Abstract
Integrated pest management (IPM) is an internationally recognized approach to pest and disease control. IPM embraces diversity, is knowledge intensive, and varies by crop, scale, and geographical location. All farmers practice IPM to some degree, including the cultural control techniques that underpin all good farming practices. In reality, most farming practice is neither IPM nor non-IPM, but can be defined at a point along the so-called IPM continuum from chemically intensive systems to bio-intensive systems. IPM was initially conceptualized to reduce dependence on pesticides and their effects on the environment. It has been built into virus control strategies from the beginning of plant virology because of the known in vivo insensitivity of viruses to chemical agents. Several methodologies are available for implementing IPM for Bemisia tabaci populations: chemical control with selective insecticides, biological control, crop plant resistance, and physical/mechanical methods. Insecticides, by their poisonous nature, are often harmful to natural enemies and therefore are disruptive to overall pest management. However, the more modern materials that are effective for B. tabaci control are relatively specific to the target pests and therefore less harmful to natural enemies and the environment; consequently, they are also more suitable for integrative combination with other methods. Conventional IPM technologies, such as intercropping, will yield mixed results with little, if any, beneficial impact on pest population in crops. This chapter reviews the known measures used for reducing populations of B. tabaci, advocating the view that only a comprehensive approach incorporating IPM programs will offer effective and sustainable strategies for managing whiteflies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Integrated pest management (IPM) is the comprehensive and coordinated use of cultural, biological, and chemical tactics to reduce a pest population below an acceptable threshold. Cultural IPM practices include nonchemical tactics, host plant resistance, planting dates, cover crops, traps, scouting, crop rotation, and sanitation. Biological IPM practices include natural enemy conservation and enhancement, whereas chemical IPM practices include pesticide selection and spray timing.
IPM is a systems-based approach designed to reduce environmental, health, and economic risks. IPM is implemented as an ongoing series of science-based pest management evaluations, decisions, and interventions. IPM practitioners use knowledge of pest biology and environmental conditions and technology to prevent, avoid, monitor, and suppress pests. IPM practices may be basic or advanced. Basic IPM practices include scouting or sampling crops for pests and pest damage (visually or with devices), monitoring weather and other conditions, and acting when pests approach economically damaging levels. Advanced IPM practices include planting pest-resistant crop varieties, rotating crops, adjusting planting times, using reduced-risk pesticides, implementing mating disruption, planting companion crops, and incorporating beneficial insects.
Some IPM practices, such as organic soil amendments with poultry refuse, mustard oil-cake, neem oil-cake, cow dung, vermicompost, and Trichoderma harzianum, can significantly reduce plant parasitic nematodes and increase or induce the growth of various beneficial fungal- and bacterial-feeding nematodes. Neither the cropping pattern nor the crop production region (e.g., Jessore and Sirajganj) influences the effects of IPM and non-IPM systems.
2 Tools for IPM in Greenhouse Production
In greenhouse production, IPM tools may include trap crops, indicator plants, and banker plants. Trap crops are most often used for insect pest control, such as perimeter trap cropping in field vegetables and trap crops interspersed in greenhouse ornamentals, with characteristics that are more attractive to pests than are crops. Because of the inability to cure plant viral diseases and the need to protect the environment from toxic pesticides, alternative indirect strategies of disease control are required. In recent decades, virologists have developed non-pesticidal, cultural control practices aimed at reducing the damage caused by these viral diseases by interrupting their epidemiological cycle. One of the most important crop improvements has been the enhancement of tolerance to biotic stresses. The identification and use of resistance sources in plant breeding programs have resulted in substantial gains in crop productivity.
Despite the ongoing efforts, India’s productivity for major crops is far below the global average, largely due to persisting problems of pests and diseases. Some IPM interventions have been used as novel strategies for overcoming the pests and diseases in India, including the following:
-
Seed treatment with chemical pesticides to avoid sucking pest attacks
-
Intercropping with legumes to augment natural enemy populations
-
Trap cropping to reduce damage to the main crop from important pests
-
Bird perches for alighting insectivorous birds to predate on insects
-
Pheromone traps for monitoring or mass trapping of moths
-
Scouting to monitor the status of pests and beneficial organisms at regular intervals
-
Augmenting biocontrol agents, such as Trichogramma/Chrysoperla
-
Spraying biopesticides, such as Helicoverpa armigera Nuclear Polyhedrosis Virus (Ha NPV) and neem seed kernel extract
-
Topping the cotton plants at the time of high oviposition with Helicoverpa
-
Periodic removal and destruction of dropped squares, dried flowers, premature bolls, and infested shoots
-
Yellow sticky traps and light traps to control sucking pests such as whiteflies, jassids, and aphids
-
Manipulation of wavelength-dependent behavior of insects to impede insects and restrict epidemics of insect-borne viruses
Using conventional practices and recent innovation techniques, scientists have designed some IPM strategies especially for tomato growers to promote and ensure the use of transplants to reduce herbicides and conserve water. In addition, the use of disease-resistant varieties can eliminate pesticide usage. Other IPM practices can reduce synthetic insecticide usage, including disease-free seeds, disease- and pest-resistant varieties, biological control (parasitic wasps), mating confusion (sex pheromones), biological pesticides, forecasting systems (TOM-CAST), risk assessment (GIS/GPS), the judicious use of synthetic pesticides, conservation tillage (which reduces fuel, dust, emission, water runoff, and soil erosion), 2–3 years of crop rotation to minimize diseases, cover cropping to improve soil texture, and habitat management, such as replanting ditches with native vegetation and preservation of wetlands.
