Abstract
The Cannabis plant has been used for many of years as a medicinal agent in the relief of pain and seizures. It contains approximately 540 natural compounds including more than 100 that have been identified as phytocannabinoids due to their shared chemical structure. The predominant psychotropic component is Δ9-tetrahydrocannabinol (Δ9-THC), while the major non-psychoactive ingredient is cannabidiol (CBD). These compounds have been shown to be partial agonists or antagonists at the prototypical cannabinoid receptors, CB1 and CB2. The therapeutic actions of Δ9-THC and CBD include an ability to act as analgesics, anti-emetics, anti-inflammatory agents, anti-seizure compounds and as protective agents in neurodegeneration. However, there is a lack of well-controlled, double blind, randomized clinical trials to provide clarity on the efficacy of either Δ9-THC or CBD as therapeutics. Moreover, the safety concerns regarding the unwanted side effects of Δ9-THC as a psychoactive agent preclude its widespread use in the clinic. The legalization of cannabis for medicinal purposes and for recreational use in some regions will allow for much needed research on the pharmacokinetics and pharmocology of medical cannabis. This brief review focuses on the use of cannabis as a medicinal agent in the treatment of pain, epilepsy and neurodegenerative diseases. Despite the paucity of information, attention is paid to the mechanisms by which medical cannabis may act to relieve pain and seizures.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
Extracts from the cannabis plant have been used medicinally for thousands of years. The first recorded use of cannabis as a medicinal compound appeared almost 5000 years ago in early Chinese texts by the Emperor Chen Nung [1, 2] when it was used as a treatment of malaria, constipation, rheumatic pain and analgesia in childbirth. Similar accounts of its use as a therapeutic agent occurred in ancient Egypt and India, around 3000 years ago [3, 4]. In more modern times it was listed in Canadian, US and British pharmacies for many years before concerns of its effects as a psychotropic agent led to it being criminalized and listed as an illicit drug of abuse in the 1970s. However, the last 15–20 years has seen a resurgence in interest of cannabis as a therapeutic agent for a range of illnesses and diseased conditions, and the decriminalization and legalization of cannabis will surely pave the way for much needed research on the therapeutic potential of this plant.
The origins of cannabis plant use can be traced back to central Asia [5, 6] with an appearance in the Western hemisphere in the 1500s [7]. There is general agreement among botanical taxonomists that more than one species of cannabis plant exists, with possibly up to 4 species in existence: Cannabis sativa, Cannabis indica, Cannabis ruderalis and Cannabis afghanica. The predominant form that is widely used in western society is Cannabis sativa, of which there are multiple chemical phenotypes (or chemotypes) which express differing chemical compositions of cannabinoids . Different chemotypes range from plants that contain Δ9-THC as the predominant cannabinoid, to plants that contain CBD as the predominant cannabinoid, to a variety of mixtures of the two [7]. There are even chemotypes that express high titers of other less known cannabinoids such as cannabidivarin or tetrahydrocannabivarin (THCV) [7]. The wide range of chemotypes is especially pertinent for medicinal forms of cannabis where producers aim to breed specific chemical phenotypes that are high in CBD and low in THC in order to minimize unwanted psychotropic effects of Δ9-THC.
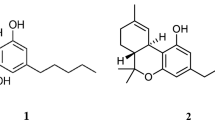
C. sativa contains approximately 540 natural compounds of which more than 100 have been identified as phytocannabinoids due to their shared chemical structure [8]. Phytocannabinoids are neutral cannabinoids that possess a lipid backbone featuring alkylresorcinol and monoteropenes in their molecules [8, 9] (Fig. 8.1). Cannabinoids are biosynthesized as cannabinoid acids and then decarboxylated into the neutral forms [8]. Phytocannabinoids can be classified into several subclasses including the tetrahydrocannabinol type, the Δ9-tetrahydrocannabivarin type, the cannabidiol type, the cannabinol type, and several others [8]. Of these, trans-Δ9-tetrahydrocannabinol (Δ9-THC) and CBD are the compounds that have been investigated to a much greater degree compared with many of the others, with CBD showing significant potential as a therapeutic agent in a several pathophysiological or diseased states.
While selective breeding of various chemotypes leads to a number of varieties that express very different titers of cannabinoids, the predominant cannabinoid in C. sativa which induces psychotropic effects is Δ9-THC. It was not until the cloning of the first cannabinoid receptor type (CB1) in 1990 that the pharmacodynamics of phytocannabinoids was initiated [10, 11]. Three years later, the second cannabinoid receptor type 2 (CB2) was cloned [12]. We now know that phytocannabinoids have the ability to influence many physiological states through their interactions with receptors and transmembrane proteins such as the prototypical CB receptors, transient receptor potential cation channels (e.g. TRPV1, TRPV2, TRPA1) and the serotonin receptors 5HT2 to only name a few. We first address some of the relevant receptor and protein interactions and then focus on therapeutic applications for pain relief, epilepsy and neurodegeneration.
8.2 Cannabinoid Receptors
It had long been thought that cannabinoids interact with receptors to produce their wide-ranging effects as psychotropic agents, analgesics or anti-emetic compounds, but it was not until 1990 that the first cannabinoid receptor was cloned from rat cerebral cortex cDNA library [10]. The translated genetic sequence gave rise to a 473 amino acid protein of the G-protein coupled family of receptors, which contained seven putative hydrophobic or membrane-spanning domains, and several potential glycosylation sites. When expressed in Chinese hamster ovary K1 cells the protein displayed cannabinoid stereo-selectivity and cannabinoid-induced inhibition of adenylate cyclase activity [10]. Consequently, the human homologue (472 amino acid protein) and mouse homologue (473 amino acid protein) were rapidly identified [11, 13]. Three years after the initial cloning of the rat CB1 receptor, a second type of G-protein coupled cannabinoid receptor was cloned from a human promyelocytic leukaemia cell line (HL60) [12]. This receptor was highly expressed in macrophages obtained from spleen and its amino acid composition exhibited significant divergence from the CB1 receptor that was cloned from rat brain. Evidence has now accumulated to show that both CB1 and CB2 receptors are negatively coupled to adenylate cyclase and are typically expressed in very different regions of the body. CB1 receptors are mainly limited to the brain and CNS, while CB2 receptors are largely confined to the peripheral nervous system and the immune system. A detailed tissue distribution of cannabinoid receptors is reviewed elsewhere [14, 15]. Radiolabeling of CB1in the brain with the tritiated CB1 receptor agonist [3H]CP55,940 showed high density expression in regions of the basal ganglia such as the substantia nigra pars reticulata and globus pallidus, as well as in the hippocampus and cerebellum [16]. However, expression was sparse in the thalamus and lower brainstem regions [16]. The subcellular location of receptors provided clues of their functional roles. Because CB1 receptors are highly localized to presynaptic membranes, they were thought to act as modulators of synaptic release. Indeed, physiological studies confirmed this hypothesis and showed that activation of CB1 altered synaptic transmission in a homeostatic manner. But how does this occur? What are the mechanisms that underlie these effects? To answer these questions we need to delve/examine the literature on the pharmacology of CB1 receptor activation (Table 8.1).
