Abstract
While it is true that only a small proportion of patients develop gastrointestinal cancer in the setting of an inherited syndrome, it is important for the medical oncologist to recognize this unique group of patients for several reasons. First, genetic testing of the tumor can have implications for the patient’s prognosis and may help tailor chemotherapy in this age of personalized cancer medicine. Second, affected patients are often at risk for other malignancies either within the gastrointestinal tract or involving other organ systems and, as such, are in need of close clinical surveillance. Lastly, the oncologist is in an excellent position to encourage genetic counseling and testing of at-risk family members. In this chapter we will review familial adenomatous polyposis (FAP) syndrome and hereditary nonpolyposis colon cancer (HNPCC), aka Lynch syndrome, in some detail. We will also discuss polyposis syndromes other than FAP, namely, Peutz-Jegher syndrome and juvenile polyposis as well as hereditary diffuse gastric cancer and inherited pancreatic cancer syndromes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Familial adenomatous polyposis syndrome (FAP)
- Hereditary nonpolyposis colon cancer syndrome (HNPCC)
- Lynch syndrome
- Genetic testing
- Peutz-Jegher syndrome
- Juvenile polyposis syndrome
- Hereditary diffuse gastric cancer syndrome (HDGC)
- Hereditary pancreatic cancer
- Mismatch repair genes
- Genetic panel testing
- Genetic counseling
Introduction
The main job of a medical oncologist is to confirm a cancer diagnosis, assure appropriate staging of the malignancy, and institute state-of-the-art treatment, typically chemotherapy for advanced disease or for adjuvant treatment for locally advanced tumors. Our mission in this chapter will be to highlight the intersection of these functions with the cancers that manifest an inherited susceptibility.
No more than about 3% to at most 5% of gastrointestinal (GI) malignancies show a clear inherited basis. As we shall see, there are only a few instances in which the presence of an underlying inherited susceptibility has an important bearing on prognosis or selection of chemotherapy. Nevertheless, the medical oncologist, as an institution’s specialist in the area of cancer, will often be called upon to help develop and coordinate a program for the identification and management of inherited cancer susceptibility.
In this chapter, we will take a somewhat historical perspective and will combine broader issues of disease management with specific areas that selectively impact the medical oncologist. As will be seen, ongoing rapid advances in molecular technology are transforming the approach to personalized therapy in the cancer patient and to the diagnosis of underlying susceptibility. In our description of specific inherited syndromes, the focus will be the traditional one of recognizing characteristic disease expressions (phenotype) in the cancer patient, along with patterns of expression in families, the combination of which may suggest a very narrow range of conditions. Counseling regarding the advantages and limitations of mutation testing is followed by such testing. Detection of a pathogenic mutation may affect cancer care and survivorship surveillance, and direct predictive testing in at-risk relatives. While all of this may prove challenging to that majority of clinicians who do not work in the field of clinical cancer genetics, even this paradigm is being supplanted by the use of broader and more powerful germline genetic “panels.” Panels are test arrays that can be readily ordered from a handful of clinical genetic testing laboratories and that offer identification of genetic susceptibility to colorectal cancer (CRC)/adenomas, breast cancer, endocrine neoplasia, and more. Other panels are not even limited to specific cancers. An entire body of literature is already developing to help guide the clinician through the range of options now available [1, 2]. The good news about panels is that the clinician needs to know very little about inherited cancer susceptibility in order to arrange testing that may provide a clear understanding of the basis for a patient’s cancer risk. The bad news is that such testing, whether informative or not, typically opens up a host of issues that will likely need to be addressed by a team that does have expertise in the management of inherited cancer risk. Powerful tools carry powerful consequences when properly used and just as powerful consequences when misused.
Familial Adenomatous Polyposis
The first evidence that inherited susceptibility might contribute to the formation of precancerous adenomas and ultimately colorectal cancer involved familial adenomatous polyposis (FAP) . Because of the very distinctive phenotype—the presence of hundreds to thousands of adenomas—the presence of such a disease phenotype clearly characterized the affected individual. As modern concepts of Mendelian inheritance evolved, it became obvious that FAP was an autosomal dominant condition. Until the discovery of the APC gene responsible for FAP, screening consisted of sigmoidoscopy in the children of affected individuals. Upon recognition of polyps, the era of prophylactic surgery began, with the performance of colectomy (with ileo-rectal anastomosis or J-pouch reconstruction) or proctocolectomy with end-ileostomy. Thus, early on there was the potential to prevent malignancy by means of surveillance and early surgical intervention. Unfortunately, about 30% of cases of FAP present as de novo cases, with no obvious antecedent family history. Often such cases presented with symptoms of bleeding, anemia, or obstruction at an early age, and commonly with a delay in diagnosis and presence of advanced disease.
There is no evidence of a unique natural history, prognosis, or patterns of response to chemotherapy or radiation that differ in FAP compared with its sporadic counterparts.
Assuming a given patient is able to benefit from surgery and survives an initial colorectal cancer, he or she remains at risk of cancer of the remaining rectum. Also, the risk of duodenum cancer remains for colorectal cancer survivors and for those undergoing prophylactic colectomy. Tumors that arise in the GI tract following an initial colectomy or proctocolectomy are generally treated in the same fashion as such tumors occurring sporadically. However, an important consideration is the fact that about 10% of FAP patients and families carry a significant risk of intra-abdominal desmoid disease. Commonly, desmoids occur within several years of colectomy and may compromise attempts at further operation for new cancers in the rectum or upper GI tract. Desmoids are almost invariably benign, but their infiltrative pattern commonly causes obstruction of the bowel, ureters, or other vital structures. Their very unpredictable natural history makes prediction of response to intervention rather challenging. Some desmoids occur as single space-occupying masses in an old surgical wound and as such are often removed surgically. Ominously, however, desmoid disease is commonly an infiltrating ill-defined mass in the small bowel mesentery. Operations to resect such desmoids are commonly very bloody, involve sacrifice of small bowel leading to short gut, and may be followed by recurring desmoid in any event.
Despite the unpredictable natural history of desmoid tumors, attempts at medical management have been undertaken. Small and poorly controlled trials have employed agents such as sulindac, the common nonsteroidal anti-inflammatory drug (NSAID), and/or antiestrogen compounds tamoxifen and toremifene. When such agents are ineffective, then more aggressive chemotherapeutic measures may include use of doxorubicin (Adriamycin®). In some cases, a favorable response to chemotherapy can be followed by surgical resection. It has been our experience that even stable, relatively asymptomatic mesenteric desmoid disease can prevent completion of duodenectomy in patients with severe dysplasia or invasive cancer of the duodenum. At our institution, all patients with evidence of intra-abdominal desmoid related to FAP undergo consultation with a medical oncologist having a special expertise in soft-tissue sarcomas.
Depending on the institutional setting, an additional role for the medical oncologist can include oversight of surveillance programs for extraintestinal disease. Having a working knowledge of the tumor spectrum of FAP can be helpful in this regard (Fig. 33.1) [3]. Patients with FAP are at increased risk of thyroid cancer and brain tumors, primarily medulloblastoma. There is not a clear consensus regarding the role of thyroid screening in FAP. The available clinical practice guidelines such as those provided by the National Comprehensive Cancer Network (NCCN) in the USA (www.nccn.org) [4] or the European Society for Medical Oncology (ESMO) do provide some guidance in this area, in addition to providing a broad and at the same time detailed overview of management strategies for FAP (www.esmo.org) [5].
