsp60 antibody production in RA (Shoda et al. 2016).
1.2 Juvenile Idiopathic Arthritis
Juvenile idiopathic arthritis (JIA), the most common pediatric chronic rheumatic disease, represents a group of disorders characterized by chronic joint inflammation (Eisenstein and Berkun 2014). The etiology and pathogenesis of JIA are not yet fully elucidated, although a few genes have been implicated (Hinks et al. 2009, 2010a, b; Yanagimachi et al. 2011). Several studies have investigated humoral responses against human Hsp60 in JIA (Table 11.1). Two studies have reported a universal presence of anti-human Hsp60 autoAbs in the sera of JIA patients (de Graeff-Meeder et al. 1993; Nguyen et al. 2006). Serum levels of anti-human Hsp60 autoAbs were elevated in JIA patients, particularly with polyarticular onset (de Graeff-Meeder et al. 1993; Nguyen et al. 2006). These results were also reported in patients with active, polyarticular JIA (Wu et al. 2011). In contrast, one study reported decreased serum antibody levels in JIA patients compared to healthy controls (Zlacka et al. 2006). Interestingly, levels of anti-human Hsp60 antibodies were much higher in the synovial fluids than matched sera, suggesting local antibody production by plasma cells in arthritic joints (de Graeff-Meeder et al. 1993).
1.3 Spondyloarthritis
Spondyloarthritis (SpA) encompasses a group of immune-mediated inflammatory diseases, including ankylosing spondylitis, psoriatic arthritis, reactive arthritis, inflammatory bowel disease-associated arthritis and undifferentiated spondyloarthritis (Terenzi et al. 2018). These diseases share clinical manifestations, such as inflammation of the spine, peripheral arthritis, enthesitis, and anterior uveitis. An early study reported elevated serum levels of IgG anti-human Hsp60 autoAbs in reactive arthritis (Handley et al. 1996) (Table 11.1). Another study examined serum IgG subclass antibodies (IgG1, IgG2, IgG3, and IgG4) against human Hsp60 and Hsp60 from Chlamydia trachomatis, Salmonella enteritidis and Campylobacter jejuni (Hjelholt et al. 2013). IgG1 and IgG3 abs against human Hsp60, but not against bacterial Hsp60, were elevated in SpA and there was only a weak association between antibodies to human and bacterial Hsp60. These findings suggest that cross-reactivity between human and bacterial Hsp60 is unlikely. Another study supported the notion reporting that different IgG subclass IgG3 and IgG1 antibodies against human and bacterial Hsp60, respectively, of the same serum sample argues against cross-reactivity (Carlsen et al. 2013).
1.4 Systemic Lupus Erythematosus
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease that can affect almost any organ system, and is characterized by a plethora of autoAbs mostly targeting nuclear antigens (Kaul et al. 2016).
In SLE patients, findings on the frequency of anti-human Hsp60 antibodies are conflicting with one study reporting increased frequency (Jamin et al. 2005), but another not (Jarjour et al. 1991) (Table 11.1). With regard to anti-human Hsp60 autoAbs serum levels, results again are conflicting, with few studies reporting elevated serum levels (Jamin et al. 2005; Yokota et al. 2000), while other do not (Dieude et al. 2004; Horvath et al. 2001; van Paassen et al. 2007).
A potential role for anti-human Hsp60 autoAbs in SLE has arisen for the vascular manifestations of the disease. SLE carries an increased cardiovascular risk, including premature or accelerated atherosclerosis (Zampieri et al. 2005). Anti-Hsp60 autoAbs have been linked to pathogenesis and prognosis of coronary heart disease, vascular disease and atherosclerosis (Kilic and Mandal 2012; Wick et al. 2004; Zhu et al. 2001), possibly through endothelial cytotoxic events (Mayr et al. 1999). For instance, anti-Hsp60 autoAbs bind to endothelial cells and induce apoptosis, an event that facilitates the generation of anti-phospholipid autoAbs and thrombosis (Dieude et al. 2004). This was likely the first study to identify autoAbs against Hsp60 as anti-endothelial cell autoAbs, a heterogeneous class of antibodies that have been detected under autoimmune conditions connected with vascular involvement (D’Cruz et al. 1991; Renaudineau et al. 2002; Salojin et al. 1996). Similarly, by using immunoblotting and immunoabsorption techniques, the complex group of anti-endothelial cell autoAbs were confirmed to contain Hsp60 autoAbs and that their binding to endothelial cells induces apoptosis (Jamin et al. 2005). The presence of anti-human Hsp60 autoAbs and their ability to promote vascular endothelial cell injury and impairment of microcirculation has also been implicated in the development of neuropsychiatric SLE (Kimura et al. 2008). However, the role of anti-human Hsp60 autoAbs in apoptosis induction was questioned, since anti-epithelial cell antibodies not directed against Hsp60 were detected to be responsible for the induction of epithelial cell apoptosis in SLE patients with glomerular vasculopathy (van Paassen et al. 2007).
