Abstract
The article is dedicated to designing the regime of Q&P (Quenching and Partitioning) heat treatment for middle-carbon high-silicon steels 60Si2CrVA and 55Si3Mn2CrVMoNbA in order to improve their mechanical properties. The temperature of suspense of quenching cooling during Q&P treatment was calculated by modeling based on the concept of “Constrained Paraequilibrium” proposed by J. Speer. The values of Ms temperature as well as the kinetics of martensitic transformation for both steels were experimentally found to be incorporated into the model. It was derived from the modeling that quenching stage should be finished when reaching the steel temperature within the range of 150–220 °C which guarantees the highest volume fraction of retained austenite in the microstructure (together with tempered martensite). The results of calculations were verified by XRD measurements of retained austenite in Q&P treated specimens being found as 17 vol% for steel 60Si2CrVA and 28.5 vol% for steel 55Si3Mn2CrVMoNbA which are lower then predicted values. The probable reasons of this discrepancy are outlined.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Achieving high strength in low-alloy steels through heat treatment is one of the main directions of saving energy and raw materials in metallurgy and mechanical engineering, which is increasingly important from an economic and environmental points of view [1, 2]. In this regard, advanced metallurgical technologies are becoming wide spread, aimed at achieving higher strength/ductility complex in low-alloyed steels [3, 4]. The steel with yield strength exceeding 550 MPa is known as AHSS (Advanced High Strength Steel) [5, 6]. At the moment there is a demand to develop new AHSSs of the third generation. Third-generation AHSSs are being designed as: (a) middle-Mn TWIP steels [7,8,9], (b) steels with carbide-free nanobanite [10,11,12], (c) steels for Q&P processing [13,14,15,16]. The latter is more promising direction allowing to get higher strength/ductility/wear resistance complex in low-alloyed steels [17,18,19].
2 Literature Review
Currently, one of the directions of designing new high-strength steels is heat treatment called “Quenching and Partitioning”, abbreviated as Q&P. This technology allows achieving a high complex strength/ductility property in relatively inexpensive low-alloyed steels. The technology is based on the concept of “Constrained Carbon Paraequilibrium” (CCE) proposed by Speer [20, 21]. Q&P treatment results in significant improvement of strength while ductility maintains at acceptable level which is beneficial for machine parts undergoing severe loadings under exploitation [22]. Q&P treatment is in focus to be applied for the steels and cast irons both [23,24,25].
According to CCE concept, if the precipitation of carbides in Fe–C alloys is suppressed, then a certain metastable (paraequilibrium) state should be established having a minimum free energy, at which the chemical potential of carbon in austenite and chemical potential of carbon in ferrite (martensite) are equal. This is ensured by a change in the amount and chemical composition of the phases. In the presence of slowly diffusing replacement elements (X) their ratio to iron (Fe/X) is almost unchanged, thus the equality of chemical potentials is achieved only due to redistribution of carbon from martensite to austenite [20]. CCE is achieved at:
-
the absence of precipitation of carbides from austenite;
-
the immobility of “martensite/austenite” boundary.
Experimental evidence of the diffusion of carbon from martensite to austenite was obtained using modern research techniques (WDS, Field-Emission EPMA, APM) for studying the processes occurring in Q&P treatment of high-Si steel [26, 27].
The Q&P process consists of several stages: austenitization followed by quenching cooling, the “partitioning” stage and final hardening. At the first stage, the steel after austenitization is rapidly cooled to a quenching temperature TQ, which is in the temperature range between Ms and Mf (temperature of martensite transformation start and finish accordingly), in order to turn part of austenite into martensite. The next stage involves heating to the “partitioning” temperature and holding at this temperature to redistribute carbon from martensite to austenite. The process is completed by final quenching (in water, oil, or in air) to room temperature [28]. As a result of carbon saturation, austenite is partially stabilized to the martensitic transformation under the final quenching, which increases the amount of retained austenite (RA) in resultant structure. After Q&P processing the structure should consist of tempered and “fresh” martensite as well as of RA of film-like or blocky morphologies [29]. This is retained austenite that is responsible for improvement of mechanical properties and wear resistance of steels due to manifestation of TRIP-effect [30].
