Abstract
The immune system plays a vital role in maintaining the balance between health and disease. Immune cells provide protection against various pathogenic organisms, protecting the host from infection and injury to health. This protection is essential for the survival of the host and contributes to the overall health and longevity of individuals. In contrast, dysregulation of the immune system, resulting in overactive immune responses or autoimmunity are detrimental to the host, and induce tissue damage and development of disease. Chronic inflammation is associated with a number of diseases such as allergy, asthma, inflammatory bowel disease, rheumatoid arthritis, and cancer. This chapter will provide a general overview of the immune system and the principles of the innate and adaptive immune responses. The various cells and molecules of the innate and adaptive immune systems will be discussed and their role in immunity will be examined. In the second half of the chapter, we will examine pharmacological approaches to treating inflammation, and will discuss the various classes of drugs that target immune cells and their mediators. These include drugs that cause immunosuppression, those that prevent the proliferation of immune cells, and those that modulate immune activity. The suggested reading list at the end of the chapter highlights major discoveries in the field of immunology during the last two centuries that have advanced our current understanding of immunology and medicine.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Immune
- Inflammation
- Innate
- Adaptive
- cytokine
- Interleukin
- Primary immune responses
- Secondary immune responses
- Immune memory
- T cell activation
- B cell activation
- Antibodies
- Glucocorticoids
- Anti-proliferative agents
- Calcineurin inhibitors
- Biologics
-
1.
Describe the process of hematopoiesis and the various types of hematopoietic cells.
-
2.
Describe the processes involved in the education and shaping of immune cells.
-
3.
Describe the role of cell surface receptors and cytokines during immune responses and explain their importance in cell-to-cell communication.
-
4.
Compare and contrast innate and adaptive immune responses in terms of cell types, humoral factors, magnitude, and kinetics.
-
5.
Explain the contribution of immune cells and their mediators to the development of primary and secondary immune responses.
-
6.
Discuss the role of primary and secondary lymphoid organs in the development and activation of immune cells.
-
7.
Describe the various classes and types of immunotherapeutic drugs and discuss their mechanism of action.
-
8.
Describe adverse reactions that can occur with the use of immunotherapeutic drugs.
-
9.
Explain the development of hypersensitivity reactions to immunological drugs.
Introduction
Since ancient times, humans across many cultures have recognized the vital role that inflammation plays in health and disease. The Jews considered blood to be the most sacred of all organs, possessing the life of an animal. Similarly, ancient Egyptians distinguished between good and bad wounds on the basis of the presence or absence of signs of inflammation, while the Hindus in India developed an early system of medicine to treat various inflammatory illnesses. In 460 B.C., the Greek physician Hippocrates first introduced terms such as edema and categorized illnesses as acute or chronic. He is also credited with further developing the concept of inflammation and correlating its presence with the resolution and healing of diseases. Based on the Hippocratic canon, the Roman writer Aulus Cornelius Celsus in the first century A.D. accurately described inflammation as consisting of four main characteristics: redness (rubor), warmth (calor), pain (dolor), and swelling (tumor). This description of inflammation has stuck with us through the centuries and modern medicine considers the development of inflammation to be critical in the battle against infection and disease.
The nineteenth and twentieth centuries significantly advanced our understanding of how inflammation affects health and disease. The advent of the compound microscope finally allowed scientists to study the various components of blood, leading to the discovery and characterization of many hematopoietic cell types. Other scientists also discovered tiny organisms called ‘microbes’ that were ubiquitous throughout nature and hypothesized to cause the development of disease. This was followed by an elegant series of experimental studies by the scientists Robert Koch and Louis Pasteur, who formally established the role of microbes in causing infectious diseases, thus paving the way for understanding the functions of blood cells such as macrophages and mast cells in fighting disease. By the mid-twentieth century, several new advances in immunology had been made including the discovery of antibodies, B cells , and T cells, and their critical role in fighting infectious organisms. Then in 1957, Frank Burnet proposed the clonal selection theory, providing an explanation for how immune cells respond to specific infectious antigens, and serving as the basis for our understanding of adaptive immunity. Collectively, these and many other findings had firmly entrenched in the minds of immunologists that inflammation is the body’s response to infection. Indeed, as the well-respected immunologist Charles Janeway famously described it several years later, “the immune system evolved to discriminate infectious nonself from noninfectious self” Immunol Today. 1992 Jan;13(1):11–6.
In recent years, work done by immunologists, has led to the discovery and identification of a number of other cell types, receptors, and soluble mediators called cytokines (a list of cytokines, their receptors and functions is provided in Table 1.1) that have shaped our current understanding of immunity and how inflammation works. These discoveries have painted a rather complex picture of inflammation that cannot be described solely in terms of the host response to infection or the cardinal characteristics of inflammation first described by Celsus. Indeed, recent studies suggest a far more complicated interplay between various players in regulating the development of inflammation. These include the hematopoietic cells of the immune system, genetic polymorphisms, epigenetic factors, microbes, and several other environmental factors that have the ability to promote or inhibit the development of inflammation. Furthermore, it has now become apparent that inflammation is not simply the body’s response to infection, but can also develop towards a host of other antigenic substances including innocuous allergens, food particles, toxic gases, environmental pollutants, and any substance with the potential to cause injury or damage to the host. Lastly, it is now well-established that while the immune system plays a vital role in conferring protection from foreign agents, it is also responsible for the induction of unmitigated inflammatory responses against normal cellular components, leading to chronic inflammatory diseases and autoimmunity. In fact, the persistence of chronic inflammation underlying many different diseases has led to the suggestion that ‘inflammation’ may be the key to unraveling the unified theory of disease. In support, chronic inflammation is now known to be causative or a co-culprit in a number of conditions not typically associated with inflammation including cardiovascular insults (atherosclerosis, coronary artery disease), neurological diseases (Alzheimer’s disease, multiple sclerosis), type 2 diabetes, and cancer.
In this book, we examine the effects of inflammation in the pathogenesis of various diseases and explore the functions of currently approved immunotherapeutic drugs used in their treatment. Specific emphasis will be placed on the roles of immune cells, membrane-bound receptors, and soluble mediators in propagating or preventing a disease and their consideration as established or putative targets for immunotherapy. In the next few sections, a brief synopsis of the immune system including its development and function is provided. This is followed by an overview of the various classes and types of drugs used in immunotherapy. The principles underlying innate and adaptive immune responses as well as therapeutic modulation of the immune system is described in detail in subsequent chapters.
Overview of the Immune Response
The primary purpose of the immune system is to defend the host against infectious organisms that may compromise the integrity of the host, leading to cellular damage and possible death of the host. Immune responses against pathogens can be compartmentalized into five stages: pathogen detection, acute inflammation, antigen presentation, adaptive immunity, and pathogen destruction (Fig. 1.1). As discussed throughout this book, various cell types are involved at each stage, with their function regulated by cell-to-cell interactions, surface receptors, and cytokines. Many of these receptors and cytokines (which include various interleukins ) are therapeutic targets for patients with inflammatory diseases.
Infection with a pathogenic organism can lead to three possible outcomes: elimination of the organism by the immune system, chronic infection that is held in check by the immune system, or death of the host due to a failure of the immune system to eliminate the pathogen. Most infections are successfully eliminated by the immune system, resulting in tissue healing and cellular memory of the infectious pathogen. A small number of pathogens may cause chronic infections that are not cleared, leading to latency of the infectious organism within the host and subsequent periods of reactivation by environmental or other stimuli. Although these infections are not completely eradicated, they are usually held in check by the immune system for long periods of time, until the immune system is either compromised or completely damaged. In the absence of treatment to restore the immune system or control the infection, this usually results in death of the host.
The immune system is also critical for human survival. In the absence of a functional immune system, the host is unable to protect itself against common environmental microorganisms, ultimately succumbing to various infections that often result in death. Severe cases of this are observed in patients born with primary immunodeficiencies, as exemplified by Severe combined immune deficiency (SCID ). In this primary immunodeficiency, patients are unable to produce the T and B cells of the adaptive immune system, and survival is not possible, unless therapy is initiated with hematopoietic stem cell transplantation (bone marrow transplantation) to restore the immune system.
In addition to initiating and propagating immune responses, the cells of the immune system play important roles in several other organ systems. Various resident and migrating populations of immune cells such as macrophages and mast cells are present in almost every organ of the body, where they contribute to the integrity of tissues and participate in maintaining organelle function.
Hematopoiesis and Cells of the Immune System
The cells of the immune system are derived and transported via blood, and hence are referred to as hematopoietic cells. The process of formation of blood cells is termed as hematopoiesis. All the populations of blood cells are derived from common progenitors termed hematopoietic stem cells (HSCs ). These cells are present throughout the adult bone marrow and are long-lasting and self-renewing. They divide in the presence of growth factors and other instructions from stromal cells into several types of progenitor populations, eventually leading to the generation of distinct lineages of red and white blood cells. Thus, HSCs are also said to be pluripotent with the ability to differentiate into many different cell types.
In the developing embryo, hematopoiesis begins in the yolk sac. This later shifts to the fetal liver and then the spleen during the third to seventh months of fetal life. During the fourth to fifth months, hematopoiesis is initiated in the fetal bone marrow, and this continues throughout the life of the host. In adults, the major sites of hematopoiesis are the skull, sternum, vertebral column, femurs, pelvis, and ribs.
Hematopoietic cells are divided into two major categories: red blood cells or erythrocytes and white blood cells or leukocytes (Fig. 1.2). Immune cells are classically referred to as white blood cells, although erythrocytes also participate in the immune response. Two distinct lineages of leukocytes are derived from hematopoiesis: the myeloid lineage, which gives rise to granulocytes, monocytes , macrophages, dendritic cells , and mast cells; and the lymphoid lineage which gives rise to natural killer (NK) cells, B cells, and various populations of T cells.
The development of immune cells through hematopoiesis. Pluripotent stem cells are self-renewing and give rise to daughter progeny with a more limited developmental potential. Hematopoiesis occurs in the bone marrow and is guided by growth factors and cell to cell interactions. The common myeloid progenitor gives rise to several innate immune cell types including granulocytes, mast cells, and monocytes. The common lymphoid progenitor gives rise to lymphocytes (T cells, B cells, NK cells) (figure contributed by Jeremy P. McAleer)
Red blood cells and megakaryocytes (which give rise to platelets) are derived from the erythroid progenitor, which is derived from a common myeloid precursor. The primary purpose of erythrocytes is to transport oxygen throughout blood. However, they also participate in the removal of immune complexes containing antibodies bound to their target proteins. Platelets maintain the integrity of blood vessels and initiate and maintain clotting reactions to promote wound healing and prevent blood loss.
The Myeloid Lineage
The myeloid progenitor gives rise to three major cell types: granulocytes, monocyte-derived cells, and mast cells. The granulocytes consist of three major populations of cells: neutrophils , eosinophils , and basophils . They are characterized by the presence of cytoplasmic granules, which house a number of toxic mediators and enzymes that are involved in immune reactions. In addition, they possess many irregular, multi-lobed nuclei, leading to the use of the term polymorphonuclear (PMN) leukocytes to describe them.
Neutrophils Are Rapidly Mobilized to Tissues During an Infection
Neutrophils are the most abundant leukocyte present in blood, accounting for up to 70% of the total leukocyte population. Their granules do not stain with acidic or basic dyes, forming the basis for the nomenclature neutrophils. Neutrophils are highly specialized cells that are adept in the capture, phagocytosis, and killing of infectious organisms. In addition, they also secrete a number of mediators that enhance inflammation. Neutrophils act as first responders during infections and are rapidly mobilized from the blood to sites of infection. Here, they help initiate and coordinate the capture and killing of microorganisms that have entered the tissue, thriving in the anaerobic conditions present in damaged tissues. Despite their intense activity, neutrophils are short-lived cells that die after their granular contents have been released, resulting in the formation of pus.
Eosinophils and Basophils Protect the Host from Parasitic Infections
Eosinophils derive their name from the pink staining of their granules when stained with the acidic dye eosin. The major function of eosinophils is to initiate defense against parasitic organisms such as helminth worms. These effects are primarily mediated in concert with parasite-specific IgE antibodies produced by the B cells of the adaptive immune system. Eosinophils are generated in the bone marrow under the control of stromal cells and several growth factors, including the interleukins (IL)-3 and IL-5 . During a parasitic infection, these cytokines, as well as chemokines such as eotaxin produced by T cells, induce their migration to tissues, where they promote their differentiation and survival. Once they reach the site of parasitic infection, eosinophils upregulate the high affinity receptor for IgE antibodies, FcεRI, and mediate antigen-induced inflammatory reactions resulting in degranulation and the release of toxic mediators such as major basic protein and eosinophil peroxidases which destroy the parasitic organism. In addition to their role in anti-parasitic responses, eosinophils are a major contributor to the development of allergic disease.
Basophils stain with the basic dye hematoxylin. They are a rare granulocyte population that like eosinophils and mast cells have been implicated in IgE-mediated reactions, but have recently been found to also contribute to other types of immune responses. Basophils are mostly present in circulation, where they constitutively express the IgE receptor, FcεRI, and participate in the development of anti-parasitic and allergic responses. Like mast cells and eosinophils, they also depend on growth factors such as IL-3 and granulocyte-macrophage colony stimulating factor (GM-CSF) for their differentiation and survival. They are prolific producers of the cytokine IL-4 , and have been hypothesized to provide the initial source of IL-4 that is required for the differentiation and activation of helper CD4 T cells of the TH2 phenotype. In addition, they have been found to express ligands for costimulatory molecules that activate T cells and have been postulated to act as antigen-presenting cells under some conditions.
