Abstract
Anal cancer commonly presents with rectal bleeding, pain, and mass sensation, although these are frequently mistaken for benign anal pathology leading to a delay in diagnosis. As the treatment of anal cancer is nonsurgical, anal cancer is clinically staged with an emphasis on physical examination, focusing on digital rectal exam (DRE), inguinofemoral lymph node evaluation, and gynecologic exam in female patients. Anoscopy or proctoscopy with biopsy confirms the diagnosis and histological type. Biopsy of suspicious inguinal nodes should be considered if indeterminate on clinical or radiographic assessment. Screening for human immunodeficiency virus (HIV) in all patients and for cervical cancer in females is generally performed, while prostate-specific antigen (PSA) screening in males and colonoscopy are optional. Proper staging workup includes computed tomography (CT) or magnetic resonance imaging (MRI) of the pelvis and CT of the chest and abdomen. The addition of whole-body positron emission tomography-computed tomography (PET/CT) leads to further nodal evaluation and informs radiotherapeutic treatment planning for curative cases. Anal canal cancers, located between the anorectal junction and the anal verge, and perianal cancers, located within 5 centimeters of the anal verge, are staged together using the American Joint Committee on Cancer (AJCC) classification scheme. About one-half of patients present with localized disease and another one-third present with regional lymphadenopathy, with the remainder found to have distant metastases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Digital rectal examination
- Nodal evaluation
- Computed tomography
- Magnetic resonance imaging
- Positron emission tomography
- Anal canal cancer
- Perianal cancer
Initial Diagnosis
Anal cancer commonly presents as a slow-growing mass that involves the anal canal or the perianal skin. The interval from symptom onset to diagnosis can be quite prolonged, exceeding 1 month in 80% of patients and 6 months in 33% of patients [1]. Up to one-half of patients report rectal bleeding, which may be mistakenly attributed to hemorrhoids or other benign anal pathology, and about one-third of patients report pain or sensation of a mass [2]. Other common symptoms include obstruction, incontinence, discharge, change in bowel habits, pruritus, non-healing ulcer formation, and, in more advanced cases, inguinal pain or lymphadenopathy. Up to 20% of patients may display no symptoms and are diagnosed incidentally during hemorrhoid evaluation or removal of anal tags [2].
An overview of the diagnostic workup of anal cancer is given in Table 3.1. A thorough history and physical examination should be performed, including history of anal sphincter continence, change in stool caliber, tenesmus, immunosuppression, and human papillomavirus (HPV)-related disease or malignancy. A full sexual history including HIV risk factors should also be performed. Patients who are smoking should be counseled to quit as smoking increases the risk of acute and late treatment toxicity. DRE and anoscopy/proctoscopy with biopsy are critical for diagnosis. Size, extent, and location of the mass (including any skin extension beyond the anal verge to the perianal skin and any sphincter involvement) should be noted along with anal sphincter tone and the presence of any fixation or involvement of adjacent organs. In females, a thorough gynecological exam should assess the relationship of the tumor to the vagina, including rectovaginal septum examination to rule out a possible fistula. Palpation of the inguinofemoral nodes is an essential component of the physical exam as well.
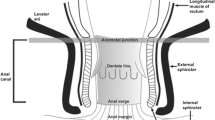
The subclassification of anal cancer as anal canal or perianal cancer guides definitive management. The anal canal is defined by a superior border at the palpable puborectalis muscles of the anorectal ring and an inferior border at the anal verge corresponding to the introitus, while the perianal area encompasses a region of five-centimeter radius around the anal verge. If a tumor in the perianal region has any extension into the anal canal, it would be more properly classified as an anal canal cancer. At our institution, for tumors with significant perianal skin involvement, we often consider a diverting colostomy to reduce the risk of infection from stool passing through non-healed skin during chemoradiation. Similarly, anovaginal or other fistulae may warrant diverting colostomy.
Pathologic Evaluation
Up to 80% of anal cancers are of squamous cell histology, including predominantly non-keratinizing, poorly differentiated tumors in the anal canal distal to the dentate line and keratinizing, well-differentiated tumors in the perianal region [3, 4]. Another histology of the anal canal includes adenocarcinomas, which most often occur in the transitional zone above the dentate line and are generally treated like rectal cancers. Rare entities of the anal canal include melanoma, sarcoma, lymphoma, neuroendocrine carcinoma, and undifferentiated cancer. Rare histologies of the perianal region include verrucous carcinoma (giant condyloma), basal cell carcinoma, Bowen’s disease, and Paget’s disease (perianal adenocarcinoma).
