Abstract
The symptoms of and disability related to decompensated heart failure are manifestations of congestion. Therefore, congestion is the main treatment target and diuretics are recommended as first line therapy. Refractory congestion refers to situations in which diuretics fail to produce the desired clinical effect. In these circumstances, ultrafiltration may be appropriate for the relief of congestion and improved outcomes. This chapter will review the concept of refractory congestion; describe the use of diuretics and ultrafiltration in the treatment of congestion; and review the importance of case selection when choosing to treat refractory congestion with ultrafiltration.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Case VignetteMrs. X is a 79 year old woman with an ischemic cardiomyopathy who was admitted to the hospital 4 days ago with signs and symptoms of volume overload. Despite full nephron blockade with intravenous acetazolamide 500 mg daily, intravenous furosemide 120 mg twice daily and metazolone 2.5 mg daily, net fluid loss is only 250 mL during the past 24 h with clinical signs of volume overload still present. The serum creatinine has bumped up from 1.68 mg/dL around admission to 2.59 mg/dL at the current. Blood pressure is 98/62 mmHg in the supine position.
FormalPara Chapter Key Points-
Indications for ultrafiltration in acute heart failure
-
Impact of ultrafiltration in acute heart failure on kidney function
-
Practical recommendations on how to prescribe ultrafiltration in acute heart failure
-
Upfront use of ultrafiltration instead of diuretics in acute heart failure
Brief Discussion of the Case
Cases like this are often encountered in clinical practice. Relief from congestion is the primary treatment goal after excluding precipitating factors such as ischemia, arrhythmia, and infection. In addition, a low cardiac output could be contributing to her clinical picture and it is important to assess the adequacy of tissue perfusion by examination and other indirect measures of cardiac output. This patient has persistent signs and symptoms of congestion despite an aggressive diuretic regimen. While clinicians depend on signs and symptoms as a surrogate for elevated cardiac filling pressures, the predictive value is limited. If there is clinical uncertainty about a patient’s cardiac filling pressures or cardiac output, further evaluation would be helpful and might include an echocardiogram, lactate levels, mixed venous oxygen saturation, non-invasive devices that estimate cardiac output based on pulse contour analysis or bioimpedance, or a right heart catheterization. For this patient, the combination of refractory congestion and acute kidney injury places her at increased risk of death or rehospitalization. Ultrafiltration is indicated and with proper monitoring and “dose” titration of fluid removal rates, can relieve congestion and improve kidney function. However, these “salvage” cases where treatment has been escalated over the course of several days without clinical improvement are at higher risk for adverse outcomes including renal failure and death. Early treatment with ultrafiltration within the first 24 h of hospital admission may result in better outcomes based on recent clinical trials.
Introduction
The importance of treating congestion is self-evident and has been covered in previous chapters. National guidelines recommend diuretics as the first-line therapy. However, in the setting of refractory congestion, ultrafiltration is recommended as a reasonable alternative. This final chapter will discuss the definitions of congestion and refractory congestion; describe the use of diuretics and ultrafiltration in the treatment of congestion; and review the importance of case selection.
Congestion
Signs and Symptoms
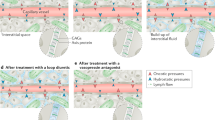
Signs and symptoms of congestion in heart failure are manifestations of ventricular diastolic pressures (Fig. 18.1). However, directly measured filling pressures are rarely available in the clinical setting and surrogates are used to determine whether congestion is present. Jugular venous distention is perhaps the best clinical indicator of elevated ventricular filling pressures. However, clinical estimates of jugular venous pressure ≥12 mmHg have relatively poor operating characteristics with a sensitivity of 65%, specificity of 64%, and positive and negative predictive values of 69% and 38%, respectively [2]. Other physical and radiographic signs of congestion cannot be reliably used to distinguish patients with from those without elevated ventricular filling pressures [2,3,4].
Pathophysiology of congestion. Abbreviations: RV right ventricular, RA right atrial, PA pulmonary artery, PCWP pulmonary capillary wedge pressure, LA left atrial, LV left ventricular, LVDP left ventricular diastolic pressure, JVD jugular venous distension. (From Gheorghiade et al. [1])
Weight Gain
Weight gain has also been used as a surrogate for congestion. A rapid increase in weight can precede decompensated heart failure and greatly increases the risk for hospitalization in patients with heart failure. However, other factors can influence weight and not all weight gain is attributable to decompensated heart failure. In addition, a large number of patients who are hospitalized for decompensated heart failure have little or no weight gain [5, 6].