To use Q&P treatment, the steel is required to have a certain alloying system (Si–Mn, Si–Al-Mn, Si–Mn-Cr) with mandatory higher amount (≥1.0 wt%) of silicon (or aluminum) which effectively suppress the precipitation of cementite from austenite during carbon partitioning [30,31,32]. Published data on the effect of Q&P treatment on the mechanical properties are mainly addressed to low carbon steel [33, 34]. The effect of Q&P treatment on the properties of steels with medium and high carbon content has been studied to a much lesser extent, and therefore requires further detailed study.
The enrichment of γ-phase with carbon depends on the ratio of the volume fractions of martensite and austenite [20]. This ratio depends on: (a) quenching temperature and (b) kinetics of martensitic transformation. Thus, when designing the Q&P treatment regime, it is important to know this kinetics in order to find the optimum temperature of quenching cooling suspense. Since such data are rarely found in the literature additional research concerning the steel of specific chemical composition is required.
3 Research Methodology
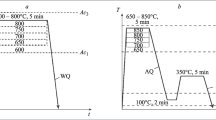
The study materials were commercial steel 60Si2CrVA (0.53 wt% C; 1.46 wt% Si; 0.44 wt% Mn; 0.95 wt% Cr; 0.10 wt% V; 0.016 wt% S; 0.013 wt% P) and experimental steel 55Si3Mn2CrVMoNb (0.56 wt% C; 2.50 wt% Si; 1.70 wt% Mn; 0.50 wt% Cr; 0.21 wt% Mo; 0.12 wt% V; 0.05 wt% Nb; 0.006 wt% S; 0.015 wt% P). The kinetics of the martensitic transformation was investigated using a magnetometer equipped with a strain gauge system for fixing the magnetization of the sample. The sample of 2 × 10 × 12 mm in size was austenized at 880 °C (60Si2CrVA) or 900 °C (55Si3Mn2CrVMoNb) for 5 min, then cooled in calm air hooked between the poles of a magnet. As the martensitic transformation developed, the sample was drawn into the magnetic field of the magnetometer, deforming the beam with strain gauges. The signal from the strain gauges was recorded using an analog-to-digital converter. The temperature was fixed using a chromel-alumel thermocouple welded to the sample (Fig. 1).
Up to 50 °C, the samples were cooled in air with average cooling rate of 13.3 K/s. Then, the sample was successively cooled outside the magnetometer to 4 °C (in ice water), to −20 °C and to −196 °C (in liquid nitrogen), after which it was placed in the magnetometer and the increase in the magnetic phase was measured. The volume fraction of retained austenite was found by XRD with Cu-Kα radiation [35]. Scanning electron microscope Ultra-55 Carl Zeiss was used for microstructure observation.
4 Results
4.1 “Constrained Paraequilibrium” Concept
The methodology for selecting the temperature of quenching suspension (first stage) during Q&P processing in order to obtain the maximum amount of retained austenite is proposed in [20, 21, 36]. This methodology is based on the assumption of the complete redistribution of carbon from fresh martensite to austenite in conditions when competitive reactions are suppressed, such as the formation of bainite, cementite or transition carbide [20, 37]. The model assumes the immobility of the interphase α/γ boundary, the flow of interstitial diffusion of carbon atoms and the absence of diffusion of iron atoms and impurity atoms which form a substitutional solid solution. Redistribution (“partitioning”) finishes when martensite (i.e. ferrite) comes to its metastable equilibrium with austenite, i.e. when at all points of the system, the same value of the chemical potential of carbon is reached. This final state is called “Constrained Paraequilibrium” (CPE), since it is based on the allowances listed above. With these assumptions, the final composition of austenite will be determined by the total carbon concentration in steel and the phase ratio during the “partitioning”.
In the conditions of interphase boundary immobility, the final state of the system can be calculated by solving equations that take into account the material balance on iron (it is assumed that the number of iron atoms in two phases during “partitioning” remains unchanged), the material balance on carbon and the condition of equality of the chemical potentials of carbon in ferrite and austenite (in this case, the influence of alloying elements is not taken into account):
where T is absolute temperature, \( X_{C}^{alloy} \) is carbon content in steel (mol%), \( f_{i}^{\gamma } \) is austenite fraction before “partitioning” (mol%), \( X_{{C_{i} }}^{\gamma } \) is initial carbon concentration in austenite, \( f_{CPE}^{\alpha } \) and \( f_{CPE}^{\gamma } \), \( X_{{C_{CPE} }}^{\alpha } \) and \( X_{{C_{CPE} }}^{\gamma } \) are α-phase and austenite fractions, as well as carbon content in α-phase and austenite, respectively, when equilibrium is reached.