1.5 Systemic Sclerosis
Systemic sclerosis (SSc) is a complex disease of the connective tissue with extensive fibrosis, microvasculopathy and production of a range of disease-related and other autoAbs (Sakkas and Bogdanos 2016). To date, no direct evidence has linked anti-human Hsp60 antibodies with SSc (Table 11.1). The frequency of IgG and IgM anti-human Hsp60 antibodies in SSc patients was “not demonstrable” (Jarjour et al. 1991), and the difference in their levels between patients and controls was not statistically significant (Horvath et al. 2001; Jamin et al. 2005). These data argue against the involvement of anti-Hsp60 autoAbs in SSc. However, elevated serum levels of anti-human-Hsp60 autoAbs were found in in patients with undifferentiated connective tissue disease (UCTD) (Horvath et al. 2001). UCTD is a systemic disorder with a mixture of clinical traits of autoimmune connective tissue diseases, such as SSc, SLE, RA or SjS (Mosca et al. 2014). Although UCTD often differentiates to one of these well-characterized diseases, most patients will maintain the diagnosis of UCTD based on their clinical and laboratory features. Anti-human Hsp60 antibodies in UCTD patients could be further investigated to determine whether they define patients who will later develop one of the connective tissue diseases or not.
Similarly, in a study of autoimmune connective tissue disease that included undifferentiated connective tissue disease, SSc and SLE, serum levels of anti-Hsp60 autoAbs were normal and not correlated with anti-H. pylori antibodies (Kalabay et al. 2002). Unfortunately, the authors did not analyze each disease group separately, so any potential disease-specific anti-Hsp60 autoAbs differences were masked. Nevertheless, the normal levels of anti-human Hsp60 antibodies in autoimmune diseases supported the author’s suggestion that these are natural autoAbs (Prohaszka et al. 2001), a class of antibodies protecting the host from both invading antigens and endogenous neo-antigens that are constantly produced (Lobo 2016).
1.6 Sjogren’s Syndrome
Sjogren’s syndrome (SjS) is an autoimmune disease characterized by inflammation of exocrine glands, mainly salivary and lacrimal glands (Tong et al. 2017). A plethora of autoAbs can be detected in SjS patients, with anti-Ro/SSA autoantibodies being the most prevalent ones. Yokota et al. were the first to examine immune responses against human Hsp60 and chaperonin containing t-complex polypeptide 1 (CCT), a member of the Hsp60 family, in SjS (Yokota et al. 2000) (Table 11.1). Serum levels of anti-CCT IgG autoAbs but not anti-Hsp60 autoAbs were elevated in SjS. Similarly, other studies reported no difference in prevalence or levels of anti-Hsp60 autoAbs between SjS patients and healthy controls (Jamin et al. 2005) or even lower levels of anti-Hsp60 IgG and IgM autoAbs (Shovman et al. 2005). Although the relevance of anti-Hsp60 antibodies to SjS pathogenesis is questionable, their lower levels could imply a protective role against the disease. Hence, larger prospective studies are necessary to define the association between particular infections and autoimmunity.
1.7 Vasculitis
Vasculitis is defined as an inflammation of blood vessel walls. Although vasculitis can be secondary to infection, here we refer to idiopathic vasculitis. Vasculitis leads to aneurysm and vessel rupture or to vessel wall thickening, stenosis and tissue ischemia and can be primary or secondary to an underlying autoimmune disease (Savage et al. 2000). Primary vasculitis can involve any type of blood vessel and can affect any organ. The clinical manifestations vary according to the size and distribution of the inflamed blood vessels.
