Abstract
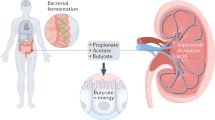
Oxalobacter formigenes is part of the bacterial flora in the large intestine of humans and many other mammalian species. It is unique in that it requires oxalate both as an energy and carbon source. A lack of colonization with O. formigenes is a risk factor for idiopathic recurrent calcium oxalate stone disease. Protection against calcium oxalate stone disease appears to be due to the oxalate degradation that occurs in the gut, particularly when calcium intake is low. There is also some evidence that suggests a possible mechanism involving intestinal oxalate secretion triggered by the bacterium itself, as O. formigenes colonization appears to lower plasma oxalate. Whether high oral doses of this organism can promote sufficient intestinal oxalate secretion to diminish the oxalate burden on the kidney in individuals with Primary Hyperoxaluria is currently being tested by OxThera, Inc. in a phase 3 clinical trial. Much still remains to be learned about how O. formigenes establishes and maintains gut colonization and the precise mechanisms by which it modifies stone risk.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Microbiology of O. formigenes
O. formigenes is a Gram-negative, obligately anaerobic, rod or curve-shaped, non-motile, non-spore forming bacterium that belongs to the Betaproteobacteria class and Burkholderiales order. Its existence was first recognized from its role in acclimating livestock to the ingestion of high-oxalate diets and preventing oxalate toxicity [1, 2]. Comparisons of the profiles of cellular fatty acids of 17 strains of O. formigenes, including strains isolated from gastrointestinal contents from humans, sheep, cattle, pigs, guinea pigs, rats and from fresh water lake sediments, support the concept of separating these strains into two main groups (currently designated as Group I and II). In Group 1 strains, a cyclic 17 carbon fatty acid predominates whereas in Group 2 a cyclic 19 carbon acid is dominant [3].
The products from oxalate metabolism are carbon-dioxide and formate, with approximately 1 mole of each produced per mole of oxalate metabolized. Energy generation is centered on the development of a proton motive force through the electrogenic exchange of oxalate (in) and formate (out) across the cell membrane together with the consumption of a proton inside the cell when the CoA-ester of oxalate is decarboxylated by oxalyl-CoA-decarboxylase [4, 5].
The availability of the genome [6] and, more recently, proteome of O. formigenes [7] has provided an opportunity to increase our understanding of the biology of this organism and how it survives in its environment. The release of the genome sequence of a Group 1 (OXCC13) and a Group 2 strain (HOxBLS) by the Broad Institute ha s provided a genetic framework for investigating important biological properties of the organism [6]. An independent sequence for OXCC13 has been published as well as the sequence of the HC-1 strain which is being used by Oxthera in clinical trials [8, 9]. With this data, molecular and functional studies can now be performed to identify important proteins and pathways that promote colonization resilience, enhance aerotolerance and increase enteric secretion of host derived oxalate. A recent review of the genomic sequences of the two strains of O. formigenes identified some interesting differences that may suggest the two strains utilize different pathways to survive and flourish within the intestine [6]. Mass spectrometry based shotgun proteomics identified 1822 proteins of the 1867 unique protein coding genes in the Group 1 O. formigenes strain OxCC13 [7]. From the protein datasets presented it is clear this organism contains a repertoire of metabolic pathways that mediate adaptation with nutrient shifts and environmental stress. For example, the proteomic analysis showed superoxide dismutase increases in stationary relative to log phase suggesting O. formigenes has the ability to persist outside the anaerobic environment of the intestine.
