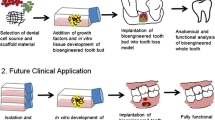
Abstract
There are several challenges in using common removable artificial dental implants, including high risk of bone loss and implant failure. Therefore, bioengineering of physiologically functional whole teeth using regenerative approaches seems essential. To a bioengineer a whole tooth, autologous stem cell-seeded scaffolds, and stem cell reassociations can be implanted at the site of the tooth loss, where they may develop and erupt similarly to a natural tooth. Successful bioengineering of a whole tooth requires appropriate cell source(s), scaffolds, and the induction of the cascade expression of special genes involved in tooth development. To achieve such tooth therapy, we need to fully understand the structure and interaction procedure of epithelial/mesenchymal stem cells during embryonic development of teeth. In this chapter, we first focus on different cell sources and cell signaling pathways through which different parts of a tooth form during tooth development. Then, we describe the recent methods employed for bioengineering a whole tooth, including the organ germ method, sheet engineering, and scaffold-based tissue engineering. Ultimately, the functionality of an engineered whole tooth and future prospects of whole tooth engineering are discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Bioengineering of whole tooth
- Epithelial/mesenchymal stem cell
- Organ germ method
- Dental stem cells
- Oral stem cells
- Whole tooth
- Tooth tissue engineering
1 Introduction
Total tooth loss due to traumatic injury , poor dental hygiene, periodontal or congenital diseases affects over 276 million people worldwide [1]. Today, removable dentures and artificial dental implants have been commonly utilized to restore occlusal function as tooth replacement therapy. Nevertheless, there are some challenges in using the artificial implants, including high risk of bone loss and fracture encircling artificial implants and high susceptibility to infection and inflammation, therefore leading to implant failure [2, 3]. Accordingly, finding alternative procedures to manufacture biologically replaced teeth is an essential demand to rehabilitate physiologically functional teeth.
Theoretically, the whole tooth would be generated by implanting the autologous stem/progenitor cell-seeded scaffolds at the site of tooth loss, where it can grow, erupt, and develop like a natural tooth. Actually, this approach would take place just in the presence of the appropriate cell source(s), scaffolds, and the cascade expression of special genes that are involved in tooth development in the presence of several growth factors. Mimicking such conditions is feasible by understanding the structure and steps of embryonic tooth development .
Interactions between dental epithelium—derived from ectoderm—and neural crest-derived mesenchymal stem cells (NC-MSCs) initiate tooth development. Briefly, at the sites of the future tooth, cascade expressions of homeobox genes—such as Barx1, Lhx8, Msc1, and Msc2—and secretion of BMPs and FGFs induce the thickening of the dental lamina and therefore initiate tooth development. After that, the dental epithelium cells in the placode, a specific dental laminar domain, proliferate and invaginate into the region wherein NC-MSCs reside and form the tooth bud. The epithelial cells proliferate and extend further into the NC-MSC-including tissue and condense the mesenchyme more to form a cap structure. In the next stage, called “bell stage,” primary, secondary, or tertiary enamel knots form—based on the number of cusps of the eventual tooth—before developing into the crown of the tooth. Then, by expression of a second cascade of genes in the epithelial cells of enamel knots (including BMPs, FGFs, Wnts, and Shh), epithelial cells differentiate into ameloblasts, producing enamel, and mesenchymal cells differentiate into the progenitor of odontoblasts, producing dentine and dental follicle. Dental follicle cells produce the periodontal ligament, cementum, and alveolar bone, and thereafter tooth eruption begins by elongation of the tooth root (Fig. 19.1).
Up until now, efforts have been made in rehabilitating lost teeth using whole tooth bioengineering and different methods of tissue engineering and organ regeneration . Recently, several techniques have been applied to engineer fully functional whole tooth from embryonic germ cells in different small and large animals, including mice, rats, pigs, and dogs [4, 5]. These studies demonstrate the feasibility of producing whole tooth at the site of tooth loss as a promising approach for tooth replacement therapy.
In this chapter, we will start by focusing on different cell sources and cell signaling through which the cells produce different parts of the tooth during development procedure. Then, current methods in whole tooth replacement will be discussed and followed by an explanation of the functionality of a whole bioengineered tooth and future prospects for whole tooth engineering.
2 Cell Sources for Whole Tooth Engineering
Tissue engineering using stem cells is a promising approach to restore lost or damaged craniofacial tissues. In whole tooth tissue engineering, different varieties of cells are used and investigated to detect the best source of cells that can be isolated, generated, and utilized for clinical applications. The findings achieved from these investigations can help us to understand how these cells can be utilized for whole tooth engineering. Recent studies, which used some scaffold-free methods for whole tooth bioengineering, manifested the importance of cell sources in the application of tooth regenerative studies [6, 7]. Two major types of stem cell sources are used in whole tooth engineering: pluripotent cells and adult stem cells. Pluripotent cells have the ability to differentiate into the cells of all three germ layers—including endoderm, mesoderm , and ectoderm. These cells, in the presence of appropriate stimuli, can differentiate into more than 200 adult cell types [8]. Adult stem cells are the other cell source, which are commonly used in engineering of different craniofacial tissues such as teeth. These cells are naturally responsible for normal tissue repair and healing after injuries [9]. Here, we will focus on the different cell types in each embryonic and adult stem cell categories and investigate their differentiation potential into different dental cells.
2.1 Embryonic Stem Cells
Embryonic stem cells (ESs) are pluripotent stem cells that can give rise to cells of the different germ layers. However, using these cells in craniofacial tissue engineering for clinical purposes is limited due to ethical issues and immune rejection reaction.
Recently, with the appearance of a new generation of pluripotent stem cells, called induced pluripotent stem cells (iPSCs) , it is possible to use pluripotent stem cells in tooth regeneration research. These cells can be generated from different patient-specific progenitor/differentiated cells including fibroblasts, gingival cells, SHED, SCAP, DSCPs, and periodontal ligament cells, therefore resolving the immune responses and rejection reactions [10,11,12,13]. Moreover, several studies have shown that iPSCs successfully differentiate into different cells of tooth tissue, such as ameloblast-like and odontoblast-like cells [14, 15].
2.2 Adult Stem Cells
As described above, pluripotent stem cells , including ESs and iPSCs, are appropriate candidates to generate a successfully bioengineered tooth. However, using human ESs for tooth regeneration is impossible due to ethical issues and potential allogeneic immune rejection. Moreover, the procedure of producing of iPSCs is difficult, and the usual procedure to generate iPSCs using genetic manipulation and different kinds of viruses makes it harmful to use in clinical application. Therefore, autologous, multipotent adult stem cells are the putative source of the cells for craniofacial tissue engineering.