Clinical Profiles
Clinical profiles of congestion have been used to provide prognostic information and to guide therapy. Characterizing heart failure patients based on the clinical indicators of perfusion and congestion as either warm, cold, wet, or dry is relatively easy to do using information available in the history and physical examination. Patients described as warm and dry after treatment in the hospital have better clinical outcomes than patients with other clinical profiles [2]. While this framework is useful in the clinical setting, the clinical indicators of congestion are often inaccurate as described above.
Fluid Compartments
While increases in left-sided filling pressures can rapidly occur due to shifts in blood compartments (this occurs largely between the splanchnic venous beds and the effective arterial circulation), this scenario is generally not the primary process in patients with refractory congestion who might be considered candidates for ultrafiltration [7].
Blood Volume
Heart failure is a sodium avid state that often leads to expansion of total body water and total blood volume. For this reason, blood volume analysis using radiolabeled Iodine-131 dilution techniques can be used as another surrogate for congestion. Physical manifestations of congestion are not associated with total blood volume and increased blood volume is significantly associated with elevated left-sided filling pressures [8]. In one study, 65% of heart failure patients who were euvolemic by physical examination were actually hypervolemic when total blood volume was measured. In another study only 37% of patients hospitalized with decompensated heart failure had an increase in total blood volume [9]. While blood volume analysis introduces a more quantitative approach to the assessment of congestion, it is only a surrogate for elevated left-sided filling pressures – increases in blood volume only explain approximately half the variation in measured wedge pressure [8]. Blood volume analysis is rarely used clinically in part because it requires handling radioactive materials and multiple blood draws to create an accurate dilution curve and because its value in directing therapeutic decisions has not yet been demonstrated.
Persistent Congestion
Relief of congestion is the primary treatment goal for patients with decompensated heart failure. Clinicians use a variety of surrogates to diagnose and monitor the regression of congestion during therapy such as physical examination, symptoms, radiographs, changes in weight, blood volume analysis, and B-type natriuretic peptide (BNP) levels. As described in the preceding paragraphs, these surrogates for elevated ventricular filling pressures are not particularly accurate [1]. Nevertheless, persistent congestion, as defined by a treating physician is associated with worse clinical outcomes (Table 18.1). Persistent congestion represents a failure to address patient symptoms, physical functioning, and quality of life. In addition, persistent congestion leads to unrelieved and ongoing neurohormonal activation which can ultimately lead to a cascade of pathologic processes including further sodium retention, renal failure, cardiac chamber dilatation, progressive mitral regurgitation, subendocardial ischemia and arrhythmia [17].
Diuretics for the Management of Congestion
Diuretics are first-line therapy for patients with heart failure and congestion [13, 18]. The goal of therapy is to relieve congestion by increasing urine output and removing excess intravascular and extravascular fluid [1]. Loop diuretics such as furosemide exert their action on the thick ascending portion of the loop of Henle to block the sodium-potassium-chloride transporter [19]. This results in an increase in urinary excretion of sodium, chloride, calcium, magnesium, and potassium. In general, plasma water follows sodium in the nephron resulting in an increase in urine production. Diuretics can sometimes be challenging to use because the dose response between individuals can be highly variable and electrolytes must be closely monitored and replaced. In addition, diuretic resistance is common, often requiring increasing doses to achieve similar degrees of urine output [20].
Diuretics produce hypotonic urine and this reduces the effective removal of excess total body sodium present in patients with heart failure. As a result, these drugs are often ineffective. In one large registry of over 100,000 patients hospitalized with acute decompensated heart failure, more than 90% received intravenous (IV) diuretics yet nearly half failed to lose any weight after treatment with IV diuretics [21]. In a clinical trial of diuretic dosing strategies, clinical decongestion was achieved in less than 20% percent of patients regardless of whether patients received high dose or low-dose diuretics, intermittent boluses or continuous intravenous infusions [22]. Vasodilators, inotropes, and other agents have been added to diuretics in an attempt to preserve renal function and improve outcomes yet these efforts have failed [23,24,25,26,27].
Refractory Congestion
There is no universally accepted definition of refractory congestion. Published guidelines are vague about this and refer to “a failure to respond to diuretics” or “after all diuretic strategies are unsuccessful”. Investigators have offered a number of working definitions for refractory congestion with varying degrees of specificity (Table 18.2). Some, but not all describe threshold doses of diuretics that must be administered before describing a patient as having refractory congestion. Other authors incorporate specific measures of sodium excretion in their definitions. However, using diuretic doses or urine sodium response to IV diuretics to define refractory congestion is problematic since less than half the variability in urine output following IV diuretics can be explained by the dose or predicted by spot urine sodium assessments [34, 35]. The common theme in all the definitions of refractory congestion is that there are persistent signs or symptoms of congestion despite therapies that include IV diuretics. The lack of a standardized definition of refractory congestion makes it difficult to conduct research and compare findings across published trials.