Clinical diagnostic algorithm based on polyp burden. CRC colorectal cancer, MMR mismatch repair, AFAP attenuated familial adenomatous polyposis, MAP MutYH associated polyposis, FAP familial adenomatous polyposis. (Reprinted with permission from Stoffel and Kastrinos [3])
Aside from the immediate oncologic management of the patient with FAP are important issues having to do with predictive testing and coordination of surveillance. Not all patients with colon cancer and multiple polyps have an APC mutation. It is now clear that a similar phenotype can occur in patients with biallelic mutations in the MUTYH gene (so-called MYH-associated polyposis or MAP). This condition is autosomal recessive. Although siblings of affected patients are at 25% risk of being biallelic carriers themselves, and thus warrant surveillance, it is quite rare for a patient with a biallelic MUTYH mutation to have a clinically affected parent. Genetic counseling is thus very important as it has implications for risk to relatives. The benefits of testing are considerably different compared to FAP. Typically, a patient with MAP presents either with a colorectal cancer in the setting of oligopolyposis, or the patient may present with a polyposis phenotype at the time of a baseline screening colonoscopy. Data from Grover et al. have shown a near 90% likelihood of an APC mutation when a patient presents with a thousand or more adenomas. However, in patients with a modest number of adenomas (20–99), the likelihood of either an APC or MUTYH biallelic mutation is in the range of 3–5% [6]. A prior probability of 5% has commonly been taken as a threshold for consideration of mutational testing. Consequently, a patient with 20 or more adenomas, with or without cancer, may be considered an appropriate candidate for APC and MUTYH testing. If a diagnosis of MAP is made, it is now clear that such patients are at risk of upper GI malignancy—though it is not clear that an increased risk of desmoid disease is present in MAP. An ongoing controversy in MAP is the question of cancer risk in mono-allelic carriers. No clear guidelines exist for the screening of siblings and children who are carriers of one mutated allele. A common approach when counseling patients with biallelic MUTYH mutations is to do mutational testing on such a patient’s spouse. If the spouse is free of an MUTYH mutation, then it can safely be concluded that all children will be mono-allelic carriers. A Spanish cohort study described MUTYH biallelic mutations in 7% of patients presenting with 10 or more colon polyps. The most frequent mutations were c.536A>G, p.Y179C and c.1187G>A, p.G396D. The authors went on to propose looking for these common mutations as the first step in their genetic testing strategy. Patients who were heterozygous for one of these mutations subsequently underwent whole-gene sequencing. There were good sensitivity and specificity when using this strategy in a Caucasian Spanish population [7]. Borras et al. proposed extrapolating this testing strategy to other Caucasian populations by including testing for founder mutations adapted for each country in the second step of testing the whole MUTYH gene analysis [8].
Hereditary Nonpolyposis Colon Cancer/“Lynch Syndrome”
Problems of Terminology
In the early twentieth century, the University of Michigan pathologist, Aldred Warthin, reported the case of the now well-known “family G” in which a constellation of early-onset colorectal cancer, uterine cancer, and gastric cancer clustered in excess. These findings remained essentially dormant until the 1960s when Henry Lynch, a medical oncology fellow with medical genetics training, began tracking another Midwest family with somewhat similar features. In addition to revisiting the pedigree of family G, he and his colleagues over the next 20 years developed a registry of families with similar features. Originally termed the “cancer family syndrome,” the clinical features of early-onset colorectal cancer, early-onset endometrial cancer, autosomal dominant transmission, apparently improved survival compared to sporadic counterparts, and a broader tumor spectrum (including ovarian cancer, uroepithelial cancer, and skin tumors) became apparent. In order to avoid confusion with the so-called cancer family syndrome of Li and Fraumeni (now called Li-Fraumeni syndrome and involving mutations in the TP53 tumor suppressor gene), the terminology for the cancer family syndrome of Lynch and Warthin was changed to the term “hereditary nonpolyposis colon cancer” or HNPCC, in order to distinguish it from familial adenomatous polyposis. This HNPCC term is somewhat clumsy and overlong, but more unfortunately, perhaps, it would lead one to believe that colorectal cancer is the only important tumor. For these reasons, Boland recommended the term “Lynch syndrome,” in recognition of the early work of Henry Lynch. Although the term has entered fairly broad acceptance, there are problems here as well. Lynch syndrome has come to be limited to families in which a pathogenic mismatch repair (MMR) variant has been found. The older term HNPCC continues to be commonly used to describe families that clinically appear to more or less have the clinical syndrome, but in which no mutation is detected. This in turn can easily be confused with the so-called familial cancer syndrome “X,” which by definition is a family that meets Amsterdam criteria for HNPCC but in which there is no evidence of microsatellite instability (MSI) in colorectal or other tumors and in which no MMR mutation is detected. To complicate matters further, the term “Lynch-like” has been coined, typically referring to families with MSI tumors but in which no MMR mutation is detected. The proliferation of terms is problematic at best for cognoscenti and likely baffling to the generalist. The reader is asked to indulge the unfortunate terms, until such time as term(s) better reflecting the underlying molecular basis is offered and comes into common parlance. Meanwhile, for purposes of this discussion, we will use the term HNPCC generically, supplemented as needed with clear modifiers.
Early Working Groups
In the 1970s, investigators in Europe interested in FAP gathered together in England for a workshop in the interest of harmonizing data collection among registries that had emerged for its tracking and management. Some of these, such as those in Denmark, were truly national registries, while others were single- or multi-institutional programs. Members of this so-called Leeds Castle Polyposis Group, or LCPG, continued to meet every 2 years and began to formulate guidelines for FAP management. Because of its narrow focus on FAP, investigators interested in HNPCC formed a parallel society termed the International Collaborative Group (ICG) on HNPCC. This group formed in 1990 and met annually. Shortly after the turn of this century, the LCPG and ICG merged and their working group is now called the International Society for Gastrointestinal Hereditary Tumors (InSiGHT). The group continues to meet biannually. Regional groups for the study of FAP, HNPCC, and other newly emerging GI polyposis and nonpolyposis GI cancers have formed in the Americas (Collaborative Group of the Americas on Inherited Colon Cancer or “CGA-ICC”) and in Europe the so-called “Mallorca” group. These working groups can be expected to collaborate in designing future studies.
Molecular Basis for Hereditary Nonpolyposis Colon Cancer: Mismatch Repair Gene Mutations and Microsatellite Instability in Tumors
The major breakthrough in understanding the genetic basis for HNPCC, which has ultimately come to guide many aspects of management, was the discovery of the locus containing the first MMR gene. This was based on a genome-wide search for evidence of linkage between disease expression typical of the HNPCC spectrum and otherwise anonymous genes. This approach was not unlike the basis for establishment of genetic linkage between breast/ovarian cancer and the BRCA genes. Only after linkage to a locus on chromosome 2 and recognition of disease-causing mutations in a gene within that region, the MSH2 gene, was it obvious that the MMR system was the basis for HNPCC. Within a very short time, additional genes within the MMR family were identified: the MLH1, MSH6, and PMS2 genes. Over the past 20 years since these genes were identified, a host of important correlations have been drawn.
Pathology
It had been known for some time that DNA mismatches occur in eukaryotes, with research into mechanisms for the identification and repair of such mismatches ongoing in yeast species. Considerable progress has been made in defining a characteristic pathology for HNPCC tumors. Colorectal cancers that occur due to an underlying MMR mutation are commonly poorly differentiated while at the same time remaining diploid. They are characterized by “tumor infiltrating lymphocytes,” that is, infiltration of the malignant epithelial cells with mature lymphocytes. In addition, a so-called Crohn’s-like reaction occurs, involving peri-tumoral lymphocytic infiltrate. Indeed, astute GI pathologists can have their index of suspicion raised for the possibility of HNPCC simply on the basis of this characteristic pathology. A huge volume of translational laboratory investigation has gradually disclosed the intricate details of the normal and abnormal workings, as well as regulation of the MMR genes [9, 10].
Genotype/Phonotype Correlations
A volume of information from large registries, including population-based registries, has yielded a wealth of information about genotype/phenotype correlations in patients with underlying MMR mutations. In most clinical series, the MLH1 and MSH2 genes are the most frequently mutated genes in HNPCC, with each one accounting for about 40% of all mutation-positive cases. MLH1 is associated with a relatively severe phenotype, with early age of onset being common. MSH2 is also associated with a severe phenotype. In addition, MSH2 generally carries the broadest range of extra-colonic tumors. The MSH6 gene tends to be associated with later age of onset, a higher tendency toward rectal cancer, and a higher risk of endometrial cancer.