Both CB1 and CB2 receptors are negatively linked to adenylate cyclase activity (Fig. 8.2). When the receptors are expressed in cell lines, they initiate a pertussis toxin mediated event that requires Gi/o signaling and that results in a reduction of cAMP production [17]. Ligand binding studies show that the endocannabinoid , anandamide , is capable of inhibiting adenylate cyclase activity in membranes possessing CB1 receptors [18, 19], but this same agonist shows markedly less efficacy on CHO cells expressing CB2 receptors, suggesting that anandamide has differential effects on CB1 vs CB2 receptors. In contrast, the other main endocannabinoid , 2-Arachidonoylglycerol (2-AG) , acts as a full agonist at the cannabinoid receptors when inhibiting forskolin-induced cAMP accumulation [20]. A critical determinant of the downstream effects of CB receptor activation is the isoform of adenylate cyclase that associates with the receptor. For instance, ligand binding to CB receptors co-expressed with adenylate cyclase isoforms 1, 3, 5, 6 or 8 leads to inhibition of cAMP, whereas co-expression with adenylate cyclase isoforms 2, 4, or 7 leads to stimulation of cAMP production [21, 22]. Thus, CB1/CB2 are capable of activating Gq in addition to Gi/o even though much of the endogenous or physiological activity appears to lead to an inhibition of cAMP. Our understanding of the mechanisms that underlie key interactions between the cannabinoid receptors and their agonists and antagonists was further increased with the elucidation of the crystal structure of the human CB1 receptor in 2016 [23].
Schematic outline for some of the possible receptors for phytocannabinoids and endocannabinoids . The prototypical G-protein coupled receptors for cannabinoids are CB1 and CB2, but GPR55 has been suggested to be a possible third cannabinoid receptor. CB1 and CB2 are negatively coupled to adenylate cyclase (AC) via Gi/o, while GPR55 is potentially linked to the IP3/DAG/Ca2+ system. Cannabinoids are also known to bind to transient receptor potential channels such as TRPV1, TRPV2 and TRPA1. Possible downstream effects include the regulation of genes and ion channel activity (A-type K+ channels)
The search for additional cannabinoid receptors led to the presentation/publication of convincing evidence in 2007 that the orphan receptor GPR55 is a cannabinoid receptor [24]. Cloning, sequencing and expression of GPR55 showed that the CB1/CB2 receptor ligand [3H]CP55940 exhibited high specificity for GPR55. Moreover, the receptor can also be activated by Δ9-THC, anandamide , 2-AG and the CB1 selective agonist noladin ether. Interestingly, 2-AG displays almost 200-fold greater potency as an agonist at GPR55 compared with the prototypical CB1and CB2 receptors, and that Δ9-THC has a greater efficacy at GPR55 compared with CB1 or CB2. GPR55 couples to Ga13 [24], but has also been linked to increases in intracellular Ca2+ via a mechanism that involves Gq, G12, RhoA, actin, phospholipase C and Ca2+ release from IP3-gated stores [25]. In other words, cannabinoid receptors are linked to multiple second messenger systems that have the potential to couple enzyme activity to ion channel behavior to gene activation and more. An investigation into the role of GPR55 at presynaptic terminals of CA3-CA1 synapses show that activation of GPR55 by L-α-lysophosphatidylinositol (LPI) transiently increases calcium release probability by elevating presynaptic Ca2+ through activation of local Ca2+ stores, implying a possible role in short-term potentiation in hippocampus [26]. Based upon these findings there have been suggestions that the GPR55 receptor could be renamed a type 3 cannabinoid receptor, CB3. Nonetheless, its current classification notwithstanding, GPR55 shows significant characteristics of a true cannabinoid type receptor and fully determining its distribution within the body, subcellular localization, temporal expression patterns and downstream signaling pathways will lead to a greater understanding of the function of endocannabinoids and effects of phytocannabinoids.
There is now significant evidence for a direct interaction between cannabinoids and transient receptor potential channels such as the transient receptor potential of vanilloid type 1 and 2 (TRPV1 and TRPV2) and transient receptor potential of ankyrin type 1 (TRPA1) [27]. TRPV1 and V2 channels are cation channels that allow the passage of Na+, K+ and Ca2+ across cell membranes and are activated by capsaicin or heat above temperatures of 40 °C and above ~50 °C respectively, whereas TRPA1 are menthol and cold activated cation channels [28]. TRPV1 are activated by the endocannabinoids 2-AG and anandamide [29], while TRPV2 and TRPA1 are activated by Δ9-THC and CBD [29,30,31]. TRPV1 are largely found in the cerebellum, basal ganglia, hippocampus, diencephalon and DRG neurons [32, 33]. TRPV2 tend to be localized to sensory neurons of the DRG, spinal cord, and trigeminal ganglia, but are also found in the cerebellum [34, 35]. TRPA1 is extensively colocalized with TRPV1 in sensory neurons [36,37,38]. Activation of these receptors typically leads to membrane depolarization and activation, but TRPV1 and TRPA1 are known to exhibit functional desensitization. In other words, activation of TRPV1 and TRPA1 by cannabinoids may lead to an immediate depolarization, but this will be followed by sensitization and subsequently inhibition because further activation by ligands, heat or cold will be muted as the channels are in a desensitized state. Some evidence exists for the direct interaction between Cannabinoids and ion channels and it has been hypothesized that some of the CB1/CB2-independent cannabinoid effects occur in this manner.
8.3 Pharmacokinetics of Cannabinoid Preparations
THC is highly lipophilic and accumulates in adipose tissue and the spleen which can act as long-term storage sites [39]. It is estimated that up to 37% of Δ9-THC present in cigarettes can be delivered to the body during smoking while up to 30% is destroyed via pyrolysis [40]. When smoked, Δ9-THC enters the blood stream extremely rapidly with rising levels detected in blood plasma within 1–2 min of the first inhalation [41]. In controlled experiments, puffs of a 3.5% Δ9-THC cigarette result in peak Δ9-THC blood plasma levels of approximately 270 ng/ml [41], and in experiments where the THC content of cigarettes was kept at either a “low” dose of 1.75% or a “high” dose of 3.55%, the blood plasma levels obtained from individuals smoking the higher dose cigarettes were variable and ranged from <90 ng/ml to >250 ng/ml [41]. These data indicate that the bioavailability varies substantially with each individual, and factors such as weight, gender, age, health and physiological background will likely impact the extent to which Δ9-THC and other cannabinoids affect an individual. Δ9-THC taken orally usually peaks in the circulation within 1–2 h, with blood plasma levels lower than those obtained during smoking [42]. Δ9-THC accumulates in fatty tissue and organs such as the heart, liver and spleen [39]. It readily crosses the blood-brain barrier and can be found in high quantities in the brain [42]. THC released from fat has a half-life of several days and in some instances may take up to several weeks to fully clear from adipose tissue [41, 43].