Monocytes, Macrophages, and Dendritic Cells Capture and Destroy Pathogens and Alert the Immune System
The second group of myeloid cells consists of monocytes, macrophages, and dendritic cells. Monocytes are a distinct group of cells with indented nuclei that are present throughout the circulation. From the blood, monocytes migrate to tissues where they mature into macrophages or dendritic cells under the control of growth factors such as GM-CSF and IL-4. Macrophages are long-lasting cells that are present in all bodily tissues. Here, they not only perform functions that are unique to the tissue such as the capture of antigens by skin-resident cells or the maintenance of bone homeostasis and integrity by bone-resident osteoclasts, but are also involved in the initiation and propagation of immune responses. A primary immune function of macrophages in tissues is to act as sentinel cells that sense and alert the immune system to the presence of infection or danger. Macrophages are well-equipped to do this by virtue of possessing a number of receptors that are adept at both capturing pathogens and initiating an inflammatory signal transduction cascade. Pathogen capture is mediated by various receptors on the surface of macrophages including C-type lectin receptors (such as dectins and mannose binding receptors) as well as scavenger receptors. This is subsequently followed by the initiation of phagocytosis or receptor-mediated endocytosis (Fig. 1.3). This involves pathogen transport via a vesicular pathway that comprises of a series of vesicles that terminate in the cellular phagosome. Here, changes in cellular pH induce the fusion of the phagosome with the lysosome, giving rise to the phagolysosome, where the pathogen is destroyed and degraded. Pathogenic peptides derived from this process may be subsequently used as antigens to induce the activation of T cells during adaptive immune responses.
Bacterial phagocytosis and destruction. Macrophages detect microbes including bacteria through pattern recognition receptors. This elicits receptor-mediated endocytosis as well as a signal transduction pathway leading to the expression of pro-inflammatory cytokines. Fusion of the phagosome with a lysosome results in acidification within the intracellular compartment and bacterial destruction (figure contributed by Jeremy P. McAleer)
In addition to receptors for antigen capture, macrophages also express receptors that initiate signal transduction pathways. A vast majority of these belong to the Toll-like receptor (TLR) family, which may be expressed either on the plasma membrane or intracellularly. Examples include the TLR4 homodimer, which binds to lipopolysaccharide , an endotoxin found in the cell wall of gram-negative bacteria. Similarly, TLR5 binds bacterial flagellin, while TLR3 binds viral nucleic acids. Binding of ligands by TLRs results in the induction of signaling cascades that culminate in the activation of various transcription factors including nuclear factor of kappa B (NF-κB), a potent transcription factor involved in the activation of a number of immune cytokine genes such as IL-2 , TNF-α, IL-1 , and IL-6 .
Dendritic cells also derive from the monocyte lineage and perform similar functions as macrophages in various tissues. In addition, they are equipped with the unique ability to induce the activation of naïve T cells in secondary lymphoid organs, serving as a link between the innate and adaptive immune responses. Once they have captured antigens in peripheral tissues such as the skin, dendritic cells migrate to secondary lymphoid organs such as the draining lymph nodes, where they activate naïve T cells and induce their differentiation into various sub-types.
Mast Cells Protect Against Parasites and Also Promote Inflammation
The third category of myeloid cells, mast cells, are present throughout vascularized tissues, where they sense the presence of pathogens and initiate immune responses. Like eosinophils and basophils, they also possess large numbers of granules containing preformed mediators such as histamine, which are released upon activation and degranulation. Mast cells also constitutively express the IgE receptor, FcεRI, and participate in IgE-mediated anti-parasitic and allergic responses. However, they are highly versatile cells that are also implicated in many other types of immune reactions, and express several other receptors including the TLRs and complement receptors.
The Lymphoid Lineage
The lymphoid lineage of cells gives rise to NK cells, T cells, and B cells. NK cells are a heterogeneous group of innate lymphocytes that express several receptors that are involved in recognizing and attacking tumors or virus-infected cells. NK cells also produce a variety of cytokines that can modulate the function of other cell types such as dendritic cells, macrophages, and T cells.
T cells are the main drivers of the adaptive immune response. They are derived from lymphoid progenitors in the bone marrow, following which they travel to the thymus, where they complete their development and maturation. In the thymus, T cells are educated to recognize the difference between host and foreign antigens under the control of thymic epithelial cells, macrophages, and dendritic cells. T cells derived their name due to their maturation in the thymus gland. Following maturation, naïve T cells exit the thymus and enter circulation, where they are poised to interact with antigens presented by antigen presenting cells such as dendritic cells. Two major types of T cells have been described: CD4 T cells , which consist of helper T cells and regulatory T cells (Treg), and CD8 T cells , also referred to as cytotoxic T cells .
Helper T cells can be differentiated into many diverse sub-types including the TH1 , TH2, TH9, TH17, TH22 , and TFH subsets depending on their phenotype. The differentiation of helper T cells is coordinated by both antigen presenting cells and other innate cell types through the release of distinct cytokines which promote their differentiation. Each type of helper T cell is associated with the expression and activation of transcription factors unique to that particular cell type. Helper T cells are critical to the immune system and perform diverse functions including the coordination of immune responses against intracellular and extracellular bacteria, viruses, parasites, and fungi. The depletion of helper T cells leads to severely compromised immune functions and death, as occurs during the development of Acquired Immune Deficiency Syndrome (AIDS ). Regulatory T cells play an important role in regulating or suppressing other cells in the immune system. They suppress unwanted responses to self or foreign antigens and prevent the development of autoimmunity.
In contrast, CD8 T cells are uniquely equipped to mediate adaptive immunity to viral infections. They kill virally-infected cells and may also enhance immune responses to other pathogens. Additionally, these cytotoxic T cells are the adaptive immune system’s primary defense against tumors and the development of cancer.
B cells also arise from lymphoid progenitors in the bone marrow and complete their maturation and development there. The origin of their name comes from where they were first discovered, the Bursa of Fabricius, a specialized hematopoietic organ which is only found in birds. The primary function of B cells is the production and secretion of various immunoglobulins or antibody isotypes. Upon maturation, B cells express the IgM antibody, which is the first antibody isotype produced during immune responses. Under the direction of T cells, B cells can be further activated to produce various other antibody isotypes such as IgG, IgA, and IgE.
The Education and Shaping of Immune Cells
Regulation of Immune Cells by Commensal Microbiota
Immune cells are conditioned by their microenvironment. Signals received during infection or other types of host injury have the potential to modulate the functions of immune cells, leading to their activation or suppression. Similarly, several environmental factors have also been shown to modulate the development of immune responses. The host microbiota in particular has an enormous influence on the immune system. During fetal development, fetal cells have no interactions with microorganisms as a result of protection provided by maternal IgG antibodies and the mother’s immune cells. This vastly changes as soon as a newborn is exposed to environmental bacteria. Upon birth, each individual is colonized by a host of commensal microorganisms, which constitute the normal microflora of the host. These bacteria reside in various mucosal tissues including the gastrointestinal tract, oral cavity, vagina, and skin. Here, they are in constant interaction with immune cells and modulate their activity and function, providing the necessary experience that is required for fighting infections. As such, the immune cells in mucosal tissues remain ever-ready to prevent the development of infection, actively monitoring the microbial population and preventing infection by opportunistic pathogens. This sentinel activity is performed in a manner that minimizes host cellular damage and prevents the development of chronic inflammation.
Commensal microorganisms perform many functions including aiding in digestion, providing metabolites and cofactors for cells, preventing the growth of opportunistic pathogens, and shaping the immune system. The interactions of immune cells with the commensal microflora play a pivotal role in providing the education and experience that is needed for future defense against infections with pathogenic organisms. Exposure to infections, especially during early life, can further modulate the development of the immune system, ensuring that immune cells develop in a healthy fashion that is geared towards host defense and not the development of unnecessary responses. For example, exposure to intracellular bacteria such as mycobacteria in early life may help to prevent a TH2 bias of the immune system, while exposure to helminth worms is thought to reduce IgE-mediated allergic sensitization.
The diversity and composition of the commensal population is determined by a host of factors including exposure to dietary components and competing species of bacteria. When changes in the microbial composition occur, either due to illness, a change in diet, or treatment with antibiotics, further modulation of the immune system may also occur. In elderly or immunocompromised patients, antibiotic treatment can disrupt the normal microbial composition and allow for the growth of opportunistic pathogens such as Clostridium difficile , which are normally held in check by the immune system and by competing bacteria such as enterobacteria.
T and B Cell Education in Primary Lymphoid Organs
Adaptive immune cells gain specific experience in primary lymphoid organs such as the thymus and bone marrow , where they develop and mature. In the thymus, T cells are taught to differentiate between ‘self’ and ‘non-self’ antigens. Under the careful coordination of thymic epithelial cells and dendritic cells, they learn to tolerate self-tissues and mediate immune responses against foreign antigens. This selection process ensures that naïve T cells will only be activated against non-self antigens that are presented by host antigen presenting cells, thereby preventing the development of autoimmunity. Immature T cells which have the potential to recognize self-antigens are eliminated and undergo death by apoptosis. In a similar manner, B cells also undergo selection processes in the bone marrow and secondary lymphoid organs. These processes ensure that self-reactive B cells with the potential to bind autoantigens are eliminated from the immune repertoire and only those that can react against non-self antigens are retained. Further selection processes in secondary lymphoid organs such as the lymph node ensure the differentiation and survival of mature B cells that have the potential to react with high affinity against specific antigens and make effective antibodies of various isotypes. Once mature naïve B and T cells enter the circulation, they are held in check by a number of regulatory processes, which are aimed at further preventing the development of autoimmunity.
Upon activation, all immune cells are programmed to differentiate into activated effector cells. Depending on the particular type of injury or antigenic trigger, effector cells mediate the active immune response to pathogens or other foreign agents, inducing the development of inflammation and destroying the foreign organism. The functions of effector cells are further conditioned by the release and activity of various cytokines, which modulate the behavior of the particular immune cell. Various types of effector cells exist depending on the type of cell and the particular immune response. In addition to giving rise to effector cells, adaptive immune cells also differentiate into memory cells. These are long-lasting cells that remember the particular antigenic exposure and are readily stimulated on antigenic re-exposure resulting in a rapid and potent immune response against the specific antigen.
The Innate Immune Response
The vast majority of infectious organisms are rapidly eliminated by the immune system soon after exposure without causing significant host damage and/or the induction of clinical symptoms. This response is mediated by a combination of both ubiquitously present anti-microbial proteins and molecules as well as the microbicidal activity of cells such as neutrophils and macrophages. This type of response is termed as the innate immune response, since it can be mobilized as soon as infection occurs, does not recognize specific antigens, and does not require a prolonged induction phase as in the case of T and B cells (Fig. 1.4).
Distinguishing characteristics of the innate and adaptive immune systems. Both innate and adaptive immune cells evolved to recognize foreign antigens. However, they differ with respect to their specificity and capacity to mediate long-lasting immune responses. Innate cells express a variety of receptors for the recognition of pathogen-associated molecular patterns. In contrast, adaptive cells are equipped with the unique ability to recognize distinct antigens by virtue of a single specific surface receptor. The effects of activated innate cells occur immediately. However, adaptive cells must go through a process of clonal selection and clonal expansion prior to activation. Activated adaptive cells can differentiate into effector and memory populations that mediate a tailored and life-long response to antigens
Pre-formed Molecules Provide an Immediate Defense Against Infectious Agents
A number of molecules present in blood and tissues possess anti-microbial activity. These include protease inhibitors such as α2-macroglobulins in the blood, acids in various tissues, lysozyme which degrades peptidoglycan, and defensins which are anti-microbial peptides. In addition to these, a unique family of serine proteases play a critical role in the elimination of pathogens and the facilitation of immune responses. These heat-labile proteins, which act on each other during the immune response, are collectively referred to as the complement system. The molecules of the complement system are constitutively produced by the liver. Upon antigenic exposure, whether due to changes in the physiological environment or the engagement of receptors, the complement pathway is activated. This results in the activation of key complement molecules such as C3b, the primary function of which is to tag pathogens for destruction and facilitate complement-initiated phagocytosis. This is referred to as complement fixation or opsonization. In addition to opsonization, complement components can directly lyse pathogenic cell membranes, mediating their destruction. They can also act as alarmin molecules, alerting the rest of the immune system to the presence of danger. C3a and C5a, formed during the cleavage of complement components can bind receptors on mast cells, basophils, and neutrophils, enhancing their recruitment and activation in tissues. A major trigger of the complement system during adaptive immune responses is antigen-specific antibody. Both IgM and IgG antibodies are very effective at complement fixation, binding complement components and inducing the phagocytosis of the pathogen. During the course of inflammation, large numbers of antigen-antibody immune complexes can be formed. Binding of these to the complement component C3b can facilitate the removal of immune complexes by erythrocytes which express receptors for C3b.
The Initial Response to Infection
In addition to immunity mediated by the complement system and other molecules, a number of leukocytes play key roles in the initial response to infection. Depending on the type and the site of infection, distinct immunological pathways are activated aimed at terminating the infection.
Early on, cells such as macrophages play a critical role in limiting the numbers of pathogens and alerting other immune cells. Damage to cells of the connective tissue such as a wound, burn, trauma, bite, or other type of injury to the skin induces the rapid activation of macrophages, which perform phagocytosis and release proinflammatory cytokines (Fig. 1.5). Of these, the cytokine, TNF-α, is a potent vasodilator, causing blood vessels to expand and inducing vascular permeability. As a result, endothelial cells lining blood vessels become leaky, allowing the exit of blood constituents including cells and other molecules. TNF-α also upregulates the expression of adhesion molecules on blood vessels, facilitating the migration of neutrophils and other cells that express corresponding ligands to tissues. The arrival of cells and other molecules to the infected tissue can cause localized swelling, resulting in the development of edema. Histamine produced by mast cells at sites of infection is also a potent vasoactivator and can act in a similar manner. Similarly, mast cells also produce high levels of TNF-α. Another important molecule produced by macrophages is the chemokine CXCL8. This molecule acts as a chemoattractant for neutrophils, driving their migration to the site of infection. Depending on the type of infection, additional cytokines may be produced by cells such as macrophages and mast cells. Most extracellular bacteria such as Staphylococcus aureus or other pyogenic (pus-inducing) bacteria are captured via phagocytosis. On the other hand, intracellular bacteria and viruses often cause infection by directly infecting the macrophages. Macrophages can also phagocytose other cells that have been infected by the pathogen. In response to viral infections, macrophages make the cytokine IL-12, which can serve to coactivate both dendritic cells and natural killer cells to the pathogen.