PMS2 appears to be the least penetrant MMR gene. It is not uncommon for patients in their 50s, 60s, or older to be found to have a PMS2 mutation, in the relative or complete absence of a family history of malignancy. In fact, rare biallelic mutations in PMS2 have been reported [11,12,13]. The phenotype is quite severe, with cancer onset in the teens or younger. When biallelic mutations are present, there is typically no immunohistochemical expression of PMS2, even in normal tissues, hence the term “constitutional” mismatch repair deficiency or “CMMRD.” Because of the very young appearance of malignancy, occasionally including brain tumors and hematologic malignancies, such patients are commonly encountered by pediatric oncologists. Biallelic mutations in carriers of MLH1 and MSH2 have not been described and are likely lethal in utero.
Population studies have been conducted in which all cases of colorectal cancer are tested for evidence of MSI, either by polymerase chain reaction (PCR)-based assay or by use of immunohistochemistry (IHC). Informative cases are then tested for the presence of an underlying MMR mutation, by means of direct exon sequencing, supplemented by assays for detection of more complex rearrangements, including deletions not detectable with sequencing or alternatively are studied for the presence of somatic methylation of the promoter of the MLH1 gene. These studies have become more robust as more powerful and nuanced technologies have emerged. The most recent studies indicate that underlying MMR mutations account for only about 1–3% of all colorectal cancers [14, 15].
Microsatellite Instability (MSI) and Distinguishing Sporadic MSI from Hereditary Nonpolyposis Colon Cancer
Microsatellite instability (MSI) is the hallmark of HNPCC tumors. As we now know, microsatellites are short repeat sequences of mono-, di-, tri-, and even tetra-nucleotides (e.g., ACACACACACAC) occurring at both coding and noncoding regions widespread in the entire genome. In malignancies caused by underlying MMR gene mutations, there is typically an increase or decrease in the length of these repeat sequences that can easily be detected by gel electrophoresis, consisting of a different, extra band occurring in the tumor compared with normal tissue. The source of normal reference tissue is typically normal mucosa taken at a surgical margin, although we prefer to take an endoscopic biopsy of normal mucosa at a distance from the tumor edge, submitted separately for the purpose of PCR-based assay. In some cases, such as archived tumor material, micro-dissected normal stroma can be used. Of course, peripheral blood or any other normal tissue can be utilized as a reference. In general, MSI is present when at least several different genes with microsatellite-containing regions are mutated. Panels of MSI markers are used, and in most cases all or nearly all such genes are mutated in HNPCC tumors. If there are no changes in the frameshift length of microsatellites, then the tumor is considered microsatellite stable (MSS). If one mutation is found, the tumor is MSI low, and if two or more microsatellite mutations or frameshift length changes are detected, the tumor is MSI high.
Use of such panels provides an easy way to distinguish HNPCC tumors from sporadic cases. HNPCC tumors virtually always show evidence of MSI, whereas sporadic cases do not. This difference is subject to one very important caveat. MSI can be caused not only by the presence of MMR mutations but also by acquired methylation of the MLH1 promoter. The frequency of MSI has been consistently determined in large unselected series of CRC at approximately 12–15% of stage II and III colorectal cancers [16, 17]. If in a given population of CRC, 15% have MSI but only 3% have an MMR mutation, then as much as 80% of all MSI cases will be found to be sporadic. Now most of these cases will be older and will have no significant family history of cancer. But if the clinical strategy at a given institution is to query all CRCs for evidence of MSI (see the “Universal Testing” section of this chapter), then some convenient method for distinguishing likely HNPCC from likely sporadic MSI must be found. Fortunately, there are features that reliably distinguish sporadic microsatellite unstable tumors from true HNPCC tumors. This is the presence, noted earlier, of MLH1 hypermethylation in the sporadic cases. This typically involves methylation of the promoter region of the MLH1 gene. HNPCC tumors virtually never show hypermethylation. At our institution, our routinely used clinical requisition form provides for the performance of methylation assay in the event MSI is detected. A surrogate for hypermethylation involves the presence of BRAF mutations. Virtually, all MSI unstable tumors that are sporadic and that manifest hypermethylation also have evidence of BRAF mutations. Conversely, germline mutation-positive HNPCC cases are virtually always wild type (WT) for somatic BRAF mutations.
An alternative or surrogate measure of MSI involves immunohistochemistry, discussed later. One problem with the reliance on MSI is the recognition that approximately 15% of all colorectal cancers show evidence of microsatellite instability. As mentioned previously, in most population series, about 80% of these tumors are in older patients with no family history. That these are indeed sporadic is demonstrated by the fact that efforts to detect mismatch repair mutations are negative. Population studies in which MSI (or IHC as a surrogate) are done on all colorectal cancers have MMR mutations detected in only about 20% of this 15% of cases that show microsatellite instability, thus yielding the final figure of 2–3% of all tumors are HNPCC.
Role of Immunohistochemistry
A simpler, cheaper, and in most cases a more informative way to evaluate for MSI is to perform IHC staining for expression of each of the MMR-associated proteins. In practice this works like any other IHC. Tumor slides are stained for proteins corresponding to MLH1, MSH2, MSH6, and PMS2 genes. Intact staining for all proteins denotes a microsatellite stable tumor. Loss of staining for one of these proteins indicates loss of expression of the corresponding gene and likelihood of underlying germline mutation. This is especially helpful as patterns of IHC expression can help prioritize and limit expensive germline testing.
There are several important cautions to be made when relying on IHC. First, loss of MLH1 protein may well be an epigenetic change that is somatic in nature. It denotes inactivation of MLH1 due to the same hypermethylation process that accounts for MSI-H tumors. Second, it is important that there be nontumorous positive control cells, typically stromal elements in the tissue section showing loss of MMR expression. Third, staining may be patchy and at least partially retained in true mutation carriers, especially MSH6. Staining may sometimes be retained in MLH1 mutation carriers, suggesting the presence of protein that is immunoreactive but not functional. Finally, because of the functional heterodimers of MLH1 with PMS2 and of MSH2 with MSH6, tumors losing expression of MLH1 will generally have an obligated, concomitant loss of PMS2, and those with MSH2 loss a corresponding MSH6 loss. Notably, population studies such as the Spanish Epicolon study have shown that the correlation between MSI when done by PCR-based assay and MSI as inferred by protein loss by IHC is not perfect. Between 5% and 10% of cases with MSI by PCR will show normal IHC, and a similar proportion with loss of protein expression by IHC will have normal MSI. This is most evident in population-based series such as Epicolon where the overall prior probability for abnormality is low, overall, such that false positives may be more prevalent.
Notwithstanding some of the limitations of IHC, it does appear that reliance on IHC alone has come to dominate the approach to clinically oriented testing. A final, practical note of caution when relying on IHC: If a patient has a high pre-test or “prior” probability of having germline MMR mutation (e.g., young, strong family history, no evidence of polyposis) but normal IHC staining, get a second pathology opinion on the staining and/or be prepared to do PCR-based MSI testing. Likewise, when the clinical picture is compelling but initial PCR-MSI is normal, consider IHC. Alternatively, if the tumor assays are normal in the setting of a compelling clinical picture, go ahead with germline mutation testing for all MMR genes (as well as EPCAM, noted later), but with counseling that stresses a low likelihood of mutation detection in the face of normal MSI/IHC.
Several additional points warrant mentioning. When tumor testing is considered, the assumption is that the tumor is, in fact, an invasive adenocarcinoma. It is possible that benign tumors can be informative when malignant tissue is not available. An example might be a patient who is undergoing clinical colonoscopy screening due to a parent with early-onset colorectal cancer. In such cases, there will commonly be no archival tumor tissue from the affected parent available for testing. The parent may be deceased and thus unavailable for direct germline mutation testing. If our patient undergoing colonoscopy is found to have an adenoma but no invasive malignancy, the question becomes the yield of doing PCR-MSI or IHC on that adenoma tissue. Little attention has been devoted to this issue, but at least one report suggests a reasonable yield, at least for large adenomas and those with severe dysplasia [18].