Jamin et al. investigated on the role of anti-human Hsp60 antibodies in vasculitis including granulomatosis with polyangiitis (GPA, formerly Wegener’s granulomatosis), eosinophilic granulomatosis with polyangiitis (EGPA, formerly Churg-Strauss syndrome), microscopic polyangiitis, polyarteritis nodosa and in vasculitis-associated autoimmune diseases (SLE) (Jamin et al. 2005) (Table 11.1). The frequency of anti-human Hsp60 autoAbs was higher in vasculitic (vasculitis and SLE) compared to non-vasculitic autoimmune rheumatic diseases (RA, SjS), particularly in anti-endothelial cell antibody (AECA)-positive patients. Furthermore, Hsp60 was an important target of AECAs in their apoptotic effect on endothelial cells and appeared to have the capacity to induce apoptosis of endothelial cells (Jamin et al. 2005). In vitro experiments have demonstrated that this apoptotic capacity occurs in endothelial cells under stress, a condition that involves Hsp60 on the cell surface, requires the interaction of Hsp60 and Hsp70 and is propagated through the chemokine receptor CCR5 (Alard et al. 2009). Of interest, a specific interaction between Hsp60 and ATP synthase has been recognized in vitro on the surface of endothelial cells and has been shown to prevent the pathogenic effect of anti-ATP synthase autoAbs on endothelial cells (Alard et al. 2011), suggesting that the diverse surface interactions of Hsp60 can affect endothelial physiology in various ways. It is also intriguing to speculate that these interactions of surface Hsp60 could form immunogenic complexes that are recognized as foreign by the immune system and may be important in the pathogenesis of vasculitis (Anders et al. 2005; Suurmond and Diamond 2015). The presence of this type of antibodies could render an individual susceptible to endothelial inflammation in the context of a primary vasculitic entity or a secondary vasculitic component of another rheumatic disease (Alard et al. 2008).
The production of anti-human Hsp60 antibodies was reported to be elevated in patients with MPO-ANCA- compared to PR3-ANCA-associated vasculitis and healthy controls (Slot et al. 2006) (Table 11.1). However, inhibition assays did not support a role for infection-triggered pathogenesis of MPO-ANCA-associated vasculitis through molecular mimicry between MPO, human Hsp60 and mycobacterial Hsp65 (Slot et al. 2006).
A link between anti-human Hsp60 autoAbs and vasculitic inflammation has been reported in Takayasu’s arteritis (TA). Both the frequency and titres of IgG, but not IgM or IgA, anti-human Hsp60 autoAbs were increased in TA (Kumar Chauhan et al. 2004) (Table 11.1). Of importance, humoral and cellular immune response against both human Hsp60 and mycobacterial Hsp65 were significantly correlated, suggesting cross-reactivity and molecular mimicry as the triggering mechanisms of autoimmunity in TA. Furthermore, anti-aortic endothelial cell antibodies comprise of anti-human Hsp60 autoAbs in the vast majority of TA patients and these autoAbs can induce the expression of adhesion molecules and pro-inflammatory cytokines, as well as apoptosis (Chauhan et al. 2006). In giant cell arteritis (GCA) a study reported elevated levels of anti-human Hsp60 autoAbs but they did not correlate with anti-Chlamydia pneumoniae Hsp60 antibodies (Lopez-Hoyos et al. 2008).
1.8 Behçet’s Disease
Behçet’s disease (BD) (also known as Adamantiades-Behcet’s disease) is a systemic, inflammatory disorder characterized by recurrent inflammation that manifests as oral apthous ulcers, genital ulcers, uveitis and acne-like skin lesions (Zeidan et al. 2016). No study thus far has investigated on the prevalence of Hsp60 autoAbs in BD. There are conflicting reports regarding serum anti-Hsp60 autoAbs levels with one study reporting elevated levels and another study reporting normal levels (Hirata et al. 1997; Jamin et al. 2005). Higher levels of anti-Hsp60 autoAbs were detected in resting saliva of BD patients with moderate disease compared to patients with mild or severe disease and higher levels were associated with stomatitis for more than 2 weeks and with gingival inflammation (Doino et al. 2017).
1.9 Idiopathic Inflammatory Myopathies
Polymyositis is an idiopathic, inflammatory rheumatic disease of the muscles with typical symptoms including symmetrical, proximal muscle weakness, difficulty arising from a seated position, dysphagia and aspiration, arthralgia Raynaud phenomenon and fever (Dalakas 2015). Of the two studies that have measured immune responses against human Hsp60 in patients with polymyositis, none of them managed to detect differences either in prevalence or serum titres of Hsp60 autoAbs (Horvath et al. 2001; Jarjour et al. 1991).
1.10 Further Prospectives
A consensus on the role of anti-human Hsp60 antibodies in autoimmune rheumatic diseases has not emerged. The discrepancies that are observed between studies on Hsp60 autoantibodies could be attributed to experimental limitations. Most studies do not measure the prevalence of anti-human Hsp60 antibodies but rather their titres on patients’ sera. In addition, almost all studies measure immune responses against human Hsp60 in numerous autoimmune rheumatic diseases, instead of focusing in a single one. This is not a de facto limitation, but it becomes one in cases where small cohorts of each disease were studied. Patient selection and numbers is a major consideration regarding interpretation of the results. Therefore, a more organized investigation of human Hsp60 humoral immune responses in larger patient groups is necessary.