Much of the metabolism of Δ9-THC occurs in the liver where it is converted to 11-hydroxy-THC or 11-nor-9-carboxy-THC [41]. This conversion is rapid and occurs within minutes of THC detection in blood plasma [43,44,45]. Whereas 11-hydroxy-THC is psychotropically active, 11-nor-9-carboxy-THC is not [46] and is the principle component found in urine analyses as a proxy for determining cannabis consumption [43]. Numerous additional oxidative metabolites occur, but in lesser quantities.
8.4 Medicinal Cannabis
Cannabis has been used as a medicinal agent and an analgesic for many years. It is sought after as an anti-emetic (anti-nausea agent), a treatment for epilepsy, muscle spasms, multiple sclerosis, neuropathic pain, neurodegenerative diseases and cancer. Cannabis-derived pharmaceuticals such as nabilone (a compound of the same general type as Δ9-THC), nabiximols and dronabinol (a synthetic Δ9-THC) are prescribed to relieve chemotherapy-induced nausea and vomiting. Sativex (a combination of Δ9-THC and CBD ) has been used to alleviate neuropathic pain . We will now explore its use as a medicinal agent.
8.5 Pain
Even though the use of cannabis for the treatment of pain can be traced back to 5000 years ago, there is still only little information on its mechanisms of action. In fact, questions still arise whether or not cannabis may alleviate certain types of pain. Cannabinoids and cannabinoid-based pharmaceuticals are prescribed to alleviate neuropathic pain, which is a severe form of chronic pain arising from lesions or disease affecting the somatosensory system [47]. Evidence is mounting that THC in particular, is somewhat effective in reducing neuropathic pain [48,49,50], however the data is inconsistent and the potential side effects are concerning. A strong desire to find alternatives to other pain medication such as opioids has pushed cannabinoids-based pain therapies to the forefront, and while there is a general lack of well-designed studies on the effects of medical cannabis as pain medications, there is data to indicate that smoking cannabis is effective for some forms of pain.
Studies designed to compare the effects of smoked cannabis against a placebo showed that participants generally reported effective pain relief with increased efficacy linked to higher THC content [51]. Overall the pain relief was modest, and not as effective as medications prescribed specifically for pain such as, the GABA receptor agonists gabapentin and pregabalin. As a general rule, more effective pain relief tends to occur when cannabinoids are taken together with existing pain medications as opposed to being taken on their own. For instance, oromucosal sprays such as Nabiximols (equal mixtures of Δ9-THC and CBD ), taken along with existing pain medication results in a significant reduction in pain intensity [49, 52, 53]. Similarly, Δ9-THC/CBD spray was found to be better than placebo when comparing mean pain relief [54].
Other studies have examined the effects of medical marijuana , which contains several hundred compounds along with approximately 100 cannabinoids [7]. Systematic reviews of randomized clinical trials on the pain relief effectiveness of medical marijuana found that medical marijuana was effective in reducing neuropathic pain only in the short term, measured in days rather than weeks or months. Interestingly, medical marijuana was better than placebo in providing a minimum pain relief of 30%, but there was no statistically significant difference between medical marijuana and placebo when comparing the mean pain relief [54].
When evaluating the effectiveness of cannabinoids for relief of visceral pain such as rheumatic disease pain, the data is inconclusive. Systematic reviews of several randomized clinical trials evaluating Δ9-THC/CBD oromucosal sprays in patients with musculoskeletal pain, fibromyalgia and rheumatoid arthritis concluded that there was insufficient evidence to recommend cannabinoids as pain relief treatment [55, 56]. However, an analysis of medical marijuana administered as a cigarette resulted in a decrease in abdominal pain and an increase in appetite of patients with Crohn’s disease compared with placebo cigarettes not containing Δ9-THC [57]. Moreover, a 3-month study on the effect of oral Δ9-THC on chronic pancreatitis led the authors to conclude that there was no significant difference between the effects of Δ9-THC compared with placebo [58]. Overall, the data is largely inconclusive in support of the idea that medical marijuana provides significant relief for chronic pain associated with cancer, rheumatoid arthritis, or fibromyalgia. Clearly, more research is needed to ascertain the use of cannabis or individual cannabinoids as effective analgesics. Of particular interest is the role of synthetic cannabinoids as analgesics. Synthetic cannabinoids (SCBs), also known as K2, spice, herbal incense and other names, are full agonists at CB1 and CB2 receptors, whereas Δ9-THC is a partial agonist. Thus, SCBs have the potential to act as pain relief agents. In fact, tail immersion assays in mice, indicate that SCBs such as JWH-018 and JWH-073 do indeed act as analgesics [59]. In these studies, the tails of mice were allowed to freely hang into 55 °C water and the time taken for the mouse to remove its tail from the painfully hot stimulus was measured. Administration of JWH-018:JWH-073 in the ratios of 2:3 and 1:1 resulted in an increase in the tail immersion time, in a manner that was additive for the 1:1 ration but synergistic for the 2:3 ratio of SCBs [59], with the tails of immobilized animals hung freely and were placed in 55 °C water.
How does medical marijuana or cannabinoids (Δ9-THC/CBD ) alleviate neuropathic pain? The answer to this is unclear but several possibilities exist. First, the use of THC as a pain relief agent is problematic because of the potential side effects as a psychoactive agent, whereas CBD offers far more promise because it does not activate CB1 receptors and indeed acts as a negative allosteric modulator of CB1, meaning that it does not induce similar psychotropic effects to that of Δ9-THC. In fact, high concentrations of CBD can be administered in vivo with relatively few complications [60]. However, care must still be taken when determining the type of patient to receive CBD based upon age, health, pregnancy status, existing illnesses etc. To act as analgesics, cannabinoids may associate with the prototypical cannabinoid receptors, CB1 and CB2Rs, but the data for CB1 is inconsistent and CBD is not an agonist of this receptor. CB1 receptors are largely limited to the CNS and not the periphery but are still associated with sensory neurons. CB1 knockouts in sensory neurons results in a reversal of cannabinoid induced anti-hyperalgesia [61], while another study found that CB1 null-mutant mice experienced significantly less anti-hyperalgesia effects, and only in the peripheral nervous system [29]. In several studies, peripheral pain responses are studied via examining capsaicin (CAP)-induced nociception. Some of these responses were found to be independent of G-protein coupled pathways [62], implying a more direct mechanism of action such as that associated with transient receptor potential channels. Indeed, cannabinoids acting via TRP channels is a very attractive hypothesis because TRP channels are highly localized to sensory neurons and they have been shown to undergo cannabinoid-induced desensitization. Moreover, their activation does not rely on G-proteins but may rely on Ca2+/calcineurin.