Clinical Decision-Making About Whom to Test
There are three basic strategies for determining which patient merits testing for a germline MMR mutation.
-
1.
Utilize clinical criteria to maximize likelihood that informative patients have tumor tissue selected for MSI/IHC (e.g., “Bethesda Guidelines”—see next section).
-
2.
Test all CRC and perhaps all endometrial cancers for MSI/IHC (“universal” testing).
-
3.
Instead of relying on tumor testing to select patients for further germline mutation testing, simply use risk prediction models to arrive at an acceptable threshold above which to offer mutational testing (e.g., PREMM1, 2, 6 and related models).
Bethesda Guidelines
In the relatively early days of testing for HNPCC, the primary role for evaluating tumors for evidence of MSI was apparent. In the absence of firm data on the yield of testing all tumors for MSI or testing all patients for germline MMR mutations, an expert panel provided recommended threshold clinical criteria that would warrant MSI/IHC. These are the Bethesda Guidelines (Table 33.1) [19].
The panel specified the mononucleotide and dinucleotide markers considered optimal for PCR-based MSI testing (MLH1, MSH2, MLH6, PMS2). Not considered directly at the time of this report was the potential for using IHC as a surrogate for PCR-MSI. Subject to the cautionary note above, it is likely that IHC would be recommended as a suitable alternative (Table 33.2) [7].
Several reports have suggested a good yield when applying Bethesda guidelines or some simplified modification of them in clinical practice. However, clinical guidelines can often have a high sensitivity for detecting a condition but at the expense of low specificity.
Universal Testing
However positive the yield may be when applying clinical criteria for selecting patients for tumor and/or germline testing, one question that persists is “Are there patients with germline MMR mutations for HNPCC that would be missed with application of clinical selection criteria? If so, how many are there and can they be predicted in any other way?”
Such questions logically led to the performance of several very important population studies. These essentially demonstrate that there are a small proportion of cases with MMR mutations that would not have been identified by Bethesda or even “relaxed” Bethesda-like guidelines. More recent series have suggested that a higher proportion of such cases are found to have MSH6 and PMS2 mutations. This should not be surprising, given the lower penetrance of these genes.
Predictive Models
A major disadvantage of clinical decision-making based on tumor testing is the need for such a tumor and the costs associated with the PCR-MSI or IHC. If suitable selection criteria existed on which to predict mutation likelihood in the absence of tumor testing, these issues disappear. One decision model, PREMM1, 2, 6, was subjected to a further modeling exercise in which it was concluded that germline mutation testing in patients with a >/+ 5% prior probability of mutation would be cost-effective [20]. The PREMM1, 2, 6 model does not use any data from tumor testing (Fig. 33.2) [21] but rather is based on personal or family history of tumors in the spectrum of HNPCC, taking age of onset and number or affected relatives into account.
Clinical diagnostic algorithm for tumor testing. CRC colorectal cancer, IHC immunohistochemistry, MSS microsatellite stable. § PREMM1,2,6 (Prediction of Mismatch Repair Gene Mutations in MLH1, MSH2, and MSH6) score can be calculated at http://premm.dfci.harvard.edu/. Other models (MMRpro, MMRpredict) may also be used with their own specified cut-off scores. ∗BRAF testing: (+), mutation present; (−), mutation absent/wild type. ∗∗Surveillance recommendations based on personal and family history. ‡Gene-specific germline mutational analysis. (Reprinted with permission from Kastrinos and Stoffel [21])
Use of predictive models can be helpful in clinic when counseling otherwise healthy patients having a family history of colorectal and other cancers. The quick bedside calculation of risk can often be reassuringly low and can help dissuade from mutational testing that has a very low yield.
Testing Algorithms and Operational Issues for Genetic Counselors
Whether selective or universal tumor testing, or risk assessment model-based testing is employed, there are reasonably straightforward algorithmic approaches to the workup of colorectal cancer patients for possible HNPCC. The details of the workup are important, but so is the clinical practice model in which the work is done.
The first step is to determine whether to test a given malignancy for MSI at all. Most clinical practice guidelines do favor use of tumor-based testing with either PCR-MSI or IHC. This is increasingly either universal (testing all CRCs) or a simple modification of universal testing (all cases below age, 70, 60, or 50, depending on local resources). In others, more narrow clinical selection criteria may be employed (modifications of Bethesda guidelines). In all such circumstances, it is essential that the clinical unit have procedures in place for routine performance of the testing. This requires clarity regarding the criteria for testing (if not strictly universal), assignment of responsibility for the ordering of testing, and an understanding of the role for genetic counselors (or otherwise suitably trained personnel). This latter point is important. Not all patients whose tumor is tested will necessarily need to see a genetic counselor. The counselor is usually in the best position to review all of the issues that are relevant: age of patient, presence or absence of family history, presence or absence of multiple polyps, and results of MSI/IHC. In the interest of efficiency, this can and should generally be accomplished through a simple review of the medical record. Thus, an older patient with unremarkable family history and either an MSS tumor or MSI-H tumor with hypermethylation or BRAF mutation requires no further consideration for underlying genetic susceptibility.
The less selective the clinical criteria, the greater the likelihood that a given case of MSI will be sporadic, as 80% of all MSI-H cases are sporadic. A simple means of further distinguishing these is thus important. Most institutions that routinely perform MSI/IHC with a low threshold (universal or near-universal testing) do also routinely perform a methylation assay or BRAF mutation assay. Only cases with no methylation and wild-type BRAF are then referred on for genetic counseling in anticipation of mutational testing.
Hereditary Nonpolyposis Colon Cancer and Microsatellite Instability in General Carry a Prognosis and Sensitivity to Chemotherapy That Differs from Microsatellite Stable Tumors
One of the earlier observations in HNPCC was a tendency toward improved survival [10, 22]. This was the case even before any of the MMR genes were discovered. The earliest observation was really before any organized screening efforts had begun that would have improved survival through early diagnosis [23]. Moreover, early diagnosis through screening would have led to an earlier average stage at diagnosis, but even the original reports on survival adjusted for stage at diagnosis.
Post-hoc analyses of large cooperative trials have now consistently shown that patients with microsatellite instability-high (MSI-H) tumors experience better stage for stage survival compared with microsatellite stable tumors [17, 24, 25]. Some of these same trials have demonstrated that within-stage differences in response to 5-fluorouracil (5-FU)-based therapies exist between MSI and MSS tumors [24].
What the post-hoc analyses from these trials have not been able to distinguish is whether there are differences in prognosis or treatment response within the MSI group. Put another way, we do not know whether there are differences between mutation-positive HNPCC patients and their sporadic counterparts, since the trial databases have not reliably distinguished these populations. Patients with colorectal cancer deficient in MMR genes had lower rates of tumor recurrence, longer time to tumor recurrence, and improved survival rates compared to those with proficient MMR genes when treated with 5-fluorouracil [24].
The MOSAIC adjuvant therapy trial (oxaliplatin/fluorouracil/leucovorin) of stage II and III CRC demonstrated that addition of oxaliplatin improved 3-year disease-free survival (DFS) and 6-year overall survival (OS) [26]. An update focusing on 10-year OS/DFS by mismatch repair status and BRAF mutation showed that while BRAF mutation status was not independently predictive of survival benefit, patients with MSI tumors treated with oxaliplatin experienced a favorable OS at a level of p = 0.014 compared with those receiving 5-FU/leucovorin alone [27]. As with other similar trial analyses, the low prevalence of patients with MSI tumors (only 9.4% in this series) limited the power to detect treatment differences.
Genetic Counseling and Testing in At-Risk Relatives
To the clinicians treating existing tumors, the emphasis rightly is placed on the management of that tumor, and our commentaries on scope of surgery decision-making, survival features, and chemotherapy responsiveness have addressed this. We have also addressed survivorship issues having to do with clinical surveillance for new colorectal, other GI, and extraintestinal tumors. However, it is incumbent upon us to reckon with the fact that, for family management purposes, our index CRC case found to have an MMR mutation is simply that: the first case found in that family. Depending on the size and composition of the family, there may be tens to hundreds of individuals potentially at risk of carrying the same pathogenic variant as that harbored by our index case. These identifiable individual relatives will benefit from knowledge of their risk, from predictive genetic testing, and from clinical surveillance essentially identical to that offered as a survivorship program for the index case.