3 Insect–Plant Communication: Visual Cues
The long evolutionary associations between insects and plants have led to mechanisms that enable insects to detect and select their preferred hosts for feeding and oviposition. Vision (color, shape, size) and olfaction (host odor) are the primary cues used by insects to orient to their plant hosts; sometimes, the two types of cues are complementary (Prokopy and Owens 1983; Dobson 1994; Terry 1997). Cues for detecting hosts may be general for polyphagous species or very specific for those that are monophagous. Once a potential host is contacted, then odor, tactile, and gustatory cues may predominate (Terry 1997).
The behavioral response of insects to colored surfaces or colored lights has been referred to as color sensation or spectral sensitivity. The first term describes a phenomenon that is governed by physical stimuli, sensorial receptors, and an integrative system. The second term refers to sensory cells or sensory organs. Visual cells may be sensitive to all wavelengths, but it is the integration of the sensorial inputs to the central nervous system that results in the specific phototactic response of a given insect species (Vaishampayan et al. 1975a).
Color and color contrasts are used by insects to distinguish between a host and the surrounding environment. From a biological perspective, there are three main parameters of color (Vaishampayan et al. 1975a; Terry 1997):
-
1.
The hue or dominant wavelength remitted by the surface (λ max).
-
2.
The color saturation or purity of the hue. For example, adding white to yellow causes a significant increase in the blue-violet region.
-
3.
Brightness (light intensity) refers to the overall reflection. Intensity affects the response when associated with a peak of a dominant wavelength.
4 Phototactic Action Spectrum for Whiteflies and Aphids
Mound (1962) suggested that the whitefly Bemisia tabaci (Gennadius) is attracted by two groups of wavelengths of transmitted light: the blue/ultraviolet and the yellow. He related the reaction to ultraviolet with the induction of migratory behavior, whereas yellow radiation induces vegetative behavior that may be a part of the host selection mechanism. It has also been found that B. tabaci has no detectable olfactory reactions. A close agreement was found between the phototactic action spectrum of the greenhouse whitefly (Trialeurodes vaporariorum (Westw.)) and the transmission spectrum of the leaf in the mid-visible wavelength (550 nm) (Macdowall 1972). Vaishampayan et al. (1975a) observed a strong positive response of T. vaporariorum to surfaces with maximum reflectance or transmittance in the yellow-green region (520–610 nm) and a moderately positive response to ultraviolet (360–380 nm). Light in the blue-violet region seemed to inhibit the response, and red (610–700 nm) may also be moderately inhibitory. Based on these findings, he suggested that the first steps in host selection, orientation, and landing of T. vaporariorum are mediated largely, if not exclusively, by a response to reflected yellow light (520–610 nm; Vaishampayan et al. 1975b).
Coombe (1982) reported that adults took off more readily and walked faster under light of 400 nm than under 500 nm. He confirmed Mound’s hypothesis that the two types of radiation are complementary, thus eliciting a balance between migratory behavior induced by ultraviolet (UV) and a landing reaction controlled by sensitivity to yellow (Mound 1962). In flying aphids, it has been suggested that the primary function of color vision lies in distinguishing plants from sky. Moericke (1955) suggested that the sensitivity of aphids to color may be related to the host range for any given species.
5 Control of Insect Vectors by Altering Their Vision Behavior
Insects communicate with their environment and host plants by light signals that elicit photoreceptors in their compound eyes. The vision behavior of insects is linked to the sequence that begins with their orientation to the plant from a distance and ends with their establishment on plants for feeding and oviposition. By interfering with different links along this pathway, contact between the vector and the plant may be prevented and, therefore, virus spread will be decreased.
6 Attracting Insects Using Color
6.1 The Use of Colored Soil Mulches to Control Whitefly-Borne Viruses
The ability of mulches to attract or to repel insects can be very important in protecting plants from virus diseases. The attraction of whiteflies to yellow was utilized successfully to protect cucumber and tomato crops from infection with the whitefly-borne viruses cucumber vein yellowing virus and tomato yellow leaf curl virus (TYLCV), respectively. These viruses were controlled by soil mulches of saw dust, straw, or yellow polythene film (Nitzany et al. 1964; Cohen and Melamed-Madjar 1978; Cohen and Berlinger 1986; Cohen and Antignus 1994). Polyethylene sheets were the most effective of these materials in reducing the incidence of TYLCV (Cohen 1982). It was suggested that this protection mechanism is associated with the preferential attraction of B. tabaci to yellow, leading to subsequent death of the insect caused by the reflected heat (Cohen 1982). No protection from TYLCV occurred when a tomato field was surrounded by a strip of yellow sticky polyethylene erected vertically 70 cm above ground level (Cohen 1982). Csizinszky et al. (1995) reported that tomato plants grown on orange plastic mulch and exposed to whitefly-transmitted tomato mottle virus performed better in terms of delayed virus symptoms and yield of marketable fruit than those grown conventionally with white or black mulches.
Grass-feeding thrips show little preference for any wavelength, whereas all anthophilous thrips are attracted to colors that match those of flowers—that is, low-UV, white, blue, and yellow, whereas a few are attracted to green, red, and black. Matteson and Terry (1992) found that the degree of the color’s attractiveness to the western flower thrips Frankliniella occidentalis (Pergande) corresponded to the brightness in the blue wavelength.