An area that is receiving more attention with regard to pain relief is that of cannabinoid anti-inflammatory effects. Since inflammation can contribute to acute and chronic pain, treatments that reduce inflammation may be effective pain relief agents. CBD has long been known as an anti-inflammatory compound and has been investigated for its ability to prevent osteoarthritic pain through its anti-inflammatory actions. For instance, local administration of CBD to male Wistar rats in which osteoarthritis was induced, resulted in a reduction in transient joint inflammation and blocked osteoarthritic pain [63]. Thus, the actions of cannabinoids as pain relief agents are still unclear. Anecdotally, patients who smoke marijuana espouse its analgesic effects on neuropathic pain, but there are only a few properly controlled, double blind, randomized clinical trials in existence and more are certainly needed if we are to have a clearer picture of medicinal marijuana and pain.
8.6 Epilepsy
Epilepsy is a disease in which neuronal networks in the brain become hyperexcitable and are capable of discharging synchronous activity. Epileptic seizures originate from various regions of the brain, usually cortical or sub-cortical structures, and can be classified as partial or generalized seizures. Epilepsy affects approximately 65 million people worldwide with an incidence rate of around 20–70 new cases per 10,000 people on an annual basis [64,65,66,67]. Approximately one third of individuals suffering from epilepsy are drug-resistant, meaning that their seizures cannot be controlled with the application of at least two anti-epileptic medications [68]. Thus, there is significant need for therapies capable of controlling epileptic seizures. It has long been thought that marijuana can reduce the severity and incidence of convulsions, epileptic seizures and spasticity. Animal epileptic model studies have shown that CBD has anticonvulsant abilities when tested in audiogenic seizure models [69, 70]; pilocarpine models [70, 71] and electroshock models [69]. Tests designed to evaluate the efficacy of Δ9-THC and CBD in animal models of epilepsy clearly indicate that both Δ9-THC and CBD have anticonvulsant effects in rodents [72]. Similarly, the endocannabinoid anandamide produces anticonvulsant effects in rodents as well [73]. Finally, synthetic agonists of CB1 receptors such as WIN55212, when used in conjunction with standard epileptic drugs, offer a greater degree of relief from seizures [74, 75]. Thus, when it comes to animal models, the evidence is overwhelmingly in support of the anticonvulsant effects of cannabinoids. But what about well-constructed, randomized clinical trials in patients? Are cannabinoids truly effective anti-seizure agents in humans?
Data from clinical trials studying the effect of CBD and CBD-enriched products on seizure frequency, safety and drug interactions is scarce and much of the information on marijuana and cannabinoid anti-seizure properties is anecdotal. One of the earliest clinical trials, reported in 1970, highlighted a randomized study of 9-patients with refractory temporal lobe epilepsy, 4 of whom received CBD for 5 weeks and 5 of whom received placebo for 5 weeks. Two of the CBD treated patients were free of seizures within 3 weeks while none of those who were administered the placebo reported relief from seizures [76]. A double-blind phase 2 study in 1980 examined 15 patients with refractory epilepsy, 8 of whom received CBD in addition to their normal anti-epileptic medication, and 7 of whom received placebo. Four of the CBD patients experienced no seizures during the study while another 3 experienced partial improvement. Only one of the placebo group showed improvement, while the others were unaffected [77]. More recently, an observational, longitudinal study examining the effect of CBD-enriched cannabis as an antiepileptic in children and adolescents was reported. The CBD-enriched cannabis oil treatment contained a ratio of CBD :THC of 20:1 and was given to children and adolescents with refractory epilepsy in addition to their baseline standard antiepileptic treatment [78]. In total, 69 patients, with a mean age of 9.6 years, received treatment with CBD-enriched cannabis oil. Overall, there was a seizure reduction of <50% in 56% of the patients and a reduction rate of >75% in 35% of patients [78].
Antiepileptic drugs work by either reducing excitation (via blocking voltage-gated Na+ channels or Ca2+ channels, usually T-type), or by increasing inhibition (often by modulating GABA related activity) in the CNS. CB1 receptors are known to regulate neuronal excitability by reducing presynaptic neurotransmitter release. In fact, CB1 receptors are considered to play homeostatic roles since increased levels of activity result in the release of endocannabinoids that feedback on presynaptic CB1 receptors. Ligand binding to these presynaptic receptors activate Gi/o or Gq which leads to a reduction in transmitter release. Activation of the CB1 receptors by endocannabinoids is involved in retrograde inhibition of transmitter release [79,80,81], the control of neuronal excitability [82] and even in the regulation of some forms of synaptic plasticity [80, 81, 83]. Therefore, it is plausible that increased levels of CB1 receptor activity might dampen neuronal excitation. The specific CB1 agonist WIN55212, and the cannabinoid d9-THC were both able to abolish spontaneous epileptic seizures in rats. Furthermore, levels of 2-AG and expression of CB1 protein increased in the hippocampus of pilocarpine-induced seizure animals [84]. In an elegant study by Monory and coworkers [85], the experimenters introduced conditional mutants lacking CB1 receptors in specific neuronal populations and used a kainic acid model of seizures to show that the CB1 receptors localized to hippocampal glutamatergic neurons are necessary for the CB1-dependent protection against kainic acid-induced acute excitotoxic seizures [85]. Interestingly, the CB1 receptors associated with GABAergic neurons did not appear to play a significant neuroprotective role against KA-induced seizures, only the CB1 receptors localized to glutamatergic neurons. Additionally, virus-mediated conditional overexpression of CB1 receptors in pyramidal and mossy fiber cells of the mouse hippocampus confers neuroprotection and reduces convulsions in an acute kainic acid seizure model [86]. The seizures induced the release of anandamide followed by activation of CB1 receptors. Thus, protection against epileptic-like synchronous activity and overexcitability in neural networks may be conferred by activation of CB1 receptors. In healthy individuals, the endocannabinoid system working through CB1 confers neuroprotection, and in those afflicted with refractory epilepsy, activation of CB1 might constitute an important avenue for medical intervention.