Clinical practice guidelines make it very clear that relatives should be offered genetic counseling and testing. The clinician most familiar with risk assessment, counseling, predictive laboratory testing, and surveillance can and should be involved in these processes. But these processes are very time-consuming and each discussion also can be very time-consuming. As such, the genetic counselor provides invaluable assistance in educating at-risk patients about their risk and the pros and cons of genetic testing. When performed properly, the elements of informed consent to undergo genetic testing call for a necessarily involved discussion [28]. In the USA, there are a host of commercial laboratories to choose from and issues of insurance coverage commonly need to be worked through. For better or worse, genetic counselors become very conversant with these issues.
The notion of starting with a disease-affected patient, testing for and finding a disease-associated mutation, and then moving on to predictive testing of at-risk relatives is termed “cascade” testing. The points referred to earlier that deal with the genetic counseling/testing process for the at-risk relative should make perfect sense, even if somewhat involved and beyond the scope of practice for the individual practitioner. Yet, this process is child’s play compared to the challenges of identifying just who in the family is at risk and communicating the existence of that risk to them.
The standard of care in the USA and other Western countries for risk notification basically consists of the counselor providing the index case with a sense of the need for them to communicate to at-risk relatives the importance of their undergoing counseling/testing. This is commonly reinforced by giving the index case printed materials about the condition in question, that they may pass this along to at-risk relatives. The reality is that this standard, even when met, is generally very ineffective in reaching very many relatives.
A host of barriers exist. Despite the counseling, the index patient may not feel an understanding of the technical information, fearing much will be lost in the translation. Many families suffer from dysfunctional communication patterns, with either the index patient or the at-risk patient being cut off. Even in families that are well educated and communicative, more distant relatives (cousins, etc.) may simply not have been contacted for decades or may not even be known at all.
If it is recognized that the index patient may not be in an ideal position to communicate such critical information to relatives, might there be a role for the provider in doing so? Just as barriers exist for the patient, barriers exist for the provider. The most obvious barrier is the simple fact that our clinical practice models do not really provide for care beyond the index patient, unless one or more relatives simply happen to become our patients themselves. We simply do not have the time or the support structure for doing so as a part of routine clinical service. Some institutions have registries that have the potential for following extended families. But until conditions such as HNPCC become more “mainstream” the resources for such efforts are harder to identify and to rationalize. Any such effort would be conducted under the notion of research, but the issues to be addressed are frankly those of clinical management. Whether considered a research or clinical undertaking, prevalent and otherwise appropriate concerns over confidentiality and privacy carry a chilling effect on even the most well intentioned of undertakings. Another chilling effect is the doctrine of “genetic exceptionalism.” This is the notion, discredited by most in the field but nevertheless prevalent in some circles, that presupposes that genetic information is somehow taboo and not something that can be managed in a routine clinical fashion, not unlike the way in which psychiatric records are sometimes regarded.
In many respects, the gap between the needs of at-risk relatives and our ability to address them suggests the need for reframing the entire conversation in terms of a public-health model. Fortunately, there are models that can be looked to. Suthers, writing on behalf of the clinical genetics service unit for the State of South Australia (Adelaide), in 2008 described an approach in which counselors offered to directly contact at-risk relatives of patients with MMR and BRCA mutations [29]. Index cases simply completed a form listing names and postcodes of at-risk relatives. The clinical services unit then corresponded with relatives, providing form letters summarizing the nature of risk and offering counseling within South Australia or referral to providers in other Australian states. The program was able to approximately double the number of at-risk relatives identified and tested. Very few complaints were lodged over “inflicted insight” or other such issues. Index cases were encouraged to talk to their relatives in whatever fashion was felt best, but such communication was to be considered as “in addition to” rather than “instead of” communication from the genetics services unit. Very few cases existed in which the index case explicitly asked that a relative not be contacted. The basic features of this program continue to the present time (Nicola Pawlowski, personal communication). A very similar program is now operating at a national level in New Zealand (Susan Parry, personal communication). It is true that these units operate as a component of the respective health ministries in these countries, are budgeted as such, and carry the respect and authority that the health ministries otherwise possess. As such, the exact model might not translate precisely to the USA or to other jurisdictions. What the programs in South Australia and New Zealand offer are models for consideration. They show the “art of the possible” and as such pose a challenge to those of us in other countries in which the lack of suitable health-delivery models or lack of will continue to compromise getting service to those in need in a way that works.
Clinical Surveillance and Clinical Practice Guidelines
Let us take the case of two MMR mutation carriers: the index patient who has undergone curative resection with or without further chemotherapy, tested on the basis of MSI status or other clinical features warranting mutational testing, and the case of the at-risk relative found to have the same mutation in the setting of predictive testing triggered by the diagnosis of the index case. Depending on age, both would be considered for essentially the same clinical surveillance. Existing clinical practice guidelines exist from several independent sources, including the National Comprehensive Cancer Network (NCCN) as well as the combined GI societies of the American Gastroenterology Association (AGA), American Society of Gastrointestinal Endoscopy (ASGE), European Society for Medical Oncology (ESMO), the American Society of Clinical Oncology (ASCO), and the American Society for Colorectal Surgery (ASCRS). Although some minor differences do exist, these are remarkably consistent with one another. They provide algorithms for the evaluation of cancer patients for MSI (some recommending universal testing, citing the EGAPP, while others making more generous provision for clinical decision-making). They all endorse predictive testing.
The various clinical practice guidelines provide recommendations for clinical surveillance in survivors and in asymptomatic mutation carriers (sometimes termed “pre-vivors” by advocates). Essentially, all provide levels of evidence for the recommended surveillance strategies. The only surveillance strategy for which support exists on the basis of well-conducted observational trials (no randomized controlled trials exist for surveillance strategy or interval) is that of optical colonoscopy [30]. The usually recommended interval for MLH1 and MSH2 mutation carriers is 1–2 years, beginning at age 20–25. Note that both the recommended ages at initiation and interval provide for a degree of discretion on the part of the provider based on various clinical considerations. In light of the lower penetrance for MSH6 and especially for PMS2, there is a growing tendency toward liberalization of the age at initiation (30–35), but not for longer intervals, as there are insufficient data on differences, by gene, in the pace of the adenoma to carcinoma sequence. The small observational trials that support these approaches are themselves supported by partly retrospective, partly prospective cohort observations from the international Cooperative Family Registry (CFR) and a European cooperative data collection depicting short cancer risks in groups under surveillance more or less according to the above guidelines [31, 32]. The European study, in particular, expresses concern about relatively high rates of interval cancers despite surveillance at these intervals. Yet the numbers of cases are small enough as to likely defy analysis over the issue of colonoscopy quality (prep quality, operator’s adenoma detection rate or ADR, etc.) versus tumor biology (aggressive growth). Such findings certainly invite innovation at the level of surveillance tools (CT colonography, mutation DNA in the stool) and intervals—ideally in randomized trials.
If controversy exists with respect to the best approach to colon neoplasia surveillance, there is much less basis for any recommendations for surveillance beyond the colon. Extra-colonic screening recommendations are predicated on lifetime risks of cancer and clinical prudence, not rigorous observational trials, much less randomized trials.
Upper Gastrointestinal Tract Surveillance
In the absence of any meaningful observational data, the NCCN recommends testing and treating for Helicobacter pylori infection of the stomach, and for periodic upper GI (UGI) endoscopy for those from high-risk geographies and for those with immediate family history of UGI cancer. There is some suggestion that stomach and small bowel cancer risk is appreciable mainly for MSH2 carriers, somewhat or much less so for MLH1, MSH6, and PMS2. Our practice is to offer UGI endoscopy to MSH2 carriers with an effort to reach at least the proximal jejunum, performed at the time of alternate colonoscopy, thus at intervals of about 2–4 years. In non-MSH2 carriers especially, any surveillance of the UGI tract has to be predicated upon individual considerations.