Because of economics, availability, limited capacity, and a lack of reliable information, synthetic pesticides have not been used extensively in small-scale cultivation worldwide. However, the limited published information available, together with analogous experience in other crops, suggests that the cost-benefit ratio of controlling pests and diseases using inorganic pesticides is favorable in some circumstances if highly standardized timing, dosing, and targeting are applied as a part of an IPM strategy. In particular, newer, more selective molecules, such as imidacloprod, which are applied as a spray or seed dressing, can be very effective at controlling sucking pests (and some disease vectors), with older molecules (principally pyrethroids) to control chewing and boring pests and low-cost, old molecules for fungal disease control. Non-target impacts on natural enemies and resistance management are important considerations in any successful regimen.
7 Bemisia tabaci: Whitefly
The whitefly Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) is a pest in many agricultural systems, including various vegetable, ornamental, and field crops (Byrne and Bellows 1991; Oliveira et al. 2001; Stansly and Naranjo 2010). It directly damages plants by feeding on phloem sap and excreting honeydew on the leaves and fruit. The sticky, sugary surface forms a substrate for the growth of black, sooty mold fungi that stains the crop and covers the leaves, thus preventing proper photosynthesis. The resulting stickiness and discoloration greatly reduce the value of agricultural crops such as ornamentals, vegetables, and cotton. In cotton, the honeydew may cause fiber stickiness that interferes with the spinning process in textile mills and greatly reduces the product’s value (Hequet et al. 2007).
B. tabaci is a vector of several important families of plant viruses (Jones 2003; Hogenhout et al. 2008). In some crops (e.g., tomatoes and cassava), the resulting viral diseases are limiting-growth factors and may cause total crop loss. Most of the important virus diseases transmitted by B. tabaci belong to the geminivirus group (Family: Geminiviridae).
B. tabaci is known for its genetic diversity, which is expressed in a complex of biotypes (Brown et al. 1995a, b; Perring 2001; De Barro et al. 2005) or, as recently suggested, a complex of separate species (Xu et al. 2010; De Barro et al. 2011). The biotypes are largely differentiated based on biochemical or molecular polymorphisms. They differ in characteristics such as host plant range, the capacity to cause plant disorders, attraction of natural enemies, expression of resistance, and plant virus-transmission capabilities (Bedford et al. 1994; Brown et al. 1995a, b; Sánchez-Campos et al. 1999; Perring 2001; Horowitz et al. 2005). Reports have suggested that the floral composition of bacterial symbionts might be specific to certain biotypes (Gottlieb et al. 2006; Chiel et al. 2007) and might confer upon them resistance to insecticides (Kontsedalov et al. 2008). The most widespread biotype, B, was recognized in the late 1980s (Costa and Brown 1991; Costa et al. 1993) following extensive outbreaks of B. tabaci in the southwestern United States, and it has a worldwide distribution. An additional widespread biotype, Q, which probably originated in the Iberian Peninsula (Guirao et al. 1997), has since spread globally (Horowitz et al. 2003; Boykin et al. 2007; Chu et al. 2010).
Management of B. tabaci populations and, in particular, management of the viral plant diseases it transmits, is difficult. This is due to the pest’s elevated population growth rates, rapid evolution of resistance to insecticides, and the relatively protected location of the individuals on the underside of the leaves. B. tabaci is highly polyphagous and is known to develop on more than 500 plant species, including a large number of fiber, vegetable, and ornamental crops (Mound and Halsey 1978; Oliveira et al. 2001). Another remarkable feature is its easy adaptation to changing environmental conditions, especially in subtropical and tropical agroecosystems and in greenhouse-grown crops, even in temperate climates (Brown 2007a, b; Castle et al. 2010). Brown (2007a, b) proposed that monoculture cropping together with year-round production practices are mostly responsible for the present whitefly and viral disease outbreaks. Because viral plant diseases transmitted by B. tabaci are not curable, the principal strategies for their management are based on prevention of transmission (Antignus 2007) and/or on utilization of host-plant resistance (Lapidot and Friedmann 2002). At present, the use of insecticides is the main approach employed to manage B. tabaci populations. This practice is greatly restricted, however, due to both environmental concerns and the widespread resistance that B. tabaci has developed to most of the insecticides in use (Palumbo et al. 2001; Horowitz et al. 2007; Castle et al. 2010). Consequently, increasing importance is being placed upon control by other methods (including cultural, mechanical, and biological) as a means of managing pest populations.
Worldwide outbreaks of B. tabaci whiteflies, especially biotype B, have facilitated the emergence of whitefly-transmitted geminiviruses (WTGs). These viruses cause economically important diseases of vegetable and fiber crops, especially in tropical and subtropical regions of the world. Because small populations of whiteflies can efficiently spread WTGs, management of these diseases is more challenging than for whiteflies alone. As the WTGs have emerged worldwide, key aspects of the biology of WTGs and B. tabaci have shaped the development of an IPM approach for these diseases. The generalized IPM package involves strategies for implementation before the growing season, such as the use of virus- and whitefly-free transplants, propagative stock, and resistant varieties. During the growing season, approaches may include whitefly population suppression, roguing virus-infected plants, floating row covers, and reflective mulches. After the growing season, strategies include region-wide sanitation, weed management, and implementation of a host-free period.