MSCs are standard stem cells that can be isolated from various tissues such as bone marrow, umbilical cord, and adipose and dental tissues [16, 17]. Postnatal dental pulp stem cells (DPSCs) , the first and the most common source of dental MSCs in tooth tissue engineering, can be isolated easily from dental pulp [18]. Studies show that DPSCs can differentiate into odontoblasts and osteoblasts and thereby form pulp, dentin, and cementum tissues, respectively [20,21,22,23]. DPSCs also can differentiate into other cell lineages, such as neurons, chondrocytes, vascular tissues, osteocytes, and adipocytes [22,23,24].
The periodontal ligament was found to be another source of dental MSC population, called periodontal ligament stem cells (PDLSCs) . The studies show that PDLSCs can differentiate into adipocytes, collagen-forming cells, cementoblast-like cells, and cementum-PDL-like structures in vitro, which contribute to periodontal tissue repair. Moreover, a study showed that co-culture of PDLSCs with DPSCs induces formation of rootlike and dentin-like structures [19].
Another promising source of stem cells is the pulp of primary human teeth (autologous baby teeth). The isolated stem cells, called human exfoliated deciduous teeth (SHEDs), can provide a sufficient number of the cells for dental tissue engineering applications. These highly proliferative cells can differentiate into neural cells, endothelium, chondrocytes, osteocytes, adipocytes, odontoblasts, and pulp-like and dentin-like tissues [20,21,22].
Dental follicle precursor cells (DFPCs) are the other type of mesenchymal stem cells that surround the developing tooth bud, which will differentiate into odontoblasts [23], cementoblasts (producing the cementum), and periodontal ligament-like tissues [24]. It has been shown that the DFPCs are remarkable undifferentiated lineage cells in the periodontium prior to, or even during, tooth eruption [24].
Stem cells from the apical papilla (SCAPs) are the other adult MSCs that are isolated from pulp tissue of developing baby teeth. They can differentiate into odontoblasts and osteoblasts, and form dentin-like structures [22]. Despite the fact that their proliferation rate is higher than DPSCs, their dentinogenic differentiation capacity is similar to DPSCs and BMSCs. Upon osteogenic differentiation, SHEDs show a higher alkaline phosphatase activity and osteocalcin expression compared with DPSC [18]. However, the studies show that their adipogenic differentiation capacity is less than BMSCs [25]. The SCAP growth factor receptor gene profiles—including FGFR1, TGFbRI, STRO-1, bone sialophosphoprotein, and osteocalcin—are similar to the gene profiles in DPSCs. Moreover, it has been shown that upon stimuli, SCAPs express a wide variety of neurogenic markers, such as nestin and neurofilament M [25].
Despite the potential of the aforementioned dental stem cells to create dental differentiated cells and structures, determining the best dental MSC for whole tooth engineering has been challenging. It is due to the difference in proliferation and clonogenicity, as well as the differentiation ability of the different types of MSCs. During distinct differentiation pathways, some specific surface markers are expressed on each dental stem cell type due to microenvironments of each cell lineage origin [26]. Even in the same population of the MSCs, the subpopulation of the cells shows heterogeneity in their differentiation potentials [27]. Moreover, to achieve an appropriate approach in whole tooth engineering, it is necessary to determine the factors that control the MSC differentiation fate during tooth development [26]. The mutual interaction between factors secreted from various types of dental epithelial cells (such as dental epithelial cell rests of Malassez (ERM), keratinocytes isolated from human foreskin and gingival epithelial cells, and factors secreted from dental MSCs) causes formation of odontoblasts, ameloblasts, cementoblasts, cementum, and enamel [28,29,30]. Combination of these epithelial cell sources with MSC sources could lead to a promising approach for effective whole tooth tissue engineering.
3 Cell Signaling
The signaling pathways are the part of a complex system of communication that organizes cell fate, activities, and interactions through cascade expressions of the genes. The signaling mechanisms in tooth development seem to be conservative among different species [31]. Investigation of tooth development in several models—such as zebra fish, snakes, lizards, ferrets, rats, mice, and humans—helps to detect the genes and signaling pathways involved in tooth development. These findings could suggest a promising approach to achieve successful whole tooth engineering [31, 32].
All signaling pathways take place in interactions between dental epithelium derived from ectoderm and NC-MSCs. The conserved signaling pathways in tooth development are Hedgehog (Hh), Wnt, fibroblast growth factor (FGF), transforming growth factor ® (Tgf ®), bone morphogenic protein (BMP), and ectodysplasin (Eda) (Fig. 19.2). There are three signaling centers in tooth development, including placodes, primary enamel knots, and secondary (or tertiary) enamel knots. Their formation is regulated by epithelial-mesenchymal interactions, and they all largely express the same array of multiple growth factors.
Epithelium and mesenchyme interactions control tooth development by some signaling pathways. At the first step of tooth development, epithelium initiates secretion of signaling molecules, which induce gene cascade expressions in the mesenchymal cells, causing a condensation in the mesenchyme and epithelial placode formation (a). The enamel knots as a signaling center which determine the cusp position and induce odontoblast differentiation (b)
Transcription factor p63 is expressed throughout the surface ectoderm, which regulates placode formation. The function of this transcription factor was discovered when its function deletion caused the lack of teeth placodes and other ectodermal appendage development [33]. P53 plays a pivotal role in Eda, Notch, BMP, and FGF signaling pathways [33]. The studies show that impairing signaling pathways at the placode stage stops tooth development before epithelial budding [34].
Ectodysplasin (Eda) , a tumor necrosis factor, affects the function of placodes and enamel knots through its receptor [35]. Human syndrome hypohidrotic ectodermal dysplasia (HED) is caused by mutations in the genes involving in Eda signaling. In this syndrome, multiple teeth are missing, and there are several defects in other ectodermal organs [35]. Impairing Eda function causes loss of third molars or incisal teeth, and the molar cusp pattern becomes abnormal [36], whereas overexpressing Eda in epithelium causes an extra tooth in front of the molars [37]. Eda signaling changes the gene expressions of the proteins involved in important signaling pathways in ectodermal organ development, including Dkk4, Fgf20, Shh, ctgf, and Follistatin [35].