Hallmarks of Inflammation. Injury to a body tissue such as the skin results in the immediate production of cytokines by epithelial cells, which serve to recruit neutrophils and other inflammatory cells from the blood. Release of TNF-α and other cytokines by tissue macrophages make blood vessels permeable, enhancing the migration of leukocytes to the injured area. The dilation of blood vessels and increased leukocyte infiltration at the injured site contributes to the redness, heat, and swelling associated with inflammation. The increase in edema causes pinching of the nerves attached to blood vessels, resulting in the induction of pain (figure contributed by Jeremy P. McAleer)
The Development of Inflammation Gives Rise to the Acute Phase Response
Signaling cascades induced by the engagement of pattern recognition receptors including TLRs on macrophages further enhance inflammation by inducing the activation of transcription factors such as NF-κB and the production of cytokines such as IL-1β and IL-6. Along with TNF-α, IL-1β and IL-6 are considered to be some of the most prominent proinflammatory cytokines produced during the innate immune response. Together, the three cytokines exert both localized and systemic effects to enhance the inflammatory response. IL-1β like TNF-α also acts on endothelial cells and promotes vasodilation. IL-6, and to a lesser effect IL-1β and TNF-α, promotes the acute phase response. This refers to a dynamic change in the profile of dozens of serum proteins that are secreted by hepatocytes in the liver. The concentrations of these proteins changes significantly, with proteins such as albumin (which is the most abundant plasma protein) decreasing while those of others such as C-reactive protein (CRP ) and serum amyloid A increasing over a hundred-fold. The latter are referred to as acute phase proteins and their levels can be directly correlated to the intensity of inflammation. One of these, CRP, is also a trigger of the classical pathway of complement activation.
IL-1β, IL-6, and TNF-α also exert systemic effects. One of these effects includes actions on the temperature control sites of the hypothalamus as well as on fat and muscle cells, with the net effect of altering energy mobilization and raising the body temperature. These cytokines are therefore also referred to as pyrogens. The raised body temperature not only inhibits the growth of pathogens, but also enhances the effects of adaptive immunity.
Macrophages and Neutrophils Destroy Pathogenic Bacteria via Phagocytosis
Phagocytosis of extracellular bacteria by macrophages induces the release of CXCL8 and the eventual recruitment of neutrophils to the infected site. Neutrophils act as first responders and are extremely effective at killing and destroying extracellular bacteria. The responding neutrophils secrete a battery of toxic mediators and degradative enzymes stored in their granules which are extremely destructive to pathogens. The process of pathogen destruction begins with phagocytosis, subsequent to which pathogens are subjected to granule contents of many different types (Fig. 1.6). One hallmark of pathogen destruction by neutrophils is the induction of the respiratory burst. This involves the activity of enzymes such as NADPH oxidase which raises the pH in the cell and induces the release of a number of toxic oxides and superoxides. These reactive oxygen species enhance the activity of several degradative enzymes and antimicrobial peptides, which cause the death of the pathogen. Eventually the pathogen is completely degraded in the phagolysosome by the action of acid hydrolases. Once the pathogen is destroyed, the neutrophil is spent and also dies. The accumulation of dead neutrophils leads to the formation of pus.
Innate Immunity to Viruses Is Mediated by Natural Killer Cells
In contrast to bacterial infections, infection of cells with viruses induces the recruitment and activation of natural killer cells. Macrophages release IL-12 , which together with TNF-α, has the ability to activate natural killer cells. However, the most potent activators of NK cells are type I interferons which are produced by all cells when infected with viruses, and produced prodigiously by epithelial cells and plasmacytoid dendritic cells. The latter, which are different from conventional dendritic cells, are also referred to as interferon producing cells, since they can produce a thousand times more type I interferon compared with epithelial cells.
The two main types of type I interferons are IFN-α and IFN-β. These are produced as soon as a cell is infected and promote what is termed as the interferon response. The net effect of the interferon response is to enhance viral resistance in infected cells, upregulate the expression of ligands that bind receptors on NK cells, and promote the activation of NK cells.
NK cells are potent killers of virally-infected cells. They are large granular lymphocytes which circulate throughout blood in a partially active state, where they are prevented from attacking the body’s cells via a delicate balance of engagement of activating and inhibitory receptors present on the NK cell surface. These receptors are stochastically expressed on the surface of NK cells, and different NK cell subsets express different combinations of receptors. Under normal conditions, inhibitory receptors on NK cells bind major histocompatibility class (MHC) I molecules which are expressed on every nucleated cell in the body. This signals the NK cell to not engage its activating receptors and prevents killing of uninfected cells. On the other hand, virally-infected cells downregulate MHC I and upregulate ligands for activating receptors on NK cells. These include stress proteins such as MHC Class I related polypeptide sequences A and B (MIC-A and MIC-B), as well as Rae 1. This alters the delicate balance of receptor engagement on NK cells and triggers their activation. Once activated, NK cells are induced to kill the virally infected cells, through a dedicated program of apoptosis mediated by perforin, granzymes, and caspases. In addition to killing, NK cells also produce a host of cytokines which have deleterious effects on infected cells as well as activate other immune cells. One of these is a potent cytokine called IFN-γ, which not only exerts anti-viral effects, but also promotes the degradation of the pathogen in antigen presenting cells, and enhances their ability to stimulate the activation of T cells. Depending on the type of response, other cytokines are also produced by specific subsets of NK cells, mimicking the types of cytokines produced by T cell subsets during the adaptive immune response.
Dendritic Cells Initiate the Development of Adaptive Immune Responses
While the major effect of cells such as macrophages, neutrophils, and NK cells is to terminate the pathogen at the site of infection and amplify the effects of the innate immune response, another innate immune cell serves a dual purpose, which is to initiate the activation of the adaptive immune response.
Like macrophages, dendritic cells (DCs) at sites of infection also conduct phagocytosis and release proinflammatory cytokines. However, their major function in immunity is to initiate the activation of naïve or uncommitted T cells to the particular pathogen. Immature DC at sites of infection phagocytose the pathogen and degrade it in either the proteasome or the phagolysosome depending on the type of infection. This results in the production of a number of peptides which may serve as antigenic triggers to activate naïve T cells. Loaded with this cargo of peptides, immature DC travel from infected sites to the draining lymph nodes or the spleen, where they encounter circulating naïve T cells that are moving through the organs. In the process of migration, DCs undergo a change in phenotype, acquiring and upregulating ligands that enhance their ability to activate a naïve T cell. Upon reaching the lymph node or spleen , the DC is now said to be a mature DC that is capable of initiating T cell activation.
The Adaptive Immune Response
While the primary purpose of the innate immune response is to limit the spread of the infection as much as possible while also paving the way for the adaptive immune response, the goal of the adaptive immune response is to mount a specific, targeted response that completely destroys the pathogen and ensures that the host is never again subjected to infection with the same pathogen (Fig. 1.7).
Kinetics of anti-viral immune responses. This diagram outlines the time course of a virus infection and the relative magnitude of subsequent immune responses. Once viruses breach epithelial barriers, there is an incubation period in which symptoms from the infection have not yet occurred. During the next few days, viruses will undergo replication in host cells and begin to infect neighboring cells. The peak of viral replication may occur 7–10 days following initial exposure (red line), with levels declining over the next week or so, until the infection is cleared. The innate immune system is the first line of defense and includes macrophages, neutrophils, dendritic cells, NK cells and complement. The innate immune system can respond within hours following infections to initiate a type I interferon response. While this is important for controlling pathogen load, the magnitude of the innate response is not sufficient to clear the infection (green line). Adaptive immune responses take about a week to develop because they depend on the outgrowth of a single antigen-specific lymphocyte (T cell, B cell; blue line). Lymphocytes that have not yet encountered their antigen in lymph nodes are referred to as naïve. Following their activation by virus antigens, lymphocytes undergo a phase of clonal expansion in which one cell proliferates several times to become a population of thousands of lymphocytes that all recognize the same antigen. Clonal expansion is linked to their effector differentiation, in which lymphocytes acquire the ability to inhibit viral replication and/or dissemination, as described in Chap. 3. Therefore, clonal expansion coincides with the initial decrease observed in viral loads. Following expansion, there is a contraction phase in which most of the effector lymphocytes undergo apoptosis. A small population of memory cells persists, capable of mounting a rapid response to a second infection with the same virus. Memory cells live for several years within the individual (figure contributed by Jeremy P. McAleer)
Principles of the Adaptive Immune Response
The adaptive immune response is mediated by T cells, B cells, and their various mediators such as cytokines and antibodies. The first time an adaptive immune response is mounted against a specific pathogen, it is called the primary immune response. Subsequent responses are termed as secondary responses. The secondary response is mediated by memory T cells and B cells which have formed a memory of the pathogen through the first encounter. The memory response is of much greater magnitude than the primary response, often resulting in termination of the infection without the development of any clinical symptoms.
The development of the adaptive response hinges on the principle of clonal selection and the expansion of T cells and B cells to the specific pathogen. Unlike innate cells, these cell types do not simply recognize the presence of patterns on pathogen surfaces. Rather, every single activated T or B cell is committed to the recognition of a unique antigen or epitope that is derived from the pathogen. Recognition of this antigen allows the T or B cell to recognize the entire pathogen and mount a targeted response. The only antigens recognized by T cells are peptides that have been derived from degradation of a pathogen in an antigen presenting cell. In contrast, B cells recognize all sorts of antigens including peptides, glycoproteins, polysaccharides, and other types of pathogen components. Clonal selection refers to the process by which naïve T cells or B cells are selected to become specific to the particular pathogen. Once a naïve cell is activated to the specific antigen, it then divides into hundreds of similarly activated clones which now act against the pathogen and mediate its destruction.
The Lymphatic System
The first step in the initiation of the adaptive immune response is the antigen-specific activation of a naïve T cell to the offending pathogen. Naïve T cells along with naïve B cells circulate throughout lymphoid organs, carried by a complex network of vessels connecting the blood and lymphatic system. The lymphatic system consists of an anastomosing network of lymphatic vessels which originate in connective tissues and collect the extracellular plasma or fluid that leaks out of blood vessels, returning it to the heart. This fluid is called the lymph and consists of a number of leukocytes which traverse throughout the lymphatic system, making regular stops at lymph nodes situated at intervening junctions in the lymphatics. At any given time, lymphocytes represent the largest population of leukocytes in the lymph, although they are only a fraction of the total lymphocyte population in the body. The vast majority of lymphocytes are present in the lymphoid organs which serve various areas of the body. Of these, the thymus and bone marrow are referred to as primary or central lymphoid organs since this is where T and B cells develop and mature. Activation of naïve lymphocytes typically occurs in the secondary or peripheral lymphoid organs, of which the lymph nodes and the spleen are the most prominent (Fig. 1.8). The lymph nodes collect the fluid draining from an infected site such as the skin or the respiratory tract and serve as an ideal location for the encounter between pathogen and naïve T and B cells. Similarly, the spleen acts as a reservoir for the filtration of blood, removing any damaged or senescent red blood cells. It is therefore uniquely poised to handle the activation of lymphocytes to blood-borne pathogens that directly enter the blood stream or eventually reach the blood. Several other organs can also act as secondary lymphoid organs including the tonsils, adenoids, Peyer’s patches, and appendix. In addition, less organized lymphoid tissues referred to as tertiary lymphoid organs are present throughout the respiratory, gastrointestinal and urogenital tracts.
Secondary lymphoid organs. Adaptive immune responses against microorganisms are initiated in lymph nodes and the spleen. These tissues contain organized regions that facilitate interactions between dendritic cells, T cells and B cells, promoting lymphocyte activation against antigens collected in peripheral tissues. In lymph nodes, the paracortical region is populated by T cells, while the outer cortex contains B cells, including germinal centers of activated B cells undergoing clonal expansion. The inner medulla of lymph nodes contains antibody-producing plasma cells and macrophages. Fluid enters lymph nodes through afferent lymphatic vessels, exits through efferent ducts, and ultimately drains into the thoracic duct where it enters the bloodstream. The spleen filters blood and has organized regions termed red pulp and white pulp. The red pulp is a site of red blood cell disposal, while the white pulp contains immune cells. Immune functions of the spleen are similar to that of lymph nodes, with the primary difference being that the spleen collects cells and antigens from blood rather than lymph fluid (figure contributed by Jeremy P. McAleer)
The Activation of Naïve Lymphocytes Occurs in Secondary Lymphoid Organs
In the absence of infection or other immune triggers, naïve lymphocytes constantly traverse the lymphatic system, surveying the lymph nodes for the presence of antigen. Antigen is detected by lymphocytes via a single unique receptor expressed on the surface of T cells and B cells. In T cells, this receptor is simply referred to as the T cell receptor or TCR. On B cells, the B cell receptor or BCR is the immunoglobulin molecule. Every T or B cell will only express one type of receptor, which is constrained by the specific antigen to which it binds, thus ensuring that activated T and B cells will only recognize the specific antigen to which it is activated.
Activation of lymphocytes is initiated in the secondary lymphoid organ. Once an infection has ensued, immature dendritic cells at the site of infection capture the offending pathogen and begin the process of pathogen destruction and migration to the secondary lymphoid organ. Depending on the type of pathogen, peptides are generated from pathogen breakdown in either the endoplasmic reticulum or the vesicular system. Intracellular pathogens such as viruses are degraded in the immunoproteasome, while extracellular pathogens such as pyogenic bacteria are destroyed in the phagolysosome. The process of pathogen destruction and the generation of peptides is termed as antigenic processing. Depending on the site of destruction, peptides generated from pathogen breakdown are eventually loaded onto the surface of ubiquitous molecules present in every nucleated cell of the body, called major histocompatibility complex or MHC molecules. Two types of MHC molecules are involved in the activation of T cells: MHC Class I molecules, which are required for the activation of CD8 T cells and MHC Class II molecules which are required for the activation of CD4 T cells. As the immature DC migrates toward the secondary lymphoid organ such as a lymph node, it begins to downregulate receptors for antigen capture and upregulate receptors that enhance activation of T cells. Of these, the upregulation of MHC molecules loaded with peptides is paramount for T cell activation to occur. In addition, a number of other receptors for ligands on naïve T cells are also highly expressed. The primary purpose of these is to costimulate the naïve T cell to the specific antigen on MHC molecules. Once the mature DC reaches the lymph node, it is now ready to make contact with a naïve T cell.