Although HPV is found in the majority of anal cancers and is prognostic of overall survival [5, 6], it is not standard to perform HPV testing on the tumor sample as it does not alter management. However, HPV testing may be recommended in cases in which it may inform additional workup, for example, if other anogenital lesions are found, or if a female has had normal cervical cancer Pap smear screening in the preceding 3–5 years and HPV testing on the tumor sample may direct if rescreening should be performed. Our preference is to perform a Pap smear at the time of workup for the anal cancer. Genetic sequencing of anal tumors is also not yet standard but may play a role in personalizing therapies in the future.
Laboratory Evaluation
Basic laboratory evaluation is required for workup of anal carcinoma, including a complete blood count (CBC), basic metabolic panel (electrolytes and creatinine), and liver function tests (LFTs). Creatinine is necessary to determine feasibility and need of any dose modification of concurrent chemotherapy. Abnormal LFTs may indicate the presence of liver metastasis, prompting further evaluation with various imaging modalities (CT, MRI, and/or ultrasound). In pre-menopausal or peri-menopausal females, pregnancy testing should be performed, as is standard before commencing radiation treatment. Finally, as detailed below, HIV testing should at a minimum be pursued in those with risk factors for infection; the preferred initial test for screening is a fourth-generation antigen/antibody combination HIV-1/2 immunoassay, followed by confirmatory testing and CD4 level if positive.
Although carcinoembryonic antigen (CEA) is elevated in ~20% of patients with anal carcinoma, it has limited diagnostic ability to detect anal cancer due to low sensitivity [7]. Specificity of CEA is also limited, given that various nonmalignant conditions can cause CEA elevation, including gastrointestinal tract infection/inflammation, liver disease, chronic obstructive pulmonary disease, diabetes, and cigarette smoking. Unlike in colorectal cancer, CEA also has no role in the assessment of prognosis or posttreatment follow-up of anal carcinoma [7, 8].
Screening for Comorbid Conditions
Colorectal Cancer Screening
Although prior studies have shown no increased risk of colorectal cancer in patients diagnosed with anal cancer, colonoscopy is often performed in the workup of anal cancer [9, 10]. The recent clinical practice guidelines developed by the American Society of Colon and Rectal Surgeons endorse colonoscopy after diagnosis of anal cancer [11], although other societies, such as the European Society of Surgical Oncology, consider it optional [12]. At a minimum, history of and time interval between any previous screening colonoscopies should be considered when making the decision to offer or refer a patient for new colonoscopy.
HIV Screening
The risk of anal cancer is significantly increased in patients with HIV infection and has been increasing over time due to improved survival from highly active antiretroviral treatment (HAART) [13,14,15]. While some advocate HIV testing for all patients with unknown HIV status, others only recommend it for patients at high risk for HIV infection. Recent evidence has shown similar treatment response and overall survival for HIV-positive patients treated with HAART compared to HIV-negative patients [16,17,18].
Multidisciplinary management with patients’ infectious disease or primary care physicians should be sought. For patients with a new diagnosis of HIV, referral to an infectious disease physician should be made for evaluation and initiation of HAART, as outcomes for those with low CD4 counts have generally been found to be inferior, although the data remain equivocal [17, 19, 20].
Gynecological Cancer Screening in Females
HPV is the common causative agent of most anogenital cancers, including 91% of cervical cancers, 75% of vaginal cancers, and 69% of vulvar cancers [21]. Field cancerization can occur leading to the synchronous or metachronous development of multiple malignant or premalignant lesions. Studies have found that a diagnosis of anal cancer carries increased risk of cervical cancer, vulvar or vaginal cancer, and cervical carcinoma in situ; likewise, invasive or in situ cervical cancer is associated with increased risk of anal cancer [22,23,24].
As previously mentioned, gynecological exam should be performed in females to assess for extent of the primary disease. Careful examination of the vulva, vaginal canal, and cervix is additionally warranted to assess for any suspicious premalignant or malignant lesions; cervical cancer screening with Pap smear and/or HPV testing should be performed at the same time.
Genitourinary Cancer Screening in Males
Similarly, HPV is found in 63% of penile cancers in males [21], and a field cancerization effect may lead to the development of multiple anogenital cancers. Indeed, condylomata acuminata (genital warts) has been found to increase the risk of both penile and anal cancers [25, 26]; as such, it may be prudent to examine the external genitalia in males for signs of papillomatous and/or malignant lesions.