Between the bud and cap stages, the primary enamel knot forms in the dental epithelium. The primary enamel knot manages crown formation and directs secondary enamel knot position, thereby determining the cusp tip position in the molar crown [38]. Wnt/b-catenin signaling in dental epithelium regulates Lef1 for FGF4 expression in the enamel knots and induces new enamel knots and placodes [39]. The enamel knots induce cell proliferation in adjacent epithelium by secreting growth factors of FGF3, FGF4, FGF9, and FGF20. Because of expression lacking of P21 and FGF receptor in the epithelial cells of enamel knots, the epithelial cells remain nonproliferative. The FGF growth factors also induce NC-MSCs to express and secrete FGF3 and Runx2. Then, FGF3 and Runx2 attach to their receptors on the epithelial cells, thereby affecting the morphogenesis of tooth [40].
Shh, another factor secreted from the enamel knot , induces mesenchyme to produce factors that regulate morphogenesis of epithelium [41]. The signaling pathways conducted by Shh, Bmp, and Tgf® induce the proliferation activity of the stem cells and ameloblast differentiation, thereby producing the enamel.
Moreover, the studies show that TGF-® proteins, including BMPs, induce odontoblastic differentiation in the canonical TGF-® pathway, modulate smads, and thereby provoke dentin formation [42].
4 Approaches for Whole Tooth Organ
The procedure of whole tooth development is a highly complicated process, where transcription and growth factors express spatiotemporally and control cusp position and number, root formation, tooth length, crown size, and tooth development. To generate a functional whole bioengineered tooth with appropriate size and morphology, the proper interaction between different aforementioned factors is necessary. Recently, several approaches for whole bioengineered teeth have been suggested, including organ germ method, cell sheets, and dental tissue engineering using scaffolds.
4.1 Organ Germ Method
A promising approach to bioengineer a whole tooth is mimicking organogenesis by inducing mutual epithelial-mesenchymal interconnection similar to what occurs in organ development. Here, we describe different approaches to bioengineer whole teeth using the organ germ method .
4.1.1 Embryonic Tooth Germ-Derived Epithelial and Mesenchymal Stem Cells
Embryonic tooth germ cells of the mouse are an appropriate candidate to develop a whole bioengineered tooth because they can make functional teeth by epithelial-mesenchymal interconnection [43]. Moreover, the organogenesis in this animal model takes place in a relatively short time frame, making it possible to achieve an accurate protocol. As described before, the primary enamel knot, acting as a second signaling center involved in the morphogenesis of the tooth crown, forms in the enamel organ, thus making it an important stage to producing functional reconstitutes of germ cells. In a study, dental mesenchyme and enamel organ were dissociated from the mouse’s first lower molars at early cap stage (E14) . Then, the dissociated cells from enamel organ were cultured and reassociated to either intact dental mesenchyme or dissociated mesenchymal cells in vitro. Although in teeth developed in both types of experiments, the tooth developed faster in intact dental mesenchyme than the dissociated mesenchymal cells due to cell history memorization in the intact mesenchymal tissue. However, progression duration in the initial steps of epithelial histogenesis is equal in both experiments, which shows that history reassociations in early stage are not memorized by mesenchymal tissue [44].
To bioengineer a whole tooth using embryonic tooth germ cells, reconstitution of tooth germ-like structures will be initiated by co-culturing the epithelial and mesenchymal stem cells in vitro for 5–7 days. In this stage, differentiation of odontoblasts, cusp morphogenesis, and crown formation will be started, and epithelial histogenesis will be completed [45, 46]. The construct will be then implanted under skin or the sub-renal capsule of adult mice, where vascular tissues, enamel and dentin, and toothlike structure will be formed [7, 47]. A study shows that this bioengineered toothlike structure with full function is produced in the case of an edentulous jaw using organ replacement therapy (Fig. 19.3) [6]. These methods can suggest a promising approach to achieve functional bioengineered whole teeth in the edentulous jaw .
Producing a bioengineered tooth in a mouse jaw. (a) Schematic image of bioengineering a tooth in the jaw using reassociation of tooth germ. (b) Oral image of transplanted bioengineered tooth before and after eruption and after occlusion (top, center, and bottom, respectively). (c) The transplanted bioengineered tooth occluded with the opposing upper tooth after 40 days. (d) Micro CT images of normal (gray, double dotted line) and no transplantation (top image) and transplanted bioengineered tooth in an extensive bone defect at day 0 (red, straight line) and after 45 days (green, dotted line). Here, each line determines the superior edges of the recipient alveolar bone. (e) H&E analysis of bioengineered tooth after occlusion, which shows that engrafted bioengineered construct has the correct tooth structure. Abbreviations: NT natural tooth, BT bioengineered tooth, PDL periodontal ligament, AB alveolar bone. (Panels B, C, D, and E are reused from Ref. [48] with permission)
There are no studies that show that human embryonic dental cells will produce bioengineered teeth, which tooth germ cells of mice produce. However, a study on the embryonic human dental epithelium (obtained from cap stage) and human embryonic lip mesenchyme shows that human embryonic dental tissues indeed possess similar tooth-inductive capability [49].
Recently, the main problems in the tooth organ method are discovering the appropriate cells and recapitulating the molecular processes of tooth development. Therefore, finding the molecular markers is necessary for detection of this molecular process. Even though the embryonic tooth cells seem to be good candidates for tooth bioengineering, their use in clinical application is challenging because of limitations, such as immunological rejection reactions and ethical concerns. Adult stem cells would be the alternative cell source for this purpose.
4.1.2 Non-embryonic Tooth Germ-Derived Epithelial and Mesenchymal Stem Cells
As described previously, tooth formation is induced by interconnections between epithelial and mesenchymal cells during the development process of a tooth [4]. Recently, different sources of cells, including non-embryonic cells, have been used based on this strategy. These cells can produce a plentiful amount of the cell population and are easy to access and isolate.
Bone marrow mesenchymal stem cells (BM-MSCs) , showing similar properties of DPSCs, would be a good candidate as an alternative cell source. These cells can be isolated and harvested in an abundant amount at all ages and differentiate into a variety of cell types, including ameloblast-like cells [45]. A study shows that interconnection between BM-MSCs and oral epithelial cells from a mouse embryo (E10) induced bioengineered-like teeth after implantation in kidney capsules [50].
iPSCs are the other alternative cell source for tooth bioengineering because of their pluripotent characteristics similar to human embryonic stem cells. However, iPSCs do not have some limitations of human embryonic stem cells, such as ethical or immune rejection reaction problems [13]. Neural crest-like cells derived from iPSCs in combination with incisor dental epithelium can undergo odontogenic differentiation [51]. A study shows co-culturing iPSCs with incisor mesenchymal cells causes toothlike structure formation with newly formed bone-like cells [52]. It has been demonstrated that integration-free human urine-derived iPSCs in combination with molar mesenchyme (form E14.5 mouse) can generate intact toothlike structures in a sub-renal culture [53].