Ultrafiltration for the Management of Congestion
Ultrafiltration is the mechanical removal of isotonic plasma water directly from the circulation. Blood is withdrawn from a vein and flows across a semipermeable membrane under pressure to separate isotonic plasma water from blood. The plasma water is discarded and the remaining blood is returned to the patient [36]. Simplified ultrafiltration devices can be used in a variety of settings without the need for central venous access. Low blood flow rates are well tolerated even in patients with advanced heart failure and plasma water removal rates can be adjusted across a range from 0 to 500 mL/h. In contrast to diuretics which produces a hypotonic urine, ultrafiltration removes isotonic plasma water which can result in greater overall sodium removal – an important objective in treating heart failure [37]. Ultrafiltration results in rapid and predictable fluid removal, restores responsiveness to diuretics in patients with diuretic resistance, has no direct effect on serum electrolytes, and does not directly stimulate the neurohormonal system [38,39,40,41].
Direct Comparisons of Diuretics and Ultrafiltration
Randomized controlled trials comparing ultrafiltration to diuretic-based strategies have been performed in the modern era of heart failure treatment. These trials contribute to a growing database of experience that suggests that ultrafiltration may be superior to diuretic-based strategies in the management of patients who fail to adequately respond to loop diuretics.
RAPID [42]
The Relief for Acutely Fluid Overloaded Patients with Decompensated Congestive Heart Failure (RAPID-CHF) trial was the first randomized controlled trial comparing diuretic-based therapies to ultrafiltration in patients with acute decompensated heart failure using a simplified ultrafiltration circuit. This feasibility study randomized 40 patients hospitalized with decompensated heart failure to usual care with intravenous diuretics versus a single 8 h course of ultrafiltration performed within the first 24 h of hospitalization. There was a trend for improved weight loss in the ultrafiltration group at 24 h and significantly greater net fluid loss (4650 mL versus 2838 mL, P = 0.001). RAPID-CHF demonstrated that ultrafiltration is well tolerated in patients with acute decompensated heart failure and may be an alternative to diuretic therapy.
UNLOAD [39]
The Ultrafiltration Versus Intravenous Diuretics for Patients Hospitalized for Acute Decompensated Heart Failure (UNLOAD) trial randomized 200 patients hospitalized with decompensated heart failure to usual care versus ultrafiltration within the first 24 h of hospitalization. Patients in the usual care group received IV diuretics at doses equal to or greater than twice their usual outpatient diuretic dose and all diuretics were stopped in the ultrafiltration group, while volume reduction therapy was managed exclusively using ultrafiltration for the first 48 h after randomization. Patients in the ultrafiltration group lost more weight in the first 48 h compared to the usual care group (5 kg versus 3.1 kg, P = 0.001). The average diuretic dose in the usual care group was 181 mg of furosemide per day and the average plasma water removal rate in the ultrafiltration group was 241 mL/h over 12.3 h. There was a slight increase in serum creatinine in the ultrafiltration group but this was not statistically or clinically significant. There was a significant reduction in the prespecified secondary endpoint of heart failure-related hospitalizations at 90 days (Fig. 18.2). While promising, this improvement in clinical outcomes came under question because there was no formal clinical events committee to adjudicate the endpoints, treatment was not blinded, and more fluid was removed during ultrafiltration raising uncertainty about the potential mechanism of benefit.
Freedom from heart failure rehospitalization. Kaplan-Meier estimate of freedom from rehospitalization for heart failure within 90 days after discharge in the ultrafiltration (red line) and standard care (blue line) groups. (From Costanzo [39])
ULTRADISCO [43]
The Effects of Ultrafiltration Versus Diuretics on Clinical, Biohumoral and Haemodynamic Variables in Patients With Decompensated Heart Failure (ULTRADISCO) study randomized 30 patients hospitalized with decompensated heart failure and congestion to a continuous IV infusion of furosemide versus ultrafiltration with a conventional renal replacement device using slow continuous ultrafiltration techniques. In the usual care group, the initial rate of furosemide infusion was 250 mg per 24 h and this dose was adjusted according to changes in creatinine, blood pressure, and heart rate. The dose was increased to 500 mg per 24 h if the initial dose did not achieve a negative fluid balance of at least 2000 mL per day. The ultrafiltration group initiated ultrafiltration with a fluid removal rate between 100–300 mL/h and this rate was adjusted according to blood pressure. Both groups achieved similar degrees of weight loss and fluid loss by the end of treatment. However, patients in the ultrafiltration group had significant improvements in cardiac performance when measured noninvasively using pulse contour analysis suggesting a possible advantage to ultrafiltration versus traditional diuretics.