The Activation and Differentiation of T Lymphocytes Requires Costimulation and Direction from Cytokines
Three steps are involved in the activation of naïve T cells. First, the TCR on naïve T cells makes contact with the peptide antigen on the surface of MHC molecules on the antigen presenting cell. This is an essential first step which commits the naïve T cell to the specific antigen and provides specific identifying information regarding the nature of the pathogen. It can only effectively be carried out by professional antigen presenting cells (APCs) such as dendritic cells, macrophages, and B cells and is referred to as antigen presentation. A fundamental criterion of antigen presentation is that the TCR on a T cell will only engage the peptide antigen in the context of an MHC molecule on the surface of an APC. In the absence of the MHC molecule, naïve T cells cannot be activated to the peptide antigen. Furthermore, presentation of MHC and peptide on the surface of an APC is critical, since only these cells can provide the necessary costimulation to fully activate the naïve T cell. The engagement of costimulatory molecules on the surface of APCs and naïve T cells serves as the second critical step in the process of activation, conveying the sense of potential danger represented by the presented antigen, and gearing the T cell to initiate a program of clonal expansion in response to the antigen. It also ensures that naïve T cells will never be activated in the absence of costimulation, thus preventing the inadvertent activation of T cells to self-antigens expressed by other cells of the body. The most important costimulatory interaction for the activation of naïve T cells occurs between CD28 on T cells and B7 family molecules such as CD80 or CD86 expressed on the surface of APCs. In addition, interactions between other costimulatory molecules play a role in the activation of effector or memory T cells. These include OX40 (CD134) and OX40 ligand (CD252) as well as ICOS (inducible costimulator on T cells) and ICOS ligand. Engagement of the TCR and costimulatory molecules is facilitated by a tight zone of contact called the immunological synapse that is established between the APC and T cell. This allows the binding of a number of other receptors that strengthen the interaction between these molecules. The provision of costimulation initiates a signaling cascade that culminates in the activation of the transcription factor nuclear factor of activated T cells (NFAT ), which activates the gene for the cytokine IL-2. In the presence of IL-2, the activated T cell is now stimulated to divide and expand, thus completing the third step involved in T cell activation.
Depending on the type of MHC molecule presenting the antigen, activation results in the initiation of either a cytotoxic or helper T cell response. MHC I molecules present to CD8 T cells initiating a cytotoxic response to viral and tumor antigens. MHC II molecules present to CD4 T cells initiating a helper T cell response to bacterial, viral, fungal, parasitic, and other antigens. The helper T cell response is further delineated by the type of helper T cell activated. In general, TH1 cells are activated to intracellular bacteria, TH2 cells to parasitic antigens, and TH17 cells to extracellular bacteria and fungi. However, in addition to their specified roles in host defense, these subsets exhibit a great degree of versatility and may be induced to perform overlapping and complementary functions depending on the type of antigenic stimulus and the cytokine microenvironment. Additional T helper subsets include follicular helper T cells (TFH) which play a critical role in the secondary lymphoid organs in initiating the activation of naïve B cells. Regulatory T cells (Tregs), another type of CD4 T cell, suppress inflammatory responses once the infection is terminated.
Activation of Naïve B Cells
The second step in the development of the adaptive immune response is the activation of naïve B cells to the respective antigen. The recognition of antigen by B cells occurs via the immunoglobulin receptor expressed on its surface. The initial process of recognition involves binding via IgM and other coreceptors expressed on the surface of a B cell, resulting in receptor-mediated endocytosis of the pathogen. Unlike T cells, B cells are not constrained by the recognition of a specific peptide antigen and can recognize various types of macromolecules. Further activation of the B cell requires interactions between the antigen-specific B cell and a similarly activated antigen-specific helper T cell in the secondary lymphoid organ. The B cell presents a peptide antigen on the surface of MHC II to the CD4 T cell and costimulation is provided in the form of CD40 molecules on the B cell which bind to CD40 ligands (CD40L) on the responding T cell. This causes the helper T cell to secrete the cytokines IL-4, IL-5, and IL-6, which stimulate the differentiation and division of B cells into effector, memory, and antibody-producing plasma B cells. Helper T cell cytokines also cause B cells to go through a program of antibody refinement, wherein B cells are induced to enhance the quality of their antibodies through repetitive mutations (somatic hypermutation), switch their antibody isotype to IgG, IgA or IgE (isotype switching), and increase the affinity of their antibodies to the antigen (affinity maturation). This ensures that activation results in the production of high affinity antibodies that are aimed at completely destroying the pathogen and terminating the infection. This is accomplished via three distinct mechanisms: neutralization, opsonization , and antibody-dependent cellular cytotoxicity (ADCC ).
Antibodies Are the Highly Specialized Weapons of Adaptive Immunity
Neutralization involves binding of antibodies to the respective antigen or pathogen, prior to cellular entry, thus preventing it from infecting or damaging the cell (Fig. 1.9). An example of neutralization is the binding of antibodies to bacterial toxins preventing their attachment and entry into a target cell. In opsonization, antigen-binding antibodies initiate phagocyte-mediated destruction by binding to antibody or complement receptors on the surfaces of macrophages, dendritic cells, or neutrophils. Lastly, ADCC is mediated by immune complexes of antigen and IgG molecules bound to IgG receptors on NK cells, triggering their activation and cytotoxic activity. Depending on the type of antigen, different types of antibodies are produced. IgM is the first antibody to be produced during an adaptive response. It is secreted as a pentamer and is very effective at complement activation. However, because of its large size, it is unable to enter many tissues. IgG is the most abundant antibody isotype present in circulation. It is very effective at neutralization, opsonization, and complement activation. Dimeric IgA is the most abundant antibody produced at mucosal surfaces and plays a critical role in mucosal immunity. Lastly, high levels of IgE are produced during infection with parasites such as helminth worms.
Role of antibodies in host defense. Antibodies produced by B cells have a variable region for antigen binding and constant region that determines antibody function. Antibodies can neutralize pathogens or toxins by preventing their attachment to host cell surfaces. Antibodies attached to bacteria can activate the classical complement pathway, leading to lysis. Macrophages and NK cells, among other cell types, express Fc receptors that recognize constant regions of antibodies. This facilitates the uptake of antibody-coated pathogens by macrophages (opsonization) and the attack of virus-infected cells by NK cells (antibody-dependent cellular cytotoxicity) (figure contributed by Jeremy P. McAleer)
The production of high quality antibodies aimed at terminating the infection represents the peak of the adaptive immune response and typically occurs approximately 1 week after the onset of infection. At this time, elevated numbers of antibodies may be found in the sera of infected individuals. Depending on the type of dominating helper T cell response, some types of antibodies may predominate over others. For example, IgG1 and IgE antibodies are typically by-products of a TH2-type response, whereas in TH1 -mediated responses, IgG2a antibodies predominate.
As the immune response progresses, the quality of antibodies continues to improve. This is a result of numerous changes in the antigen-binding portion or variable region of the antibody molecule, and its primary purpose is to increase the overall binding and effectiveness of the antibody. One process called somatic hypermutation involves the generation of successive mutations in the complementarity determining regions (CDR)s of the antigen-binding site. The purpose of this mutation is to increase the binding of the antibody for the antigen. Similarly, transcriptional changes in IgM molecules result in switching of the IgM isotype of antibody to either the IgG, IgA, or IgE isotype. Lastly, processes such as affinity maturation increase the individual affinity of the antibody molecule and contribute to the strength or avidity of the overall binding. As such, successive exposures with the antigen exponentially increase the overall number and quality of the antibodies produced.
The Successful Outcome of the Adaptive Immune Response Results in Termination of the Infection
The combined actions of antibodies as well as effector T and B cells serves to eventually clear the infection from the patient. This changes the dynamics of the infection from one in which the pathogen predominates to one controlled by the immune system. As the numbers and effects of the pathogens subside, a dramatic improvement in the patient’s condition occurs and the patient begins to experience relief from infection-associated symptoms. Control of the infection is also accompanied by the induction of several populations of regulatory cells, whose purpose is to aid in the resolution of inflammation, prevent inadvertent responses, and spur on the process of healing. Of these, CD4-positive regulatory T cells play a pivotal role in the suppression of unwanted T cell responses and the resolution of inflammation. Similarly, as the populations of effector T cells and B cells dwindle, subsets of antigen-specific memory T and B cell populations continue to persist and clones of these cells are retained interminably, poised to encounter a repeated strike by the same pathogen. When such a strike does occur, these cells are readily activated, mounting a rapid, potent memory T or B cell response that completely eliminates the pathogen before it has had a chance to initiate the process of infection.
Pharmacological Approaches to Treating Inflammation
Although the singular purpose of inflammation is to target and eliminate a perceived threat such as a pathogen, injury, or danger to the host, at the height of inflammation, most individuals are left feeling utterly feeble and helpless. In the midst of a high fever, patients often experience weakness, lethargy, fatigue and other symptoms that are geared toward encouraging the patient to rest so that the body’s resources and energy may be expended toward the goal of eliminating the infection. This is also accompanied by other symptoms such as chills, muscle weakness, shivering, and organ-associated symptoms that contribute to the deterioration of the patient’s health and overall malaise. In the presence of chronic inflammation, inflammation often continues to persist at a subclinical level, and contributes to fibromyalgia, pain, and decline in health of the patient. As such, a number of drugs have been developed with the aim of subduing inflammation or reducing some of the symptoms associated with it, without sacrificing the overall effectiveness of the immune response.
Drugs such as non-steroidal anti-inflammatory drugs (NSAIDs ) including aspirin, ibuprofen, and naproxen have been used for quite some time to bring about an overall reduction in pains and aches all over the body. These drugs act by typically targeting intermediaries in the inflammatory pathways such as enzymes that act on nerve cells and induce pain. Similarly, other drug classes such as corticosteroids mediate an overall suppression of the immune response, resulting in attenuation of inflammation-associated pain and pathology. More recently, advances in basic science research have resulted in fundamental breakthroughs regarding our understanding of the immunologic basis of many diseases, elucidating several new and selective targets for therapeutic purposes. These include among others cytokines, chemokines and their receptors, signaling molecules, mediators involved in leukocyte trafficking, and cellular targets such as activated T and B cells. Similarly, a number of drugs that can stimulate the immune response have also been developed for some diseases. A short description of the different types of drugs is provided below. Table 1.2 summarizes the present list of immunotherapeutic drugs approved by the Food and Drug Administration (FDA) for the treatment of many diseases.
Inhibitors of Inflammation
Glucocorticoids
Glucocorticoids (corticosteroids) have remained the mainstay of immunotherapy for several decades and are widely prescribed for the treatment of many chronic inflammatory diseases such as asthma and rheumatoid arthritis. They work by inducing a general suppression of the immune system and thereby preventing inflammation-associated pathology. The mechanism of action of glucocorticoids is described in detail in Chaps. 4 and 6. Since glucocorticoids are broadly effective, long-term treatment with systemic corticosteroids is not recommended and is associated with several adverse effects including susceptibility to infections and the development of cancer. However, the use of selective formulations such as inhaled corticosteroids for chronic asthma have proven to be extremely beneficial and effective in the treatment of inflammatory conditions.
Anti-histamines (Histamine Receptor Antagonists)
These are widely available over the counter and used in the symptomatic treatment of diverse conditions including allergies, acidity, sleeplessness and anxiety. They include both competitive inhibitors of histamine as well as inverse agonists of histamine receptors and work primarily by targeting histamine produced by cells such as mast cells, preventing its binding to one of four types of histamine receptors. Induction of histamine signaling can result in the development of vasodilation, vascular permeability and smooth muscle constriction, resulting in symptoms such as coughing, sneezing or itching. Thus, anti-histamines provide a temporary resolution of these symptoms by curtailing histamine-induced effects. The mechanism of action of anti-histamines is described in Chap. 4.
Anti-eicosanoids
Derivatives of the arachidonic acid pathway such as prostaglandins and leukotrienes play a vital role in inflammation, inducing a variety of physiological responses such as vasodilation, vascular permeability, smooth muscle reactivity, recruitment of immune cells, thrombosis and gastrointestinal secretion. These derivatives along with other arachidonate metabolites such as thromboxane A2, lipoxins, and hepoxilins are collectively referred to as eicosanoids. In addition to the above effects, eicosanoids such as prostaglandins contribute to the development of inflammation by inducing both pyrogenic and neurological effects such as fever and pain. A number of widely available agents have been developed to target the effects of both prostaglandins and leukotrienes. These include inhibitors of the enzymes that are necessary for their derivation from arachidonic acid as well as specific leukotriene receptor antagonists. Prostaglandins are synthesized via the cyclooxygenase (COX ) pathway of arachidonic acid metabolism, whereas leukotrienes are derived via the 5-lipoxygenase pathway. Both COX 1 and COX2 inhibitors as well as selective COX2 inhibitors are widely available as over-the-counter agents. These include several NSAIDs such as aspirin, ibuprofen, acetaminophen and COX2 inhibitors such as celecoxib (Celebrex®). The NSAIDS and COX2 inhibitors are not discussed further in this book. Inhibitors of leukotriene synthesis and function are also available and are commonly prescribed for the treatment of chronic inflammatory diseases such as asthma. The mechanism of action of leukotriene antagonists is described in detail in Chap. 4.
Calcineurin Inhibitors (Specific Inhibitors of T Cell Function)
The calcineurin inhibitors cyclosporine and tacrolimus revolutionized the treatment of solid organ and hematopoietic stem cell or bone marrow transplantation. In fact, the advent of cyclosporine in the therapeutics of transplantation is referred to as the cyclosporine era of transplantation. Both cyclosporine and tacrolimus act by suppressing the proliferation of T cells via the inhibition of NFAT activation and the blockade of IL-2 release. Cyclosporine is a peptide antibiotic that binds to cyclophilin, a member of a class of intracellular proteins termed as immunophilins. The cyclosporine-cyclophilin complex then inhibits the phosphatase calcineurin, which is required for the activation of the T cell specific transcription factor NFAT (nuclear factor of activated T cells). Tacrolimus (FK 506), a macrolide antibiotic, produced by Streptomyces tsukubaensis, acts in a similar manner by binding to the immunophilin, FK binding protein. Calcineurin inhibitors are widely used in solid organ and hematopoietic stem cell transplantation and along with glucocorticoids remain the mainstay of therapy. Topical calcineurin inhibitors are also used in the treatment of dermatologic diseases such as atopic dermatitis and psoriasis. The mechanism of action of calcineurin inhibitors is described in Chaps. 5, 7, and 8.