Growth of O. formigenes in culture occurs under anaerobic conditions, with optimal growth at pH between 6 and 7 in a carbonate–bicarbonate buffered medium that contains minerals, oxalate, acetate, and a small amount of yeast extract. It requires a low concentration of acetate (0.5 mM) to grow, but acetate alone cannot support growth [3]. Oxalate serves as both the energy yielding substrate and the major source of carbon for growth [10, 11]. Smaller amounts of carbon are also assimilated from acetate and carbon dioxide. The energy yield from oxalate is low, but sufficient to support growth. The low yield of O. formigenes in culture and its sensitivity to oxygen has implications for the preparation of O. formigenes for probiotic use. A recent study examining some common processes and conditions associated with manufacturing of probiotic strains highlighted the resilience of the Group 1 O. formigenes strain OxCC13 to lyophilization and storage in yogurt. In light of this work, it would be of interest to test if individuals can be colonized with either lyophilized O. formigenes or O. formigenes mixed in with yogurt. Previous in vitro work examining various biological properties of O. formigenes showed that different strains of O. formigenes have different tolerances to environmental stress such as acid and air exposure. This work would suggest that it will be prudent to identify an O. formigenes strain more resilient to common processes associated with probiotic preparation before embarking on a colonization study in humans.
A recent in vitro study indicated that O. formigenes culture conditioned medium stimulates oxalate secretion in human intestinal Caco-2-BBE cells [12]; however, these findings were not replicated by a different group [13] and warrants further investigation. Arvans et al. also showed that rectal administration of O. formigenes culture conditioned medium resulted in a reduction in urinary oxalate excretion in a mouse model of PH1; however, a direct measurement of enteric oxalate secretion utilizing oxalate isotopes administered intravenously and then measured in the gut is still needed to quantify the extent of this pathway. Of interest was the recent filing of a patent by OxThera Pharmaceuticals that covers the invention of the isolation and administration of secretagogues derived from O. formigenes that may enhance oxalate secretion into the intestinal lumen (http://www.google.com/patents/WO2015002588A1?cl=en). Although the work described in this patent did not identify any compelling secretagogue candidates, the identification of a bioactive factor or factors secreted by O. formigenes that induces oxalate secretion may be an effective therapy to reduce the oxalate burden in patients with Primary Hyperoxaluria .
O. formigenes in the Human Gut
Because of O. formigenes dependency on oxalate for growth, its intestinal numbers are sensitive to both dietary oxalate and dietary calcium intake. This was highlighted in a study where O. formigenes numbers were measured in the stool of healthy subjects equilibrated to diets controlled in oxalate, calcium and other nutrients, as shown in Fig. 9.1 [14]. In this study, bacterial numbers were shown to increase 12-fold on average as dietary oxalate increased 15-fold. Interestingly, the availability of oxalate was also shown to influence bacterial numbers as a fivefold increase in dietary calcium, which will limit the bioavailability of oxalate due to the high affinity of calcium for oxalate, decreased bacterial numbers approximately fivefold. The dependency for oxalate and the inverse relationship between dietary calcium and O. formigenes numbers may lead to a loss of colonization in stone formers who are recommended to maintain an adequate calcium and low oxalate intake and warrants further investigation.
Number of fecal O. formigenes with changes in dietary oxalate (■) or dietary calcium (□). Daily calcium intake was 1000 mg on the varied oxalate dietary phase and daily oxalate was 250 mg on the varied calcium dietary phase. Real-time PCR was used to quantitate O. formigenes numbers. 5.5 × 104 CFU/ng DNA was used to convert qPCR data to number of O. formigenes per g feces. (Modified from [14])
Enumeration in human stool from healthy non-kidney stone forming individuals suggests O. formigenes represents a tiny fraction of the total intestinal microbiota [6]. Many low abundance bacteria are thought to survive in the intestines by occupying specific nutrient niches where competition for their food source is limited [15]. Indeed, both in vitro culture studies [16] and a recent human study [14] show that O. formigenes utilizes oxalate more efficiently than many other bacteria. Thus, an important factor in the survival of this organism in the intestines is its unique ability to outcompete other bacteria for its food source [14], highlighting the highly efficient oxalate degrading capacity of O. formigenes relative to other microbiota. These data also show that the oxalate degrading capacity of the microbiome of non-colonized individuals is negligible at low oxalate intake, but increases with adaptation to ingestion of higher levels of dietary oxalate, as the dietary oxalate recovered in stool with a daily intake of 250 and 750 mg dietary oxalate was ~80% and ~60%, respectively (Fig. 9.2). The impact of these “generalist” oxalate degrading bacteria in calcium oxalate stone disease is not known, and warrants careful investigation [17].