Although there appears to be little etiological similarity between anal and prostate cancers, prostate cancer is the most common cancer in males, and thus, a considerable portion of male patients with anal cancer can be expected to harbor a simultaneous prostate cancer. This relationship has not been systematically studied due to the relative rarity of anal cancer and its predominance in females; however, in a prospective study of 20 male patients with colorectal cancer who were screened for prostate cancer, it was found that 16% had biopsy-proven prostate malignancy [27]. Given that any treatment of prostate cancer, whether surgical or radiotherapeutic, would be impacted by radiation treatment to the anal canal and pelvis, some consider it prudent to screen for prostate malignancy with PSA, although there are insufficient data to make this standard practice.
Radiographic Assessment of Primary Tumor
CT or MRI of the pelvis is an essential component of workup and provides additional characteristics of the primary tumor (e.g., involvement of adjacent organ or structures, such as vaginal canal or external anal sphincter). However, CT scan alone is often not sufficient in assessing the primary tumor due to limitations of CT in delineating the anatomy of the anal region. Studies have reported sensitivity of CT scan in detecting the primary tumor of ~60%, in contrast to a detection rate of >90% with the addition of PET [28,29,30]. Similarly, MRI provides higher resolution of the location, size, and extent of disease of the primary tumor compared to CT, especially with regard to adjacent organ and soft tissue involvement (Fig. 3.1a, b) [31, 32]. As definitive chemoradiotherapy is the mainstay of treatment for anal carcinoma, more accurate tumor delineation with MRI may be beneficial in treatment planning, most notably for T4 disease (adjacent organ involvement). Lastly, there is generally no role for endoscopic ultrasound, as the depth of invasion is not used in anal cancer staging and does not dictate management, in contrast to that of other gastrointestinal cancers.
(a–f) CT pelvis with IV contrast (a) demonstrating heterogeneously enhancing bulky mass with necrosis in the anal canal. T2-weighted axial pelvic MRI (b) depicting the same anal carcinoma, characterized by hyperintense diffusely enhancing anal mass with mild infiltration of the posterior perirectal soft tissue. Left (black arrow) and right (white arrow) common iliac lymph node metastases detected on CT pelvis with IV contrast (c) and PET/CT (d), with disease extension superiorly involving the para-aortic lymph nodes (black-dashed arrow) on CT pelvis with IV contrast (e) and PET/CT (f)
Assessment of Nodal Metastases
Anal carcinoma can metastasize to the perirectal and internal iliac lymph nodes if located superior to the dentate line and to the superficial inguinal and external iliac lymph nodes if located inferior to the dentate line. CT or MRI of the pelvis is an integral part of staging evaluation to assess both primary and nodal diseases. While older studies have demonstrated similar efficacy of CT and MRI in lymph node evaluation [33], improvement in MR technology has allowed greater sensitivity in detecting smaller lymph nodes (<5 mm) that may also harbor cancer cells [34]. Nevertheless, both techniques rely on nonspecific characteristics of size and morphologic criteria to differentiate between benign and malignant lymph nodes, which could often lead to false-positive or false-negative interpretations [35].
Increasingly, PET/CT has been used by clinicians as a complementary study to the staging pelvic CT/MRI. Multiple studies over the past decade have evaluated the value of PET/CT in the staging for anal cancer, which are summarized in Table 3.2. PET/CT allows for enhanced evaluation of the primary tumor and increased detection of inguinal lymph node involvement compared to CT and physical exam. As such, studies have reported significant upstaging of disease with the addition of PET/CT, with rates ranging from 5.1% to 37.5% [28,29,30, 36,37,38,39,40]. Interestingly, several of these studies also demonstrate downstaging of nodal disease in some patients, wherein enlarged lymph nodes detected on CT or MRI are not metabolically active on PET; however, the overall changes in staging with PET/CT still distinctly trend toward upstaging [30, 36, 37, 40]. Additionally, various studies report changes in the radiation planning (prescribed dose and treatment field) due to changes in nodal staging from PET/CT, ranging from 12.5% to 59.3% of patients treated [29, 36,37,38, 40].