Because the risk of small bowel tumors is increased, the question is raised regarding possible approaches to the jejunum and ileum, beyond the reach of conventional UGI endoscopy. If such assessment were to be done, capsule enteroscopy would be the tool of choice. Indeed, one trial does exist, albeit essentially negative [33].
The risk of pancreatic cancer is at least marginally increased in HNPCC. However, major limitations exist for screening even in those at highest risk, such as use of magnetic resonance pancreatography (MRP) and endoscopic ultrasound in those with Peutz-Jegher syndrome and CDKN2A mutation carriers [34]. Consequently, there can be no recommendation at present for pancreas screening in general for MMR mutation carriers. Exceptions may exist for those with an immediate family history of pancreatic cancer, but even here any decision in favor would have to be entirely empiric, likely in response to major patient anxiety.
Gynecologic Surveillance
The risk of endometrial cancer is second only to that of colorectal cancer in HNPCC. In fact, many HNPCC patients will present with endometrial cancer as their sentinel event. As such, the uterus should be an important target of clinical attention, both for surveillance in at-risk patients and for universal tumor testing in patients with endometrial cancer. Risk of ovarian cancer is also increased in HNPCC, and in general is a much more feared malignancy than is endometrial. The cumulative risk of uterine cancer varies with the specific MMR mutation present and ranges from 15 up to 70% (in patients with MSH6 who have the lowest risk). The cumulative risk of ovarian cancer can be as high as 22% (patients with MSH2 and MSH6 at higher risk) [35]. The most commonly employed tool for screening for both diseases is annual transvaginal ultrasound (TVUS). However, interpretation can be challenging in premenopausal women resulting in poor sensitivity and specificity with this modality.
Surveillance is recommended for endometrial cancer in patients meeting Bethesda criteria or those patients with identified MMR mutations with an annual combined imaging and biopsy approach. The best data on ovarian cancer screening come from trials in BRCA mutation carriers, where the risk of ovarian cancer far exceeds that in HNPCC. Several observational studies have evaluated the impact of screening for ovarian and endometrial cancer in patients with HNPCC and data have been disappointing. Let us take one example: 175 women with HNPCC were enrolled in a screening program. Eleven cases of endometrial cancer were diagnosed through screening with only nine diagnosed/suspected on biopsy and four with suspicious findings on TVUS. Four women were diagnosed with ovarian cancer, none of them through the screening tests [36]. Being enrolled in a screening program should translate to a survival benefit for patients. However, this has not been demonstrated with endometrial cancer and there is a scarcity of studies even evaluating the effectiveness of ovarian cancer screening.
Tissue sampling is, of course, the gold standard. Toward that end we have piloted a so-called “combined screening” program. Women with MMR mutations who are otherwise undergoing periodic colonoscopy were invited to undergo endometrial biopsy while under sedation for the colonoscopy procedure, eliminating the need for a separate visit and procedure, and offering sedation that would otherwise be difficult to rationalize. Our data showed that this was very well received by the women engaged in the program. Biopsy yield data are not yet mature, but early findings suggest a good yield for hyperplasia and atypical hyperplasia. No cancers have yet been detected, but no interval cancers have been observed either [37].
In light of the limitations of both endometrial and ovarian cancer surveillance, an obvious question is the role of prophylactic total abdominal hysterectomy/bilateral salpingo-oophorectomy (TAH/BSO). Since the risk of primary peritoneal carcinoma is not the concern in HNPCC that it is in HBOC, outcome data on our institutional series of women undergoing TAH/BSO showed no risk of postoperative endometrial or ovarian cancer. Current recommendations are for women who are carriers of MMR mutations to undergo TAH/BSO once they have completed childbearing.
Polyposis Syndromes Other than Familial Adenomatous Polyposis
Peutz-Jegher Syndrome
Peutz-Jegher syndrome (PJS) is inherited as an autosomal dominant disorder. It is much rarer than FAP and should never be confused with FAP. It is caused by the STK11 gene and the great majority of patients will be found to have a pathogenic variant in this gene. The most characteristic distinguishing clinical feature is the presence of small pigmented freckling of the lips and buccal mucosa or fingers. Since these are fairly subtle in most cases and cause no symptoms, they can commonly be overlooked. These patients are at risk for cancers of the breast, pancreas, colon, small intestine, and stomach.
The most common presenting symptoms generally do involve the GI tract. A typical presentation will be abdominal pain due to obstruction in the teenage years or younger, commonly due to intussusception of the small bowel related to the presence of a large polyp. These polyps, hamartomas, are the other characteristic defining feature of PJS along with the noted freckling. These hamartomas may at times be difficult to distinguish from juvenile polyps (see “Juvenile Polyposis” in next section), but the pathology characteristically involves smooth muscle strands extending in finger-like projections interdigitated between exuberant glands. The glandular epithelium itself may show areas of cystic dilation akin to those seen in juvenile polyps and, like juvenile polyps, are nondysplastic. However, foci of dysplasia may emerge and form the basis for risk of adenocarcinoma in any part of the colon, small bowel, or stomach. The polyps can involve any part of the GI tract. Considerable variability can exist between members of the same family with respect to the severity of polyp involvement and the area of the gut involved.
Peutz-Jegher hamartomas typically have a very long stalk. This makes even very large polyps fairly easy to remove endoscopically. We generally do not undertake to aggressively remove small polyps unless they are few in number, preferring to focus on larger polyps that have formed a stalk. This is a reasonable approach both in the colon and small bowel.
Of particular importance to the oncologist is the risk of extraintestinal malignancy, most notably involving the breast, pancreas, and reproductive organs. While most PJS patients will be followed by endoscopists concerned with the GI polyps, as described earlier, the care of such patients really requires a multidisciplinary approach and it may fall to the oncologist to coordinate such care.
Surveillance guidelines in PJS do exist and are rather draconian (Table 33.3) [35, 38]. Surveillance is ideally overseen by clinicians in a high genetic-risk breast and gynecology clinic, and typically involves aggressive breast and pelvic imaging. No recommendations exist for mastectomy. However, consideration may be given to oophorectomy based on considerations similar to those for women with BRCA mutations—namely, increased risk of cancer with high mortality and suboptimal measures for early detection.
Surveillance for pancreatic cancer poses special challenges. Patients with PJS carry a lifetime risk of pancreatic cancer that may be as high as 20% [39]. The notion of prophylactic pancreatectomy raises the extraordinary issue of surgical risk and postoperative diabetes and exocrine pancreatic insufficiency. Historically, measures for early pancreatic cancer detection have been entirely unsatisfactory. Recent improvements in imaging, involving magnetic resonance targeting the pancreas, complemented by endoscopic ultrasound have shown some promise [34].
In summary, the key principles of management of PJS include regular endoscopic surveillance augmented by a multidisciplinary approach to surveillance of extraintestinal organs at risk, in the interest of early cancer detection and prevention. We are not aware of any data suggesting that the natural history or management of locoregional or advanced malignancy in PJS tumors differs appreciably from sporadic counterparts.
Juvenile Polyposis
Juvenile polyps may occur sporadically in infants, children, and adults. Considerable histologic overlap exists between juvenile and inflammatory polyps, with the main feature being prominent cystic dilation of nondysplastic but exuberant glands. When sufficiently numerous, extending beyond early childhood, or particularly when associated with any family history of similar involvement, the presence of juvenile polyposis syndrome (JPS) should be suspected. JPS is most commonly caused by pathologic mutations in the SMAD4 gene. Less commonly, mutations in the BMPR1A gene cause a nearly identical clinical picture. It is likely that other genes yet to be identified can also cause JPS. As with Peutz-Jegher syndrome, marked variation in severity (age at onset, polyp count) may exist within the same family. As with PJS, the polyps themselves are nondysplastic, but foci of dysplasia with attendant cancer risk can occur. The tendency toward dysplasia and cancer in polyps seems more typical in some families than others.