Inhibitors of Proliferation
Sirolimus (Rapamycin) and Everolimus
These drugs also inhibit T cell activation and block the release of IL-2 and other T cell-derived cytokines. Although, like the calcineurin inhibitors, they also complex with an immunophilin, FK506-binding protein 12, their mechanism of action is dependent on the inhibition of the protein kinase, mTOR (molecular target of rapamycin), which is essential for cell growth, proliferation, and metabolism. Both these drugs are mainly used in transplantation therapy.
Mycophenolate Mofetil (MMF)
MMF is semisynthetic derivative of mycophenolic acid (MPA), which is produced by the mold Penicillium glaucus. It is a pro-drug that is converted to its active metabolite MPA in vivo, which selectively and reversibly inhibits inosine monophosphate dehydrogenase, an enzyme required for the synthesis of guanine nucleotides in T and B cells. The inosine monophosphate dehydrogenase-dependent de novo pathway of guanine synthesis is essential for T and B cell proliferation and function, due to the absence of alternative salvage pathways which exist in other cell types. As a result, inhibition of this enzyme by MPA leads to the suppression of T and B cell proliferation and function. MMF is mainly used in transplantation therapy, but is also being considered for therapeutic purposes in rheumatoid arthritis, inflammatory bowel disease, and lupus nephritis.In addition, it is also commonly prescribed off-label for the treatment of systemic lupus erythematosus.
Azathioprine
Azathioprine is a cytotoxic drug that is mainly used for the treatment of solid-organ transplantation. It is a pro-drug that is converted in vivo to 6-mercaptopurine, which is subsequently converted to other derivatives that inhibit de novo purine synthesis. This results in the death of stimulated lymphocytes, dampening the induction of T and B cell mediated immunity. Due to its cytotoxic effects, the major toxicity associated with azathioprine is bone marrow suppression resulting in leukopenia, thrombocytopenia, and/or anemia. It is used for the maintenance of renal allografts, and also sometimes in the treatment of rheumatoid arthritis, Crohn’s disease, and acute glomerulonephritis associated with systemic lupus erythematosus.
Other Anti-proliferative Drugs
Other anti-proliferative and cytotoxic drugs include thalidomide , which inhibits angiogenesis, has anti-inflammatory effects, and is used in the treatment of multiple myeloma and other cancers; cyclophosphamide , a cytotoxic agent, which destroys immune cells by alkylating resting and proliferating cells and is used in the treatment of some autoimmune disorders; and pyrimidine synthesis inhibitors such as leflunomide and teriflunomide , which inhibit the mitochondrial enzyme dihydroorotate dehydrogenase required for pyrimidine synthesis and immune cell function, and are used in the treatment of rheumatoid arthritis and relapsing-remitting multiple sclerosis. In addition, other cytotoxic drugs such as methotrexate are also commonly used for various therapeutic purposes.
Biologics
Over the last few decades, revolutionary advances in the understanding of the immunological basis of diseases and molecular and cell biology techniques, have resulted in the development of several new biologics that specifically target components of immune-mediated pathways such as immune cells, cytokines, cell signaling molecules, and various receptors and their associated ligands. These classes of drugs include monoclonal antibodies, small molecule inhibitors, fusion proteins, and various receptor antagonists.
Monoclonal Antibodies
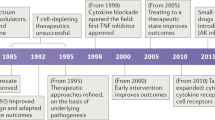
Since the advent of recombinant DNA technology, various monoclonal antibodies are now therapeutically used for the treatment of a number of diseases. Based on whether they incorporate the variable and/or complementarity -determining regions (CDRs) of mouse antibodies, they are referred to as chimeric (composed of mouse variable chains), humanized (composed of mouse CDR sequences), or fully human (consisting of no mouse chains or sequences). The first monoclonal antibodies were produced using hybridomas derived from myeloma cell lines, although they are now frequently produced commercially using recombinant DNA technology. The different classes of therapeutic monoclonal antibodies are described more extensively in Chap. 3. Examples of prototypic antibodies belonging to the different classes include rituximab (Rituxan®), a chimeric anti-CD20 antibody used in the treatment of non-Hodgkin’s lymphoma, omalizumab (Xolair®), a humanized anti-IgE antibody used in the treatment of asthma, and adalimumab (Humira®), a fully human anti-TNFα antibody used in the treatment of rheumatoid arthritis.
In addition to treatment of inflammatory diseases, a number of monoclonal antibodies targeting various cell surface molecules and pathways have also been developed for the treatment of diverse types of cancers. These include inhibitors of angiogenesis, proliferation, and cell signaling. Examples include alemtuzumab , an anti-CD52 antibody that depletes B and T cells and is used for the treatment of B-cell chronic lymphocytic leukemia, bevacizumab , which inhibits vascular endothelial growth factor (VEGF ) and blocks angiogenesis in tumors, and trastuzumab , which blocks the extracellular domain of HER-2/neu in breast cancer patients and prevents signaling in HER-2/neu-positive tumors. Several monoclonal antibodies are also used to specifically deliver toxins and radioisotopes to tumor cells.
Similarly, monoclonal antibodies that target infectious organisms have also been approved by the FDA. Palivizumab (Synagis®) is a humanized neutralizing IgG antibody that is used to prevent respiratory syncytial virus (RSV) infections in children at increased risk of severe disease. This includes children who were born prematurely and are under 6 months of age, and children who may be at high risk of RSV infection due to bronchopulmonary dysplasia or congenital heart disease. The drug is given once a month during RSV season and provides protection by binding an epitope in the F protein subunit of RSV. This results in the inhibition of viral fusion and the prevention of syncytia within the lungs, which are necessary for the intercellular spread of the virus.
T Cell Checkpoint Inhibitors
More recently, a number of T cell specific inhibitors have been developed that selectively inhibit or enhance the activation of T cells for the treatment of non-immune cell cancers. Checkpoint inhibitors such as anti-PD1 ( pembrolizumab and nivolumab ) and anti-CTLA-4 ( ipilimumab ) enhance T cell activity against tumors by presumably increasing their costimulatory activity (Figs. 10.2 and 10.3). In contrast, the fusion proteins abatacept and belatacept , which consist of the extracellular domain of human CTLA-4 fused with the constant regions of IgG, inhibit the costimulation of T cells and dampen T cell activity. The 2018 Nobel Prize in Physiology or Medicine was awarded to Drs. James P. Allison and Tasuko Honjo for their work on T cell checkpoint inhibitors. In addition to the drugs mentioned above, several others have been approved by the FDA for the treatment of various cancers and inflammatory diseases, and others are currently in development.
Cytokine Inhibitors
Insights into immune signaling mechanisms and the cross-talk between various immune cells have led to the development of several drugs that block cytokine function by inhibiting their binding, signaling, or activity. The first cytokine antagonists to be developed included both monoclonal antibodies and fusion proteins targeting the pro-inflammatory cytokine, TNF-α. These include infliximab , etanercept , and certolizumab pegol . More recently, a number of other cytokine antagonists have been developed targeting various cytokines including IL-1, IL-2, IL-5, IL-6, IL-12 and IL-23, and IL-17. In addition, inhibitors that target the JAK-STAT pathway of cytokine signaling have also been approved by the FDA and others are currently in development. It is also anticipated that several new drugs targeting various cytokines and their signaling pathways will be approved by the FDA in the near future. Presently FDA-approved drugs targeting various cytokines are described throughout this textbook.
Immune Cell Recruitment Inhibitors
The migration of immune cells to various organs is a critical component of inflammatory reactions during chronic inflammatory diseases. As such, a number of drugs have been developed that inhibit the expression or function of adhesion molecules on leukocytes or blood vessels and prevent the migration of effector cells to target tissues. Examples of these drugs include the integrin inhibitors natalizumab and vedolizumab used in the treatment of multiple sclerosis and Crohn’s diseases as well as leukocyte function-associated antigen-1 (LFA-1) inhibitors such as efalizumab, which was previously used for the treatment of Crohn’s disease, but was subsequently withdrawn.
Immunomodulatory Drugs
Recombinant Cytokines
Interferons
Interferons have been used for therapeutic purposes since as early as the 1980s. While initially studied mainly in the context of anti-viral responses, increasing evidence suggests that they have a number of immunomodulatory effects. These include the enhancement of antigen presentation, phagocytosis, upregulation of MHC Class I and II , and the induction of the immunoproteasome. They are also potent inducers of the antiviral cytotoxic response by both NK cells and cytotoxic T cells. The two main types of interferon that have been extensively studied in humans are both used for immunotherapeutic purposes. These include the type I interferons IFN-α and IFN-β as well as the type II interferon, IFN-γ. The type I interferons are produced by cells such as epithelial cells and plasmacytoid dendritic cells soon after infection and both activate NK cells as well as increase the intensity of anti-viral resistance through a series of molecular and cellular events, which are collectively termed as the interferon response. IFN-γ is produced by both activated NK cells and T cells (TH1 and CD8 T cells) and further contributes to the antiviral response by inhibiting viral replication and activating various immune cells such as phagocytes. A number of interferon drugs comprising of all three types have been developed for the treatment of several diseases. While their mechanism of action is not always clear, they are therapeutically effective.
IFN-α is used for the treatment of a variety of cancers. These include hairy cell leukemia , malignant melanoma, follicular lymphoma, and Kaposi’s sarcoma (seen in patients with AIDS). It is also used for the treatment of chronic hepatitis B and until recently, was also commonly used in conjunction with ribavirin for the treatment of chronic hepatitis C. IFN-β is currently FDA-approved for the treatment of relapsing multiple sclerosis and is used to reduce the frequency of clinical exacerbations. IFN-γ is currently used to reduce the frequency and severity of infections associated with chronic granulomatous disease, which is an immune deficiency resulting in defective phagocytic function due to the absence of NADPH oxidase activity.
Adverse effects associated with interferon therapy include flu-like symptoms such as fever, chills and headaches, gastrointestinal reactions, rash, myalgia, and injection site reactions.
Interleukin-2
IL-2 is a cytokine that is critical for the proliferation and division of T cells and induces their function and activity. Human recombinant IL-2 ( aldesleukin or Proleukin®) is used for the treatment of metastatic renal cell carcinoma and melanoma. It stimulates lymphocytes and enhances their activity against the tumor. Adverse effects include flu-like symptoms as well as a serious but uncommon cardiovascular toxicity resulting from capillary leak syndrome.
Immunization
Immunization used in the prevention of various diseases can be classified as active or passive. Active immunization involves the induction of systemic immune responses to specific antigens, and often results in the acquisition of life-long immunity. The most commonly utilized application of active immunization has been the vaccination of a population against prevalent infectious pathogens. A number of vaccines described in Chap. 9 are currently in use against a wide variety of bacterial and viral infections. These include diphtheria, pertussis, tetanus, measles, mumps, polio, meningitis (bacterial and viral), chicken pox, shingles, rota viruses, influenza, and human papilloma virus, among others. In addition to protection from infectious organisms, therapeutic vaccines have also been developed or are being considered for other types of diseases. This includes cancer vaccines that utilize various types of cancer antigens (as described in Chap. 10) and vaccines to prevent the development of allergic disorders (as described in Chap. 4).
Passive immunization has also been used for the treatment of diseases and historically has included the transfer of antibodies from both animals and humans to patients. The passive transfer of antibodies is indicated when individuals are unable to make antibodies due to a congenital or acquired deficiency, when exposed to lethal doses of an infectious or toxic agent such as venoms and there is no time for the development of adaptive immunity, or when a particular disease can be ameliorated with the passive transfer of antibodies. Depending on the disease, both non-specific and highly-specific immunoglobulins may be used. Protection typically lasts for 1–3 months, by which time many immune-competent individuals should be able to generate adaptive immune responses. Specific immunoglobulin preparations are available for several diseases including rabies, tetanus, botulism, and respiratory syncytial virus. Rho(D) is a specific IgG preparation given to Rh-negative pregnant women for the prophylaxis of hemolytic disease of the newborn in the developing fetus.
Intravenous Immunoglobulin (IVIG)
Non-specific immune globulin is derived from the pooled plasma of thousands of adults and is used for the treatment of a variety of diseases. It mainly consists of IgG molecules and its initial application was primarily for the treatment of antibody deficiencies such as agammaglobulinemia . However, more recently, it has been considered for the treatment of several autoimmune diseases, both FDA-approved and off-label. The effects of IVIG are thought to be mediated through a combination of factors. This includes the saturation of both activating and inhibitory Fc receptors for IgG as well as the FcRn receptor which enhances the half-life of IgG in serum. This prevents autoantibodies from engaging Fc receptors and inducing phagocytosis. The pooled preparation is also thought to contain anti-idiotypic antibodies, i.e. antibodies that bind to other antibodies such as the autoantibodies that exist within the patient. In addition, they may also contain helpful antibodies against B cell proliferation and survival factors, thus shutting down the production of autoantibodies. Lastly, they are thought to downregulate antigen presentation and complement activation.
Allergen Immunotherapy
Allergen immunotherapy or allergen desensitization is an immunotherapeutic procedure that has existed since the early 1900s. It is mostly used in the treatment of allergic diseases with the aim of shifting the immunologic response from a pathologic IgE-dominated response to a protective IgG4-dominant response. The induction of the tolerogenic response is thought to be dependent on the production of immunoregulatory cytokines such as IL-10.