The degree of true sensitivity and specificity of PET/CT in detecting nodal metastases in anal cancer remains controversial. A meta-analysis of seven retrospective and five prospective studies demonstrated pooled estimates of sensitivity of 56% and specificity of 90% [39]. The low sensitivity may be at least partially attributed to decreased sensitivity of PET for lymph node sizes of <8 mm [41]. However, a more recent meta-analysis of 17 studies reported pooled sensitivity and specificity of 93% and 76%, respectively, for the detection of nodal metastases [40]. Overall, PET/CT appears to provide valuable assessment of nodal status in anal cancer, and should be used in conjunction with, rather than as a replacement of, pelvic CT or MRI, as per NCCN guidelines.
While PET/CT has higher rates of lymph node detection than CT, there is also concern regarding its high false-positive rates, which is likely in part due to increased FDG uptake from inflammatory reaction. Histological confirmation with needle biopsy of FDG-avid inguinal nodes is recommended if the lymph node is of sufficient size and is otherwise indeterminate on radiographic or clinical assessment; biopsy is also recommended for any suspicious inguinal nodes that lack FDG avidity. However, surgical data demonstrate that pelvic lymph node metastases for anal cancer are often <5 mm in diameter, suggesting that many early inguinal node metastases may not be amenable to biopsy [42]. Demonstrating the false positivity of PET/CT, Mistrangelo and colleagues compared PET/CT to sentinel lymph node biopsy (SLNB) in the detection of inguinal node metastases in 27 patients with anal cancer and showed that of seven patients with positive PET scans, four had negative SLNBs [43]. This evidence suggests that PET/CT may lead to overdiagnosis of nodal metastases, and thus, overtreatment of the nodal regions. Indeed, a recent meta-regression and simulation study observed that modern clinical series of anal cancer reported much higher rates of lymph node positivity than predicted, which is likely attributable to overdiagnosis by modern imaging studies [44]. Notably, nodal stage migration with the advent of PET/CT staging was associated with improved survival in both node-positive and node-negative patients and decreased survival differences by nodal status, while proportions of T staging remained unchanged, suggesting misclassification of node-negative patients to the node-positive cohort [44].
The false positivity of PET/CT in nodal staging is especially important to consider when assessing an HIV-positive patient. Patients with HIV often have diffuse lymph node activation resulting in low-level uptake of FDG in lymph nodes at baseline, likely driven by inflammatory changes [45]. Correspondingly, Cotter and colleagues showed that HIV-positive patients were more likely to have positive PET findings in the inguinal (44% vs. 34%) and pelvic lymph nodes (44% vs. 16%) compared to HIV-negative patients, although the number of patients studied was small [28]. Of the four HIV-positive patients with FDG-avid inguinal lymph nodes, two underwent biopsy which revealed only diffuse inflammatory changes. There was also a higher rate of FDG-avid distant lymph nodes, which were again felt to be reflective of the heightened inflammatory state rather than distant metastatic deposits. Thus, biopsy of suspicious FDG-avid lymph nodes in HIV-positive patients is of particular importance before making management decisions.
To circumvent the overdiagnosis and overtreatment of inguinal lymph nodes, further efforts have been made to investigate the efficacy of inguinal SLNB. As most studies that evaluated SLNB had small sample sizes, two meta-analyses were carried out to provide important insight. Noorani and colleagues showed that SLN detection rate ranges from 47% to 100% across 17 studies [46], while Tehranian and colleagues demonstrated pooled inguinal SLN detection rate of 86.2% among 16 studies [47]. In seven studies of the former meta-analysis, patients with negative inguinal SLNB underwent inguinal-sparing radiotherapy and the rate of inguinal nodal recurrence (a surrogate of false-negative rate of SLNB) ranged from 0% to 18.75% [46]. Although inguinal SLNB prior to definitive chemoradiotherapy appears to be a promising strategy, it is unclear if it will change practice, as the toxicity of overtreating questionable lymph nodes with chemoradiation is currently low with modern techniques such as intensity-modulated radiation treatment.
Assessment of Distant Metastases
Anal carcinoma can spread via both the portal venous system and systemic circulation to result in distant metastases. The blood supply of anal lesions located above the dentate line drains into the portal venous system and provides a direct conduit for metastases to the liver, which is the most common site of distant spread for anal carcinoma. Conversely, lesions below the dentate line drain directly into the systemic circulation, which can lead to lung metastases. As such, proper workup for anal cancer includes CT abdomen with intravenous (IV) and oral contrast for evaluation of liver and abdominal metastases as well as CT chest for pulmonary metastases. An additional benefit to CT chest is the potential detection of new primary lung cancers, which occur at increased rates in patients with anal cancer due to the common risk factor of smoking. Whole-body PET/CT, which is increasingly utilized for nodal evaluation, will also further increase the sensitivity of detecting distant disease.