Because it is not possible to cure plants of WTG infections, efforts must be taken to keep plants from becoming infected or to manage the rate, timing, and severity of the infection to protect crop health. Growers emphasize whitefly management with insecticides to control WTGs; however, in most cases, this does not provide adequate protection. IPM approaches have been more successful in the management of WTGs (Jones 2003), using multiple strategies that target different levels of the plant–WTG–whitefly interaction. The first widely recognized concept of IPM stressed a combination of chemical, biological, and other control methods for insect pest management (Stern et al. 1959).
A number of very effective strategies can provide effective management of diseases caused by WTGs when combined into an IPM package. The specific strategies used for the IPM package in a given agroecosystem are dependent on knowledge of the crop plant, the cropping system, climatic conditions, and the biology of the virus and the vector.
A generalized scheme for the IPM of a whitefly-transmitted virus is divided into three parts: before the growing season, during the growing season, and after the growing season. Before the growing season, advance preparation in terms of the cultivar of the crop, the source of the planting material (seed, transplants, or propagative material), and field location are very important. The cultivar and seed selection of the proper cultivar are important for many reasons. However, in the case of IPM of WTGs, the key points are related to the availability of virus resistance or tolerance and certain horticultural aspects. Host plant resistance to whiteflies or WTGs provides an ideal pest management tool, with little or no environmental impact. Unfortunately, host plant resistance is not available for many whitefly-transmitted begomoviruses, and there are even fewer examples of resistance to the vector.
Coping with plant diseases in the field is relatively difficult because the causal organisms (bacteria, MLO, fungi, virus and nematodes) are very small and cannot be seen moving around like insects or rats. The most important first step in thinking about diseases is to realize that diseases must be managed and not controlled. What is the difference? Management means a complete set of activities that support each other. Management means that these activities are carefully planned and are implemented over several seasons, not controlled within a single season. Management includes control methods for prevention and control methods to slow down epidemics; diseases will never be completely eradicated; only populations reduced to very low levels. Management usually needs the cooperation of several farmers working together to reduce overall disease in an area. Management requires someone who can observe larger areas of disease incidence and levels of infection.
Many WTGs are important in developing countries where subsistence farmers are involved in vegetable production. In general, the earlier that a plant is infected with a virus, the more severe the disease symptoms and the greater the yield loss. Thus, it is critical to establish new plantings with virus-free and whitefly-free transplants or propagative stocks. The first step is to keep transplant propagation facilities free of whiteflies. Greenhouses should have induced positive airflow, double-door airlock entrances, and roofs covered with UV-absorbing films. All vents and other openings should be covered with whitefly-resistant, fine-mesh screening with 0.25 × 0.8 mm openings or less. Sanitation within and around the propagation facilities is also important. Potential whitefly or virus host plants must be eliminated in and around the facility, with discarded plant materials sealed in whitefly-proof containers or destroyed.
In addition, systemic neonicotinoid class insecticides (e.g., imidacloprid or thiamethoxam) applied as soil drenches, along with foliar insecticide sprays, can be used in greenhouse operations to suppress whitefly populations. Monitoring of whitefly adults with yellow sticky traps can be used to know when foliar insecticides need to be applied (Gillespe and Quiring 1987). One approach is to place one trap per 80 plants, or at least one per 6 m2, at the beginning of the transplant production to be used as a monitoring and control measure.
8 Location and Time of Planting
New plantings should be established following a host-free period or during periods when virus and whitefly pressure are low. If there is a good information available on the seasonal patterns of whitefly populations and virus pressure, planting times can be modified to avoid periods of high pressure. If multiple staggered plantings are planned, barrier crops can be planted prior to the establishment of the plantings, which are established upwind of earlier plantings and in blocks such that minimal area of the field is exposed to wind. However, under heavy virus pressure, these approaches alone are unlikely to substantially reduce virus infection in the field.
Whitefly management during the growth season leads to the suppression of whitefly populations with insecticides. Especially in areas with histories of whitefly outbreaks, this is an important component of a successful IPM package for WTGs. Insecticides are most commonly applied as foliar sprays or injected into the soil, but may also be applied via chemigation through drip irrigation. Soil applications are typically systemic insecticides, mostly in the neonicotinoid chemical class. The prophylactic use of soil-applied systemic insecticides has been documented to slow, reduce, or delay virus transmission by whiteflies. However, the use of insecticides alone often does not deliver sufficient protection from WTGs to prevent economically important crop damage.
9 Roguing
A roguing strategy involves the physical removal of virus-infected plants over the course of the growing season. Roguing needs to be done soon after plots are established and is most helpful if the incidence of the virus is low (<5%). After roguing, it is also important that there is a minimal level of virus spread in the field, as well as a limited amount of introduction of virus form outside the field. If whitefly populations are high, plants should be treated with an insecticide to kill whitefly adults prior to roguing. If nymphs are present, rogued plants should be removed in plastic bags and disposed of well away from production fields.
Vegetables can be protected from whitefly damage by an exclusion method using protected culture in greenhouses and screen houses for virus infection by physical means (i.e., preventing the insects from contacting susceptible plants). In the most extreme case, the entire crop is grown in a greenhouse or screen house, and plants are protected from whiteflies for the entire production cycle. When these structures are kept free of whiteflies (e.g., through the use of glass, plastic, or screening; vents covered with screening; double doors with positive pressure), excellent management of whiteflies and WTGs can be achieved.