Recently, in another study, epithelial cells obtained from adult human gingiva are used as a source of epithelial cells to engineer a whole bioengineered tooth. These cells, in combination with embryonic tooth mesenchymal cells of a mouse, produce a bioengineered tooth with different parts of a tooth, such as enamel with ameloblast-like cells, dentine, and the ERM of human origin after transplantation in kidney capsules [53].
It seems human keratinocytes can be a good source of epithelial cells, which in combination with embryonic mouse mesenchyme can differentiate into enamel-secreting ameloblasts [54].
Human umbilical cord mesenchymal stem cells (hUCMSCs) are another potential cell source to bioengineer the whole tooth with characteristics similar to those of pulp tissue stem cells. These cells can differentiate into odontoblast-like cells expressing dentine-related proteins, such as dentine sialoprotein and dentine matrix protein-1 [55].
4.2 Scaffolds for Tooth Bioengineering Approach
Organ substitution brings to light the whole tissue replacement of an impaired organ using in vitro cell-cultured 3D structures. It is supposed that future technologies will reconstruct the whole organ in vitro to replace the dysfunctional tissue. Whole tooth regeneration necessitates the accompaniment of the cells with proper scaffolding to regenerate the whole tooth to functional recovery of the lost tooth (Fig. 19.4). In this method, natural and synthetic polymers have been utilized to reconstruct the whole tissue, which clinical experiments have reported. In this section, whole tissue regeneration using scaffold will be discussed.
4.2.1 Synthetic Polymeric Scaffolds
Biomaterials play an important role in tissue engineering, in which incorporation of cells exhibits a synergistic effect on damaged tissue regeneration. Appropriate material selection has a strong impact on dental regeneration. For instance, scaffold modulus affects cellular adhesion, growth, proliferation, differentiation, and fate. Young et al. seeded cells on the PLGA scaffolds, which are appropriate for controlling the shape and size of the tooth, and successfully regenerated a tooth from dissociated tooth tissues, involving enamel and dentin [56]. However, scaffold residue in the tissue hindered the whole tooth regeneration. Precise arrangement of cells—such as ameloblasts, odontoblasts, and cementoblasts similar to native teeth—resulted in proper connection of enamel, dentin, and cementum, which leads to full regeneration of teeth [57]. In whole tooth engineering, root regeneration is the most important aspect. After root regeneration, the crown can regenerate on the constructed root. Bopp et al. loaded Cyclosporine A (CsA) in PLGA nanoparticles and embedded these cells into PCL electrospun scaffolds in which the local and sustained release of the nanoparticles was achieved.
It was reported that the in vivo implantation of such scaffolds did not alter tooth regeneration, and that 88% of the regenerated teeth were innervated [58]. Chen et al. electrospun the PLGA and gelatin to achieve aligned nanofibers and treated the nanofibers using dentin matrix and native dental pulp extracellular matrix for periodontal and dental pulp regeneration. Such scaffolding can simulate the ECM properties, which facilitates the odontogenic differentiation of dental stem cells after seeding with stem cells transplanted within the porcine jaws. It is observed that in dentin, an odontoblast-like layer forms between the predentin matrix and dental pulp-like tissues, along with blood vessel formation; moreover, in the periodontium, cellular cementum and periodontal ligament (PDL)-like tissues are formed [59]. Rasperini et al. bioprinted the PCL for periodontal repair. Adjustable degradation rate and high porosity results in tissue ingrowth and vascularization [60]. Zhang et al. synthesized chitosan−/collagen-containing growth factor for periodontal reconstruction. This scaffold induced the cellular proliferation and upregulation of collagen expression, and surrounding tissues grew within the scaffold, as well [61].
4.2.2 Decellularized Scaffold as Natural Scaffolds
Even though synthetic scaffolds can be constructed with desired properties, it is hard to perfectly recapitulate the ECM in dental tissues. In this regard, decellularized scaffolding can be utilized to enhance such simulation. Moreover, decellularized scaffolding reduces the inflammation, immune rejection, and foreign body rejection. Such scaffolds can maintain the structure, mechanical feature, shape, and molecular gradient to enhance cellular activities. Various methods such as physical, chemical, and enzymatic methods have been utilized for decellularization, in which the cell membranes were disrupted and rinsed away to achieve the decellularized scaffold. It is theorized that decellularized scaffolds can enhance tooth regeneration.
Zhang et al. utilized decellularized scaffolds for whole tissue regeneration. In their study, a porcine decellularized tooth bud is utilized to regenerate the whole tooth. A decellularized scaffold is seeded with porcine dental epithelial cells, human dental pulp cells, and human umbilical vein endothelial cells. The constructed scaffold exhibits a high degree of cellular activity, which was beneficial for whole tooth regeneration [62].
It is supposed that endodontic regeneration is an alternative procedure to treat the root canal of immature teeth. Song et al. decellularized human dental pulp and recellularized it with the stem cells of the apical papilla to regenerate the tooth. The decellularized scaffold supports the proliferation and differentiation of the stem cells of the apical papilla [63]. Traphagen et al. decellularized porcine molar tooth buds, maintaining the ECM proteins such as collagen, laminin, and fibronectin. A reseeded decellularized tooth contains higher content of collagen than decellularized tooth tissue. It was concluded that the natural decellularized scaffolds are proper for tooth regeneration to mimic the native tissue [64].
Hu et al. decellularized the swine dental pulp from the mandibular anterior teeth of swine and seeded with human dental pulp stem cells for pulp regeneration. It is observed that the bioscaffold maintained the natural shape and ECM components, which enhanced cellular activities, such as growth and proliferation [65]. Precisely controlling the bioengineered tooth shape and size, forming the functional tooth root, and removing abnormal mineralized tissue formation are the challenging issues in whole tissue engineering. Based on the reported studies, decellularized scaffolds can provide a niche-like environment with minimal immunological response.
4.3 Cell Sheets for Tooth Regeneration
Sheet engineering has been developed as a new effective approach to produce tissues in vitro. In sheet engineering, cell sheets are detached from culture plates using scrapers, thermos-responsive polymer coatings, magnetic force, ionic-induced dissolution, electrochemical polarization, electrochemically induced pH decrease, and UV illumination [66]. Using these techniques, the extracellular matrix formed by the cells perseveres. Herein, following implantation, the cells in the sheets can attach to the recipient tissues without any additional materials, which increases the survival rate of implanted tissues [67]. In the case of bioengineering a whole tooth, the cell sheet technology can be applied to investigate and establish an epithelial-mesenchymal interconnection. Moreover, studies show that it is possible to generate a functional bioengineered tooth using this technique in combination with scaffold-based tissue engineering.