Para-aortic lymph node spread may also occur. Although spread of disease to the para-aortic lymph nodes is staged as distant metastases, recent retrospective studies have shown that patients with distant disease limited to the para-aortic nodes can be treated curatively with extended-field chemoradiation (Fig. 3.1c–f) [48, 49].
Staging
The AJCC anal cancer staging manual (eighth edition) is used to stage all anal cancers, including anal canal and perianal cancers [50], and is outlined in Box 3.1. All histologies are staged as anal cancers, except for melanoma, sarcoma, and well-differentiated neuroendocrine carcinoma. Assessment of the primary tumor (T) focuses on tumor size, rather than depth of invasion as in the remainder of the luminal gastrointestinal tract. Assessment of nodal involvement (N) is based on the nodal region involved rather than the number of lymph nodes involved. Assessment of distant metastasis (M) evaluates for the presence or absence of distant spread.
Box 3.1 AJCC Staging for Anal Cancer, Eighth Edition (2017)
Primary tumor (T) | |||
TX | Primary tumor (T) | ||
T0 | No evidence of primary tumor | ||
Tis | High-grade squamous intraepithelial lesion | ||
T1 | Tumor ≤2 cm | ||
T2 | Tumor >2 cm but ≤5 cm | ||
T3 | Tumor >5 cm | ||
T4 | Tumor of any size invading adjacent organ(s), such as the vagina, urethra, or bladder | ||
Regional lymph nodes (N) | |||
NX | Regional lymph nodes cannot be assessed | ||
N0 | No regional lymph node metastasis | ||
N1 | Metastasis in inguinal, mesorectal, internal iliac, or external iliac nodes | ||
N1a | Metastasis in inguinal, mesorectal, or internal iliac lymph nodes | ||
N1b | Metastasis in external iliac lymph nodes | ||
N1c | Metastasis in external iliac with any N1a nodes | ||
Distant metastasis (M) | |||
M0 | No distant metastasis | ||
M1 | Distant metastasis | ||
Prognostic stage groups | |||
0 | Tis | N0 | M0 |
I | T1 | N0 | M0 |
IIA | T2 | N0 | M0 |
IIB | T3 | N0 | M0 |
IIIA | T1-2 | N1 | M0 |
IIIB | T4 | N0 | M0 |
IIIC | T3-4 | N1 | M0 |
IV | Any T | Any N | M1 |
Changes were made from the AJCC staging manual seventh edition (2009) to the eighth edition (2017), including removal of N1, N2, and N3 categories and addition of N1a, N1b, and N1c categories, based on similar prognosis with any level of nodal involvement [50]. The stage groups were also revised to accommodate the new N categories. Additionally, perianal cancers were previously termed anal margin cancers and staged as skin cancers, but because many of these tumors involve the anal canal, they are now staged as anal cancers.
The TNM staging assessment carries prognostic significance. In a secondary analysis of 620 patients on the Radiation Therapy Oncology Group 98-11 trial, the best overall survival (OS), disease-free survival, and locoregional failure outcomes were found in those with T2-3N0 tumors and the poorest outcomes were found in those with T4N0 and T3-4N+ tumors [51]. Similarly, the need for future colostomy, a surrogate for local recurrence, was lowest for patients with T2N0 and T2N+ tumors and highest in those with T4N0 and T3-4N+ tumors.
In a Surveillance, Epidemiology, and End Results (SEER) study, between 2008 and 2014, 48% of anal cancers were localized at initial diagnosis, 32% had spread to the regional lymph nodes, and 13% presented with distant metastases; 5-year OS was 82%, 64%, and 30%, respectively [52]. By stage, the AJCC has reported 5-year OS of 77% and 71% for Stage I squamous and non-squamous anal cancers, respectively, 67% and 59% for Stage II cancers, 58% and 50% for Stage IIIA cancers, 51% and 35% for Stage IIIB cancers, and 15% and 7% for Stage IV cancers [50].
Conclusion
Initial evaluation of anal cancer consists of a comprehensive history and physical examination, including digital rectal exam and palpation of inguinofemoral lymph nodes, followed by tumor biopsy with or without biopsy of any suspicious inguinal lymphadenopathy. CT or MRI of the pelvis and CT of the chest/abdomen are required to complete staging; PET/CT has also become widely implemented as it provides valuable additional information and guides radiation therapy planning to better delineate the primary tumor. Anal cancer is clinically staged primarily using primary tumor size and extent of regional lymphadenopathy according to AJCC guidelines with an overall very favorable prognosis. Subclassification of anal cancer as anal canal or perianal cancer has implications for definitive management.