2 Conclusions
In conclusion, anti-hsp60 autoantibodies are prevalent in various autoimmune rheumatic and other autoimmune diseases, as well as other chronic inflammatory diseases. Whether are of pathogenic significance or not remains to be seen. Detailed, well-designed studies regarding their prevalence, epitope specificity and IgG subclass distribution is missing and should be performed in large multi-centre studies.
Molecular mimicry and immunological cross-reactivity involving human Hsp60 and bacterial Hsp60 mimics has been implicated in the production of cross-reactive autoantibodies but solid data are still absent, and is premature to connect initiation of infectious triggered hsp60 autoreactivity infection with autoimmunity and autoimmune disease.
Abbreviations
- ACPAs:
-
Anti-citrullinated peptide antibodies
- AECAs:
-
Anti-endothelial cell antibodies
- AIRDs:
-
Autoimmune and inflammatory rheumatic diseases
- autoAbs:
-
Autoantibodies
- BD:
-
Behçet’s disease
- CCT:
-
Chaperonin containing t-complex polypeptide 1
- E. coli :
-
Escherichia coli
- EGPA:
-
Eosinophilic granulomatosis with polyangiitis
- GCA:
-
Giant cell arteritis
- GPA:
-
Granulomatosis with polyangiitis
- H. pylori :
-
Helicobacter pylori
- Hsp:
-
Heat shock protein
- IL:
-
Interleukin
- JIA:
-
Juvenile idiopathic arthritis
- MPO-ANCA:
-
Myeloperoxidase-antineutrophil cytoplasmic antibodies
- PR3-ANCA:
-
Peroxidase 3- antineutrophil cytoplasmic antibodies
- RA:
-
Rheumatoid arthritis
- SjS:
-
Sjogren’s syndrome
- SLE:
-
Systemic lupus erythematosus
- SpA:
-
Spondyloarthritis
- SSc:
-
Systemic sclerosis
- TA:
-
Takayasu’s arteritis
- TLR:
-
Toll-like receptor
- UCTD:
-
Undifferentiated connective tissue disease
References
Alard JE, Dueymes M, Youinou P, Jamin C (2007) Modulation of endothelial cell damages by anti-Hsp60 autoantibodies in systemic autoimmune diseases. Autoimmun Rev 6:438–443
Alard JE, Dueymes M, Youinou P, Jamin C (2008) HSP60 and anti-HSP60 antibodies in vasculitis: they are two of a kind. Clin Rev Allergy Immunol 35:66–71
Alard JE, Dueymes M, Mageed RA, Saraux A, Youinou P, Jamin C (2009) Mitochondrial heat shock protein (HSP) 70 synergizes with HSP60 in transducing endothelial cell apoptosis induced by anti-HSP60 autoantibody. FASEB J 23:2772–2779
Alard JE, Hillion S, Guillevin L et al (2011) Autoantibodies to endothelial cell surface ATP synthase, the endogenous receptor for hsp60, might play a pathogenic role in vasculatides. PLoS One 6:e14654
Anders HJ, Zecher D, Pawar RD, Patole PS (2005) Molecular mechanisms of autoimmunity triggered by microbial infection. Arthritis Res Ther 7:215–224
Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK (2000) Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol 12:1539–1546
Bogdanos DP, Sakkas LI (2017) From microbiome to infectome in autoimmunity. Curr Opin Rheumatol 29:369–373
Bogdanos DP, Choudhuri K, Vergani D (2001) Molecular mimicry and autoimmune liver disease: virtuous intentions, malign consequences. Liver 21:225–232
Bogdanos DP, Smyk DS, Invernizzi P et al (2013a) Infectome: a platform to trace infectious triggers of autoimmunity. Autoimmun Rev 12:726–740
Bogdanos DP, Smyk DS, Invernizzi P et al (2013b) Tracing environmental markers of autoimmunity: introducing the infectome. Immunol Res 56:220–240
Bogdanos DP, Smyk DS, Rigopoulou EI, Sakkas LI, Shoenfeld Y (2015) Infectomics and autoinfectomics: a tool to study infectious-induced autoimmunity. Lupus 24:364–373
Boog CJ, de Graeff-Meeder ER, Lucassen MA et al (1992) Two monoclonal antibodies generated against human hsp60 show reactivity with synovial membranes of patients with juvenile chronic arthritis. J Exp Med 175:1805–1810
Carlsen TG, Hjelholt A, Jurik AG et al (2013) IgG subclass antibodies to human and bacterial HSP60 are not associated with disease activity and progression over time in axial spondyloarthritis. Arthritis Res Ther 15:R61