Stool oxalate of O. formigenes colonized (□) and non-colonized healthy subjects (■) on nutrient controlled diets varying in oxalate. Daily calcium intake was 1000 mg. (Modified from [14])
O. formigenes Colonization
Little is known about how and when individuals become colonized or how O. formigenes persists over time. The source of O. formigenes that colonizes the gut is not known. Studies to date suggest it occurs early in childhood [18] and based on what we know about O. formigenes transmission from animal experiments it is obtained from the environment, not directly from the mother [19].
A review of the colonization frequencies conducted worldwide indicated that 38–77% of a normal population is colonized and it was consistently observed that the colonization frequency in stone formers was about half that in normal subjects [20,21,22,23]. Several studies have indicated that the intake of antibiotics can result in the loss of colonization [21, 24, 25], and this is supported by lower prevalence of O. formigenes in both cystic fibrosis patients [26], and calcium oxalate stone formers who are frequently prescribed antibiotics [27, 28]. It is also possible that a lower rate of colonization in stone formers is due to patients restricting dietary oxalate intake. To date, there has only been one study to examine factors that impact colonization, and in this study [21] only a slight (non-significant) trend was observed between prevalence of colonization (simply whether or not a person was colonized with O. formigenes) in normal subjects and dietary oxalate intake.
Recent analyses of the American Gut Project (AGP) large-scale datasets (>8000 samples) has provided novel insights into O. formigenes colonization of the human gastrointestinal tract [22]. These analyses support the finding that individuals residing in countries with strong healthcare programs and/or higher economic life-styles tend to have significantly less O. formigenes colonization, an observation consistent with the higher general use of antibiotics within these more affluent societies and populations. The AGP sequence analysis also revealed humans may be co-colonized by Group 1 and Group 2 O. formigenes strains, and Group 1 strains may be the most prevalent and abundant strains in the human gastrointestinal tract, while group 2 strains are less common; however, studies still need to be performed to determine if colonization with both strains is possible and offers any advantages regarding oxalate handling. The AGP and a recent study of gut microbiota in stone formers and controls [29] emphasize that biodiversity in the communal structure supporting O. formigenes is a key feature in oxalate degradation.
The ability to re-colonize individuals lacking O. formigenes has previously been addressed by a study in which two healthy adults not colonized with O. formigenes became colonized following the ingestion of cultured O. formigenes [30], and subsequently remained colonized for 9 months. However, other studies where O. formigenes was provided in the form of an enteric coated capsule or as a frozen paste to patients suffering from Primary Hyperoxaluria , resulted in only a minority of the patients remaining colonized post-treatment [31, 32]. Therefore, although it seems quite possible that O. formigenes colonization of non-colonized stone formers may be a cheap and effective way to help minimize stone risk in calcium oxalate stone formers, long term colonization studies are required.
O. formigenes Colonization and Risk of Calcium Oxalate Stone Disease
Since the discovery of O. formigenes in 1985 and the recognition that it resides in the human gut and degrades oxalate, a role for the organism in stone disease has been considered. Initial case–control studies with small numbers of subjects suggested colonization may be protective against stone disease [27, 33, 34], as measurements of urinary oxalate excretion was lower in colonized compared to non-colonized individuals despite a large variability in oxalate excretion and a lack of dietary oxalate and calcium control during urine collections. In addition, a recent study showed 24 h urinary oxalate excretion and plasma oxalate were significantly lower in O. formigenes colonized patients compared to O. formigenes negative patients on a standardized diet [35]. Colonization was also found to be significantly inversely associated with the number of stone episodes.