Abbreviations
- AJCC:
-
American Joint Committee on Cancer
- BMP:
-
Basic metabolic panel
- CBC:
-
Complete blood count
- CEA:
-
Carcinoembryonic antigen
- CT:
-
Computed tomography
- DRE:
-
Digital rectal exam
- HAART:
-
Highly active antiretroviral treatment
- HIV:
-
Human immunodeficiency virus
- HPV:
-
Human papillomavirus
- IV:
-
Intravenous
- LFTs:
-
Liver function tests
- MRI:
-
Magnetic resonance imaging
- NCCN:
-
National Comprehensive Cancer Network
- OS:
-
Overall survival
- PET/CT:
-
Positron emission tomography-computed tomography
- PSA:
-
Prostate-specific antigen
- SEER:
-
Surveillance, epidemiology, and end results
- SLNB:
-
Sentinel lymph node biopsy
References
Tanum G, Tveit K, Karlsen KO. Diagnosis of anal carcinoma – doctor’s finger still the best. Oncology. 1991;48(5):383–6.
Ryan DP, Compton CC, Mayer RJ. Carcinoma of the anal canal. N Engl J Med. 2000;342(11):792–800.
Myerson RJ, Karnell LH, Menck HR. The National Cancer Data Base report on carcinoma of the anus. Cancer. 1997;80(4):805–15.
Oliver GC, Labow SB. Neoplasms of the anus. Surg Clin North Am. 1994;74(6):1475–90.
Serup-Hansen E, Linnemann D, Skovrider-Ruminski W, Høgdall E, Geertsen PF, Havsteen H. Human papillomavirus genotyping and p16 expression as prognostic factors for patients with American joint committee on cancer stages I to III carcinoma of the anal canal. J Clin Oncol. 2014;32(17):1812–7.
Rödel F, Wieland U, Fraunholz I, Kitz J, Rave-Fränk M, Wolff HA, et al. Human papillomavirus DNA load and p16 INK4a expression predict for local control in patients with anal squamous cell carcinoma treated with chemoradiotherapy. Int J Cancer. 2015;136(2):278–88.
Tanum G, Stenwig AE, Børmer OP, Tveit KM. Carcinoembryonic antigen in anal carcinoma. Acta Oncol. 1992;31(3):333–5.
Indinnimeo M, Reale MG, Cicchini C, Stazi A, Fiori E, Izzo P. CEA, TPA, CA 19-9, SCC and CYFRA at diagnosis and in the follow-up of anal canal tumors. Int Surg. 1997;82(3):275–9.
Wasvary HJ, Barkel DC, Klein SN. Is total colonic evaluation for anal cancer necessary? Am Surg. 2000;66(6):592–4.
Klompje J, Petrelli NJ, Herrera L, Mittelman A. Synchronous and metachronous colon lesions in squamous cell carcinoma of the anal canal. J Surg Oncol. 1987;35(2):86–8.
Stewart DB, Gaertner WB, Glasgow SC, Herzig DO, Feingold D, Steele SR, et al. The American Society of Colon and Rectal Surgeons clinical practice guidelines for anal squamous cell cancers (revised 2018). Dis Colon Rectum. 2018;61(7):755–74.
Glynne-Jones R, Nilsson PJ, Aschele C, Goh V, Peiffert D, Cervantes A, et al. Anal cancer: ESMO–ESSO–ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Radiother Oncol. 2014;111(3):330–9.
Patel P, Hanson DL, Sullivan PS, Novak RM, Moorman AC, Tong TC, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148(10):728–36.
Frisch M, Biggar RJ, Goedert JJ. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J Natl Cancer Inst. 2000;92(18):1500–10.
Chiao EY, Krown SE, Stier EA, Schrag D. A population-based analysis of temporal trends in the incidence of squamous anal canal cancer in relation to the HIV epidemic. J Acquir Immune Defic Syndr. 2005;40(4):451–5.
Fraunholz I, Rabeneck D, Gerstein J, Jäck K, Haberl A, Weiss C, et al. Concurrent chemoradiotherapy with 5-fluorouracil and mitomycin C for anal carcinoma: are there differences between HIV-positive and HIV-negative patients in the era of highly active antiretroviral therapy? Radiother Oncol. 2011;98(1):99–104.