The Adverse Effects Associated with Immunotherapy
As in the case of any type of pharmacological treatment, there exists the potential for the development of both minor and serious, or even life-threatening adverse effects. The development of adverse effects is often correlated with the dose, duration, and route of therapy, but other factors also play a role such as age, gender, and the immune status of the patient. Short-term treatment often results in minimal side effects, which are reversible after the end of treatment. However, long-term treatment may be associated with longer-lasting adverse effects. The immune specificity of the drug is also an important factor. For example, short term treatment with the glucocorticoid, oral prednisone, may result in weight gain, insomnia, fluid retention, and mood changes. However, long-term treatment is associated with osteoporosis, hypertension, weight gain, glaucoma, diabetes, muscle weakness and Cushing syndrome. In contrast, the major effect associated with long-term treatment with calcineurin inhibitors is the development of irreversible nephrotoxicity and impaired renal function. The route of treatment is also an important factor. For example, topical treatment with calcineurin inhibitors is associated with limited toxicities such as the development of rashes associated with herpes simplex virus infections. Similarly, one adverse effect of inhaled corticosteroids is the potential for development of oral candidiasis.
Inhibition of specific types of immune cells or their mediators such as cytokines is often associated with adverse effects relative to the functions of the specific target. For example, inhibition of TNF-α may result in the activation of latent tuberculosis, while the inhibition of IL-17 may result in the occurrence of upper respiratory tract infections or fungal infections. Similarly, enhancement of T cell costimulation for cancer treatment may be associated with the risk for developing autoimmunity. Chronic treatment with almost all immunosuppressive drugs is associated with the risk of development of infections. This is further enhanced in the case of transplantation recipients receiving long-term treatment with a wide variety of immunosuppressive drugs. As such, patients are often advised about the potential for developing infections and closely monitored throughout the duration of therapy. Long-term treatment over several years can also increase the risk of development of cancers such as carcinomas of the skin and genital tract and Kaposi’s sarcoma. Transplantation recipients in particular are two to four times more likely to develop these cancers compared to the general population.
Hypersensitivity Reactions to Drugs
Drug hypersensitivities are immune-mediated reactions to drugs. Depending on the type of drug, they may be mediated by IgE or IgG antibodies and often involve the activation of T cells, B cells and phagocytes. Symptoms may range from mild to severe, and may include the development of rash, serum sickness, or in rare cases anaphylaxis.
Many drugs act as haptens that bind to cell-surface molecules, resulting in the formation of immunogenic peptides that stimulate the immune system. For example, in some individuals, penicillin can conjugate with erythrocytes resulting in the production of modified epitopes that stimulate TH2 cells and induce the production of IgE antibodies. Subsequently treatment with penicillin can induce severe IgE-mediated anaphylactic reactions (type I hypersensitivity reactions) that can have fatal consequences. Conversely, the induction of IgG antibodies can trigger the complement-mediated phagocytic destruction of erythrocytes resulting in anemia (type II hypersensitivity reactions). Recent findings implicate a single, specific receptor, MrgprX2, on mast cells that is often involved in pseudo-allergic reactions to various drugs.
Some drugs such as large proteins or therapeutic monoclonal antibodies can directly stimulate antibodies resulting in type III hypersensitivity reactions and the development of serum sickness. This is often accompanied by fever, chills, rash and vasculitis in the target tissues. The reactions are mediated by immune complexes of the drug with IgG antibodies and subside when the immune complexes are cleared.
Steven-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) is a severe reaction to some types of drugs that induces skin necrosis and detachment. The mucus membranes of the eyes, mouth, and genitals are often affected. The onset of SJS/TEN is a significant medical emergency and is life-threatening. The first symptoms include fever and flu-like symptoms, which is followed 1–3 days later by various rashes leading to blistering and peeling of the skin, starting with the face and spreading to other areas of the body. The reaction is triggered by certain medications such as allopurinol , anti-epileptics, cancer therapies, and antibiotics and often occur 1–3 weeks after therapy. It may also be triggered by infections. People with a weakened immune system, HIV, a family history of SJS/TEN, and certain polymorphisms in HLA-B are particularly at risk. The reaction is thought to be mediated by activated CD8 T cells which cause the blistering and is considered a type IV hypersensitivity reaction. Treatment includes supportive therapy and treatment with cyclosporine, IVIG, plasma exchange, and corticosteroids.
Summary
The immune system plays a critical role in host defense and is required for human survival. The cells of the immune system are generated through the process of hematopoiesis in the bone marrow. Innate immune responses are triggered immediately after infection, injury, or other trauma and involve the activity of various cell types including myeloid cells, granulocytes, and mast cells. These cells in coordination with a number of proinflammatory cytokines initiate host defense and alert the immune system. Neutrophils are efficient defenders of the host against bacterial infections, while NK cells are quick to respond to viral infections. Dendritic cells survey the infectious site for pathogens and act as an important link between the innate and adaptive immune responses. Adaptive immune responses are established several days after infection and mediate specific, potent, and long-lasting responses against pathogenic antigens. Helper CD4 T cells drive the adaptive immune response and differentiate into various subsets depending on the specific type of pathogen. Cytotoxic CD8 T cells are activated against viral and tumor antigens. The activation of T cells and their effector function is dependent on dendritic cells, other antigen presenting cells, and cytokines, which regulate their function. B cells are also activated to pathogenic antigens and produce various antibodies, which can destroy the pathogen. When the infection begins to clear, the adaptive immune response wanes, and memory lymphocytes are generated that persist during life and provide protection against future attacks.
Recent advances in immunology research have significantly enhanced our understanding of the immunological basis of many diseases. Immune cells and their mediators are not only involved in host defense against foreign pathogens, but also play a vital role in maintaining homeostatic integrity and participate in reactions that contribute to the development of either immunity or tolerance. Further investigation of the mechanisms involved in these processes have resulted in the elucidation of several new immune-mediated targets for therapeutic purposes, leading to the development of novel drugs that target the immune system. These drugs belong to various classes inducing immunosuppression, inhibiting proliferation of immune cells, and modulating immune activity. The latter category includes several monoclonal antibodies and other biologics that target specific immune cells and molecules. In addition, several types of drugs also stimulate the immune system. These include various vaccines as well as recombinant cytokines that can activate immune activity against specific targets. It is anticipated that in future years, the complexity of the various interactions between immune cells and their mediators will be further uncovered, leading to the development of even more immunotherapeutic drugs.
Practice Questions
-
1.
Provide three examples each of an innate and an adaptive immune cell and list at least one function of each cell type.
-
2.
Provide examples of how microbiota influence the development of the immune response.
-
3.
Describe the cardinal characteristics of inflammation and explain the immunological mechanisms underlying their development.
-
4.
_______ is a cytokine produced by macrophages that causes the dilation of blood vessels and promotes the recruitment of leukocytes to injured tissues.
-
(a)
TNF-α
-
(b)
IL-6
-
(c)
IL-1β
-
(d)
IL-5
-
(a)
-
5.
_______ is a chemokine produced by macrophages that enhances the recruitment of neutrophils to injured tissues.
-
(a)
CXCL8
-
(b)
Eotaxin
-
(c)
TNF-α
-
(d)
IL-5
-
(a)
-
6.
Which of the following represent examples of innate cells that are involved in IgE-mediated immune responses to parasites? Select all that apply.
-
(a)
TH2 cells
-
(b)
Eosinophils
-
(c)
Basophils
-
(d)
Neutrophil
-
(a)
-
7.
The_________ is involved in the phagocytosis-mediated killing of bacterial cells by neutrophils.
-
(a)
activation of NF-κB
-
(b)
respiratory burst
-
(c)
release of cytokines
-
(d)
induction of costimulation
-
(a)
-
8.
______ is a growth factor required for the generation of eosinophils in the bone marrow. Select all that apply.
-
(a)
IL-3
-
(b)
IL-6
-
(c)
IL-1β
-
(d)
IL-5
-
(a)
-
9.
What do you mean by the interferon response? How does it affect the activation of NK cells?
-
10.
Explain how cell surface receptors contribute to the NK cell-mediated killing of virally-infected cells.
-
11.
Explain the process involved in the activation of naïve T cells to a novel antigen.
-
12.
Which of the following molecules is expressed by dendritic cells as they migrate towards draining lymph nodes after capture of an antigen?
-
(a)
CD80
-
(b)
MHC I
-
(c)
IL-6 receptor
-
(d)
CD28
-
(a)
-
13.
The engagement of ___________ molecules is a critical second step in the activation of naïve T cells to an antigen.
-
(a)
TLR
-
(b)
adhesion
-
(c)
costimulatory
-
(d)
inhibitory
-
(a)
-
14.
Describe the various subsets of helper T cells and give one example of their immunological function.
-
15.
Explain the mechanisms by which antibodies combat infectious organisms.
-
16.
________ is a costimulatory molecule expressed on B cells required for the induction of isotype switching in antibody molecules.
-
(a)
CD28
-
(b)
CD40
-
(c)
ICOS
-
(d)
PD-1
-
(a)
-
17.
How does somatic hypermutation contribute to the development of the B cell repertoire?
-
18.
___________ is an example of an eicosanoid produced by mast cells after IgE-mediated activation.
-
(a)
Histamine
-
(b)
Leukotriene
-
(c)
Tryptase
-
(d)
IL-6
-
(a)
-
19.
Which of the drugs below complexes with FK506BP12 and inhibits the actions of mTOR?
-
(a)
Tacrolimus
-
(b)
Everolimus
-
(c)
Cyclosporine
-
(d)
Abatacept
-
(a)
-
20.
_______ is a drug that inhibits purine synthesis via derivatives of 6-mercaptopurine.
-
(a)
Azathioprine
-
(b)
Methotrexate
-
(c)
Cyclophosphamide
-
(d)
IL-5
-
(a)
-
21.
_______ is an example of a drug that inhibits T cell-mediated responses by inhibiting T cell costimulation.
-
(a)
Abatacept
-
(b)
Ipilimumab
-
(c)
Pembrolizumab
-
(d)
Nivolumab
-
(a)
-
22.
_________ is a chimeric monoclonal antibody used in the treatment non-Hodgkin’s lymphoma.
-
(a)
Rituxan
-
(b)
Xolair
-
(c)
Humira
-
(d)
Cosentyx
-
(a)
-
23.
Which of the following are examples of inhibitors of TNF-α? Select all that apply.
-
(a)
Etanercept
-
(b)
Infliximab
-
(c)
Adalimumab
-
(d)
Secukinumab
-
(a)
-
24.
Which of the following is an example of an immunotherapeutic drug used in the treatment of metastatic renal carcinoma?
-
(a)
Rituximab
-
(b)
Aldesleukin
-
(c)
Nivolumab
-
(d)
Alemtuzumab
-
(a)
-
25.
__________ is a receptor on mast cells that is involved in pseudo-allergic reactions to drugs
-
(a)
MrgprX2
-
(b)
FcεRI
-
(c)
TLR
-
(d)
Leukotriene receptor D4
-
(a)
-
26.
___________ is an example of a severe hypersensitivity reaction that can develop in some people who are treated with allopurinol.
-
(a)
Type II hypersensitivity reactions
-
(b)
Anaphylaxis.
-
(c)
Steven Johnson syndrome
-
(d)
Serum sickness
-
(a)
Notes
- 1.
The suggested reading list below was created with the aim of highlighting classic papers in immunology that describe seminal findings in the field of immunology. Many of these discoveries have advanced our current understanding of immunology and medicine and several have resulted in the receipt of the Nobel Prize.
Suggested Reading
The suggested reading list below was created with the aim of highlighting classic papers in immunology that describe seminal findings in the field of immunology. Many of these discoveries have advanced our current understanding of immunology and medicine and several have resulted in the receipt of the Nobel Prize.
Jenner E. An inquiry into the causes and effects of variolae vaccinae: a disease discovered in some Western Counties of England. London: Sampson Low; 1798. p. 75.
Jenner E. Letter addressed to the medical profession generally, relative to vaccination. Lond Med Phys J. 1821;45:277–80.
Pasteur L. Sur les maladies virulentes, et en particulier sur la maladie appelee vulgairement cholera des poules. C R Acad Sci. 1880;90:248–9.
Pasteur LC, Roux E. Compte rendu sommaire des experiences faites a Pouilly-Le-Fort, pres de Melun, sur la vaccination charnonneuse. C R Acad Sci. 1881;92:1378–83.
Koch R. A further communication on a remedy for tuberculosis. Br Med J. 1891;1:125–7.
Koch R. An address on the fight against tuberculosis in the light of the experience that has been gained in the successful combat of other infectious diseases. Br Med J. 1901;2:189–93.
Weil R. Studies in anaphylaxis. XIV. On the relation between precipitin and sensitizin. J Immunol. 1916;1:1–18.
Lurie MB. A correlation between the histological changes and the fate of living tubercule bacilli in the organs of infected rabbits. J Exp Med. 1933;57:31–54.
Gibson T, Medawar PB. The fate of skin homografts in man. J Anat. 1943;77:299–310 294.
Chase MW. The cellular transfer of cutaneous sensitivity to tuberculin. Proc Soc Exp Biol Med. 1945;59:134.
Coombs RR, Mourant AE, Race RR. A new test for the detection of weak and incomplete Rh agglutinins. Br J Exp Pathol. 1945;26:255–66.
Owen RD. Immunogenetic consequences of vascular anastomoses between bovine twins. Science. 1945;102:400–1.
Fagraeus A. Plasma cellular reaction and its relation to the formation of antibodies in vitro. Nature. 1947;159:499.
Fagraeus A. The plasma cellular reaction and its relation to the formation of antibodies in vitro. J Immunol. 1948;58:1–13.
Snell GD. Methods for the study of histocompatibility genes. J Genet. 1948;49:87–108.
Bordley JE, Carey RA, et al. Preliminary observations on the effect of adrenocorticotropic hormone in allergic diseases. Bull Johns Hopkins Hosp. 1949;85:396–8.
Hench PS, Kendall EC, Slocumb CH, Polley HF. Effects of cortisone acetate and pituitary ACTH on rheumatoid arthritis, rheumatic fever and certain other conditions. Arch Intern Med (Chic). 1950;85:545–666.
Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–6.
Jerne NK. The natural-selection theory of antibody formation. Proc Natl Acad Sci U S A. 1955;41:849–57.