Hanna, et al. [44]
This is the only randomized controlled study of ultrafiltration versus usual care in which all patients underwent invasive hemodynamic monitoring. Thirty-six patients, all with pulmonary arterial wedge pressure ≥24 mmHg were randomized to usual care with IV diuretics at the discretion of the treating physician or slow continuous ultrafiltration using a standard renal replacement device. The primary endpoint was the time required for the pulmonary arterial wedge pressure to fall ≤18 mmHg for at least four consecutive hours. Both groups experienced significant decreases in central venous pressure and pulmonary arterial wedge pressure and there was a trend favoring ultrafiltration for achieving the primary endpoint (22 h versus 34.8 h, P = 0.081). Despite more fluid removal in the ultrafiltration group (5213 mL versus 2167 mL, P = 0.041), there was no significant change in renal function. Length of hospital stay was lower in the ultrafiltration group (4.53 days versus 9.61 days, P < 0.001).
CUORE [45]
The Continuous Ultrafiltration for Congestive Heart Failure (CUORE) study randomized 56 patients hospitalized with decompensated heart failure and significant congestion to usual care involving IV diuretics (average dose of diuretics at initiation of therapy was 153 mg per day) or ultrafiltration for an average of 19 h with a mean plasma water removal of 4254 mL. Interestingly, diuretics were continued in patients randomized to the ultrafiltration group. There was no significant difference in weight loss achieved in the two groups (7.5 kg for ultrafiltration versus 7.9 kg for usual care, P = 0.75). There was no difference in length of hospital stay. However, 6 months after discharge, patients in the usual care group gained more weight, required higher doses of diuretics, and had higher creatinine compared to the ultrafiltration group. Ultrafiltration patients had fewer heart failure readmissions after 12 months of follow-up compared to usual care (hazard ratio 0.14, P = 0.002).
CARRESS-HF [32]
The Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF) randomized 188 patients with acute kidney injury and persistent congestion after failing standard treatment with escalating doses of diuretics to a diuretic-based, stepped pharmacologic care treatment protocol designed to achieve 3–5 L of urine output per day or ultrafiltration (average treatment duration 40 h, target plasma water removal rate of 200 mL/h). The primary endpoint was change in weight and change in creatinine measured 96 h after randomization. There was no significant difference in weight loss and a transient increase in creatinine at 96 h which resolved 30 days after discharge. There were no differences in clinical outcomes at 60 days. Due to a significant number of dropouts and crossovers from the ultrafiltration arm of the trial to the diuretic-based arm of the trial, an analysis was performed comparing subjects who actually received their assigned treatment after randomization. In this per-protocol analysis, patients receiving ultrafiltration had significantly greater net fluid loss and weight reduction than patients receiving pharmacologic therapy [46] Ultrafiltration was associated with higher creatinine and blood urea nitrogen values, lower serum sodium concentrations, and increased plasma renin activity; pharmacologic therapy was associated with higher serum bicarbonate. However, there were no significant differences in 60-day outcomes suggesting that transient increases in serum creatinine associated with ultrafiltration are not clinically significant [46].
AVOID-HF [47]
The Aquapheresis versus Intravenous Diuretics and Hospitalizations for Heart Failure (AVOID-HF) trial randomized patients hospitalized for decompensated heart failure and congestion to IV diuretics or ultrafiltration within 24 h of hospital admission. Both treatment strategies included protocols for adjusting the rate of fluid removal based on response to therapy and other clinical parameters. The primary endpoint was time to heart failure readmission, or treatment with IV diuretics or ultrafiltration during an unscheduled outpatient or emergency room visit. The sample size of 810 patients was based on the ability to detect a 37.5% reduction in 90 day heart failure events with 90% power (Fig. 18.3). Unfortunately, this study was terminated for nonclinical reasons after enrolling 224 patients. Patients randomized to IV diuretics received an average daily dose of 271 mg furosemide during an average of 100 h. Patients randomized to ultrafiltration had an average rate of plasma water removal of 138 mL/h and underwent treatment for an average of 80 h. There was a 37% reduction in the risk of heart failure events with ultrafiltration versus diuretic therapy but this failed to reach statistical significance (hazard ratio 0.663, confidence interval 0.402–1.092). There was a non-significant trend favoring ultrafiltration for time to heart failure event (62 days versus 34 days, P = 0.106). Patients in the ultrafiltration group had fewer heart failure and cardiovascular events at 30 days, and no significant changes in serum creatinine. There were more serious adverse events felt to be related to study therapy in the ultrafiltration group than in the diuretic group (14.6% versus 5.4%, P = 0.026) and more adverse events of special interest including infection, bleeding, hypotension, and acute coronary syndrome (31% versus 17% P = 0.018).