But exactly how does activation of CB1 lead to a downregulation of neural activity? This could happen via a number of mechanisms. For instance, presynaptic activation of CB1 reduces presynaptic Ca2+ entry through N-type Ca2+ channels and lowers glutamate release [87]. Activation of CB1 also leads to an enhancement of A-type voltage gated K+ channels [88] as well as an enhancement of inward rectifying K+ channels conductance [89]. The overall effect of activation of either of these K channel types could lead to a reduction in excitation.
8.7 Neurodegenerative Diseases
While medical marijuana and cannabinoids have been proposed to act as antiepileptics and analgesics, the evidence is mounting for use to alleviate a number of neurodegenerative diseases such as multiple sclerosis and Alzheimer’s disease. Additionally, a role in schizophrenia and other psychiatric conditions has been proposed. Multiple sclerosis shares a number of pathological features with other neurodegenerative diseases such as a link with neurodegeneration, neuroinflammation and excitotoxicity. It is an autoimmune disease that is characterized by demyelination and degeneration of motor neurons, often associated with neuropathic pain, aberrant neuronal activity and debilitating and painful muscle spasms. Cannabis plant extracts have been used with some success to relieve the symptoms of MS [90], while application of a 1:1 ratio of Δ9-THC and CBD (sativex) via the oral-mucosal route has analgesic effects and limits neuropathic pain while also reducing muscle spasms [52]. Indeed, CBD has been shown to be capable of relieving neuropathic pain associated with MS [91]. In patients with MS, endocannabinoid levels in the circulating plasma are increased [92, 93] whereas in an experimental animal model for MS, known as experimental autoimmune encephalomyelitis (EAE), the endocannabinoid levels in the brain have actually been downregulated [94]. In fact, animals in which CB1 receptors are deficient and are then induced with EAE tend to develop neurodegeneration more rapidly than those that express CB1 receptors [95] implying a neuroprotective role for CB1.
Well-constructed, randomized, double blind clinical trials using whole plant cannabis-based medicinal extracts containing equal amounts of Δ9-THC and CBD, on a cohort of 160 patients with MS resulted in improved scores on symptoms such as spasticity, spasms, tremor, pain and bladder control, however statistical significance was lacking [96]. A meta-analysis of three studies evaluated a total of over 660 patients with spasticity, to determine if nabiximols (Δ9-THC:CBD extract) alleviated these symptoms [97]. The authors concluded that nabiximols reduced spasticity beyond what would occur by placebo alone.
Alzheimer’s disease is an age-related neurodegenerative disease in which a pathological hallmark is the onset of neurofibrillary tangles and amyloid beta plaques in the brain. Neurodegeneration occurs and the individual presents with a progressive decline in cognition and memory. There is a concomitant activation of microglia in plaque filled regions along with neuroinflammation and oxidative stress. Cell death occurs via multiple mechanisms but in large part due to excitotoxicity. CB1 receptor expression is high in basal ganglia and hippocampus, where β-amyloid plaques tend to occur most often in AD. Neuronal CB1 expression is reduced in these two regions [98] while expression of CB1 and CB2 expressing microglia is increased [99]. These studies suggest that medications that protect from excitotoxicity and neuroinflammation have the potential to offer therapeutic benefits to individuals afflicted with AD because they relieve secondary pathologies rather than the direct cause of the disease. Links between the endocannabinoid system and Alzheimer’s disease have been reported [100, 101], and evidence exists that THC may actively inhibit Aβ aggregation [102]. For instance, Δ9-THC has been shown to be directly linked to AD [102]. In this study, Eubanks and colleagues found that Δ9-THC competitively inhibits Acetylcholinesterase activity and reduces Aβ aggregation in vitro. Moreover, The CB1 receptor agonists anandamide and noladin ether are capable of inhibiting Aβ toxicity in a differentiated human teratocarcinoma cell line Ntera 2/cl-D1 neurons [103].
As described in a previous section this may be linked to a reduction in glutamate release through downregulation of N-type Ca channel activity, or an upregulation of K-channel activity, both of which are associated with reduced synaptic transmitter release.
8.8 Conclusions
It is clear that medicinal cannabis has the potential to play a significant role in the treatment of ailments from neuropathic pain to epilepsy, nausea, cancer and neurodegenerative diseases. Until now much of the evidence for its use as a medicinal agent has been anecdotal and limited in power. We are at the dawn of a period where legalization of cannabis for medicinal use and recreational purposes will ease the restrictions for research. In this exciting time, we stand to make significant progress in our understanding of the pharmacological basis of the actions of cannabinoids. But there are still obstacles to overcome. For instance, the unwanted psychotropic side effects of THC limit its capacity as a therapeutic agent. Moreover, the cannabinoid receptor sites need to be fully identified and properly characterized. One can imagine that a wide array of effects such as an analgesic, anti-epileptic agent, anti-emetic or anti-inflammatory compound could occur through the action of highly selective cannabimimetics. This can only be realized following intensive research identifying the molecular targets and signaling mechanisms of cannabinoids. Indeed, there is much to learn.