The concentration of iron (carbon) in austenite is taken as for a binary alloy:
During the “partitioning”, the volume fraction of the phases must be changed (i.e. \( f_{CPE}^{\gamma } \ne f_{i}^{\gamma } \)) despite the fact that the interface is fixed). The fact is that the redistribution of carbon atoms between the phases must be accompanied by a small mutual adjustment of atoms in each phase, as well as a change in the density and parameter of the crystal lattice (accompanied by elastic deformation), which ultimately should lead to a slight change in the volume fraction of the phases.
4.2 The Determination of Martensite Transformation Kinetics
The magnetometric analyze was used to investigate the kinetics of athermal martensitic transformation in studied steels. In steel 55Si3Mn2CrMVNb, the ferromagnetic phase appears when cooled to 240 °C, which corresponds to the point Ms of this steel. A feature of steel 60SiCrVA is that when the sample is cooled in air, austenite transformation begins in the bainite region at 465 °C; upon further cooling, the amount of the ferromagnetic phase stabilizes up to 257 °C. Starting from 257 °C, the amount of the ferromagnetic phase increases sharply, indicating the beginning of the martensitic transformation. Thus, the point Ms for steel 60SiCrVA is fixed at 257 °C.
It was assumed that cooling in liquid nitrogen ensures the reaching of the temperature of martensitic transformation finish for both steels. Based on this, and taking into account the partial transformation of austenite in the bainitic region in 60SiCrVA steel, the obtained magnetometric curves were recalculated into the kinetic curves of the martensitic transformation presented in Fig. 2.
As follows from Fig. 2, for the studied steels the martensitic transformation has a kinetics close to explosive type, i.e. the main fraction of martensite is formed within the temperature range of 120°–150° below Ms. Thus, for steel 55Si3Mn2CrMVNb, the cooling to 200 °C leads to the appearance of 22 vol% martensite, and at 100 °C its amount increases to 80 vol%. At lower temperatures, the intensity of the martensitic transformation sharply decreases. At room temperature, about 7 vol% of austenite is retained in the structure of steel 55Si3Mn2CrMVNb. For steel 60SiCrVA, the most considerable increase in martensite amount corresponds to the interval Ms—200 °C, when 45 vol% of martensite is formed. At lower temperatures, the growth rate of martensite decreases. When cooled to 20 °C, the structure of steel 60SiCrVA contains about 4% of RA.
Many empiric equations were previously proposed to describe the kinetics of martensitic transformation in steels. It was found that equation of Koistinen-Marburger (K-M) [37] with a parameter am equal 0.012 is most suitable for the accurate prediction of martensite volume fraction (f) formed in steels 60SiCrVA and 55Si3Mn2CrMVNb:
4.3 The Calculation of “Quenching” Temperature for Q&P Process
The choice of the quenching temperature, ensuring the achievement of the maximum amount of retained austenite, was carried out in three stages. At first, the microstructural state after austenitization was evaluated. Assuming heating to the austenitic region, 100% austenite was taken with a carbon concentration equal to the total carbon concentration in the steel. At the second stage, the amount of martensite and austenite was calculated at a specific quenching temperature. For this purpose, the K-M equation (6) was used.
At the third stage, the final chemical composition of austenite was calculated according to Eqs. (1)–(5) for partitioning finish. Using these data, the fraction of carbon-saturated austenite which transforms into fresh martensite at the final stage of Q&P processing was determined at the fourth stage. For this purpose, the K-M equation was used again, and Ms point for austenite was calculated depending on its elemental composition (wt%) using the equation of Andrews (7) [38] for steel 60SiCrVA and using the equation of Kunitake (8) [38] for steel 55Si3Mn2CrMV:
The calculated results are presented in Fig. 3. According to these results, the RA amount after Q&P treatment should vary along the curve with a maximum corresponding to quenching temperature 196 °C for steel 60SiCrVA and to quenching temperature 177 °C for steel 55Si3Mn2CrMVNb. After the quenching to this temperature and after the subsequent carbon partitioning, the carbon content in austenite will increase to 1.08 and 1.12 wt% for the steels 60SiCrVA and 55Si3Mn2CrMVNb, respectively. With such carbon content, the Ms point of the steels decreases to room temperature, and, accordingly, fresh martensite does not form at the final cooling resulting in 48.4 vol% of RA for steel 60SiCrVA and 47.4 vol% of RA for steel 55Si3Mn2CrMVNb. As the quenching temperature further decreases, the carbon content in RA continuously increases (at 100 °C: to 3.48 wt% in steel 60SiCrVA and to 2.84 wt% in steel 55Si3Mn2CrMVNb), which is associated with an increase in amount of fresh martensite and, accordingly, with a decrease in amount of austenite, in which carbon can be partitioned.