Primary endpoint: time to heart failure event after discharge from the AVOID-HF study. Log-rank analysis of the time to first heart failure (HF) event after discharge from index hospitalization up to 90 days in the adjustable ultrafiltration (AUF) (squares) and adjustable loop diuretics (ALD) (triangles) groups. The difference in time to first heart failure event within 90 days after discharge from the index hospitalization was not statistically significant at the 0.05 alpha level (p = 0.106) due to the smaller than originally planned sample size. AQ aquapheresis, LD loop diuretics. (From Costanzo [47])
Treatment Pearls for the Case Vignette
Importance of Case Selection
Despite the promising results described in some of the randomized trials above, case selection clearly plays a key role in determining the outcomes of patients undergoing ultrafiltration therapy. Small, uncontrolled case series involving patients with refractory heart failure have been associated with very poor outcomes. In one series of 12 patients treated with vasopressors and high doses of furosemide for 22 days prior to initiating hemofiltration or hemodialysis, the median survival was 24 days [31]. A larger series of 63 patients with advanced heart failure in the intensive care unit were treated with slow continuous ultrafiltration for an average of 3 days. Prior to ultrafiltration therapy, all of these patients had oliguria or worsening renal function with persistent congestion and the majority were receiving high-dose IV loop diuretics and IV vasoactive drugs. The mean pulmonary arterial wedge pressure was 30 mmHg, central venous pressure 20 mmHg, and cardiac index 1.8 L/min/m2. After ultrafiltration, hemodynamic parameters improved, weight loss occurred, and there was a significant negative fluid balance. However, there were no improvements in renal function and 30% of patients died during the index hospitalization with an additional 10% discharged to hospice care [48].
There is currently no consensus regarding the optimal selection criteria for ultrafiltration. Factors that need to be considered include the patient’s volume status, the patient’s clinical response to diuretics, and the patient’s severity of illness prior to consideration of ultrafiltration therapy. When ultrafiltration is used as rescue or salvage therapy, it appears that the underlying disease processes involving the heart and the kidneys are so advanced that overall outcomes are poor. The randomized trials of ultrafiltration demonstrating more favorable outcomes targeted patients for early ultrafiltration therapy usually within the first 24 h of hospitalization.
Volume Status
Ultrafiltration effectively removes extracellular volume and can only be recommended in patients with volume overload and congestion. However, the clinical assessment of volume status and filling pressures is challenging even for experienced clinicians. Common elements include symptoms of congestion such as orthopnea; physical exam findings such as edema, jugular venous distention, pulmonary rales, and the presence of a 3rd heart sound; radiographic evidence of pulmonary congestion; direct or indirect measures of elevated filling pressures including central venous pressure, pulmonary artery pressure, pulmonary arterial wedge pressure, and left ventricular diastolic pressure; non-invasive assessments of hemodynamic status using ultrasound, pulse contour analysis, bio impedance; wireless intravascular or intracardiac pressure measurement, and others.
The CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients (CHAMPION) trial placed a wireless implantable hemodynamic monitor in eligible ambulatory patients with class III heart failure symptoms and then randomized them to usual clinical care with no hemodynamic data available to guide therapy versus an active treatment group in which the treating medical team had access to hemodynamic data to assist in titration of medications to achieve a prespecified pulmonary artery pressure. Patients in the treatment group had a 28% reduction in heart failure related hospitalizations in 6 months (rate 0.32 versus 0.44, P = 0.0002) [15]. This study demonstrates the potential value in accurate measurements of filling pressures and the importance of clinical decongestion on outcomes.
In another study, heart failure medications were titrated to achieve a prespecified total blood volume estimated using an iodine 131 labeled albumin indicator dilution technique. In this retrospective analysis performed at a single community hospital, targeted decongestion based on estimates of total blood volume was associated with improved 30 day readmission rates (12.2% versus 27.7%, P < 0.001) and a significant reduction in 30 day mortality (2% versus 11.1%, P < 0.001) when compared to propensity score-matched controls from a CMS limited data set.