Chauhan SK, Tripathy NK, Nityanand S (2006) Antigenic targets and pathogenicity of anti-aortic endothelial cell antibodies in Takayasu arteritis. Arthritis Rheum 54:2326–2333
Chiba S, Yokota S, Yonekura K et al (2006) Autoantibodies against HSP70 family proteins were detected in the cerebrospinal fluid from patients with multiple sclerosis. J Neurol Sci 241:39–43
Choy EH, Panayi GS (2001) Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med 344:907–916
D’Cruz DP, Houssiau FA, Ramirez G et al (1991) Antibodies to endothelial cells in systemic lupus erythematosus: a potential marker for nephritis and vasculitis. Clin Exp Immunol 85:254–261
Dalakas MC (2015) Inflammatory muscle diseases. N Engl J Med 373:393–394
Davies EL, Bacelar MM, Marshall MJ et al (2006) Heat shock proteins form part of a danger signal cascade in response to lipopolysaccharide and GroEL. Clin Exp Immunol 145:183–189
de Graeff-Meeder ER, Voorhorst M, van Eden W et al (1990) Antibodies to the mycobacterial 65-kd heat-shock protein are reactive with synovial tissue of adjuvant arthritic rats and patients with rheumatoid arthritis and osteoarthritis. Am J Pathol 137:1013–1017
de Graeff-Meeder ER, Rijkers GT, Voorhorst-Ogink MM et al (1993) Antibodies to human HSP60 in patients with juvenile chronic arthritis, diabetes mellitus, and cystic fibrosis. Pediatr Res 34:424–428
Dieude M, Senecal JL, Raymond Y (2004) Induction of endothelial cell apoptosis by heat-shock protein 60-reactive antibodies from anti-endothelial cell autoantibody-positive systemic lupus erythematosus patients. Arthritis Rheum 50:3221–3231
Doino M, Yokoyama M, Sasaki Y, Kondo K, Yasuda Y, Arakawa S (2017) Evaluation of the relationship between salivary concentration of anti-heat shock protein immunoglobulin and clinical manifestations of Behcet’s disease. Scand J Rheumatol 46:381–387
Dolasik I, Birtas Atesoglu E, Tarkun P et al (2015) Decreased serum heat shock protein 60 levels in newly diagnosed immune thrombocytopenia patients. Platelets 26:220–223
Dubaniewicz A (2010) Mycobacterium tuberculosis heat shock proteins and autoimmunity in sarcoidosis. Autoimmun Rev 9:419–424
Efthymiou G, Dardiotis E, Liaskos C et al (2016) Anti-hsp60 antibody responses based on Helicobacter pylori in patients with multiple sclerosis: (ir)relevance to disease pathogenesis. J Neuroimmunol 298:19–23
Ehser J, Holdener M, Christen S et al (2013) Molecular mimicry rather than identity breaks T-cell tolerance in the CYP2D6 mouse model for human autoimmune hepatitis. J Autoimmun 42:39–49
Eisenstein EM, Berkun Y (2014) Diagnosis and classification of juvenile idiopathic arthritis. J Autoimmun 48–49:31–33
Elfaitouri A, Herrmann B, Bolin-Wiener A et al (2013) Epitopes of microbial and human heat shock protein 60 and their recognition in myalgic encephalomyelitis. PLoS One 8:e81155
Getts DR, Chastain EM, Terry RL, Miller SD (2013) Virus infection, antiviral immunity, and autoimmunity. Immunol Rev 255:197–209
Goeb V, Thomas-L’Otellier M, Daveau R et al (2009) Candidate autoantigens identified by mass spectrometry in early rheumatoid arthritis are chaperones and citrullinated glycolytic enzymes. Arthritis Res Ther 11:R38
Gupta RS (1990) Sequence and structural homology between a mouse T-complex protein TCP-1 and the ‘chaperonin’ family of bacterial (GroEL, 60–65 kDa heat shock antigen) and eukaryotic proteins. Biochem Int 20:833–841
Handley HH, Yu J, Yu DT, Singh B, Gupta RS, Vaughan JH (1996) Autoantibodies to human heat shock protein (hsp)60 may be induced by Escherichia coli groEL. Clin Exp Immunol 103:429–435
Hinks A, Ke X, Barton A et al (2009) Association of the IL2RA/CD25 gene with juvenile idiopathic arthritis. Arthritis Rheum 60:251–257