The calculated results depict the quenching temperature range which should be in a focus when choosing Q&P parameters (150–220 °C). An excessively high (above 220 °C) and an excessively low (below 150 °C) temperature will not ensure an increased volume fraction of RA, i.e. the target of Q&P processing will not be achieved. Based on this finding Q&P heat treatment was performed for according to the regimes: (a) for steel 60SiCrVA—quenching to 160 °C, partitioning at 300 °C for 5 min; (b) for steel 55Si3Mn2CrMVNb—quenching to 160 °C, partitioning at 300 °C for 120 min.
After the treatments XRD measurements were fulfilled revealing dual-phase “martensite/retained austenite” microstructure (Fig. 4) with RA of 17.0 vol% in steel 60SiCrVA and 28.5 vol% in steel 55Si3Mn2CrMVNb (see dots on Fig. 3a, b). Thus, the real RA amount is significantly lower that of predicted by modeling. This finding is in accordance with data presented in [20]. The main reason of discrepancy is that not all carbon escaped from martensite into austenite under partitioning. Some of carbon was bond with dislocations as “atmospheres” while some part was consumed by ε-carbides precipitated from martensite during partitioning. Nevertheless the calculation of quenching temperature gives useful information for designing Q&P heat treatment to ensure the best mechanical performance of steel.
Microstructure of Q&P treated steels 55Si3Mn2CrMVNb (a) and 60SiCrVA (b) (the designations are the same as in Fig. 3).
Q&P heat treatment is already applied for low-carbon steel sheet production [21], though this technology is perspective to be used for machine parts and tools (especially for cold forming) made of steel with higher carbon content (0.3–0.7 wt% C).
5 Conclusions
The martensite start temperature and the kinetics of athermal martensitic transformation are experimentally defined for the steels 60SiCrVA and 55Si3Mn2CrMVNb. It was found that this kinetics can be adequately predicted by equation of Koistinen-Marburger with a parameter am equal 0.012. The model based on “Constrained Paraequilibrium” concept was applied to calculate the temperature of quenching suspension at the first stage of Q&P heat treatment. As seen, the quenching temperature for both steels should fall in the range of 150–220 °C in order to obtain the maximum fraction of retained austenite in Q&P-treated steels. The performance of Q&P treatment with TQ = 160 °C and partitioning at 300 °C provided retained austenite of 17 and 28 vol% in steels 60SiCrVA and 55Si3Mn2CrMVNb, respectively, which is beneficial for steels mechanical properties.
References
Fonstein, N.: Advanced high strength sheet steels: physical metallurgy, design, processing, and properties. Springer, Berlin (2015)
Billur, E., Dykeman, J., Altan, T.: Three generations of advanced high strength steels for automotive applications. Stamp. J., 12–13 (2014 Mar/Apr)
Kuziak, R., Kawalla, R., Waengler, S.: Advanced high strength steels for automotive industry. Arch. Civ. Mech. Eng. 8(2), 103–117 (2008)
Grushko, A.V., Kukhar, V.V., Slobodyanyuk, YuO: Phenomenological model of low-carbon steels hardening during multistage drawing. Solid State Phenom. 265, 114–123 (2017)
Karelova, A., Krempaszky, C., Werner, E., Tsipouridis, P., Hebesberger, T., Pichler, A.: Hole expansion of dual-phase and complex-phase AHS steels—effect of edge conditions. Steel Res. Int. 80(1), 71–77 (2009)
Nie, Y.H., Fu, W.T., Hui, W.J., Dong, H., Weng, Y.Q.: Very high cycle fatigue behaviour of 2000-MPa ultra-high-strength spring steel with bainite–martensite duplex microstructure. Fatigue Fract. Eng. Mater. Struct. 32(3), 189–196 (2009)