Response to Diuretics
The above two trials demonstrate improved clinical outcomes by targeting directly measured pulmonary artery pressures and total blood volume. However, not every patient with elevated filling pressures and/or expanded extracellular volume will require or benefit from ultrafiltration therapy. In the CHAMPION trial, ambulatory patients with elevated filling pressures were successfully treated in the outpatient setting with oral medications, especially diuretics, to optimize filling pressures [15]. Therefore, only patients who fail oral medications in the ambulatory setting could be considered candidates for ultrafiltration. IV diuretics are often effective in managing the symptoms of congestion in decompensated heart failure – especially in patients without previous exposure to diuretics or in those who are taking diuretics at lower doses. The threshold at which ultrafiltration therapy is favored over intravenous diuretics has yet to be determined and will likely be defined by clinical outcomes. In the Diuretic Optimization Strategies Evaluation trial in Acute Heart Failure (DOSE-AHF) trial, a threshold of 80 mg of oral furosemide per day was used to define eligibility in a randomized clinical trial testing different dosing strategies of furosemide in patients hospitalized with decompensated heart failure. Clinical outcomes were poor (42% died, were rehospitalized, or had an emergency department visit within the 60-day follow-up period) regardless of the dosing strategy used (high intensity versus low intensity, continuous infusion versus intermittent bolus) [22]. A similar threshold of 80 mg of furosemide was used in the UNLOAD [39] and AVOID [47] trials and in these clinical trials there was a trend towards improved outcomes in patients undergoing ultrafiltration therapy versus IV diuretics.
Severity of Illness
Severity of illness is another consideration in selecting candidates for ultrafiltration. When ultrafiltration is used as a rescue or salvage procedure in patients with cardiogenic shock, often days or weeks after the initial hospitalization, outcomes are poor. [31, 48,49,50] Ultrafiltration performed early in the course of therapy in congested patients not requiring vasoactive drugs for support appears to result in improved outcomes compared to diuretics in randomized trials [39, 43, 45, 47, 51]. Based on the above considerations and the results of randomized controlled trials, ultrafiltration may be particularly useful in patients hospitalized with decompensated heart failure and significant congestion with failed or inadequate response to IV diuretics equal to at least 80 mg of oral furosemide per day. The best outcomes following ultrafiltration therapy occur when ultrafiltration is initiated within 24–48 h of the first dose of intravenous diuretics (Table 18.3). Successful ultrafiltration therapy depends on careful and appropriate patient selection. Once the decision to perform ultrafiltration has been made, it should be administered by clinicians with experience using ultrafiltration. The initial rate of plasma water removal should be carefully considered based on blood pressure, creatinine, and degree of left versus right ventricular dysfunction. Patients should be closely monitored during ultrafiltration for clinical response with special attention to heart rate, blood pressure, creatinine, urine output and signs of congestion [52].
References
Gheorghiade M, Follath F, Ponikowski P, Barsuk JH, Blair JE, Cleland JG, et al. Assessing and grading congestion in acute heart failure: a scientific statement from the acute heart failure committee of the heart failure association of the European Society of Cardiology and endorsed by the European Society of Intensive Care Medicine. Eur J Heart Fail. 2010;12(5):423–33.
Drazner MH, Hellkamp AS, Leier CV, Shah MR, Miller LW, Russell SD, et al. Value of clinician assessment of hemodynamics in advanced heart failure: the ESCAPE trial. Circ Heart Fail. 2008;1(3):170–7.
Chakko S, Woska D, Martinez H, de Marchena E, Futterman L, Kessler KM, et al. Clinical, radiographic, and hemodynamic correlations in chronic congestive heart failure: conflicting results may lead to inappropriate care. Am J Med. 1991;90(3):353–9.
Stevenson LW, Perloff JK. The limited reliability of physical signs for estimating hemodynamics in chronic heart failure. JAMA. 1989;261(6):884–8.
Chaudhry SI, Wang Y, Concato J, Gill TM, Krumholz HM. Patterns of weight change preceding hospitalization for heart failure. Circulation. 2007;116(14):1549–54.
Zile MR, Bennett TD, St John SM, Cho YK, Adamson PB, Aaron MF, et al. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation. 2008;118(14):1433–41.
Fallick C, Sobotka PA, Dunlap ME. Sympathetically mediated changes in capacitance: redistribution of the venous reservoir as a cause of decompensation. Circ Heart Fail. 2011;4(5):669–75.
Androne AS, Hryniewicz K, Hudaihed A, Mancini D, Lamanca J, Katz SD. Relation of unrecognized hypervolemia in chronic heart failure to clinical status, hemodynamics, and patient outcomes. Am J Cardiol. 2004;93(10):1254–9.
Strobeck JE, Feldschuh J, Miller WL. Heart failure outcomes with volume-guided management. JACC Heart Fail. 2018;6(11):940–8.
Lucas C, Johnson W, Hamilton MA, Fonarow GC, Woo MA, Flavell CM, et al. Freedom from congestion predicts good survival despite previous class IV symptoms of heart failure. Am Heart J. 2000;140(6):840–7.