Abbreviations
- Δ9-THC:
-
tetrahydrocannabinol
- 2-AG:
-
2-arachiodonoylglycerol
- AEA:
-
anandamide
- AD:
-
Alzheimer’s disease
- cAMP:
-
cyclic adenosine monophosphate
- CB1:
-
cannabinoid receptor 1
- CB2:
-
cannabinoid receptor 2
- CB3:
-
cannabinoid receptor 3
- CBD:
-
cannabidiol
- CBN:
-
cannabinol
- CNS:
-
central nervous system
- CHO:
-
Chinese hamster ovary
- DRG:
-
dorsal root ganglion
- EAE:
-
experimental autoimmune encephalomyelitis
- GABA:
-
gamma-aminobutyric acid, or γ-aminobutyric acid
- GPCR55:
-
G protein-coupled receptor 55
- IP3:
-
Inositol trisphosphate
- KA:
-
kainic acid
- LPI:
-
L-α-lysophosphatidylinositol
- MS:
-
multiple sclerosis
- SCBs:
-
synthetic cannabinoids
- TRPA1:
-
transient receptor potential cation channel, subfamily A, member 1
- TRPV1:
-
transient receptor potential cation channel, subfamily V, member 1
- TRPV2:
-
transient receptor potential cation channel, subfamily V, member 2
References
Friedman D, Sirven JI (2017) Historical perspective on the medical use of cannabis for epilepsy: ancient times to the 1980s. Epilepsy Behav 70(Pt B):298–301
Russo EB (2017) Cannabis and epilepsy: an ancient treatment returns to the fore. Epilepsy Behav 70(Pt B):292–297
Aggarwal SK, Carter GT, Sullivan MD, ZumBrunnen C, Morrill R, Mayer JD (2009) Medicinal use of cannabis in the United States: historical perspectives, current trends, and future directions. J Opioid Manag 5(3):153–168
Epstein HA (2010) A natural approach to soothing atopic skin. Skinmed 8(2):95–97
Stevens CJ, Murphy C, Roberts R, Lucas L, Silva F, Fuller DQ (2016) Between China and South Asia: a middle Asian corridor of crop dispersal and agricultural innovation in the Bronze Age. The Holocene 26(10):1541–1555
Jiang HE, Li X, Zhao YX, Ferguson DK, Hueber F, Bera S et al (2006) A new insight into Cannabis sativa (Cannabaceae) utilization from 2500-year-old Yanghai Tombs, Xinjiang, China. J Ethnopharmacol 108(3):414–422
Bonini SA, Premoli M, Tambaro S, Kumar A, Maccarinelli G, Memo M et al (2018) Cannabis sativa: a comprehensive ethnopharmacological review of a medicinal plant with a long history. J Ethnopharmacol 227:300–315
Hanus LO, Meyer SM, Munoz E, Taglialatela-Scafati O, Appendino G (2016) Phytocannabinoids: a unified critical inventory. Nat Prod Rep 33(12):1357–1392
Hill AJ, Williams CM, Whalley BJ, Stephens GJ (2012) Phytocannabinoids as novel therapeutic agents in CNS disorders. Pharmacol Ther 133(1):79–97
Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI (1990) Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346(6284):561–564
Gerard C, Mollereau C, Vassart G, Parmentier M (1990) Nucleotide sequence of a human cannabinoid receptor cDNA. Nucleic Acids Res 18(23):7142
Munro S, Thomas KL, Abu-Shaar M (1993) Molecular characterization of a peripheral receptor for cannabinoids. Nature 365(6441):61–65
Gerard CM, Mollereau C, Vassart G, Parmentier M (1991) Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochem J 279. (Pt 1:129–134
Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA et al (2002) International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev 54(2):161–202
Jordan CJ, Xi ZX (2019) Progress in brain cannabinoid CB2 receptor research: from genes to behavior. Neurosci Biobehav Rev 98:208–220
Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR et al (1990) Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A 87(5):1932–1936
Pertwee RG (2008) The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol 153(2):199–215
Howlett AC, Mukhopadhyay S (2000) Cellular signal transduction by anandamide and 2-arachidonoylglycerol. Chem Phys Lipids 108(1–2):53–70
Childers SR, Sexton T, Roy MB (1994) Effects of anandamide on cannabinoid receptors in rat brain membranes. Biochem Pharmacol 47(4):711–715
Gonsiorek W, Lunn C, Fan X, Narula S, Lundell D, Hipkin RW (2000) Endocannabinoid 2-arachidonyl glycerol is a full agonist through human type 2 cannabinoid receptor: antagonism by anandamide. Mol Pharmacol 57(5):1045–1050
Rhee MH, Vogel Z, Barg J, Bayewitch M, Levy R, Hanus L et al (1997) Cannabinol derivatives: binding to cannabinoid receptors and inhibition of adenylylcyclase. J Med Chem 40(20):3228–3233
Rhee MH, Bayewitch M, Avidor-Reiss T, Levy R, Vogel Z (1998) Cannabinoid receptor activation differentially regulates the various adenylyl cyclase isozymes. J Neurochem 71(4):1525–1534
Hua T, Vemuri K, Pu M, Qu L, Han GW, Wu Y et al (2016) Crystal structure of the human cannabinoid receptor CB1. Cell 167(3):750–62 e14
Ryberg E, Larsson N, Sjogren S, Hjorth S, Hermansson NO, Leonova J et al (2007) The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol 152(7):1092–1101
Lauckner JE, Jensen JB, Chen HY, Lu HC, Hille B, Mackie K (2008) GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc Natl Acad Sci U S A 105(7):2699–2704
Sylantyev S, Jensen TP, Ross RA, Rusakov DA (2013) Cannabinoid- and lysophosphatidylinositol-sensitive receptor GPR55 boosts neurotransmitter release at central synapses. Proc Natl Acad Sci U S A 110(13):5193–5198
Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V et al (1999) Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400(6743):452–457
Zheng J (2013) Molecular mechanism of TRP channels. Compr Physiol 3(1):221–242
Akopian AN, Ruparel NB, Patwardhan A, Hargreaves KM (2008) Cannabinoids desensitize capsaicin and mustard oil responses in sensory neurons via TRPA1 activation. J Neurosci 28(5):1064–1075
Kim D, Cavanaugh EJ, Simkin D (2008) Inhibition of transient receptor potential A1 channel by phosphatidylinositol-4,5-bisphosphate. Am J Physiol Cell Physiol 295(1):C92–C99
Qin N, Neeper MP, Liu Y, Hutchinson TL, Lubin ML, Flores CM (2008) TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J Neurosci 28(24):6231–6238
Cristino L, de Petrocellis L, Pryce G, Baker D, Guglielmotti V, Di Marzo V (2006) Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience 139(4):1405–1415
Ahluwalia J, Urban L, Capogna M, Bevan S, Nagy I (2000) Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience 100(4):685–688
Caterina MJ, Julius D (1999) Sense and specificity: a molecular identity for nociceptors. Curr Opin Neurobiol 9(5):525–530
Kowase T, Nakazato Y, Yoko OH, Morikawa A, Kojima I (2002) Immunohistochemical localization of growth factor-regulated channel (GRC) in human tissues. Endocr J 49(3):349–355
Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR et al (2003) ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112(6):819–829
Diogenes A, Akopian AN, Hargreaves KM (2007) NGF up-regulates TRPA1: implications for orofacial pain. J Dent Res 86(6):550–555
Diogenes MJ, Assaife-Lopes N, Pinto-Duarte A, Ribeiro JA, Sebastiao AM (2007) Influence of age on BDNF modulation of hippocampal synaptic transmission: interplay with adenosine A2A receptors. Hippocampus 17(7):577–585
Nahas GG, Frick HC, Lattimer JK, Latour C, Harvey D (2002) Pharmacokinetics of THC in brain and testis, male gametotoxicity and premature apoptosis of spermatozoa. Hum Psychopharmacol 17(2):103–113
Perez-Reyes M (1990) Marijuana smoking: factors that influence the bioavailability of tetrahydrocannabinol. NIDA Res Monogr 99:42–62
Huestis MA (2007) Human cannabinoid pharmacokinetics. Chem Biodivers 4(8):1770–1804
McGilveray IJ (2005) Pharmacokinetics of cannabinoids. Pain Res Manag 10(Suppl A):15A–22A
Huestis MA, Mitchell JM, Cone EJ (1996) Urinary excretion profiles of 11-nor-9-carboxy-delta 9-tetrahydrocannabinol in humans after single smoked doses of marijuana. J Anal Toxicol 20(6):441–452
Huestis MA, Henningfield JE, Cone EJ (1992) Blood cannabinoids. I. Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. J Anal Toxicol 16(5):276–282
Huestis MA, Sampson AH, Holicky BJ, Henningfield JE, Cone EJ (1992) Characterization of the absorption phase of marijuana smoking. Clin Pharmacol Ther 52(1):31–41
Perez-Reyes M, Timmons MC, Lipton MA, Davis KH, Wall ME (1972) Intravenous injection in man of 9 -tetrahydrocannabinol and 11-OH- 9 -tetrahydrocannabinol. Science 177(4049):633–635
Alles SRA, Smith PA (2018) Etiology and pharmacology of neuropathic pain. Pharmacol Rev 70(2):315–347
Abrams DI, Jay CA, Shade SB, Vizoso H, Reda H, Press S et al (2007) Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology 68(7):515–521
Ellis RJ, Toperoff W, Vaida F, van den Brande G, Gonzales J, Gouaux B et al (2009) Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology 34(3):672–680
Wilsey B, Marcotte T, Tsodikov A, Millman J, Bentley H, Gouaux B et al (2008) A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain 9(6):506–521
Borgelt LM, Franson KL, Nussbaum AM, Wang GS (2013) The pharmacologic and clinical effects of medical cannabis. Pharmacotherapy 33(2):195–209
Rog DJ, Nurmikko TJ, Young CA (2007) Oromucosal delta9-tetrahydrocannabinol/cannabidiol for neuropathic pain associated with multiple sclerosis: an uncontrolled, open-label, 2-year extension trial. Clin Ther 29(9):2068–2079
Nurmikko TJ, Serpell MG, Hoggart B, Toomey PJ, Morlion BJ, Haines D (2007) Sativex successfully treats neuropathic pain characterised by allodynia: a randomised, double-blind, placebo-controlled clinical trial. Pain 133(1–3):210–220
Hauser W, Fitzcharles MA, Radbruch L, Petzke F (2017) Cannabinoids in pain management and palliative medicine. Deutsches Arzteblatt Int 114(38):627–634
Fitzcharles MA, Baerwald C, Ablin J, Hauser W (2016) Efficacy, tolerability and safety of cannabinoids in chronic pain associated with rheumatic diseases (fibromyalgia syndrome, back pain, osteoarthritis, rheumatoid arthritis): a systematic review of randomized controlled trials. Schmerz 30(1):47–61
Fitzcharles MA, Ste-Marie PA, Hauser W, Clauw DJ, Jamal S, Karsh J et al (2016) Efficacy, tolerability, and safety of cannabinoid treatments in the rheumatic diseases: a systematic review of randomized controlled trials. Arthritis Care Res (Hoboken) 68(5):681–688
Volz MS, Siegmund B, Hauser W (2016) Efficacy, tolerability, and safety of cannabinoids in gastroenterology: a systematic review. Schmerz 30(1):37–46
de Vries M, van Rijckevorsel DCM, Vissers KCP, Wilder-Smith OHG, van Goor H (2017) Tetrahydrocannabinol does not reduce pain in patients with chronic abdominal pain in a phase 2 placebo-controlled study. Clin Gastroenterol Hepatol 15(7):1079–1086. e4
Brents LK, Zimmerman SM, Saffell AR, Prather PL, Fantegrossi WE (2013) Differential drug-drug interactions of the synthetic cannabinoids JWH-018 and JWH-073: implications for drug abuse liability and pain therapy. J Pharmacol Exp Ther 346(3):350–361
Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R (2009) Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci 30(10):515–527
Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ et al (2007) Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci 10(7):870–879
Patwardhan AM, Jeske NA, Price TJ, Gamper N, Akopian AN, Hargreaves KM (2006) The cannabinoid WIN 55,212-2 inhibits transient receptor potential vanilloid 1 (TRPV1) and evokes peripheral antihyperalgesia via calcineurin. Proc Natl Acad Sci U S A 103(30):11393–11398
Philpott HT, O’Brien M, McDougall JJ (2017) Attenuation of early phase inflammation by cannabidiol prevents pain and nerve damage in rat osteoarthritis. Pain 158(12):2442–2451
Barrese V, Miceli F, Soldovieri MV, Ambrosino P, Iannotti FA, Cilio MR et al (2010) Neuronal potassium channel openers in the management of epilepsy: role and potential of retigabine. Clin Pharmacol 2:225–236
Korczyn AD, Schachter SC, Amlerova J, Bialer M, van Emde Boas W, Brazdil M et al (2015) Third international congress on epilepsy, brain and mind: Part 1. Epilepsy Behav 50:116–137
Rektor I, Schachter SC, Arya R, Arzy S, Braakman H, Brodie MJ et al (2015) Third international congress on epilepsy, brain, and mind: part 2. Epilepsy Behav 50:138–159
Hesdorffer DC, Beck V, Begley CE, Bishop ML, Cushner-Weinstein S, Holmes GL et al (2013) Research implications of the institute of medicine report, epilepsy across the Spectrum: promoting health and understanding. Epilepsia 54(2):207–216
Tang F, Hartz AMS, Bauer B (2017) Drug-resistant epilepsy: multiple hypotheses, few answers. Front Neurol 8:301
Hill AJ, Mercier MS, Hill TD, Glyn SE, Jones NA, Yamasaki Y et al (2012) Cannabidivarin is anticonvulsant in mouse and rat. Br J Pharmacol 167(8):1629–1642
Hill TD, Cascio MG, Romano B, Duncan M, Pertwee RG, Williams CM et al (2013) Cannabidivarin-rich cannabis extracts are anticonvulsant in mouse and rat via a CB1 receptor-independent mechanism. Br J Pharmacol 170(3):679–692
Jones NA, Glyn SE, Akiyama S, Hill TD, Hill AJ, Weston SE et al (2012) Cannabidiol exerts anti-convulsant effects in animal models of temporal lobe and partial seizures. Seizure 21(5):344–352