Although polyps may involve the small bowel, risk of intussusception appears much lower than in PJS. Several significant clinical features have emerged in recent years as particular sources of concern to those managing patients with JPS. In some patients, the number, size, and confluence of juvenile polyps of the stomach are associated with refractory anemia. In such cases, prophylactic gastrectomy may be required. While it is not clear that gastric cancer risk is limited to such cases of severe gastric polyposis, the difficulty in aggressively sampling polyps already causing problems of anemia makes it easier to arrive at a decision in favor of prophylactic gastrectomy.
Another clinical complication in some families with JPS is the concomitant presence of hereditary hemorrhagic telangiectasia. The Cleveland Clinic group has written an excellent review of the association and the surveillance and management measures to be undertaken [35, 40].
Hereditary Gastric Cancer
Gastric cancer is more common worldwide than in North America and is associated with several environmental risk factors, the most recognized being Helicobacter pylori infection. Familial clustering of gastric cancer is seen in 10% of cases in the general population where gastric cancer in a first-degree relative confers a two- to threefold risk to an individual [41]. Up to 3% of familial gastric cancer occurs in the setting of hereditary diffuse gastric cancer (HDGC) [42]. HDGC is associated with a mutation in the CDH1 gene that codes for E-cadherin, a protein responsible for cell-to-cell adhesion and epithelial integrity. The mutation detection rate can be up to 50% when multiple family members have diffuse gastric cancer under the age of 50 [43]. Mutation in the CDH1 gene confers a cumulative risk of gastric cancer of 80% by age 80 with a mean age of diagnosis at age 40 [44]. Women with a CDH1 mutation are uniquely at risk for lobular breast cancer with a 60% lifetime risk [44]. Clinical criteria for testing individuals for CDH1 germline mutations are described [44].
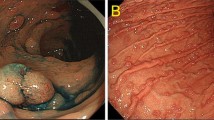
The only way to eliminate risk of gastric cancer among patients with a CDH1 mutation is prophylactic total gastrectomy. The timing of referral to a surgeon is individualized to each particular patient. Grossly normal gastrectomy specimens will often show microscopic foci of signet ring cells on histopathology. Patients may opt for annual surveillance endoscopy while they are considering gastrectomy. Upper endoscopy should be performed by an experienced gastroenterologist with sufficient time taken to examine all segments of the gastric body and antrum. In our experience, greater than 50 biopsy specimens should be obtained from different segments of the stomach with special attention paid to any mucosal abnormalities. Even perfectly normal exams can reveal signet ring adenocarcinoma on histopathology prompting referral for surgery (Figs. 33.3, 33.4, 33.5, 33.6, 33.7, and 33.8).
Apart from HDFC, there is also an increased risk for gastric cancer—both diffuse and intestinal types—in many other hereditary cancer syndromes including HNPCC, FAP, PJS, Li-Fraumeni, and Cowden syndrome (Table 33.4). Patients affected by these syndromes who are living in areas of high incidence of gastric cancer carry a greater risk, suggesting the possible influence of environmental factors [42].
Hereditary Pancreatic Cancer
As in gastric cancer, the majority of pancreatic cancer cases is sporadic, with 5–10% related to either familial clustering, inherited risk for pancreatitis predisposing one to cancer, or in the setting of a hereditary cancer syndrome. Individuals with two to three relatives with pancreatic cancer (one of whom is a first-degree relative) or two first-degree relatives with pancreatic cancer should undergo screening themselves [34]. There is no consensus on the appropriate age to start screening. However, it is suggested to begin at age 40 or at 10 years younger than the youngest affected relative. Pancreatic adenocarcinoma is associated with many hereditary cancer syndromes with PJS conferring the greatest lifetime risk (36%), familial atypical multiple mole melanoma syndrome (16%), and HNPCC (9%) [45,46,47]. Familial breast and ovarian cancer are associated with germline mutations of BRCA1 and BRCA2 and are at risk for pancreatico-biliary and gastric cancer. Specifically, BRCA2 carriers carry a higher risk for pancreatic cancer (up to tenfold) compared to BRCA1 carriers (up to fourfold) [48]. Patients with hereditary pancreatitis have a PRSS1 mutation that predisposes them to early-onset and chronic pancreatitis as well as a significant lifetime risk of up to 50% by age 75 [49].
While the importance of screening for early detection of pancreatic cancer is recognized, there is no single ideal screening method. Annual endoscopic ultrasound or MRI with magnetic resonance cholangiopancreatography (MRCP) is commonly used. While endoscopic ultrasound is operator dependent, it has demonstrated a higher diagnostic yield in some studies [48]. While abdominal CT scans can be used, the sensitivity of this modality is lower than the others and there can be cumulative exposure to radiation when used in a screening program [50]. Patients should be encouraged to eliminate modifiable risk factors for pancreatic cancer including tobacco use and follow a low-fat diet [35].
References
Hall MJ, Forman AD, Pilarski R, Wiesner G, Giri VN. Gene panel testing for inherited cancer risk. J Natl Compr Cancer Netw. 2014;12(9):1339–46.
Vockley JG, Niederhuber JE. Diagnosis and treatment of cancer using genomics. BMJ. 2015;350:h1832.
Stoffel EM, Kastrinos F. Familial colorectal cancer, beyond Lynch syndrome. Clin Gastroenterol Hepatol. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review]. 2014;12(7):1059–68.
Network NCC. Nccn guidelines®. [May 18, 2018]; Available from: https://www.nccn.org/professionals/physician_gls/default.aspx.
ESMO. ESMO clinical practice guidelines. European Society for Medical Oncology; [May 18, 2018]; Available from: http://www.esmo.org/Guidelines.
Grover S, Kastrinos F, Steyerberg EW, Cook EF, Dewanwala A, Burbidge LA, et al. Prevalence and phenotypes of APC and MUTYH mutations in patients with multiple colorectal adenomas. JAMA. 2012;308(5):485–92.
Guarinos C, Juarez M, Egoavil C, Rodriguez-Soler M, Perez-Carbonell L, Salas R, et al. Prevalence and characteristics of MUTYH-associated polyposis in patients with multiple adenomatous and serrated polyps. Clin Cancer Res. 2014;20(5):1158–68.
Borras E, Taggart MW, Lynch PM, Vilar E. Establishing a diagnostic road map for MUTYH-associated polyposis. Clin Cancer Res. 2014;20(5):1061–3.
Greenson JK, Huang SC, Herron C, Moreno V, Bonner JD, Tomsho LP, et al. Pathologic predictors of microsatellite instability in colorectal cancer. Am J Surg Pathol. 2009;33(1):126–33.
Vilar E, Gruber SB. Microsatellite instability in colorectal cancer-the stable evidence. Nat Rev Clin Oncol. 2010;7(3):153–62.
Bodo S, Colas C, Buhard O, Collura A, Tinat J, Lavoine N, et al. Diagnosis of constitutional mismatch repair-deficiency syndrome based on microsatellite instability and lymphocyte tolerance to methylating agents. Gastroenterology. 2015;149(4):1017–29 e1013.
Mork ME, Borras E, Taggart MW, Cuddy A, Bannon SA, You YN, et al. Identification of a novel PMS2 alteration c.505C>G (R169G) in trans with a PMS2 pathogenic mutation in a patient with constitutional mismatch repair deficiency. Familial Cancer. 2016;15(4):587–91.
Wimmer K, Kratz CP, Vasen HF, Caron O, Colas C, Entz-Werle N, et al. Diagnostic criteria for constitutional mismatch repair deficiency syndrome: suggestions of the European consortium ‘care for CMMRD’ (C4CMMRD). J Med Genet. 2014;51(6):355–65.
Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, et al. Screening for the lynch syndrome (hereditary nonpolyposis colorectal cancer). N Engl J Med. 2005;352(18):1851–60.
Pinol V, Castells A, Andreu M, Castellvi-Bel S, Alenda C, Llor X, et al. Accuracy of revised Bethesda guidelines, microsatellite instability, and immunohistochemistry for the identification of patients with hereditary nonpolyposis colorectal cancer. JAMA. 2005;293(16):1986–94.