Wattad M, Darawsha W, Solomonica A, Hijazi M, Kaplan M, Makhoul BF, et al. Interaction between worsening renal function and persistent congestion in acute decompensated heart failure. Am J Cardiol. 2015;115(7):932–7.
Aoki S, Okumura T, Sawamura A, Kitagawa K, Morimoto R, Sakakibara M, et al. Usefulness of the combination of in-hospital poor diuretic response and systemic congestion to predict future cardiac events in patients with acute decompensated heart failure. Am J Cardiol. 2017;119(12):2010–6.
Lala A, McNulty SE, Mentz RJ, Dunlay SM, Vader JM, AbouEzzeddine OF, et al. Relief and recurrence of congestion during and after hospitalization for acute heart failure: insights from Diuretic Optimization Strategy Evaluation in Acute Decompensated Heart Failure (DOSE-AHF) and Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARESS-HF). Circ Heart Fail. 2015;8(4):741–8.
Kociol RD, McNulty SE, Hernandez AF, Lee KL, Redfield MM, Tracy RP, et al. Markers of decongestion, dyspnea relief, and clinical outcomes among patients hospitalized with acute heart failure. Circ Heart Fail. 2013;6(2):240–5.
Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377(9766):658–66.
Darawsha W, Chirmicci S, Solomonica A, Wattad M, Kaplan M, Makhoul BF, et al. Discordance between hemoconcentration and clinical assessment of decongestion in acute heart failure. J Card Fail. 2016;22(9):680–8.
Mentz RJ, Kjeldsen K, Rossi GP, Voors AA, Cleland JG, Anker SD, et al. Decongestion in acute heart failure. Eur J Heart Fail. 2014;16(5):471–82.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):e240–327.
Dikshit K, Vyden JK, Forrester JS, Chatterjee K, Prakash R, Swan HJ. Renal and extrarenal hemodynamic effects of furosemide in congestive heart failure after acute myocardial infarction. N Engl J Med. 1973;288(21):1087–90.
Brater DC. Diuretic therapy. N Engl J Med. 1998;339(6):387–95.
Gheorghiade M. Reassessing treatment of acute heart failure syndromes: the ADHERE registry. Eur Heart J. 2005;7(Suppl):B13–9.
Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364(9):797–805.
Chen HH, Anstrom KJ, Givertz MM, Stevenson LW, Semigran MJ, Goldsmith SR, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA. 2013;310(23):2533–43.
Massie BM, O'connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, et al. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N Engl J Med. 2010;363(15):1419–28.
O’Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365(1):32–43.
Packer M, O'Connor C, McMurray JJV, Wittes J, Abraham WT, Anker SD, et al. Effect of ularitide on cardiovascular mortality in acute heart failure. N Engl J Med. 2017;376(20):1956–64.
Teerlink JR. RELAXin in acute heart failure-2-RELAX-AHF-2. 2017.
Ellison DH. Diuretic therapy and resistance in congestive heart failure. Cardiology. 2001;96(3–4):132–43.
Sackner-Bernstein JD, Obeleniene R. How should diuretic-refractory, volume-overloaded heart failure patients be managed? J Invasive Cardiol. 2003;15(10):585–90.
Simpson IA, Rae AP, Simpson K, Gribben J, Boulton Jones JM, Allison ME, et al. Ultrafiltration in the management of refractory congestive heart failure. Br Heart J. 1986;55(4):344–7.
Dormans TP, Huige RM, Gerlag PG. Chronic intermittent haemofiltration and haemodialysis in end stage chronic heart failure with oedema refractory to high dose frusemide. Heart. 1996;75(4):349–51.
Bart BA, Goldsmith SR, Lee KL, Givertz MM, O'connor CM, Bull DA, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med. 2012;367(24):2296–304.
ter Maaten JM, Valente MA, Damman K, Hillege HL, Navis G, Voors AA. Diuretic response in acute heart failure-pathophysiology, evaluation, and therapy. Nat Rev Cardiol. 2015;12(3):184–92.
Aronson D, Burger AJ. Diuretic response: clinical and hemodynamic predictors and relation to clinical outcome. J Card Fail. 2016;22(3):193–200.
Brinkley DM Jr, Burpee LJ, Chaudhry SP, Smallwood JA, Lindenfeld J, Lakdawala NK, et al. Spot urine sodium as triage for effective diuretic infusion in an ambulatory heart failure unit. J Card Fail. 2018;24(6):349–54.
Ronco C, Ricci Z, Bellomo R, Bedogni F. Extracorporeal ultrafiltration for the treatment of overhydration and congestive heart failure. Cardiology. 2001;96(3–4):155–68.