In studies, it has been shown that human dental follicle cell sheets combined with dentin matrix scaffolds and autologous fibroblast multilayer cell sheets regenerate bio-root structures [68, 69]. 3D SCAP sheet-derived pellets also regenerate roots when they were implanted in the back of immunodeficient mice. Here, SCAP sheets induce generation of odontoblast-like cells and mineralized dentine-like tissue [70]. In another study, Vc-induced periodontal ligament stem cell sheets cover dental pulp stem cell-seeded root-shaped hydroxy-apatite scaffolds, which are then implanted into jaw bone implant sockets. After 6 months, by installing a crown on the bio-root, the whole functional tooth is generated [71].
5 Investigation the Functionality the Whole Bioengineered Teeth
The ultimate goal of tooth engineering is to achieve a fully functional bioengineered tooth. Recently, several studies report producing whole bioengineered teeth using cell aggregation methods [69], cell sheet engineering , and biocompatible scaffolds [71]. For successful whole tooth replacement therapy, the bioengineered teeth must be able to integrate with the bone and periodontal ligament tissues in the edentulous jaw area. Moreover, the bioengineered teeth should have sufficient strength against mechanical load during mastication and respond well to noxious stimulations in the maxillofacial region.
5.1 Successful Transplantation
The main concern about whole tooth bioengineering using the organ germ method is whether the implanted construct can erupt and occlude with the neighbor and opposing teeth in the oral environment. During tooth development, cell signaling and genetic and molecular mechanisms regulate tooth eruption and occlusion in the region of jaw bone from where the teeth will be erupted [72, 73]. Thus far, several studies indicate that transplanted in vitro germ constructs can erupt in an edentulous region of the oral cavity [6, 43, 50]. It seems that the bioengineered teeth, generated using the organ germ method, could erupt through bone/gingival remodeling induced by the genetic/molecular mechanisms similar to the process of natural tooth eruption. Moreover, the studies show that the bioengineered teeth generated by organ germ method occluded with the neighbor and opposing teeth after transplantation [6].
5.2 Integration with Periodontal Ligament Tissues
The tooth germ is surrounded by dental follicular cells during tooth development. These follicular cells will differentiate into osteoblasts, cementoblasts, and fibroblasts, and therefore produce alveolar bone, cementum, and the periodontal ligament [74]. One of the main concerns in bioengineering tooth therapy is whether transplanting an engineered tooth or construct will successfully fix and create periodontal ligament tissue around the implant in the edentulous area. Regarding transplantation of bioengineered immature tooth using the organ germ method, because the whole process of developing tooth will be established in the transplanted germ construct in the edental region, it is obviously the periodontal ligament that will be generated during tooth development in the appropriate area. But this is a bit different for transplantation of bioengineered mature tooth. The bioengineered mature tooth has a higher priority compared with bioengineered immature tooth, as the mature one would exhibit an in vivo immediate functional operation [75]. In a study, it has been shown that when a bioengineered mature tooth unit is transplanted into a murine jawbone, the bioengineered tooth (with its periodontal ligament tissues) was successfully engrafted and integrated into the jaw after 40 days [7]. Therefore, both the bioengineered mature and immature teeth would successfully restore the masticatory function related to integration of the periodontal ligament after transplantation.
5.3 Responses to Mechanical Load of Bioengineered Teeth
Biological response to mechanical stresses in the bioengineered whole tooth is another important factor that should be investigated. Oral function necessitates the harmonized cooperation of the maxillofacial region and teeth using the periodontal ligament. The physiological properties of the tooth—such as absorption of occlusal loadings, preserving the alveolar bone height, and orthodontic movement of the tooth—affect the periodontal properties. It has been theorized that preserving the periodontal tissue of the tooth root is crucial for ankylosis prevention. The PDL connection plays an important role in tooth function, as its absence in synthetic implants results in major drawbacks in tooth functionality. In this regards, biological therapies attract more attention than artificial options in tooth restoration [76, 77].
Since the periodontal ligament is an important component for implant restoration, the neural responses also need to be considered. The tissue-implant interface is an important region, which in proper conditions results in osseointegration. The fibro-osseous interface can be developed by micromovement of the implant. Bone not only provides strength, but also provides regulation of calcium homeostasis. It is hypothesized that the mechanical load on bone causes a chain of events that results in a biological response. In this regard, properly designing the implant provides an appropriate mechanical transfer to the bone and results in activity of bone cells [78]. Direction, degree, duration, and rate of loading to the tooth determine the biomechanical response. Mechano-transduction includes (1) mechanocoupling, which transduces the mechanical loading to the biosignal to be detectable for sensor cells; (2) biochemical coupling, which converts the mechanical signals to the biochemical signals to illuminate the cell response; (3) transfer the sensor cell biosignals to effector cells; and (4) final response of effector cell.
Osteocytes sense the mechanical forces and assist the translation of the mechanical forces to the biochemical signals. These cells are located in the lacunae of the bony matrix and are more resistant to mechanical forces than osteoblasts [79, 80]. Recent studies on bioengineered teeth show that functional tooth movement and bone regeneration have been attained [6, 7]. These results determine that bioengineered teeth could appropriately accommodate the mechanical forces, similar to natural teeth. This is because of PDL formation and integration in bioengineered teeth—in contrast to dental implants, where the lack of PDL tissue causes their lack of response to mechanical loads and subsequent failure of the whole implant.
5.4 Perceptive Potential for Noxious Stimuli in Bioengineered Teeth
Sympathetic, parasympathetic, and sensory nerves innervate the teeth, like other peripheral organs. Afferent nerves regulate the physiological function of tooth and noxious stimulation comprehension [81]. Moreover, it seems that the nervous system plays an important role in tooth development. In a study, it was determined that tooth regeneration with a lesioned nerve did not occur [82]. This proves that there is a close correlation between tooth formation and peripheral innervation. It is supposed that nerves produce signaling molecules that affect the interaction between mesenchyme and dental epithelium. During tooth development, trigeminal ganglion sensory endings near dental MCSs release Shh, which acts as a key signaling molecule in tooth growth [83].
Hence, it seems that after transplantation of a bioengineered or autologous tooth, the neuronal regeneration is necessary for successful whole tooth bioengineering therapy [84]. Here, neuronal regeneration causes nerve fibers to enter into the bioengineered tooth, which innervate the pulp cavity, odontoblastic layer, and periodontal ligament with blood vessel reconstruction [6, 7]. Lack of innervation in the periodontal tissues and the pulp cavity after transplantation of conventional dental implants causes them to not comprehend peripheral stimuli, such as injuries, excessive loading, and orthodontic movement [85].