Another common method used for the protection of plants in the field with floating row covers is the covering of young plants, either those emerging from seeds or that have been transplanted, with protective netting. This netting is a spun-bonded polyester material (commercially available as Agribon or Agril) that is placed directly over the rows of emerging seedings or transplants. The covers are typically placed over the plants without any type of support, such that it is a floating row cover and moves with the growth of the plants. In other cases, semi-circular lengths of wire or piping are used to provide support and keep the netting from directly contacting the leaves. These materials allow passage of adequate amounts of light for normal plant growth, although there have been some reports that the microclimate formed under the row covers can favor the development of foliar diseases caused by bacteria and fungi. In general, the row covers are left on for 30 days or until pollination, such as in the case of cucurbits.
It is well established that the use of row covers can protect plants from whiteflies and reduce the spread of WTGs in crops such as cucurbits, pepper, and tomato (Natwick and Durazo 1985; Natwick and Laemmlen 1993; Orozco-Santos et al. 1995; Webb and Linda 1992). In cases of severe whitefly and virus pressure, this protection can make the difference in whether a marketable crop is produced. This approach has been shown to slow the spread of geminivirus and is being used in Guatemala to protect tomato and peppers form infection with various WTGs. Row covers have also been successfully used in Guatemala to protect melons from WTGs and the whitefly-transmitted crinivirus. However, the use of floating row covers is expensive; it should be used to protect plants during periods where whitefly and virus pressure are known to be high. Small-scale farmers can use row covers to protect seed beds or to produce small tunnels in which seedlings can be protected from whiteflies during this critical stage of growth (Hilje et al. 2001).
10 Barrier Crops and Mulches
A number of other cultural practices can be used to protect crops from whiteflies and thus slow the spread of WTGs. Physical barriers can be designed to prevent the movement of whiteflies into fields of susceptible crops. Barriers may be non-living, such as plastic (yellow plastic with sticky material to trap insects) or screening, or living, such as the planting of a tall plant species (non-hosts of the whitefly and WTGs) between fields of susceptible crops. The best barrier plants for WTGs are monocots such as corn, sorghum, and elephant grass. There is little evidence that barriers effectively reduce whitefly migration or virus spread because whiteflies can fly or be wind-carried over barriers and transmit WTGs for long periods of time, due to the persistent nature of transmission (Hilje et al. 2001). Thus, barriers are generally not an essential component of the IPM package for WTGs.
Mulches are designed to prevent insects from recognizing and landing on a crop that is susceptible to virus infection. Like barriers, mulches can be non-living (plastic or some other material) or living (plants grown among the susceptible crop). In terms of non-living mulches, the most effective materials are colored or UV-reflective plastic. These have been reported to have some success in reducing whitefly population densities as well as the incidence of WTGs (Antignus 2000). Living mulches involve planting low-growing ground cover-type plants, which are non-hosts for whiteflies and WTGs, among a susceptible crop. These living mulches reduce whitefly populations by causing the insects to leave the field due to the presence of the non-host plants (Hilje et al. 2001).
11 IPM Package for Tomato-Infecting Geminiviruses: Preplant Activities
11.1 Use of Virus-Free and Whitefly-Free Transplants
Tomato-infecting begomoviruses are not seed-transmitted, so transplants will not become infected via contaminated or infected seeds. However, whiteflies can transmit the virus to plants in the seedling stage; establishing fields with virus-infected transplants will lead to rapid spread of the virus within the field. Furthermore, infection of plants at such an early stage of growth will lead to the greatest economic losses. Therefore, an essential component of the IPM package is the use of virus-free and whitefly-free transplants. In the case of WTGs, this means keeping whiteflies physically separated from transplants.
11.2 Whitefly Monitoring and Management
Whitefly monitoring and management is costly and not good for the health of farmers or the environment. It is important to monitor whitefly populations to understand the population dynamics on a regional basis, especially to detect the build-up of populations early in the crop production cycle. This can be done by monitoring adult populations with yellow sticky cards or with the leaf turn method, in which adults and/or nymphs are directly counted on the undersurfaces of leaves. However, as mentioned earlier, with WTGs, the challenge is developing threshold populations that can trigger pesticide applications that will slow the spread of the virus.
During the growing season in row covers, the crop is being transplanted into the field in the presence of viruliferous whiteflies; plants can be physically protected with floating row covers. These materials are placed over the rows of plants, leaving the ground between rows uncovered. The covers can only be left over plants for approximately 30 days; if viruliferous whiteflies are still present, these plants will become infected. Cultivated tomato has been a good host for the evolution of new WTGs, and this has been facilitated by the worldwide dissemination of the polyphagous B. tabaci biotype B. In many cases, these viruses cause diseases of considerable economic importance, particularly in tropical and subtropical regions. Many components of the IPM package for these viruses do not require specific knowledge of the begomovirus(es) involved, but identification of the WTGs involved in a region may influence the selection of resistant varieties.
It is critical to start with virus-free and whitefly-free transplants and, if possible, resistant varieties. Ideally, transplants are planted during a period of low virus pressure, such as following a host-free period or away from established fields. If viruliferous whiteflies are present, then additional measures may be taken, such as floating row covers or management of whitefly populations with insecticides. Roguing infected plants early in the season may slow down spread of the virus, as may the use of reflective mulches. Following harvest, it is critical to uproot and destroy old plants through removal or tillage. In tropical and sub-tropical regions, the implementation of a 1- to 3-month tomato or whitefly host-free period can substantially reduce virus and whitefly pressure for the next crop. The free period provides an effective approach that is not based on pesticides, but this requires regional cooperation. By implementing this IPM package, there is a high probability that effective management of any tomato-infecting begomovirus can be accomplished.