Hinks A, Eyre S, Ke X et al (2010a) Association of the AFF3 gene and IL2/IL21 gene region with juvenile idiopathic arthritis. Genes Immun 11:194–198
Hinks A, Martin P, Flynn E et al (2010b) Association of the CCR5 gene with juvenile idiopathic arthritis. Genes Immun 11:584–589
Hirata D, Hirai I, Iwamoto M et al (1997) Preferential binding with Escherichia coli hsp60 of antibodies prevalent in sera from patients with rheumatoid arthritis. Clin Immunol Immunopathol 82:141–148
Hjelholt A, Carlsen T, Deleuran B et al (2013) Increased levels of IgG antibodies against human HSP60 in patients with spondyloarthritis. PLoS One 8:e56210
Horvath L, Czirjak L, Fekete B et al (2001) Levels of antibodies against C1q and 60 kDa family of heat shock proteins in the sera of patients with various autoimmune diseases. Immunol Lett 75:103–109
Horvath L, Cervenak L, Oroszlan M et al (2002) Antibodies against different epitopes of heat-shock protein 60 in children with type 1 diabetes mellitus. Immunol Lett 80:155–162
Jamin C, Dugue C, Alard JE et al (2005) Induction of endothelial cell apoptosis by the binding of anti-endothelial cell antibodies to Hsp60 in vasculitis-associated systemic autoimmune diseases. Arthritis Rheum 52:4028–4038
Jarjour WN, Jeffries BD, Davis JS, Welch WJ, Mimura T, Winfield JB (1991) Autoantibodies to human stress proteins. A survey of various rheumatic and other inflammatory diseases. Arthritis Rheum 34:1133–1138
Jones DB, Coulson AF, Duff GW (1993) Sequence homologies between hsp60 and autoantigens. Immunol Today 14:115–118
Kalabay L, Fekete B, Czirjak L et al (2002) Helicobacter pylori infection in connective tissue disorders is associated with high levels of antibodies to mycobacterial hsp65 but not to human hsp60. Helicobacter 7:250–256
Karlsson-Parra A, Soderstrom K, Ferm M, Ivanyi J, Kiessling R, Klareskog L (1990) Presence of human 65 kD heat shock protein (hsp) in inflamed joints and subcutaneous nodules of RA patients. Scand J Immunol 31:283–288
Kaufmann SH, Schoel B, van Embden JD et al (1991) Heat-shock protein 60: implications for pathogenesis of and protection against bacterial infections. Immunol Rev 121:67–90
Kaul A, Gordon C, Crow MK et al (2016) Systemic lupus erythematosus. Nat Rev Dis Primers 2:16039
Kilic A, Mandal K (2012) Heat shock proteins: pathogenic role in atherosclerosis and potential therapeutic implications. Autoimmune Dis 2012:502813
Kimura A, Sakurai T, Tanaka Y et al (2008) Proteomic analysis of autoantibodies in neuropsychiatric systemic lupus erythematosus patient with white matter hyperintensities on brain MRI. Lupus 17:16–20
Kivity S, Agmon-Levin N, Blank M, Shoenfeld Y (2009) Infections and autoimmunity – friends or foes? Trends Immunol 30:409–414
Kumar Chauhan S, Kumar Tripathy N, Sinha N, Singh M, Nityanand S (2004) Cellular and humoral immune responses to mycobacterial heat shock protein-65 and its human homologue in Takayasu’s arteritis. Clin Exp Immunol 138:547–553
Leung PS, Gershwin ME (1991) The immunobiology of heat shock proteins. J Investig Allergol Clin Immunol 1:23–30
Lobo PI (2016) Role of natural autoantibodies and natural IgM anti-leucocyte autoantibodies in health and disease. Front Immunol 7:198
Lopez-Hoyos M, Alvarez L, Ruiz Soto M, Blanco R, Jose Bartolome M, Martinez-Taboada VM (2008) Serum levels of antibodies to Chlamydia pneumoniae and human HSP60 in giant cell arteritis patients. Clin Exp Rheumatol 26:1107–1110
Lu MC, Yu CL, Yu HC, Huang HB, Koo M, Lai NS (2016) Anti-citrullinated protein antibodies promote apoptosis of mature human Saos-2 osteoblasts via cell-surface binding to citrullinated heat shock protein 60. Immunobiology 221:76–83
Mandal K, Foteinos G, Jahangiri M, Xu Q (2005) Role of antiheat shock protein 60 autoantibodies in atherosclerosis. Lupus 14:742–746