Wallace MJ, Wiley JL, Martin BR, DeLorenzo RJ (2001) Assessment of the role of CB1 receptors in cannabinoid anticonvulsant effects. Eur J Pharmacol 428(1):51–57
Wallace MJ, Martin BR, DeLorenzo RJ (2002) Evidence for a physiological role of endocannabinoids in the modulation of seizure threshold and severity. Eur J Pharmacol 452(3):295–301
Luszczki JJ, Andres-Mach M, Barcicka-Klosowska B, Florek-Luszczki M, Haratym-Maj A, Czuczwar SJ (2011) Effects of WIN 55,212-2 mesylate (a synthetic cannabinoid) on the protective action of clonazepam, ethosuximide, phenobarbital and valproate against pentylenetetrazole-induced clonic seizures in mice. Prog Neuro-Psychopharmacol Biol Psychiatry 35(8):1870–1876
Luszczki JJ, Misiuta-Krzesinska M, Florek M, Tutka P, Czuczwar SJ (2011) Synthetic cannabinoid WIN 55,212-2 mesylate enhances the protective action of four classical antiepileptic drugs against maximal electroshock-induced seizures in mice. Pharmacol Biochem Behav 98(2):261–267
Mechoulam R (1970) Marihuana chemistry. Science 168(3936):1159–1166
Cunha JM, Carlini EA, Pereira AE, Ramos OL, Pimentel C, Gagliardi R et al (1980) Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology 21(3):175–185
Hausman-Kedem M, Menascu S, Kramer U (2018) Efficacy of CBD-enriched medical cannabis for treatment of refractory epilepsy in children and adolescents – an observational, longitudinal study. Brain Dev 40(7):544–551
Alger BE (2002) Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol 68(4):247–286
Chevaleyre V, Takahashi KA, Castillo PE (2006) Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci 29:37–76
Marsicano G, Lutz B (2006) Neuromodulatory functions of the endocannabinoid system. J Endocrinol Investig 29(3 Suppl):27–46
Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A et al (2003) CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 302(5642):84–88
Gerdeman GL, Lovinger DM (2003) Emerging roles for endocannabinoids in long-term synaptic plasticity. Br J Pharmacol 140(5):781–789
Wallace MJ, Blair RE, Falenski KW, Martin BR, DeLorenzo RJ (2003) The endogenous cannabinoid system regulates seizure frequency and duration in a model of temporal lobe epilepsy. J Pharmacol Exp Ther 307(1):129–137
Monory K, Massa F, Egertova M, Eder M, Blaudzun H, Westenbroek R et al (2006) The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron 51(4):455–466
Guggenhuber S, Monory K, Lutz B, Klugmann M (2010) AAV vector-mediated overexpression of CB1 cannabinoid receptor in pyramidal neurons of the hippocampus protects against seizure-induced excitoxicity. PLoS One 5(12):e15707
Mackie K, Hille B (1992) Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells. Proc Natl Acad Sci U S A 89(9):3825–3829
Hampson RE, Evans GJ, Mu J, Zhuang SY, King VC, Childers SR et al (1995) Role of cyclic AMP dependent protein kinase in cannabinoid receptor modulation of potassium “A-current” in cultured rat hippocampal neurons. Life Sci 56(23–24):2081–2088
Mackie K, Lai Y, Westenbroek R, Mitchell R (1995) Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J Neurosci 15(10):6552–6561
Lakhan SE, Rowland M (2009) Whole plant cannabis extracts in the treatment of spasticity in multiple sclerosis: a systematic review. BMC Neurol 9:59
Iskedjian M, Bereza B, Gordon A, Piwko C, Einarson TR (2007) Meta-analysis of cannabis based treatments for neuropathic and multiple sclerosis-related pain. Curr Med Res Opin 23(1):17–24
Centonze D, Bari M, Rossi S, Prosperetti C, Furlan R, Fezza F et al (2007) The endocannabinoid system is dysregulated in multiple sclerosis and in experimental autoimmune encephalomyelitis. Brain 130(Pt 10):2543–2553
Centonze D, Rossi S, Finazzi-Agro A, Bernardi G, Maccarrone M (2007) The (endo)cannabinoid system in multiple sclerosis and amyotrophic lateral sclerosis. Int Rev Neurobiol 82:171–186
Jean-Gilles L, Feng S, Tench CR, Chapman V, Kendall DA, Barrett DA et al (2009) Plasma endocannabinoid levels in multiple sclerosis. J Neurol Sci 287(1–2):212–215
Pryce G, Ahmed Z, Hankey DJ, Jackson SJ, Croxford JL, Pocock JM et al (2003) Cannabinoids inhibit neurodegeneration in models of multiple sclerosis. Brain 126(Pt 10):2191–2202
Wade DT, Makela P, Robson P, House H, Bateman C (2004) Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult Scler 10(4):434–441
Wade DT, Collin C, Stott C, Duncombe P (2010) Meta-analysis of the efficacy and safety of Sativex (nabiximols), on spasticity in people with multiple sclerosis. Mult Scler 16(6):707–714
Westlake TM, Howlett AC, Bonner TI, Matsuda LA, Herkenham M (1994) Cannabinoid receptor binding and messenger RNA expression in human brain: an in vitro receptor autoradiography and in situ hybridization histochemistry study of normal aged and Alzheimer’s brains. Neuroscience 63(3):637–652
Ramirez BG, Blazquez C, Gomez del Pulgar T, Guzman M, de Ceballos ML (2005) Prevention of Alzheimer’s disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J Neurosci 25(8):1904–1913
Benito C, Nunez E, Tolon RM, Carrier EJ, Rabano A, Hillard CJ et al (2003) Cannabinoid CB2 receptors and fatty acid amide hydrolase are selectively overexpressed in neuritic plaque-associated glia in Alzheimer’s disease brains. J Neurosci 23(35):11136–11141
Pazos MR, Nunez E, Benito C, Tolon RM, Romero J (2004) Role of the endocannabinoid system in Alzheimer’s disease: new perspectives. Life Sci 75(16):1907–1915
Eubanks LM, Rogers CJ, AEt B, Koob GF, Olson AJ, Dickerson TJ et al (2006) A molecular link between the active component of marijuana and Alzheimer’s disease pathology. Mol Pharm 3(6):773–777
Milton NG (2002) Anandamide and noladin ether prevent neurotoxicity of the human amyloid-beta peptide. Neurosci Lett 332(2):127–130
Acknowledgements
This research was supported by the Natural Sciences and Engineering Research Council of Canada Discovery Grant to DWA.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Amin, M.R., Ali, D.W. (2019). Pharmacology of Medical Cannabis. In: Bukiya, A. (eds) Recent Advances in Cannabinoid Physiology and Pathology. Advances in Experimental Medicine and Biology, vol 1162. Springer, Cham. https://doi.org/10.1007/978-3-030-21737-2_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-21737-2_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-21736-5
Online ISBN: 978-3-030-21737-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)