Innervation of the bioengineered teeth from the alveolar nerve after transplantation is one of the main challenges in producing a functional tooth implant. Studies show that if bioengineered immature constructs, including mesenchymal-epithelial cells , implant in the correct position in the jaw, the regeneration of nerves will be conducted [6, 7, 86]. To investigate innervation of embryonic dental epithelium and neural crest-derived MSCs, the reassociations of the cells obtained from embryonic day 14 mouse molars were implanted underneath the skin along with dorsal root ganglia. The results show that the innervation of the dental mesenchyme was not observed. Then, cell reassociation implantation along with trigeminal ganglia caused extension of axon growth to surround the forming teeth. However, the axons are detected in the dental mesenchyme in just 2.5% of samples, showing a specific defect in entering trigeminal ganglia into the dental mesenchyme. It has been shown that inhibition of T cells using immunosuppressive reagents such as cyclosporin A improves axonal regeneration. Cyclosporin A also has a direct effect on axonal regeneration by enhancing growth associated protein-43 expression [87]. The coimplantation of cell reassociations and trigeminal ganglia in cyclosporin A-treated ICR and immunocompromised nude mice shows the innervation of the dental mesenchyme in both strains similarly. These results demonstrate that immunosuppression can impair the innervation process in the dental mesenchyme [88].
Despite previous studies showing that the bioengineered whole tooth would potentially restore neuronal responses, more research needs to be done in order to reach practically this achievement with high performance.
6 Summary
Although clinical prosthetics, such as dental implants, have been used for tooth replacement, there are many disadvantages in their use. Currently, whole tooth engineering can provide a promising alternative approach. The research attempts in the bioengineering field of teeth should focus on the molecular processes during the tooth development, finding a promising autologous cell source, appropriate bioactive materials, and other barriers that limit the development of clinically functional bioengineered whole teeth. Despite the high complexity of the tooth organ component, the accomplishments of previous studies show that whole tooth engineering for humans is possible and a solution is on the horizon.
References
Kassebaum, N., et al. (2017). Global, regional, and national prevalence, incidence, and disability-adjusted life years for oral conditions for 195 countries, 1990–2015: A systematic analysis for the global burden of diseases, injuries, and risk factors. Journal of Dental Research, 96(4), 380–387.
Greenstein, G., Cavallaro, J., Romanos, G., & Tarnow, D. (2008). Clinical recommendations for avoiding and managing surgical complications associated with implant dentistry: A review. Journal of Periodontology, 79(8), 1317–1329.
Jung, R. E., Pjetursson, B. E., Glauser, R., Zembic, A., Zwahlen, M., & Lang, N. P. (2008). A systematic review of the 5-year survival and complication rates of implant-supported single crowns. Clinical Oral Implants Research, 19(2), 119–130.
Lai, W.-F., Lee, J.-M., & Jung, H.-S. (2014). Molecular and engineering approaches to regenerate and repair teeth in mammals. Cellular and Molecular Life Sciences, 71(9), 1691–1701.
Zhang, Y. D., Zhi, C., Song, Y. Q., Chao, L., & Chen, Y. P. (2005). Making a tooth: Growth factors, transcription factors, and stem cells. Cell Research, 15(5), 301.
Ikeda, E., et al. (2009). Fully functional bioengineered tooth replacement as an organ replacement therapy. Proceedings of the National Academy of Sciences, 106(32), 13475–13480.
Oshima, M., et al. (2011). Functional tooth regeneration using a bioengineered tooth unit as a mature organ replacement regenerative therapy. PLoS One, 6(7), e21531.
Gao, Z., et al. (2016). Bio-root and implant-based restoration as a tooth replacement alternative. Journal of Dental Research, 95(6), 642–649.
Young, C. S., et al. (2005). Tissue-engineered hybrid tooth and bone. Tissue Engineering, 11(9–10), 1599–1610.
Egusa, H., et al. (2010). Gingival fibroblasts as a promising source of induced pluripotent stem cells. PLoS One, 5(9), e12743.
Wada, N., Wang, B., Lin, N. H., Laslett, A. L., Gronthos, S., & Bartold, P. M. (2011). Induced pluripotent stem cell lines derived from human gingival fibroblasts and periodontal ligament fibroblasts. Journal of Periodontal Research, 46(4), 438–447.
Yan, X., Qin, H., Qu, C., Tuan, R. S., Shi, S., & Huang, G. T.-J. (2010). iPS cells reprogrammed from human mesenchymal-like stem/progenitor cells of dental tissue origin. Stem Cells and Development, 19(4), 469–480.
Amirabad, L. M., et al. (2017). Enhanced cardiac differentiation of human cardiovascular disease patient-specific induced pluripotent stem cells by applying unidirectional electrical pulses using aligned electroactive nanofibrous scaffolds. ACS Applied Materials & Interfaces, 9(8), 6849–6864.
Liu, L., Liu, Y. F., Zhang, J., Duan, Y. Z., & Jin, Y. (2016). Ameloblasts serum-free conditioned medium: Bone morphogenic protein 4-induced odontogenic differentiation of mouse induced pluripotent stem cells. Journal of Tissue Engineering and Regenerative Medicine, 10(6), 466–474.
Ozeki, N., et al. (2013). Mouse-induced pluripotent stem cells differentiate into odontoblast-like cells with induction of altered adhesive and migratory phenotype of integrin. PLoS One, 8(11), e80026.
Zamanlui, S., Amirabad, L. M., Soleimani, M., & Faghihi, S. (2018). Influence of hydrodynamic pressure on chondrogenic differentiation of human bone marrow mesenchymal stem cells cultured in perfusion system. Biologicals, 56, 1–8.
Amari, A., et al. (2015). In vitro generation of IL-35-expressing human Wharton’s jelly-derived mesenchymal stem cells using lentiviral vector. Iranian Journal of Allergy, Asthma, and Immunology, 14(4), 416–426.
Gronthos, S., Mankani, M., Brahim, J., Robey, P. G., & Shi, S. (2000). Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proceedings of the National Academy of Sciences, 97(25), 13625–13630.
Seo, B.-M., et al. (2004). Investigation of multipotent postnatal stem cells from human periodontal ligament. The Lancet, 364(9429), 149–155.
Miura, M., et al. (2003). SHED: Stem cells from human exfoliated deciduous teeth. Proceedings of the National Academy of Sciences, 100(10), 5807–5812.
Cordeiro, M. M., et al. (2008). Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. Journal of Endodontics, 34(8), 962–969.