Mayr M, Metzler B, Kiechl S et al (1999) Endothelial cytotoxicity mediated by serum antibodies to heat shock proteins of Escherichia coli and Chlamydia pneumoniae: immune reactions to heat shock proteins as a possible link between infection and atherosclerosis. Circulation 99:1560–1566
McInnes IB, Schett G (2011) The pathogenesis of rheumatoid arthritis. N Engl J Med 365:2205–2219
Mosca M, Tani C, Vagnani S, Carli L, Bombardieri S (2014) The diagnosis and classification of undifferentiated connective tissue diseases. J Autoimmun 48–49:50–52
Nguyen TT, Zlacka D, Vavrincova P, Sedlacek P, Hromadnikova I (2006) Detection of antibodies against 60-, 65- and 70-kDa heat shock proteins in paediatric patients with various disorders using Western blotting and ELISA. Clin Chem Lab Med 44:442–449
Pathak JL, Bakker AD, Verschueren P et al (2015) CXCL8 and CCL20 enhance osteoclastogenesis via modulation of cytokine production by human primary osteoblasts. PLoS One 10:e0131041
Pfister G, Stroh CM, Perschinka H et al (2005) Detection of HSP60 on the membrane surface of stressed human endothelial cells by atomic force and confocal microscopy. J Cell Sci 118:1587–1594
Polymeros D, Bogdanos DP, Day R, Arioli D, Vergani D, Forbes A (2006) Does cross-reactivity between mycobacterium avium paratuberculosis and human intestinal antigens characterize Crohn’s disease? Gastroenterology 131:85–96
Prohaszka Z, Duba J, Horvath L et al (2001) Comparative study on antibodies to human and bacterial 60 kDa heat shock proteins in a large cohort of patients with coronary heart disease and healthy subjects. Eur J Clin Investig 31:285–292
Rai R, Chauhan SK, Singh VV, Rai M, Rai G (2015) Heat shock protein 27 and its regulatory molecules express differentially in SLE patients with distinct autoantibody profiles. Immunol Lett 164:25–32
Renaudineau Y, Dugue C, Dueymes M, Youinou P (2002) Antiendothelial cell antibodies in systemic lupus erythematosus. Autoimmun Rev 1:365–372
Res P, Thole J, de Vries R (1991) Heat-shock proteins and autoimmunity in humans. Springer Semin Immunopathol 13:81–98
Richter W, Mertens T, Schoel B et al (1994) Sequence homology of the diabetes-associated autoantigen glutamate decarboxylase with coxsackie B4-2C protein and heat shock protein 60 mediates no molecular mimicry of autoantibodies. J Exp Med 180:721–726
Saeki Y, Ishihara K (2014) Infection-immunity liaison: pathogen-driven autoimmune-mimicry (PDAIM). Autoimmun Rev 13:1064–1069
Sakkas LI, Bogdanos DP (2016) Systemic sclerosis: new evidence re-enforces the role of B cells. Autoimmun Rev 15:155–161
Sakkas LI, Bogdanos DP, Katsiari C, Platsoucas CD (2014) Anti-citrullinated peptides as autoantigens in rheumatoid arthritis-relevance to treatment. Autoimmun Rev 13:1114–1120
Salojin KV, Le Tonqueze M, Nassovov EL et al (1996) Anti-endothelial cell antibodies in patients with various forms of vasculitis. Clin Exp Rheumatol 14:163–169
Savage CO, Harper L, Cockwell P, Adu D, Howie AJ (2000) ABC of arterial and vascular disease: vasculitis. BMJ 320:1325–1328
Schultz DR, Arnold PI (1993) Heat shock (stress) proteins and autoimmunity in rheumatic diseases. Semin Arthritis Rheum 22:357–374
Shaker O, Ay El-Deen MA, El Hadidi H, Grace BD, El Sherif H, Abdel Halim A (2007) The role of heat shock protein 60, vascular endothelial growth factor and antiphospholipid antibodies in Behcet disease. Br J Dermatol 156:32–37
Shoda H, Hanata N, Sumitomo S, Okamura T, Fujio K, Yamamoto K (2016) Immune responses to mycobacterial heat shock protein 70 accompany self-reactivity to human BiP in rheumatoid arthritis. Sci Rep 6:22486
Shovman O, Sherer Y, Gilbourd B et al (2005) Low levels of heat shock proteins-60 and -65 autoantibodies in Sjogren’s syndrome. Isr Med Assoc J: IMAJ 7:778–780