Schrier RW. Role of diminished renal function in cardiovascular mortality: marker or pathogenetic factor? J Am Coll Cardiol. 2006;47(1):1–8.
Ali SS, Olinger CC, Sobotka PA, Dahle TG, Bunte MC, Blake D, et al. Loop diuretics can cause clinical natriuretic failure: a prescription for volume expansion. Congest Heart Fail. 2009;15(1):1–4.
Costanzo MR, Guglin ME, Saltzberg MT, Jessup ML, Bart BA, Teerlink JR, et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol. 2007;49(6):675–83.
Guazzi MD, Agostoni P, Perego B, Lauri G, Salvioni A, Giraldi F, et al. Apparent paradox of neurohumoral axis inhibition after body fluid volume depletion in patients with chronic congestive heart failure and water retention. Br Heart J. 1994;72(6):534–9.
Marenzi G, Guazzi M, Lauri G, Perego GB, Sganzerla P, Agostoni P. Body fluid withdrawal with isolated ultrafiltration effects persistent improvement of functional capacity in patients with chronic congestive heart failure. Furosemide does not produce the same result. Cardiologia. 1994;39(11):763–72.
Bart BA, Boyle A, Bank AJ, Anand I, Olivari MT, Kraemer M, et al. Ultrafiltration versus usual care for hospitalized patients with heart failure: the Relief for Acutely Fluid-Overloaded Patients With Decompensated Congestive Heart Failure (RAPID-CHF) trial. J Am Coll Cardiol. 2005;46(11):2043–6.
Giglioli C, Landi D, Cecchi E, Chiostri M, Gensini GF, Valente S, et al. Effects of ULTRAfiltration vs. DIureticS on clinical, biohumoral and haemodynamic variables in patients with deCOmpensated heart failure: the ULTRADISCO study. Eur J Heart Fail. 2011;13(3):337–46.
Hanna MA, Tang WH, Teo BW, O'Neill JO, Weinstein DM, Lau SM, et al. Extracorporeal ultrafiltration vs. conventional diuretic therapy in advanced decompensated heart failure. Congest Heart Fail. 2012;18(1):54–63.
Marenzi G, Muratori M, Cosentino ER, Rinaldi ER, Donghi V, Milazzo V, et al. Continuous ultrafiltration for congestive heart failure: the CUORE trial. J Card Fail. 2014;20(5):378–9.
Grodin JL, Carter S, Bart BA, Goldsmith SR, Drazner MH, Tang WHW. Direct comparison of ultrafiltration to pharmacological decongestion in heart failure: a per-protocol analysis of CARRESS-HF. Eur J Heart Fail. 2018;20:1148–56.
Costanzo MR, Negoianu D, Jaski BE, Bart BA, Heywood JT, Anand IS, et al. Aquapheresis versus intravenous diuretics and hospitalizations for heart failure. JACC Heart Fail. 2016;4(2):95–105.
Patarroyo M, Wehbe E, Hanna M, Taylor DO, Starling RC, Demirjian S, et al. Cardiorenal outcomes after slow continuous ultrafiltration therapy in refractory patients with advanced decompensated heart failure. J Am Coll Cardiol. 2012;60(19):1906–12.
Liang KV, Hiniker AR, Williams AW, Karon BL, Greene EL, Redfield MM. Use of a novel ultrafiltration device as a treatment strategy for diuretic resistant, refractory heart failure: initial clinical experience in a single center. J Card Fail. 2006;12(9):707–14.
Ramos R, Salem BI, DePawlikowski MP, Tariq M, Haikal M, Pohlman T, et al. Outcome predictors of ultrafiltration in patients with refractory congestive heart failure and renal failure. Angiology. 1996;47(5):447–54.
Siddiqui WJ, Kohut AR, Hasni SF, Goldman JM, Silverman B, Kelepouris E, et al. Readmission rate after ultrafiltration in acute decompensated heart failure: a systematic review and meta-analysis. Heart Fail Rev. 2017;22(6):685–98.
Costanzo MR, Ronco C, Abraham WT, Agostoni P, Barasch J, Fonarow GC, et al. Extracorporeal ultrafiltration for fluid overload in heart failure: current status and prospects for further research. J Am Coll Cardiol. 2017;69(19):2428–45.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bart, B.A. (2020). Refractory Congestion: When to Use Ultrafiltration?. In: Tang, W., Verbrugge, F., Mullens, W. (eds) Cardiorenal Syndrome in Heart Failure. Springer, Cham. https://doi.org/10.1007/978-3-030-21033-5_18
Download citation
DOI: https://doi.org/10.1007/978-3-030-21033-5_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-21032-8
Online ISBN: 978-3-030-21033-5
eBook Packages: MedicineMedicine (R0)