Billingham RE, Brent L, Medawar PB. The antigenic stimulus in transplantation immunity. Nature. 1956;178:514–9.
Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci. 1957;147:258–67.
Isaacs A, Lindenmann J, Valentine RC. Virus interference. II. Some properties of interferon. Proc R Soc Lond B Biol Sci. 1957;147:268–73.
Nossal GJ, Lederberg J. Antibody production by single cells. Nature. 1958;181:1419–20.
Porter RR. Separation and isolation of fractions of rabbit gamma-globulin containing the antibody and antigenic combining sites. Nature. 1958;182:670–1.
Burnet FM. The clonal selection theory of acquired immunity. Cambridge: Cambridge University Press; 1959.
Nowell PC. Phytohemagglutinin: an initiator of mitosis in cultures of normal human leukocytes. Cancer Res. 1960;20:462–6.
Yalow RS, Berson SA. Immunoassay of endogenous plasma insulin in man. J Clin Invest. 1960;39:1157–75.
Dresser DW. Effectiveness of lipid and lipidophilic substances as adjuvants. Nature. 1961;191:1169–71.
Burnet FM. The immunological significance of the thymus: an extension of the clonal selection theory of immunity. Australas Ann Med. 1962;11:79–91.
Dresser DW. Specific inhibition of antibody production. II. Paralysis induced in adult mice by small quantities of protein antigen. Immunology. 1962;5:378–88.
Mackaness GB. Cellular resistance to infection. J Exp Med. 1962;116:381–406.
Mackaness GB. The immunological basis of acquired cellular resistance. J Exp Med. 1964;120:105–20.
Cooper MD, Peterson RD, Good RA. Delineation of the thymic and bursal lymphoid systems in the chicken. Nature. 1965;205:143–6.
Brenner S, Milstein C. Origin of antibody variation. Nature. 1966;211:242–3.
Cooper MD, Raymond DA, Peterson RD, South MA, Good RA. The functions of the thymus system and the bursa system in the chicken. J Exp Med. 1966;123:75–102.
Ishizaka K, Ishizaka T. Physicochemical properties of reaginic antibody. 1. Association of reaginic activity with an immunoglobulin other than gammaA- or gammaG-globulin. J Allergy. 1966a;37:169–85.
Ishizaka K, Ishizaka T. Physicochemical properties of reaginic antibody. 3. Further studies on the reaginic antibody in gamma-A-globulin preparations. J Allergy. 1966b;38:108–19.
Ishizaka K, Ishizaka T, Lee EH. Physiochemical properties of reaginic antibody. II. Characteristic properties of reaginic antibody different from human gamma-A-isohemagglutinin and gamma-D-globulin. J Allergy. 1966a;37:336–49.
Ishizaka K, Ishizaka T, Hornbrook MM. Physico-chemical properties of human reaginic antibody. IV. Presence of a unique immunoglobulin as a carrier of reaginic activity. J Immunol. 1966b;97:75–85.
Ishizaka K, Ishizaka T, Hornbrook MM. Physicochemical properties of reaginic antibody. V. Correlation of reaginic activity wth gamma-E-globulin antibody. J Immunol. 1966c;97:840–53.
Edelman GM, Gally JA. Somatic recombination of duplicated genes: an hypothesis on the origin of antibody diversity. Proc Natl Acad Sci U S A. 1967;57:353–8.
Henney CS, Ishizaka K. An antigenic determinant of human gamma-globulin susceptible to papain digestion. J Immunol. 1967;99:695–702.
Ishizaka K, Ishizaka T. Identification of gamma-E-antibodies as a carrier of reaginic activity. J Immunol. 1967;99:1187–98.
Ishizaka K, Ishizaka T, Menzel AE. Physicochemical properties of reaginic antibody. VI. Effect of heat on gamma-E-, gamma-G- and gamma-A-antibodies in the sera of ragweed sensitive patients. J Immunol. 1967a;99:610–8.
Ishizaka K, Ishizaka T, Terry WD. Antigenic structure of gamma-E-globulin and reaginic antibody. J Immunol. 1967b;99:849–58.
Johansson SG, Bennich H. Immunological studies of an atypical (myeloma) immunoglobulin. Immunology. 1967;13:381–94.
Mintz B, Silvers WK. “Intrinsic” immunological tolerance in allophenic mice. Science. 1967;158:1484–6.
Mishell RI, Dutton RW. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967;126:423–42.
Edelman GM, et al. The covalent structure of an entire gammaG immunoglobulin molecule. Proc Natl Acad Sci U S A. 1969;63:78–85.
Klinman NR. Antibody with homogeneous antigen binding produced by splenic foci in organ culture. Immunochemistry. 1969;6:757–9.
McDevitt HO, Chinitz A. Genetic control of the antibody response: relationship between immune response and histocompatibility (H-2) type. Science. 1969;163:1207–8.
Raff MC. Two distinct populations of peripheral lymphocytes in mice distinguishable by immunofluorescence. Immunology. 1970;19:637–50.
Wu TT, Kabat EA. An analysis of the sequences of the variable regions of Bence Jones proteins and myeloma light chains and their implications for antibody complementarity. J Exp Med. 1970;132:211–50.
Armstrong JA, D’Arcy Hart P. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971;134:713–40.
Craig SW, Cebra JJ. Peyer’s patches: an enriched source of precursors for IgA-producing immunocytes in the rabbit. J Exp Med. 1971;134:188–200.
Cudkowicz G, Bennett M. Peculiar immunobiology of bone marrow allografts. I. Graft rejection by irradiated responder mice. J Exp Med. 1971a;134:83–102.
Cudkowicz G, Bennett M. Peculiar immunobiology of bone marrow allografts. II. Rejection of parental grafts by resistant F 1 hybrid mice. J Exp Med. 1971b;134:1513–28.
Engvall E, Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971;8:871–4.
Jerne NK. The somatic generation of immune recognition. Eur J Immunol. 1971;1:1–9.
Mitchison NA. The carrier effect in the secondary response to hapten-protein conjugates. I. Measurement of the effect with transferred cells and objections to the local environment hypothesis. Eur J Immunol. 1971a;1:10–7.
Mitchison NA. The carrier effect in the secondary response to hapten-protein conjugates. II. Cellular cooperation. Eur J Immunol. 1971b;1:18–27.
Sprent J, Miller JF, Mitchell GF. Antigen-induced selective recruitment of circulating lymphocytes. Cell Immunol. 1971;2:171–81.
Klinman NR. The mechanism of antigenic stimulation of primary and secondary clonal precursor cells. J Exp Med. 1972;136:241–60.
Babior BM, Kipnes RS, Curnutte JT. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973;52:741–4.
Cotton RG, Milstein C. Letter: fusion of two immunoglobulin-producing myeloma cells. Nature. 1973;244:42–3.
Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–62.
Strander H, Cantell K, Carlstrom G, Jakobsson PA. Clinical and laboratory investigations on man: systemic administration of potent interferon to man. J Natl Cancer Inst. 1973;51:733–42.
Bottazzo GF, Florin-Christensen A, Doniach D. Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet. 1974;2:1279–83.
Brandtzaeg P. Mucosal and glandular distribution of immunoglobulin components: differential localization of free and bound SC in secretory epithelial cells. J Immunol. 1974;112:1553–9.
MacCuish AC, Irvine WJ, Barnes EW, Duncan LJ. Antibodies to pancreatic islet cells in insulin-dependent diabetics with coexistent autoimmune disease. Lancet. 1974;2:1529–31.
Owen JJ, Cooper MD, Raff MC. In vitro generation of B lymphocytes in mouse foetal liver, a mammalian ‘bursa equivalent’. Nature. 1974;249:361–3.
Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. II. Functional properties in vitro. J Exp Med. 1974;139:380–97.
Zinkernagel RM, Doherty PC. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974;248:701–2.
Bevan MJ. The major histocompatibility complex determines susceptibility to cytotoxic T cells directed against minor histocompatibility antigens. J Exp Med. 1975;142:1349–64.
Cantor H, Boyse EA. Functional subclasses of T-lymphocytes bearing different Ly antigens. I. The generation of functionally distinct T-cell subclasses is a differentiative process independent of antigen. J Exp Med. 1975a;141:1376–89.
Cantor H, Boyse EA. Functional subclasses of T lymphocytes bearing different Ly antigens. II. Cooperation between subclasses of Ly+ cells in the generation of killer activity. J Exp Med. 1975b;141:1390–9.
Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–7.
Burnet FM. A modification of Jerne’s theory of antibody production using the concept of clonal selection. CA Cancer J Clin. 1976;26:119–21.
Hozumi N, Tonegawa S. Evidence for somatic rearrangement of immunoglobulin genes coding for variable and constant regions. Proc Natl Acad Sci U S A. 1976;73:3628–32.
Solley GO, Gleich GJ, Jordon RE, Schroeter AL. The late phase of the immediate wheal and flare skin reaction. Its dependence upon IgE antibodies. J Clin Invest. 1976;58:408–20.
Robinson JH, Owen JJ. Generation of T-cell function in organ culture of foetal mouse thymus. II. Mixed lymphocyte culture reactivity. Clin Exp Immunol. 1977;27:322–7.
Ruscetti FW, Morgan DA, Gallo RC. Functional and morphologic characterization of human T cells continuously grown in vitro. J Immunol. 1977;119:131–8.
Silverton EW, Navia MA, Davies DR. Three-dimensional structure of an intact human immunoglobulin. Proc Natl Acad Sci U S A. 1977;74:5140–4.
Brack C, Hirama M, Lenhard-Schuller R, Tonegawa S. A complete immunoglobulin gene is created by somatic recombination. Cell. 1978;15:1–14.
Baker PE, Gillis S, Smith KA. Monoclonal cytolytic T-cell lines. J Exp Med. 1979;149:273–8.
Kung P, Goldstein G, Reinherz EL, Schlossman SF. Monoclonal antibodies defining distinctive human T cell surface antigens. Science. 1979;206:347–9.
Murphy RC, Hammarstrom S, Samuelsson B. Leukotriene C: a slow-reacting substance from murine mastocytoma cells. Proc Natl Acad Sci U S A. 1979;76:4275–9.
Parks DR, Bryan VM, Oi VT, Herzenberg LA. Antigen-specific identification and cloning of hybridomas with a fluorescence-activated cell sorter. Proc Natl Acad Sci U S A. 1979;76:1962–6.
Reinherz EL, Kung PC, Goldstein G, Schlossman SF. Separation of functional subsets of human T cells by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979;76:4061–5.
Silverstein AM. History of immunology. Cell Immunol. 1979a;42:1–2.
Silverstein AM. History of immunology. Cellular versus humoral immunity: determinants and consequences of an epic 19th century battle. Cell Immunol. 1979b;48:208–21.
Davis MM, et al. An immunoglobulin heavy-chain gene is formed by at least two recombinational events. Nature. 1980;283:733–9.
Early P, Huang H, Davis M, Calame K, Hood L. An immunoglobulin heavy chain variable region gene is generated from three segments of DNA: VH, D and JH. Cell. 1980;19:981–92.
Silverstein AM, Bialasiewicz AA. History of immunology. A history of theories of acquired immunity. Cell Immunol. 1980;51:151–67.
Van Wauwe JP, De Mey JR, Goossens JG. OKT3: a monoclonal anti-human T lymphocyte antibody with potent mitogenic properties. J Immunol. 1980;124:2708–13.
Kappler JW, Skidmore B, White J, Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981;153:1198–214.
Silverstein AM, Miller G. History of immunology. The royal experiment on immunity: 1721-1722. Cell Immunol. 1981;61:437–47.
Steiner H, Hultmark D, Engstrom A, Bennich H, Boman HG. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature. 1981;292:246–8.
Ziegler K, Unanue ER. Identification of a macrophage antigen-processing event required for I-region-restricted antigen presentation to T lymphocytes. J Immunol. 1981;127:1869–75.
Allison JP, McIntyre BW, Bloch D. Tumor-specific antigen of murine T-lymphoma defined with monoclonal antibody. J Immunol. 1982;129:2293–300.
Butcher EC, et al. Surface phenotype of Peyer’s patch germinal center cells: implications for the role of germinal centers in B cell differentiation. J Immunol. 1982;129:2698–707.
Howard M, et al. Identification of a T cell-derived b cell growth factor distinct from interleukin 2. J Exp Med. 1982;155:914–23.
Isakson PC, Pure E, Vitetta ES, Krammer PH. T cell-derived B cell differentiation factor(s). Effect on the isotype switch of murine B cells. J Exp Med. 1982;155:734–48.
Silverstein AM. History of immunology. Development of the concept of immunologic specificity, I. Cell Immunol. 1982a;67:396–409.
Silverstein AM. History of immunology: development of the concept of immunologic specificity: II. Cell Immunol. 1982b;71:183–95.
Takahashi N, et al. Structure of human immunoglobulin gamma genes: implications for evolution of a gene family. Cell. 1982;29:671–9.
Turkeltaub PC, Rastogi SC, Baer H, Anderson MC, Norman PS. A standardized quantitative skin-test assay of allergen potency and stability: studies on the allergen dose-response curve and effect of wheal, erythema, and patient selection on assay results. J Allergy Clin Immunol. 1982;70:343–52.
Haskins K, et al. The major histocompatibility complex-restricted antigen receptor on T cells. I. Isolation with a monoclonal antibody. J Exp Med. 1983;157:1149–69.
Kappler J, et al. The major histocompatibility complex-restricted antigen receptor on T cells in mouse and man: identification of constant and variable peptides. Cell. 1983;35:295–302.
Tonegawa S. Somatic generation of antibody diversity. Nature. 1983;302:575–81.
Auron PE, et al. Nucleotide sequence of human monocyte interleukin 1 precursor cDNA. Proc Natl Acad Sci U S A. 1984;81:7907–11.
Gough NM, et al. Molecular cloning of cDNA encoding a murine haematopoietic growth regulator, granulocyte-macrophage colony stimulating factor. Nature. 1984;309:763–7.
Hedrick SM, Cohen DI, Nielsen EA, Davis MM. Isolation of cDNA clones encoding T cell-specific membrane-associated proteins. Nature. 1984;308:149–53.
Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–5.
McKean D, et al. Generation of antibody diversity in the immune response of BALB/c mice to influenza virus hemagglutinin. Proc Natl Acad Sci U S A. 1984;81:3180–4.
Morrison SL, Johnson MJ, Herzenberg LA, Oi VT. Chimeric human antibody molecules: mouse antigen-binding domains with human constant region domains. Proc Natl Acad Sci U S A. 1984;81:6851–5.
Rock KL, Benacerraf B, Abbas AK. Antigen presentation by hapten-specific B lymphocytes. I. Role of surface immunoglobulin receptors. J Exp Med. 1984;160:1102–13.
Townsend AR, McMichael AJ, Carter NP, Huddleston JA, Brownlee GG. Cytotoxic T cell recognition of the influenza nucleoprotein and hemagglutinin expressed in transfected mouse L cells. Cell. 1984;39:13–25.
Babbitt BP, Allen PM, Matsueda G, Haber E, Unanue ER. Binding of immunogenic peptides to Ia histocompatibility molecules. Nature. 1985;317:359–61.
Beutler B, Milsark IW, Cerami AC. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985;229:869–71.
Jenner E, Pasteur L. [From Jenner to Pasteur or the development of ideas on vaccination]. Bull Acad Natl Med. 1985;169:771–8.
Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314:537–9.
Nakano T, et al. Fate of bone marrow-derived cultured mast cells after intracutaneous, intraperitoneal, and intravenous transfer into genetically mast cell-deficient W/Wv mice. Evidence that cultured mast cells can give rise to both connective tissue type and mucosal mast cells. J Exp Med. 1985;162:1025–43.
Brenner MB, et al. Identification of a putative second T-cell receptor. Nature. 1986;322:145–9.
Clark EA, Ledbetter JA. Activation of human B cells mediated through two distinct cell surface differentiation antigens, Bp35 and Bp50. Proc Natl Acad Sci U S A. 1986;83:4494–8.
Dustin ML, Rothlein R, Bhan AK, Dinarello CA, Springer TA. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986;137:245–54.
Jones PT, Dear PH, Foote J, Neuberger MS, Winter G. Replacing the complementarity-determining regions in a human antibody with those from a mouse. Nature. 1986;321:522–5.
Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986;319:675–8.
Kehrl JH, et al. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986;163:1037–50.
Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–57.
Rothlein R, Dustin ML, Marlin SD, Springer TA. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J Immunol. 1986;137:1270–4.
Royer-Pokora B, et al. Cloning the gene for an inherited human disorder—chronic granulomatous disease—on the basis of its chromosomal location. Nature. 1986;322:32–8.
Sen R, Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986a;47:921–8.
Sen R, Baltimore D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell. 1986b;46:705–16.
Bjorkman PJ, et al. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987a;329:506–12.
Bjorkman PJ, et al. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987b;329:512–8.
Brunet JF, et al. A new member of the immunoglobulin superfamily—CTLA-4. Nature. 1987;328:267–70.
Cher DJ, Mosmann TR. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987;138:3688–94.
Doyle C, Strominger JL. Interaction between CD4 and class II MHC molecules mediates cell adhesion. Nature. 1987;330:256–9.
Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med. 1987;165:302–19.
Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–80.
Orme IM. The kinetics of emergence and loss of mediator T lymphocytes acquired in response to infection with Mycobacterium tuberculosis. J Immunol. 1987;138:293–8.
Snapper CM, Paul WE. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–7.
Yoshimura T, Matsushima K, Oppenheim JJ, Leonard EJ. Neutrophil chemotactic factor produced by lipopolysaccharide (LPS)-stimulated human blood mononuclear leukocytes: partial characterization and separation from interleukin 1 (IL 1). J Immunol. 1987a;139:788–93.
Yoshimura T, et al. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci U S A. 1987b;84:9233–7.
Bird RE, et al. Single-chain antigen-binding proteins. Science. 1988;242:423–6.
Goodnow CC, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–82.
Schall TJ, et al. A human T cell-specific molecule is a member of a new gene family. J Immunol. 1988;141:1018–25.
Shaw JP, et al. Identification of a putative regulator of early T cell activation genes. Science. 1988;241:202–5.
Coffman RL, Lebman DA, Shrader B. Transforming growth factor beta specifically enhances IgA production by lipopolysaccharide-stimulated murine B lymphocytes. J Exp Med. 1989;170:1039–44.
Emmel EA, et al. Cyclosporin A specifically inhibits function of nuclear proteins involved in T cell activation. Science. 1989;246:1617–20.
Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–95.
Janeway CA Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13.
Lefrancois L, Goodman T. In vivo modulation of cytolytic activity and Thy-1 expression in TCR-gamma delta+ intraepithelial lymphocytes. Science. 1989;243:1716–8.
Porcelli S, et al. Recognition of cluster of differentiation 1 antigens by human CD4-CD8-cytolytic T lymphocytes. Nature. 1989;341:447–50.
Schatz DG, Oettinger MA, Baltimore D. The V(D)J recombination activating gene, RAG-1. Cell. 1989;59:1035–48.
Sonoda E, et al. Transforming growth factor beta induces IgA production and acts additively with interleukin 5 for IgA production. J Exp Med. 1989;170:1415–20.
Townsend A, et al. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature. 1989;340:443–8.
Wright AE, Douglas SR, Sanderson JB. An experimental investigation of the role of the blood fluids in connection with phagocytosis. 1903. Rev Infect Dis. 1989;11:827–34.
Conrad DH, Ben-Sasson SZ, Le Gros G, Finkelman FD, Paul WE. Infection with Nippostrongylus brasiliensis or injection of anti-IgD antibodies markedly enhances Fc-receptor-mediated interleukin 4 production by non-B, non-T cells. J Exp Med. 1990;171:1497–508.
Hombach J, Tsubata T, Leclercq L, Stappert H, Reth M. Molecular components of the B-cell antigen receptor complex of the IgM class. Nature. 1990;343:760–2.
Koller BH, Marrack P, Kappler JW, Smithies O. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science. 1990;248:1227–30.
Le Gros G, Ben-Sasson SZ, Seder R, Finkelman FD, Paul WE. Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J Exp Med. 1990;172:921–9.
Linsley PS, Clark EA, Ledbetter JA. T-cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB-1. Proc Natl Acad Sci U S A. 1990;87:5031–5.
Monaco JJ, Cho S, Attaya M. Transport protein genes in the murine MHC: possible implications for antigen processing. Science. 1990;250:1723–6.
Moore KW, et al. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science. 1990;248:1230–4.
Oettinger MA, Schatz DG, Gorka C, Baltimore D. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science. 1990;248:1517–23.
Ruddle NH, et al. An antibody to lymphotoxin and tumor necrosis factor prevents transfer of experimental allergic encephalomyelitis. J Exp Med. 1990;172:1193–200.
Zijlstra M, et al. Beta 2-microglobulin deficient mice lack CD4-8+ cytolytic T cells. Nature. 1990;344:742–6.
Falk K, Rotzschke O, Stevanovic S, Jung G, Rammensee HG. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991;351:290–6.
Fiorentino DF, et al. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–51.
Holmes WE, Lee J, Kuang WJ, Rice GC, Wood WI. Structure and functional expression of a human interleukin-8 receptor. Science. 1991;253:1278–80.
Murphy PM, Tiffany HL. Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science. 1991;253:1280–3.
Pearce EJ, Caspar P, Grzych JM, Lewis FA, Sher A. Downregulation of Th1 cytokine production accompanies induction of Th2 responses by a parasitic helminth, Schistosoma mansoni. J Exp Med. 1991;173:159–66.
Rudensky A, Preston-Hurlburt P, Hong SC, Barlow A, Janeway CA Jr. Sequence analysis of peptides bound to MHC class II molecules. Nature. 1991;353:622–7.
Todd JA, et al. Genetic analysis of autoimmune type 1 diabetes mellitus in mice. Nature. 1991;351:542–7.
von Behring E, Kitasato S. [The mechanism of diphtheria immunity and tetanus immunity in animals. 1890]. Mol Immunol. 1991;28(1317):1319–20.
Carter RH, Fearon DT. CD19: lowering the threshold for antigen receptor stimulation of B lymphocytes. Science. 1992;256:105–7.
Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–95.
Janeway CA Jr. The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today. 1992;13:11–6.
Karlhofer FM, Ribaudo RK, Yokoyama WM. MHC class I alloantigen specificity of Ly-49+ IL-2-activated natural killer cells. Nature. 1992;358:66–70.
Noelle RJ, et al. A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proc Natl Acad Sci U S A. 1992;89:6550–4.
Schindler C, Shuai K, Prezioso VR, Darnell JE Jr. Interferon-dependent tyrosine phosphorylation of a latent cytoplasmic transcription factor. Science. 1992;257:809–13.
Velazquez L, Fellous M, Stark GR, Pellegrini S. A protein tyrosine kinase in the interferon alpha/beta signaling pathway. Cell. 1992;70:313–22.
Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–7.
Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–9.
Brown JH, et al. Three-dimensional structure of the human class II histocompatibility antigen HLA-DR1. Nature. 1993;364:33–9.
Gay D, Saunders T, Camper S, Weigert M. Receptor editing: an approach by autoreactive B cells to escape tolerance. J Exp Med. 1993;177:999–1008.
Heinzel FP, Rerko RM, Hatam F, Locksley RM. IL-2 is necessary for the progression of leishmaniasis in susceptible murine hosts. J Immunol. 1993;150:3924–31.
Hsieh CS, et al. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–9.
Noguchi M, et al. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73:147–57.
Shinkai Y, et al. Restoration of T cell development in RAG-2-deficient mice by functional TCR transgenes. Science. 1993;259:822–5.
Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. J Exp Med. 1993;177:1009–20.
De Togni P, et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703–7.
Hogquist KA, et al. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27.
Iwashima M, Irving BA, van Oers NS, Chan AC, Weiss A. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science. 1994;263:1136–9.
Jardetzky TS, et al. Three-dimensional structure of a human class II histocompatibility molecule complexed with superantigen. Nature. 1994;368:711–8.
Lau LL, Jamieson BD, Somasundaram T, Ahmed R. Cytotoxic T-cell memory without antigen. Nature. 1994;369:648–52.
Saint-Ruf C, et al. Analysis and expression of a cloned pre-T cell receptor gene. Science. 1994;266:1208–12.
Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–18.
Surh CD, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994;372:100–3.
Walunas TL, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–13.
Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–65.
McBlane JF, et al. Cleavage at a V(D)J recombination signal requires only RAG1 and RAG2 proteins and occurs in two steps. Cell. 1995;83:387–95.
Robey E, Allison JP. T-cell activation: integration of signals from the antigen receptor and costimulatory molecules. Immunol Today. 1995;16:306–10.
Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64.
Yao Z, et al. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3:811–21.
Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–7.
Garboczi DN, et al. Structure of the complex between human T-cell receptor, viral peptide and HLA-A2. Nature. 1996;384:134–41.
Garcia KC, et al. An alphabeta T cell receptor structure at 2.5 A and its orientation in the TCR-MHC complex. Science. 1996;274:209–19.
Larsen CP, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–8.
Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–83.
Medzhitov R, Preston-Hurlburt P, Janeway CA Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7.
Muzio M, Ni J, Feng P, Dixit VM. IRAK (Pelle) family member IRAK-2 and MyD88 as proximal mediators of IL-1 signaling. Science. 1997;278:1612–5.
Wesche H, Henzel WJ, Shillinglaw W, Li S, Cao Z. MyD88: an adapter that recruits IRAK to the IL-1 receptor complex. Immunity. 1997;7:837–47.
Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–96.
Butz EA, Bevan MJ. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 1998;8:167–75.
Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–6.
Murali-Krishna K, et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–87.
Poltorak A, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–8.
Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1998;101:273–81.
Bauer S, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–9.
Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–9.
Forster R, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33.
Grakoui A, et al. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–7.
Hoshino K, et al. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–52.
Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–51.
Nutt SL, Heavey B, Rolink AG, Busslinger M. Commitment to the B-lymphoid lineage depends on the transcription factor Pax5. Nature. 1999;401:556–62.
Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12.
Muramatsu M, et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–63.
Szabo SJ, et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–69.
Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565–94.
Girardi M, et al. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294:605–9.
Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–7.
Shankaran V, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–11.
Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–6.
Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6.
Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61.
Pentcheva-Hoang T, Egen JG, Wojnoonski K, Allison JP. B7-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity. 2004;21:401–13.
Silverstein AM. Paul Ehrlich, archives and the history of immunology. Nat Immunol. 2005;6:639.
Khanna KM, McNamara JT, Lefrancois L. In situ imaging of the endogenous CD8 T cell response to infection. Science. 2007;318:116–20.
Kaufmann SH. Immunology’s foundation: the 100-year anniversary of the Nobel Prize to Paul Ehrlich and Elie Metchnikoff. Nat Immunol. 2008a;9:705–12.
Kaufmann SH. Paul Ehrlich: founder of chemotherapy. Nat Rev Drug Discov. 2008b;7:373.
Robinson JH, Owen JJ. Pillars article: generation of T-cell function in organ culture of foetal mouse thymus I. Mitogen responsiveness. 1975. J Immunol. 2008;181:7437–44.
Xu Z, Zan H, Pone EJ, Mai T, Casali P. Immunoglobulin class-switch DNA recombination: induction, targeting and beyond. Nat Rev Immunol. 2012;12:517–31.
Cooper MD. The early history of B cells. Nat Rev Immunol. 2015;15:191–7.
Gitlin AD, Nussenzweig MC. Immunology: fifty years of B lymphocytes. Nature. 2015;517:139–41.
Akdis M, et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor β, and TNF-α: receptors, functions, and roles in disease. J Allergy Clin Immunol. 2016;138:984–1010.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Mathias, C.B. (2020). Overview of the Immune System and Its Pharmacological Targets. In: Mathias, C., McAleer, J., Szollosi, D. (eds) Pharmacology of Immunotherapeutic Drugs. Springer, Cham. https://doi.org/10.1007/978-3-030-19922-7_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-19922-7_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-19921-0
Online ISBN: 978-3-030-19922-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)