Slot MC, Theunissen R, van Paassen P, Damoiseaux JG, Tervaert JW (2006) Evaluation of antibodies against human HSP60 in patients with MPO-ANCA associated glomerulonephritis: a cohort study. J Autoimmune Dis 3:4
Suurmond J, Diamond B (2015) Autoantibodies in systemic autoimmune diseases: specificity and pathogenicity. J Clin Invest 125:2194–2202
Terenzi R, Monti S, Tesei G, Carli L (2018) One year in review 2017: spondyloarthritis. Clin Exp Rheumatol 36:1–14
Tong L, Koh V, Thong BY (2017) Review of autoantigens in Sjogren’s syndrome: an update. J Inflamm Res 10:97–105
van der Zee R, Anderton SM, Prakken AB, Liesbeth Paul AG, van Eden W (1998) T cell responses to conserved bacterial heat-shock-protein epitopes induce resistance in experimental autoimmunity. Semin Immunol 10:35–41
van Eden W, van der Zee R, Prakken B (2005) Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat Rev Immunol 5:318–330
van Eden W, Jansen MAA, Ludwig I, van Kooten P, van der Zee R, Broere F (2017) The enigma of heat shock proteins in immune tolerance. Front Immunol 8:1599
van Halm VP, Slot MC, Nurmohamed MT, Tervaert JW, Dijkmans BA, Voskuyl AE (2006) Antibodies against human 60 kDa heat shock protein are not associated with cardiovascular disease in patients with rheumatoid arthritis. Ann Rheum Dis 65:590–594
van Paassen P, Duijvestijn A, Debrus-Palmans L, Damoiseaux J, Vroomen M, Tervaert JW (2007) Induction of endothelial cell apoptosis by IgG antibodies from SLE patients with nephropathy: a potential role for anti-endothelial cell antibodies. Ann N Y Acad Sci 1108:147–156
Wick G, Knoflach M, Xu Q (2004) Autoimmune and inflammatory mechanisms in atherosclerosis. Annu Rev Immunol 22:361–403
Wu CT, Ou LS, Yeh KW, Lee WI, Huang JL (2011) Serum heat shock protein 60 can predict remission of flare-up in juvenile idiopathic arthritis. Clin Rheumatol 30:959–965
Yanagimachi M, Miyamae T, Naruto T et al (2011) Association of HLA-A∗02:06 and HLA-DRB1∗04:05 with clinical subtypes of juvenile idiopathic arthritis. J Hum Genet 56:196–199
Yokota SI, Hirata D, Minota S et al (2000) Autoantibodies against chaperonin CCT in human sera with rheumatic autoimmune diseases: comparison with antibodies against other Hsp60 family proteins. Cell Stress Chaperones 5:337–346
Zampieri S, Iaccarino L, Ghirardello A et al (2005) Systemic lupus erythematosus, atherosclerosis, and autoantibodies. Ann N Y Acad Sci 1051:351–361
Zeidan MJ, Saadoun D, Garrido M, Klatzmann D, Six A, Cacoub P (2016) Behcet’s disease physiopathology: a contemporary review. Auto Immun Highlights 7:4
Zhu J, Quyyumi AA, Rott D et al (2001) Antibodies to human heat-shock protein 60 are associated with the presence and severity of coronary artery disease: evidence for an autoimmune component of atherogenesis. Circulation 103:1071–1075
Zlacka D, Vavrincova P, Hien Nguyen TT, Hromadnikova I (2006) Frequency of anti-hsp60, −65 and −70 antibodies in sera of patients with juvenile idiopathic arthritis. J Autoimmun 27:81–88
Zugel U, Kaufmann SH (1999) Role of heat shock proteins in protection from and pathogenesis of infectious diseases. Clin Microbiol Rev 12:19–39
Acknowledgements
G.E. is supported by a PhD scholarship funded by the Hellenic State Scholarship Foundation (IKY, Greece).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Efthymiou, G., Sakkas, L.I., Bogdanos, D.P. (2019). Anti-human Hsp60 Autoantibodies in Autoimmune and Inflammatory Rheumatic Diseases. In: Asea, A., Kaur, P. (eds) Heat Shock Protein 60 in Human Diseases and Disorders. Heat Shock Proteins, vol 18. Springer, Cham. https://doi.org/10.1007/978-3-030-23154-5_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-23154-5_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-23153-8
Online ISBN: 978-3-030-23154-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)