11.3 Cassava Mosaic Disease
Cassava mosaic disease (CMD) is one of the most damaging diseases of cassava (Fargette et al. 2006; Thresh and Cooter 2005). It can cause substantial yield reductions and is very difficult to manage. CMD is characterized by a striking light to dark green mosaic of leaves, various degrees of leaf and stem distortion, and reduced numbers and weights of tubers. CMD is caused by a complex of whitefly-transmitted begomoviruses, including African cassava mosaic virus and East African cassava mosaic virus. Biological considerations for an effective IPM strategy for CMD must consider the perennial nature of the crop and the fact that is vegetatively propagated (Thresh and Cooter 2005). Also, the viruses that cause CMD have a relatively narrow host range, infecting only members of the plant family Euphorbiaceae, including cassava, castor bean, and certain wild hosts and weeds.
An IPM package for CMD includes preplanting activities, such as disease-free cuttings, resistant varieties, and cultural practices. A number of cultural practices can also be considered, although these may have only limited beneficial effects or be too difficult for farmers to utilize (Thresh and Cooter 2005). Elongated plots that are exposed to prevailing winds should be avoided, because this is where the highest infection rates tend to occur. Intercropping of cassava with other crops such as banana, sweet potato, and legumes can reduce virus spread through the reduction of whitefly populations. Finally, cassava should be grown under favorable conditions, as CMD spreads slower in fields with healthy plants.
During the growing season, the physical removal of virus-infected plants over the course of the growing season can be useful, particularly when disease incidences are relatively low (<5%). Thus, roguing will be most effective when used in combination with preplanting measures, such as planting disease-free cuttings. It is also an important method for the amplification of sources of disease-free cuttings; it needs to be done soon after plots are established. Fields should be monitored once or twice shortly after planting as cuttings show symptoms in newly emerging leaves.
With respect to whitefly management, although there is a correlation between the number of whiteflies and the rate of spread of CMD, managing the disease with insecticide sprays has not been effective, nor is it practical. This relates to the fact that cassava is grown on small plots by subsistence farmers who often lack an understanding of CMD, the training and equipment to apply pesticides, and the resources to purchase the appropriate insecticides. However, the suppression of whitefly populations, either via biological control or with natural or synthetic insecticides, may help to slow the spread of the CMD in certain situations.
After the growing season ,cassava is the main host of the viruses that cause CMD, It is critical to destroy cassava plants promptly after harvest, as well as any other known host plants. This should be done within and around fields (for reservoir hosts) following harvest and, if necessary, before establishing new plantings. Ideally, the planting and harvest times in defined regions or localities should be coordinated to avoid periods of high disease pressure (e.g. high populations of viruliferous whiteflies) and to possibly allow a period with minimal plantings of cassava to help cleanse the agroecosystem of the virus.
12 Summary
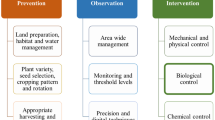
Different IPM strategies can be followed for different periods of implementation (Fig. 1). Before the growing season, IPM strategies include the use of virus-free propagative material, the use of resistant cultivars, modification of planting dates, and avoidance of the planting of new fields near old fields. During the growing season, IPM includes the roguing of plants showing mosaic symptoms, monitoring for whitefly populations using established means of sampling, application of insecticides only when necessary, and rotation of insecticides to minimize development of resistance (e.g., no more than two uses of any material per season). After the growing season, IPM requires the prompt removal of crops following harvest. Certain cultural practices can also help to reduce the incidence or spread of disease, as can a systematic roguing program. Extensive sanitation, in the form of prompt removal of other hosts of the virus, can reduce inoculum pressure for subsequent plantings.
An IPM program or its components can provide effective disease management, but IPM has not been widely implemented. This is related to a lack of understanding of the disease by farmers and a lack of extension programs to deliver the IPM package to farmers. It will take a major effort to develop regional coordination to implement relevant IPM packages. These packages may differ depending on the region or locality. IPM may require decentralized and participatory breeding efforts to generate resistant varieties that provide the desired horticultural properties preferred by local growers and consumers.