Ge, J., et al. (2013). Distal C terminus of CaV1. 2 channels plays a crucial role in the neural differentiation of dental pulp stem cells. PLoS One, 8(11), e81332.
Guo, W., et al. (2009). The use of dentin matrix scaffold and dental follicle cells for dentin regeneration. Biomaterials, 30(35), 6708–6723.
Morsczeck, C., et al. (2005). Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biology, 24(2), 155–165.
Sonoyama, W., et al. (2008). Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: A pilot study. Journal of Endodontics, 34(2), 166–171.
Saito, M. T., Silvério, K. G., Casati, M. Z., Sallum, E. A., & Nociti, F. H., Jr. (2015). Tooth-derived stem cells: Update and perspectives. World Journal of Stem Cells, 7(2), 399.
Okamoto, T., et al. (2002). Clonal heterogeneity in differentiation potential of immortalized human mesenchymal stem cells. Biochemical and Biophysical Research Communications, 295(2), 354–361.
Shinmura, Y., Tsuchiya, S., Hata, K., & Honda, M. J. (2008). Quiescent epithelial cell rests of Malassez can differentiate into ameloblast-like cells. Journal of Cellular Physiology, 217(3), 728–738.
Honda, M., Shinohara, Y., Hata, K., & Ueda, M. (2007). Subcultured odontogenic epithelial cells in combination with dental mesenchymal cells produce enamel–dentin-like complex structures. Cell Transplantation, 16(8), 833–847.
Liu, Y., et al. (2013). Skin epithelial cells as possible substitutes for ameloblasts during tooth regeneration. Journal of Tissue Engineering and Regenerative Medicine, 7(12), 934–943.
Jussila, M., Juuri, E., & Thesleff, I. (2013). Tooth morphogenesis and renewal. In Stem cells in craniofacial development and regeneration (pp. 109–134). Hoboken: Wiley-Blackwell.
Wu, P., et al. (2013). Specialized stem cell niche enables repetitive renewal of alligator teeth. Proceedings of the National Academy of Sciences, 110(22), E2009–E2018.
Laurikkala, J., Mikkola, M. L., James, M., Tummers, M., Mills, A. A., & Thesleff, I. (2006). p63 regulates multiple signalling pathways required for ectodermal organogenesis and differentiation. Development, 133(8), 1553–1563.
Bei, M. (2009). Molecular genetics of tooth development. Current Opinion in Genetics & Development, 19(5), 504–510.
Mikkola, M. L. (2009). Molecular aspects of hypohidrotic ectodermal dysplasia. American Journal of Medical Genetics Part A, 149(9), 2031–2036.
Pispa, J., et al. (1999). Cusp patterning defect in Tabby mouse teeth and its partial rescue by FGF. Developmental Biology, 216(2), 521–534.
Mustonen, T., et al. (2003). Stimulation of ectodermal organ development by Ectodysplasin-A1. Developmental Biology, 259(1), 123–136.
Jernvall, J., Keränen, S. V., & Thesleff, I. (2000). Evolutionary modification of development in mammalian teeth: Quantifying gene expression patterns and topography. Proceedings of the National Academy of Sciences, 97(26), 14444–14448.
Kratochwil, K., Galceran, J., Tontsch, S., Roth, W., & Grosschedl, R. (2002). FGF4, a direct target of LEF1 and Wnt signaling, can rescue the arrest of tooth organogenesis in Lef1−/− mice. Genes & Development, 16(24), 3173–3185.
Klein, O. D., et al. (2006). Sprouty genes control diastema tooth development via bidirectional antagonism of epithelial-mesenchymal FGF signaling. Developmental Cell, 11(2), 181–190.
Gritli-Linde, A., Bei, M., Maas, R., Zhang, X. M., Linde, A., & McMahon, A. P. (2002). Shh signaling within the dental epithelium is necessary for cell proliferation, growth and polarization. Development, 129(23), 5323–5337.
Nakashima, M., & Reddi, A. H. (2003). The application of bone morphogenetic proteins to dental tissue engineering. Nature Biotechnology, 21(9), 1025.
Nakao, K., et al. (2007). The development of a bioengineered organ germ method. Nature Methods, 4(3), 227.
Hu, B., Nadiri, A., Bopp-Kuchler, S., Perrin-Schmitt, F., Wang, S., & Lesot, H. (2005). Dental epithelial histo-morphogenesis in the mouse: Positional information versus cell history. Archives of Oral Biology, 50(2), 131–136.
Hu, B., Nadiri, A., Kuchler-Bopp, S., Perrin-Schmitt, F., Peters, H., & Lesot, H. (2006). Tissue engineering of tooth crown, root, and periodontium. Tissue Engineering, 12(8), 2069–2075.
Ikeda, E., & Tsuji, T. (2008). Growing bioengineered teeth from single cells: Potential for dental regenerative medicine. Expert Opinion on Biological Therapy, 8(6), 735–744.
Lechguer, A. N., et al. (2011). Cell differentiation and matrix organization in engineered teeth. Journal of Dental Research, 90(5), 583–589.
Oshima, M., & Tsuji, T. (2015). Whole tooth regeneration as a future dental treatment. In Engineering Mineralized and Load Bearing Tissues (pp. 255–269). Cham: Springer.
Hu, X., et al. (2014). Conserved odontogenic potential in embryonic dental tissues. Journal of Dental Research, 93(5), 490–495.
Ohazama, A., Modino, S., Miletich, I., & Sharpe, P. (2004). Stem-cell-based tissue engineering of murine teeth. Journal of Dental Research, 83(7), 518–522.
Otsu, K., et al. (2011). Differentiation of induced pluripotent stem cells into dental mesenchymal cells. Stem Cells and Development, 21(7), 1156–1164.
Wen, Y., et al. (2012). Application of induced pluripotent stem cells in generation of a tissue-engineered tooth-like structure. Tissue Engineering Part A, 18(15–16), 1677–1685.
Cai, J., et al. (2013). Generation of tooth-like structures from integration-free human urine induced pluripotent stem cells. Cell Regeneration, 2(1), 6.
Wang, B., et al. (2010). Induction of human keratinocytes into enamel-secreting ameloblasts. Developmental Biology, 344(2), 795–799.
Chen, Y., et al. (2015). Human umbilical cord mesenchymal stem cells: A new therapeutic option for tooth regeneration. Stem Cells International, 2015, 1.
Young, C., Terada, S., Vacanti, J., Honda, M., Bartlett, J., & Yelick, P. (2002). Tissue engineering of complex tooth structures on biodegradable polymer scaffolds. Journal of Dental Research, 81(10), 695–700.