De Cooman, B.C., Estrin, Y., Kim, S.K.: Twinning-induced plasticity (TWIP) steels. Acta Mater. 142, 283–362 (2018)
Lee, S., Lee, S.J., Kumar, S., Lee, K., De Cooman, B.C.: Localized deformation in multiphase, ultra-fine-grained 6 pct Mn transformation-induced plasticity steel. Metall. Mater. Trans. A 42(12), 3638–3651 (2011)
Heo, Y.U., Song, Y.Y., Park, S.J.: Influence of silicon in low density Fe-C-Mn-Al steel. Metall. Mater. Trans. A 43(6), 1731–1735 (2012)
Morales-Rivas, L., Garcia-Mateo, C., Sourmail, T., Rementeria, R., Caballero, F.G.: Ductility of nanostructured bainite. Metals 6(12), 302 (2016)
Caballero, F.G., Bhadeshia, H.K.D.H., Mawella, K.J., Jones, D.G., Brown, P.: A very strong low temperature bainite. Mater. Sci. Technol. 18, 279–284 (2002)
Garcia-Mateo, C., Caballero, F.G., Sourmail, T., Kuntz, M., Cornide, J., Elvira, R.: Tensile behaviour of a nanocrystalline bainitic steel containing 3wt% silicon. Mater. Sci. Eng., A 549, 185–192 (2012)
Han, X., Zhong, Y., Xin, P., Chen J.: Research on one-step quenching and partitioning treatment and its application in hot stamping process. Proc. Inst. Mech. Eng. Part B: J. Eng. Manuf. 231(11), 1972–1982 (2017)
Nayak, S.S., Anumolu, R., Misra, R.D.K., Kim, K.H., Lee, D.L.: Microstructure–hardness relationship in quenched and partitioned medium-carbon and high-carbon steels containing silicon. Mater. Sci. Eng. A 498, 442–456 (2008)
Zhou, S., Zhang, K., Wang, Y., Gu, J.F., Rong, Y.H.: High strength-elongation product of Nb-microalloyed low-carbon steel by a novel quenching–partitioning–tempering process. Mater. Sci. Eng., A 528, 8006–8012 (2011)
Seo, E.J., Cho, L., Estrin, Y., De Cooman, B.C.: Microstructure-mechanical properties relationships for quenching and partitioning (Q&P) processed steel. Acta Mater. 113, 124–139 (2016)
Speer, J.G., Rizzo, F.C., Matlock, D.K., Edmonds, D.V.: The “quenching and partitioning” process: background and recent progress. Mater. Res. 8(4), 417–423 (2005)
Efremenko, V.G., Zurnadzhi, V.I., Chabak, Y.G., Tsvetkova, O.V., Dzherenova, A.V.: Application of the Q-n-P-treatment for increasing the wear resistance of low-alloy steel with 0.75% C. Mater. Sci. 53, 67–75 (2017)
Seo, E.J., Cho, L., De Cooman, B.C.: Application of quenching and partitioning (Q&P) processing to press hardening steel. Metall. Mater. Trans. A 45, 4022–4037 (2014)
Speer, J.G., Matlock, D.K., De Cooman, B.C., Schroth, J.G.: Carbon partitioning into austenite after martensite transformation. Acta Mater. 51(9), 2611–2622 (2003)
Wang, L., Speer, J.G.: Quenching and partitioning steel heat treatment. Metallogr. Microstruct. Anal. 2(4), 268–281 (2013)
Ostash, O.P., Kulyk, V.V., Poznyakov, V.D., Haivorons’kyi, O.A., Markashova, L.I., Vira, V.V., Duriagina, Z.A., Tepla, T.L.: Fatigue crack growth resistance of welded joints simulating the weld-repaired railway wheels metal. Arch. Mater. Sci. Eng. 86(2), 49–55 (2017)
Jia, X., Zuo, X., Liu, Y., Chen, N., Rong, Y.: High wear resistance of white cast Iron treated by novel process: principle and mechanism. Metall. Mater. Trans. A 46(12), 5514–5525 (2015)
Efremenko, V.G., Chabak, Y.G., Lekatou, A., Karantzalis, A.E., Efremenko, A.V.: High-temperature oxidation and decarburization of 14.55 wt pct Cr-cast iron in dry air atmosphere. Metall. Mater. Trans. A 47, 1529–1543 (2016)
Efremenko, V.G., Shimizu, K., Pastukhova, T.V., Chabak, YuG, Kusumoto, K., Efremenko, A.V.: Effect of bulk heat treatment and plasma surface hardening on the microstructure and erosion wear resistance of complex-alloyed cast irons with spheroidal vanadium carbides. J. Frict. Wear 38(1), 58–64 (2017)