References
Antignus Y (2000) Manipulation of wavelength dependent behavior of insects: an IPM tool to impede epidemics and restrict spread of insect-borne viruses. Virus Res 71:213–220
Antignus Y (2007) The management of tomato yellow leaf curl virus in greenhouses and the open field, a strategy of manipulation. In: Czosnek H (ed) Tomato yellow leaf curl virus disease. Springer, Dordrecht, pp 263–278
Bedford ID, Briddon RW, Brown JK, Rosell RC, Markham PG (1994) Geminivirus transmission and biological characterisation of Bemisia tabaci Gennadius from different geographic regions. Ann Appl Biol 125:311–325
Boykin LM, Shatters RG Jr, Rosell RC, McKenzie CL, Bagnall RN, De Barro P, Frohlich DR (2007) Global relationships of Bemisia tabaci (Hemiptera: Aleyrodidae) revealed using Bayesian analysis of mitochondrial COI DNA sequences. Mol Phylogenet Evol 44:1306–1319
Brown JK (2007a) The Bemisia tabaci complex: genetic and phenotypic variability drives begomovirus spread and virus diversification. APSnetvFeature
Brown JK (2007b) The Bemisia tabaci complex: genetic and phenotypic variation and relevance to TYLCV-vector interactions. In: Czosnek H (ed) Tomato yellow leaf curl virus disease: management, molecular biology, breeding for resistance. Springer, Dordrecht, pp 25–56
Brown JK, Bird J, Banks G, Kiesler SM et al (1995a) First report of an epidemic in tomato caused by two whitefly-transmitted geminiviruses in Puerto Rico. Plant Dis 79:1250
Brown JK, Bird J, Banks G, Kiesler SM et al (1995b) First report of an epidemic in tomato caused by two whitefly-transmitted geminiviruses in Puerto Rico. Plant Dis 79:1250
Byrne DN, Bellows TS (1991) Whiteflies biology. Annu Rev Entomol 36:431–457
Castle SJ, Palumbo JC, Prabhaker N, Horowitz AR, Denholm I (2010) Ecological determinants of Bemisia tabaci resistance to insecticides. In: Stansly PA, Naranjo SE (eds) Bemisia: bionomics and management of a global pest. Springer, Dordrecht
Chiel E, Gottlieb Y, Zchori-Fein E, Mozes-Daube N, Katzir N, Inbar M, Ghanim M (2007) Biotype-dependent secondary symbiont communities in sympatric populations of Bemisia tabaci. Bull Entomol Res 97:407–413
Chu D, Wan FH, Zhang YJ, Brown JK (2010) Change in the biotype composition of Bemisia tabaci in Shandong Province of China from 2005 to 2008. Environ Entomol 39:1028–1036
Cohen S (1982) Control of whitefly vectors of viruses by color mulches. In: Harris KF, Maramorosch K (eds) Pathogens, vectors and plant diseases, approaches to control. Academic, New York, pp 45–56
Cohen S, Antignus Y (1994) Tomato yellow leaf curl virus, a whitefly-borne geminivirus of tomatoes. In: Harris KS (ed) Advances in disease vector research, vol 10. Springer-Verlag, New York, pp 259–288
Cohen S, Berlinger MJ (1986) Transmission and cultural control of whitefly-borne viruses. Agric Ecosyst Environ 17:89–97
Cohen S, Melamed-Madjar V (1978) Prevention by soil mulching of the spread of tomato yellow leaf curl virus transmitted by Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) in Israel. Bull Entomol Res 68:465–470
Coombe PE (1982) Visual behavior of the greenhouse whitefly, Trialeurodes vaporariorum. Physiol Entomol 7:243–251
Costa H, Brown JK (1991) Variation in biological characteristics and esterase patterns among populations of Bemisia tabaci and the association of one population with silver leaf symptom induction. Entomol Exp Appl 61:211–219
Costa HS, Brown JK, Sivasupramaniam S, Bird J (1993) Regional distribution, insecticide resistance, and reciprocal crosses between the ‘A’ and ‘B’ biotypes of Bemisia tabaci. Insect Sci Appl 14:255–266
Csizinszky AA, Schuster DJ, Kring JB (1995) Color mulches influence yield and insect pest populations in tomatoes. J Am Soc Hortic Sci 120:778–784
De Barro PJ, Trueman JWH, Frohlich DR (2005) Bemisia argentifolii is a race of B. tabaci (Hemiptera: Aleyrodidae): the molecular genetic differentiation of B. tabaci populations around the world. Bull Entomol Res 95:193–203
De Barro PJ, Liu SS, Boykin LM, Dinsdale A (2011) Bemisia tabaci: a statement of species status. Annu Rev Entomol 56:1–19
Dobson HE (1994) Floral volatiles in insect biology. In: Bernays EA (ed) Insect–plant interactions, vol 5. CRC Press, Boca Raton, FL, pp 47–81
Fargette D, Konate G, Fauquet C, Muller E, Peterschmitt M, Thresh JM (2006) Molecular ecology and emergence of tropical plant viruses. Annu Rev Phytopathol 44:235–260
Gillespe DR, Quiring D (1987) Yellow sticky traps for detecting and monitoring greenhouse whitefly (Homoptera: Aleyrodidae) adults on greenhouse tomato crops. J Econ Entomol 80:675–679
Gottlieb Y, Ghanim M, Chiel E, Gerling D, Portnoy V, Steinberg S, Tzuri G, Horowitz AR, Belausov E, Mozes-Daube N, Kontsedalov S, Gershon M, Gal S, Katzir N, Zchori-Fein E (2006) Identification and localization of a Rickettsia sp in Bemisia tabaci (Homoptera: Aleyrodidae). Appl Environ Microbiol 72:3646–3652
Guirao P, Beitia F, Cenis JL (1997) Biotype determination of Spanish populations of Bemisia tabaci (Hemiptera: Aleyrodidae). Bull Entomol Res 87:587–593