Buchanan DD, Clendenning M, Rosty C, Eriksen SV, Walsh MD, Walters RJ, et al. Tumour testing to identify lynch syndrome in two australian colorectal cancer cohorts. J Gastroenterol Hepatol. 2016.
Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23(3):609–18.
Yurgelun MB, Goel A, Hornick JL, Sen A, Turgeon DK, MTt R, et al. Microsatellite instability and DNA mismatch repair protein deficiency in lynch syndrome colorectal polyps. Cancer Prev Res (Phila). 2012;5(4):574–82.
Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Ruschoff J, et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96(4):261–8.
Dinh TA, Rosner BI, Atwood JC, Boland CR, Syngal S, Vasen HF, et al. Health benefits and cost-effectiveness of primary genetic screening for Lynch syndrome in the general population. Cancer Prev Res (Phila). 2011;4(1):9–22.
Kastrinos F, Stoffel EM. History, genetics, and strategies for cancer prevention in Lynch syndrome. Clin Gastroenterol Hepatol. [Historical Article Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t Review]. 2014;12(5):715–27; quiz e741–713.
Gryfe R, Kim H, Hsieh ET, Aronson MD, Holowaty EJ, Bull SB, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med. 2000;342(2):69–77.
Lynch HT, Kimberling W, Albano WA, Lynch JF, Biscone K, Schuelke GS, et al. Hereditary nonpolyposis colorectal cancer (Lynch syndromes I and II). I. Clinical description of resource. Cancer. 1985;56(4):934–8.
Sinicrope FA, Foster NR, Thibodeau SN, Marsoni S, Monges G, Labianca R, et al. DNA mismatch repair status and colon cancer recurrence and survival in clinical trials of 5-fluorouracil-based adjuvant therapy. J Natl Cancer Inst. 2011;103(11):863–75.
Sargent DJ, Marsoni S, Monges G, Thibodeau SN, Labianca R, Hamilton SR, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28(20):3219–26.
Andre T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the mosaic trial. J Clin Oncol. 2009;27(19):3109–16.
Andre T, de Gramont A, Vernerey D, Chibaudel B, Bonnetain F, Tijeras-Raballand A, et al. Adjuvant fluorouracil, leucovorin, and oxaliplatin in stage II to III colon cancer: updated 10-year survival and outcomes according to BRAF mutation and mismatch repair status of the mosaic study. J Clin Oncol. 2015;33(35):4176–87.
Robson ME, Storm CD, Weitzel J, Wollins DS, Offit K, American Society of Clinical O. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2010;28(5):893–901.
Ratnayake P, Wakefield CE, Meiser B, Suthers G, Price MA, Duffy J, et al. An exploration of the communication preferences regarding genetic testing in individuals from families with identified breast/ovarian cancer mutations. Familial Cancer. 2011;10(1):97–105.
Dove-Edwin I, Sasieni P, Adams J, Thomas HJ. Prevention of colorectal cancer by colonoscopic surveillance in individuals with a family history of colorectal cancer: 16 year, prospective, follow-up study. BMJ. 2005;331(7524):1047.
Choi YH, Briollais L, Win AK, Hopper J, Buchanan D, Jenkins M, et al. Modeling of successive cancer risks in Lynch syndrome families in the presence of competing risks using copulas. Biometrics. 2016.
Moller P, Seppala T, Bernstein I, Holinski-Feder E, Sala P, Evans DG, et al. Cancer incidence and survival in Lynch syndrome patients receiving colonoscopic and gynaecological surveillance: first report from the prospective Lynch syndrome database. Gut. 2015.
Haanstra JF, Al-Toma A, Dekker E, Vanhoutvin SA, Nagengast FM, Mathus-Vliegen EM, et al. Prevalence of small-bowel neoplasia in Lynch syndrome assessed by video capsule endoscopy. Gut. 2015;64(10):1578–83.
Canto MI, Harinck F, Hruban RH, Offerhaus GJ, Poley JW, Kamel I, et al. International cancer of the pancreas screening (caps) consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut. 2013;62(3):339–47.
Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW, et al. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015;110(2):223–62; quiz 263.
Renkonen-Sinisalo L, Butzow R, Leminen A, Lehtovirta P, Mecklin JP, Jarvinen HJ. Surveillance for endometrial cancer in hereditary nonpolyposis colorectal cancer syndrome. Int J Cancer. 2007;120(4):821–4.
Nebgen DR, Lu KH, Rimes S, Keeler E, Broaddus R, Munsell MF, et al. Combined colonoscopy and endometrial biopsy cancer screening results in women with Lynch syndrome. Gynecol Oncol. 2014;135(1):85–9.
Beggs AD, Latchford AR, Vasen HF, Moslein G, Alonso A, Aretz S, et al. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut. 2010;59(7):975–86.
Korsse SE, Harinck F, van Lier MG, Biermann K, Offerhaus GJ, Krak N, et al. Pancreatic cancer risk in Peutz-Jeghers syndrome patients: a large cohort study and implications for surveillance. J Med Genet. 2013;50(1):59–64.
Aytac E, Sulu B, Heald B, O’Malley M, LaGuardia L, Remzi FH, et al. Genotype-defined cancer risk in juvenile polyposis syndrome. Br J Surg. 2015;102(1):114–8.
Goldgar DE, Easton DF, Cannon-Albright LA, Skolnick MH. Systematic population-based assessment of cancer risk in first-degree relatives of cancer probands. J Natl Cancer Inst. 1994;86(21):1600–8.
Vogelaar IP, van der Post RS, Bisseling TM, van Krieken JH, Ligtenberg MJ, Hoogerbrugge N. Familial gastric cancer: detection of a hereditary cause helps to understand its etiology. Hered Cancer Clin Pract. 2012;10(1):18.
Oliveira C, Senz J, Kaurah P, Pinheiro H, Sanges R, Haegert A, et al. Germline CDH1 deletions in hereditary diffuse gastric cancer families. Hum Mol Genet. 2009;18(9):1545–55.
Fitzgerald RC, Hardwick R, Huntsman D, Carneiro F, Guilford P, Blair V, et al. Hereditary diffuse gastric cancer: updated consensus guidelines for clinical management and directions for future research. J Med Genet. 2010;47(7):436–44.
de Snoo FA, Bishop DT, Bergman W, van Leeuwen I, van der Drift C, van Nieuwpoort FA, et al. Increased risk of cancer other than melanoma in CDKN2A founder mutation (p16-Leiden)-positive melanoma families. Clin Cancer Res. 2008;14(21):7151–7.
Giardiello FM, Brensinger JD, Tersmette AC, Goodman SN, Petersen GM, Booker SV, et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology. 2000;119(6):1447–53.
Kastrinos F, Mukherjee B, Tayob N, Wang F, Sparr J, Raymond VM, et al. Risk of pancreatic cancer in families with Lynch syndrome. JAMA. 2009;302(16):1790–5.
Chang MC, Wong JM, Chang YT. Screening and early detection of pancreatic cancer in high risk population. World J Gastroenterol. 2014;20(9):2358–64.
Lowenfels AB, Maisonneuve P, DiMagno EP, Elitsur Y, Gates LK Jr, Perrault J, et al. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J Natl Cancer Inst. 1997;89(6):442–6.
Canto MI, Hruban RH, Fishman EK, Kamel IR, Schulick R, Zhang Z, et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology. 2012;142(4):796–804; quiz e714–795.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Thirumurthi, S., Vilar, E., Lynch, P.J. (2019). Hereditary Gastrointestinal Cancers. In: Yalcin, S., Philip, P. (eds) Textbook of Gastrointestinal Oncology. Springer, Cham. https://doi.org/10.1007/978-3-030-18890-0_33
Download citation
DOI: https://doi.org/10.1007/978-3-030-18890-0_33
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-18888-7
Online ISBN: 978-3-030-18890-0
eBook Packages: MedicineMedicine (R0)