Santofimia, M.J., Zhao, L., Petrov, R., Kwakernaak, C., Sloof, W.G., Sietsma, J.: Microstructural development during the quenching and partitioning process in a newly designed low-carbon steel. Acta Mater. 59, 6059–6068 (2011)
Toji, Y., Miyamoto, G., Raabe, D.: Carbon partitioning during quenching and partitioning heat treatment accompanied by carbide precipitation. Acta Mater. 86, 137–147 (2015)
Huang, X., Liu, W., Huang, Y., Chen, H., Huang, W.: Effect of a quenching–long partitioning treatment on the microstructure and mechanical properties of a 0.2 C% bainitic steel. J. Mater. Process. Technol. 222, 181–187 (2015)
Xiong, X.C., Chen, B., Huang, M.X., Wang, J.F., Wang, L.: The effect of morphology on the stability of retained austenite in a quenched and partitioned steel. Scripta Mater. 68(5), 321–324 (2013)
Hesse, O., Merker, J., Brykov, M., Efremenko, V.: Zur Festigkeit niedriglegierter Stäble mit erhöhtem Kohlenstoffgehalt gegen abrasiven Verschleiß (On the strength of low-alloy steels with increased carbon content against abrasive wear). Tribol. + Schmier. 60(6), 37–43 (2013)
Mašek, B., Jirková, H., Hauserova, D., Kučerová, L., Klauberová, D.: The effect of Mn and Si on the properties of advanced high strength steels processed by quenching and partitioning. Mater. Sci. Forum 654, 94–97 (2010)
Hesse, O., Liefeith, J., Kunert, M., Kapustyan, A., Brykov, M., Efremenko, V.: Bainite in steels with high resistance against abrasive wear [Bainit in Stählen mit hohem Widerstand gegen Abrasivverschleiß]. Tribol. + Schmier. 63(2), 5–13 (2015)
Maheswari, N., Chowdhury, S.G., Hari Kumar, K.C., Sankaran, S.: Influence of alloying elements on the microstructure evolution and mechanical properties in quenched and partitioned steels. Mater. Sci. Eng., A 600, 12–20 (2014)
Zhang, J., Ding, H., Wang, C., Zhao, J., Ding, T.: Work hardening behaviors of a low carbon Nb-microalloyed Si–Mn quenching–partitioning steel with different cooling styles after partitioning. Mater. Sci. Eng. A 585, 132–138 (2013)
Efremenko, V.G., Chabak, YuG, Lekatou, A., Karantzalis, A.E., Shimizu, K., Fedun, V.I., Azarkhov, AYu., Efremenko, A.V.: Pulsed plasma deposition of Fe-C-Cr-W coating on high-Cr-cast iron: Effect of layered morphology and heat treatment on the microstructure and hardness. Surf. Coat. Technol. 304, 293–305 (2016)
Santofimia, M.J., Nguyen-Minh, T., Zhao, L., Petrov, R., Sabirov, I., Sietsma, J.: New low carbon Q&P steels containing film-like intercritical ferrite. Mater. Sci. Eng., A 527, 6429–6439 (2010)
Clarke, A.J., Speer, J.G., Miller, M.K., Hackenberg, R.E., Edmonds, D.V., Matlock, D.K., De Moor, E.: Carbon partitioning to austenite from martensite or bainite during the quench and partition (Q&P) process: A critical assessment. Acta Mater. 56, 16–22 (2008)
Gorni, A.: Steel forming and heat treating book. (2012) http://www.chte.org/d/file/p/2013-01-22/bbf30bc70b449796e79d568fe864a802.pdf
Acknowledgements
The support of Ministry of Education and Science of Ukraine (project number 0117U002270) is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this paper
Cite this paper
Zurnadzhy, V., Zaichuk, N., Sergeev, A., Chabak, Y., Efremenko, V. (2020). Optimal Parameters of Q&P Heat Treatment for High-Si Steels Found by Modeling Based on “Constrained Paraequilibrium” Concept. In: Ivanov, V., et al. Advances in Design, Simulation and Manufacturing II. DSMIE 2019. Lecture Notes in Mechanical Engineering. Springer, Cham. https://doi.org/10.1007/978-3-030-22365-6_49
Download citation
DOI: https://doi.org/10.1007/978-3-030-22365-6_49
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-22364-9
Online ISBN: 978-3-030-22365-6
eBook Packages: EngineeringEngineering (R0)