Hequet E, Henneberry TJ, Nichols RL (eds.) (2007) Sticky cotton: causes, effects, and prevention. USDA-ARS Technical Bulletin No 1915
Hilje L, Costa HS, Stansly PA (2001) Cultural practices for managing Bemisia tabaci and associated viral diseases. Crop Prot 20:801–812
Hogenhout SA, Ammar ED, Whitfield AE, Redinbaugh MG (2008) Insect vector interactions with persistently transmitted viruses. Annu Rev Phytopathol 46:327–359
Horowitz AR, Denholm I, Gorman K, Cenis JL, Kontsedalov S, Ishaaya I (2003) Biotype Q of Bemisia tabaci identified in Israel. Phytoparasitica 31:94–98
Horowitz AR, Kontsedalov S, Khasdan V, Ishaaya I (2005) Biotypes B and Q of Bemisia tabaci and their relevance to neonicotinoid and pyriproxyfen resistance. Arch Insect Biochem Physiol 58:216–225
Horowitz AR, Denholm I, Morin S (2007) Resistance to insecticides in the TYLCV vector, Bemisia tabaci. In: Czosnek H (ed) Tomato yellow leaf curl virus disease. Springer, Dordrecht, pp 305–325
Jones DR (2003) Plant viruses transmitted by whiteflies. Eur J Plant Pathol 109:195–219
Kontsedalov S, Zchori-Fein E, Chiel E, Gottlieb Y, Inbar M, Ghanim M (2008) The presence of Rickettsia is associated with increased susceptibility of Bemisia tabaci (Homoptera: Aleyrodidae) to insecticides. Pest Manag Sci 64:789–792
Lapidot M, Friedmann M (2002) Breeding for resistance to whitefly-transmitted geminiviruses. Ann Appl Biol 140:109–127
Macdowall FDH (1972) Phototactic action spectrum for whitefly and the question of colour vision. Can Entomol 104:299–307
Matteson N, Terry LI (1992) Response to colour by male and female Frankliniella occidentalis. Entomol Exp Appl 63:187–201
Moericke V (1955) U8 on the lifestyle of the winged leaf louse (Aphidina) with special consideration of behavior in the country. Z Angew Entomol 37:29–91
Mound L (1962) Studies on the olfaction colour sensitivity of Bemisia tabaci Genn. Aleyrodidae. Entomol Exp Appl 5(2):99–104
Mound LA, Halsey SH (1978) Whitefly of the world: a systematic catalogue of the Aleyrodidae (Homoptera) with host plant and natural enemy data. British Museum (Natural History), Chichester
Natwick ET, Durazo A III (1985) Polyester covers protect vegetables from whiteflies and virus disease. Calif Agric 39:21–22
Natwick E, Laemmlen FF (1993) Protection from phytophagous insects and virus vectors in honeydew melons using row covers. Flo Entomol 76:120. https://doi.org/10.2307/3496020
Nitzany FE, Geisenberg H, Koch B (1964) Tests for the protection of cucumbers from a whitefly-borne virus. Phytopathology 54:1059–1061
Oliveira MRV, Henneberry TJ, Anderson P (2001) History, current status, and collaborative research projects for Bemisia tabaci. Crop Prot 20:709–723
Orozco-Santos M, Perez-Zamora O, Lopez-Arriaga M (1995) Floating row cover and transparent mulch to reduce insect population, virus diseases and increase yield in cantaloupe. Fla Entomol 78:493–501
Palumbo JC, Horowitz AR, Prabhaker N (2001) Insecticidal control and resistance management for Bemisia tabaci. Crop Prot 20:739–765
Perring TM (2001) The Bemisia tabaci species complex. Crop Prot 20:725–737
Prokopy RJ, Owens ED (1983) Visual detection of plants by herbivorous insects. Annu Rev Entomol 28:337–364
Sánchez-Campos S, Navas-Castillo J, Camero R, Saria C, Díaz JA, Moriones E (1999) Displacement of Tomato yellow leaf curl virus (TYLCV)-Sr by TYLCV-is in tomato epidemics in Spain. Phytopathology 89:1038–1043
Stansly PA, Naranjo SE (eds) (2010) Bemisia: bionomics and management of a global pest. Springer, Dordrecht
Stern VM, Smith RF, van den Bosch R, Hagen KS (1959) The integrated control concept. Hilgardia 29:81–101
Terry LI (1997) Host selection, communication and reproductive behaviour. In: Lewis T (ed) Thrips as crop pests. CAB International, New York, pp 65–118
Thresh JM, Cooter RJ (2005) Strategies for controlling cassava mosaic virus disease in Africa. Plant Pathol 54:587–614
Vaishampayan SM, Kogan M, Waldbauer GP, Wooley JT (1975a) Spectral specific responses in the visual behaviour of the greenhouse whitefly, Trialeurodes 6aporariorum (Homoptera: Aleurodidae). Entomol Exp Appl 18:344–356
Vaishampayan SM, Waldbauer GP, Kogan M (1975b) Visual and olfactory responses in orientation to plants by the greenhouse whitefly, Trialeurodes vaporariorum (Homoptera: Aleurodidae). Entomol Exp Appl 18:412–422
Webb SE, Linda SB (1992) Evaluation of spun-bounded polyethylene row covers as a method of excluding insects and viruses affecting fall-grown squash in Florida. J Econ Entomol 85:2344–2352
Xu J, De Barro PJ, Liu SS (2010) Reproductive incompatibility among genetic groups of Bemisia tabaci supports the proposition that the whitefly is a cryptic species complex. Bull Entomol Res 100:359–366
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Riyaz, S.U.M., Kathiravan, K. (2019). Integrated Pest Management Approaches. In: Kumar, R. (eds) Geminiviruses. Springer, Cham. https://doi.org/10.1007/978-3-030-18248-9_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-18248-9_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-18247-2
Online ISBN: 978-3-030-18248-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)