Angelova Volponi, A., Kawasaki, M., & Sharpe, P. (2013). Adult human gingival epithelial cells as a source for whole-tooth bioengineering. Journal of Dental Research, 92(4), 329–334.
Kuchler-Bopp, S., et al. (2017). Promoting bioengineered tooth innervation using nanostructured and hybrid scaffolds. Acta Biomaterialia, 50, 493–501.
Chen, G., et al. (2015). Combination of aligned PLGA/Gelatin electrospun sheets, native dental pulp extracellular matrix and treated dentin matrix as substrates for tooth root regeneration. Biomaterials, 52, 56–70.
Rasperini, G., et al. (2015). 3D-printed bioresorbable scaffold for periodontal repair. Journal of Dental Research, 94(9_suppl), 153S–157S.
Zhang, Y., et al. (2006). Novel chitosan/collagen scaffold containing transforming growth factor-β1 DNA for periodontal tissue engineering. Biochemical and Biophysical Research Communications, 344(1), 362–369.
Zhang, W., Vazquez, B., Oreadi, D., & Yelick, P. (2017). Decellularized tooth bud scaffolds for tooth regeneration. Journal of Dental Research, 96(5), 516–523.
Song, J., Takimoto, K., Jeon, M., Vadakekalam, J., Ruparel, N., & Diogenes, A. (2017). Decellularized human dental pulp as a scaffold for regenerative endodontics. Journal of Dental Research, 96(6), 640–646.
Traphagen, S. B., et al. (2012). Characterization of natural, decellularized and reseeded porcine tooth bud matrices. Biomaterials, 33(21), 5287–5296.
Hu, L., et al. (2017). Decellularized swine dental pulp as a bioscaffold for pulp regeneration. BioMed Research International, 2017, 9342714.
Owaki, T., Shimizu, T., Yamato, M., & Okano, T. (2014). Cell sheet engineering for regenerative medicine: Current challenges and strategies. Biotechnology Journal, 9(7), 904–914.
Okano, T. (2014). Current progress of cell sheet tissue engineering and future perspective. Tissue Engineering Part A, 20(9–10), 1353–1354.
Yang, B., et al. (2012). Tooth root regeneration using dental follicle cell sheets in combination with a dentin matrix-based scaffold. Biomaterials, 33(8), 2449–2461.
Zhou, Y., Li, Y., Mao, L., & Peng, H. (2012). Periodontal healing by periodontal ligament cell sheets in a teeth replantation model. Archives of Oral Biology, 57(2), 169–176.
Na, S., et al. (2016). Regeneration of dental pulp/dentine complex with a three-dimensional and scaffold-free stem-cell sheet-derived pellet. Journal of Tissue Engineering and Regenerative Medicine, 10(3), 261–270.
Wei, F., et al. (2013). Functional tooth restoration by allogeneic mesenchymal stem cell-based bio-root regeneration in swine. Stem Cells and Development, 22(12), 1752–1762.
Wise, G., & King, G. (2008). Mechanisms of tooth eruption and orthodontic tooth movement. Journal of Dental Research, 87(5), 414–434.
Wise, G., Frazier-Bowers, S., & D’souza, R. (2002). Cellular, molecular, and genetic determinants of tooth eruption. Critical Reviews in Oral Biology & Medicine, 13(4), 323–335.
Hou, L.-T., et al. (1999). Characterization of dental follicle cells in developing mouse molar. Archives of Oral Biology, 44(9), 759–770.
Gridelli, B., & Remuzzi, G. (2000). Strategies for making more organs available for transplantation. New England Journal of Medicine, 343(6), 404–410.
Bas, O., De-Juan-Pardo, E. M., Catelas, I., & Hutmacher, D. W. (2018). The quest for mechanically and biologically functional soft biomaterials via soft network composites. Advanced Drug Delivery Reviews, 132, 214–234.
Nanci, A. (2017). Ten Cate’s oral histology: Development, structure, and function. Elsevier Health Sciences, St. Louis, Missouri.
Jamal, H. (2016). Tooth organ bioengineering: Cell sources and innovative approaches. Dentistry Journal, 4(2), 18.
Saxena, S. (2015). Tissue response to mechanical load in dental implants. International Journal of Oral & Maxillofacial Pathology, 6(3), 02–06.
Xiu, P., et al. (2016). Tailored surface treatment of 3D printed porous Ti6Al4V by microarc oxidation for enhanced osseointegration via optimized bone in-growth patterns and interlocked bone/implant interface. ACS Applied Materials & Interfaces, 8(28), 17964–17975.
Luukko, K., Kvinnsland, I. H., & Kettunen, P. (2005). Tissue interactions in the regulation of axon pathfinding during tooth morphogenesis. Developmental Dynamics, 234(3), 482–488.
Tuisku, F., & Hildebrand, C. (1994). Evidence for a neural influence on tooth germ generation in a polyphyodont species. Developmental Biology, 165(1), 1–9.
Zhao, H., et al. (2014). Secretion of shh by a neurovascular bundle niche supports mesenchymal stem cell homeostasis in the adult mouse incisor. Cell Stem Cell, 14(2), 160–173.
Kjaer, M., Beyer, N., & Secher, N. (1999). Exercise and organ transplantation. Scandinavian Journal of Medicine & Science in Sports, 9(1), 1–14.
Burns, D. R., Beck, D. A., & Nelson, S. K. (2003). A review of selected dental literature on contemporary provisional fixed prosthodontic treatment: report of the Committee on Research in Fixed Prosthodontics of the Academy of Fixed Prosthodontics. The Journal of Prosthetic Dentistry, 90(5), 474–497.
Luukko, K., et al. (2008). Secondary induction and the development of tooth nerve supply. Annals of Anatomy-Anatomischer Anzeiger, 190(2), 178–187.
Ibarra, A., et al. (2007). Cyclosporin-A enhances non-functional axonal growing after complete spinal cord transection. Brain Research, 1149, 200–209.
Kökten, T., Bécavin, T., Keller, L., Weickert, J.-L., Kuchler-Bopp, S., & Lesot, H. (2014). Immunomodulation stimulates the innervation of engineered tooth organ. PLoS One, 9(1), e86011.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Mohammadi Amirabad, L., Zarrintaj, P., Lindemuth, A., Tayebi, L. (2020). Whole Tooth Engineering. In: Tayebi, L. (eds) Applications of Biomedical Engineering in Dentistry. Springer, Cham. https://doi.org/10.1007/978-3-030-21583-5_19
Download citation
DOI: https://doi.org/10.1007/978-3-030-21583-5_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-21582-8
Online ISBN: 978-3-030-21583-5
eBook Packages: EngineeringEngineering (R0)