Seo Y, Kinsella MT, Reynolds HL, Chipman G, Remick SC, Kinsella TJ. Outcomes of chemoradiotherapy with 5-fluorouracil and mitomycin C for anal cancer in immunocompetent versus immunodeficient patients. Int J Radiat Oncol. 2009;75(1):143–9.
Salama JK, Mell LK, Schomas DA, Miller RC, Devisetty K, Jani AB, et al. Concurrent chemotherapy and intensity-modulated radiation therapy for anal canal cancer patients: a multicenter experience. J Clin Oncol. 2007;25(29):4581–6.
Wexler A, Berson AM, Goldstone SE, Waltzman R, Penzer J, Maisonet OG, et al. Invasive anal squamous-cell carcinoma in the HIV-positive patient: outcome in the era of highly active antiretroviral therapy. Dis Colon Rectum. 2008;51(1):73–81.
Edelman S, Johnstone PAS. Combined modality therapy for HIV-infected patients with squamous cell carcinoma of the anus: outcomes and toxicities. Int J Radiat Oncol. 2006;66(1):206–11.
Saraiya M, Unger ER, Thompson TD, Lynch CF, Hernandez BY, Lyu CW, et al. US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines. J Natl Cancer Inst. 2015;107(6):djv086.
Rabkin CS, Biggar RJ, Melbye M, Curtis RE. Second primary cancers following anal and cervical carcinoma: evidence of shared etiologic factors. Am J Epidemiol. 1992;136(1):54–8.
Melbye M, Sprøgel P. Aetiological parallel between anal cancer and cervical cancer. Lancet. 1991;338(8768):657–9.
Ebisch RMF, Rutten DWE, IntHout J, Melchers WJG, Massuger LFAG, Bulten J, et al. Long-lasting increased risk of human papillomavirus-related carcinomas and premalignancies after cervical intraepithelial neoplasia grade 3: a population-based cohort study. J Clin Oncol. 2017;35(22):2542–50.
Nordenvall C, Chang ET, Adami HO, Ye W. Cancer risk among patients with condylomata acuminata. Int J Cancer. 2006;119(4):888–93.
Blomberg M, Friis S, Munk C, Bautz A, Kjaer SK. Genital warts and risk of Cancer: a Danish study of nearly 50,000 patients with genital warts. J Infect Dis. 2012;205(10):1544–53.
Terris MK, Wren SM. Results of a screening program for prostate cancer in patients scheduled for abdominoperineal resection for colorectal pathologic findings. Urology. 2001;57(5):943–5.
Cotter SE, Grigsby PW, Siegel BA, Dehdashti F, Malyapa RS, Fleshman JW, et al. FDG-PET/CT in the evaluation of anal carcinoma. Int J Radiat Oncol. 2006;65(3):720–5.
Nguyen BT, Joon DL, Khoo V, Quong G, Chao M, Wada M, et al. Assessing the impact of FDG-PET in the management of anal cancer. Radiother Oncol. 2008;87(3):376–82.
Jones M, Hruby G, Solomon M, Rutherford N, Martin J. The role of FDG-PET in the initial staging and response assessment of anal cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2015;22(11):3574–81.
Kochhar R, Plumb AA, Carrington BM, Saunders M. Imaging of anal carcinoma. AJR Am J Roentgenol. 2012;199(3):W335–44.
Ciombor KK, Ernst RD, Brown G. Diagnosis and diagnostic imaging of anal canal cancer. Surg Oncol Clin N Am. 2017;26(1):45–55.
Dooms GC, Hricak H, Crooks LE, Higgins CB. Magnetic resonance imaging of the lymph nodes: comparison with CT. Radiology. 1984;153(3):719–28.
Saokar A, Islam T, Jantsch M, Saksena MA, Hahn PF, Harisinghani MG. Detection of lymph nodes in pelvic malignancies with computed tomography and magnetic resonance imaging. Clin Imaging. 2010;34(5):361–6.
Hedgire SS, Pargaonkar VK, Elmi A, Harisinghani AM, Harisinghani MG. Pelvic nodal imaging. Radiol Clin N Am. 2012;50(6):1111–25.
de Winton E, Heriot AG, Ng M, Hicks RJ, Hogg A, Milner A, et al. The impact of 18-fluorodeoxyglucose positron emission tomography on the staging, management and outcome of anal cancer. Br J Cancer. 2009;100(5):693–700.
Mistrangelo M, Pelosi E, Bellò M, Ricardi U, Milanesi E, Cassoni P, et al. Role of positron emission tomography-computed tomography in the management of anal cancer. Int J Radiat Oncol Biol Phys. 2012;84(1):66–72.
Sveistrup J, Loft A, Berthelsen AK, Henriksen BM, Nielsen MB, Engelholm SA. Positron emission tomography/computed tomography in the staging and treatment of anal cancer. Int J Radiat Oncol. 2012;83(1):134–41.
Caldarella C, Annunziata S, Treglia G, Sadeghi R, Ayati N, Giovanella L. Diagnostic performance of positron emission tomography/computed tomography using fluorine-18 fluorodeoxyglucose in detecting locoregional nodal involvement in patients with anal canal cancer: a systematic review and meta-analysis. ScientificWorldJournal. 2014;2014:196068.
Mahmud A, Poon R, Jonker D. PET imaging in anal canal cancer: a systematic review and meta-analysis. Br J Radiol. 2017;90(1080):20170370.
Scher ED, Ahmed I, Yue NJ, Jabbour SK. Technical aspects of radiation therapy for anal cancer. J Gastrointest Oncol. 2014;5(3):198–211.
Wade DS, Herrera L, Castillo NB, Petrelli NJ. Metastases to the lymph nodes in epidermoid carcinoma of the anal canal studied by a clearing technique. Surg Gynecol Obstet. 1989;169(3):238–42.
Mistrangelo M, Pelosi E, Bellò M, Castellano I, Cassoni P, Ricardi U, et al. Comparison of positron emission tomography scanning and sentinel node biopsy in the detection of inguinal node metastases in patients with anal cancer. Int J Radiat Oncol Biol Phys. 2010;77(1):73–8.
Sekhar H, Zwahlen M, Trelle S, Malcomson L, Kochhar R, Saunders MP, et al. Nodal stage migration and prognosis in anal cancer: a systematic review, meta-regression, and simulation study study. Lancet Oncol. 2017;18(10):1348–59.
Scharko AM, Perlman SB, Pyzalski RW, Graziano FM, Sosman J, Pauza CD. Whole-body positron emission tomography in patients with HIV-1 infection. Lancet. 2003;362(9388):959–61.
Noorani A, Rabey N, Durrani A, Walsh SR, Davies RJ. Systematic review of sentinel lymph node biopsy in anal squamous cell carcinoma. Int J Surg. 2013;11(9):762–6.
Tehranian S, Treglia G, Krag DN, Dabbagh Kakhki VR, Zakavi SR, Sadeghi R, et al. Sentinel node mapping in anal canal cancer: systematic review and meta-analysis. J Gastrointestin Liver Dis. 2013;22(3):321–8.
Holliday EB, Lester SC, Harmsen WS, Eng C, Haddock MG, Krishnan S, et al. Extended-field chemoradiation therapy for definitive treatment of anal canal squamous cell carcinoma involving the Para-aortic lymph nodes. Int J Radiat Oncol Biol Phys. 2018;102(1):102–8.
Hodges JC, Das P, Eng C, Reish AG, Beddar AS, Delclos ME, et al. Intensity-modulated radiation therapy for the treatment of squamous cell anal cancer with Para-aortic nodal involvement. Int J Radiat Oncol Biol Phys. 2009;75(3):791–4.
Welton ML, Steele SR, Goodman KA, et al. Anus. In: Amin MB, editor. AJCC Cancer staging manual. 8th ed. New York: Springer; 2017. p. 275–84.
Gunderson LL, Moughan J, Ajani JA, Pedersen JE, Winter KA, Benson AB 3rd, et al. Anal carcinoma: impact of TN category of disease on survival, disease relapse, and colostomy failure in US gastrointestinal intergroup RTOG 98-11 phase 3 trial. Int J Radiat Oncol. 2013;87(4):638–45.
Noone AM, Howlader N, Krapcho M, et al. SEER cancer statistics review [Internet]. Bethesda: National Cancer Institute; 2018. [Updated 2018 Sept 10; cited 2018 Sept 24]. Available at: https://seer.cancer.gov/csr/1975_2015/.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Gupta, A., Wang, SJ., Jabbour, S.K. (2019). Staging and Initial Evaluation of Anal Cancer. In: Meyer, J., Kachnic, L. (eds) Anal Cancer. Springer, Cham. https://doi.org/10.1007/978-3-030-20253-8_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-20253-8_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-20252-1
Online ISBN: 978-3-030-20253-8
eBook Packages: MedicineMedicine (R0)