Similarly, the association of recurrent calcium oxalate stone disease and a lack of O. formigenes was assessed in a study of 247 calcium oxalate stone formers and 259 matched controls [20]. The odds ratio for forming a recurrent stone when colonized was found to be 0.3, which indicates a 70% reduction in stone risk. Surprisingly, there was no difference in urinary oxalate excretion between colonized and non-colonized individuals in either group, which may be due to highly variable oxalate excretion results despite a large enough sample size, as well as the fact that dietary oxalate and calcium levels were not controlled. The discordance in results may be partially explained by our study in healthy subjects that illustrated that the beneficial oxalate degrading activity of O. formigenes is highly dependent on diet [14]. In this study, urinary oxalate excretion was only found to be significantly lower in colonized subjects compared to non-colonized individuals when subjects were administered a low calcium (400 mg/day) and moderate oxalate (250 mg/day) diet, indicating that the efficiency of this bacterium is not maximal at all calcium and/or oxalate concentrations. Further controlled dietary studies are needed to examine what levels of dietary oxalate and calcium intake are required for successful colonization of non-colonized calcium oxalate stone formers with O. formigenes.
Patients subjected to Roux-en-Y gastric bypass (RYGB) are at risk of hyperoxaluria and calcium oxalate kidney stone disease [36], most probably due to increased net gastrointestinal absorption. Of note, rates of O. formigenes colonization have been shown to be lower in morbibly obese individuals being evaluated for or just after bariatric surgery [37, 38]. Recent work by Canales and Hatch using a RYGB rat model showed that colonization with O. formigenes lowered 24 h urinary oxalate excretion 74% in RYGB animals, suggesting patients who exhibit hyperoxaluria after malabsorptive bariatric surgery may benefit from colonization with O. formigenes [39]. O. formigenes has been demonstrated to induce gastrointestinal oxalate secretion in animal models, which may be a second mechanism by which this organism decreases oxalate levels within the circulation and the kidney [39,40,41,42]. A recent controlled dietary study with 11 O. formigenes calcium oxalate stone formers and 26 non-colonized calcium oxalate stone formers, showed absorption of a 13C2-oxalate load was not significantly different between the groups, but plasma oxalate concentrations were significantly higher in non-colonized (5.79 μmol/l) compared to O. formigenes colonized stone formers (1.70 μmol/l) [35]. These data support the findings in rodent models that O. formigenes induces enteric secretion of endogenously produced oxalate, thereby decreasing plasma oxalate concentration. Whether the modification of host oxalate transport properties by O. formigenes colonization underlies the reduction of risk for calcium oxalate stone formation is currently being tested by OxThera, Inc., in a Phase 3 clinical trial with Primary Hyperoxaluria patients.
Conclusions
Much still remains to be learned about how O. formigenes establishes and maintains gut colonization. Unraveling these mechanisms is especially important with respect to the colonization of non-colonized stone formers. Further studies on the factors involved in colonization resilience and enteric secretion of host derived oxalate are warranted in light of this. The range of conditions where O. formigenes lowers stone risk and the role the composition of the gut microbiome plays in this remain to be clearly defined.
References
Allison MJ, Cook HM. Oxalate degradation by microbes of the large bowel of herbivores: the effect of dietary oxalate. Science. 1981;212:675–6.
Allison MJ, Littledike ET, James LF. Changes in ruminal oxalate degradation rates associated with adaptation to oxalate ingestion. J Anim Sci. 1977;45:1173–9.
Allison MJ, Dawson KA, Mayberry WR, Foss JG. Oxalobacter formigenes gen. nov., sp. nov.: oxalate-degrading anaerobes that inhabit the gastrointestinal tract. Arch Microbiol. 1985;141:1–7.
Anantharam V, Allison MJ, Maloney PC. Oxalate:formate exchange. The basis for energy coupling in Oxalobacter. J Biol Chem. 1989;264:7244–50.
Kuhner CH, Hartman PA, Allison MJ. Generation of a proton motive force by the anaerobic oxalate-degrading bacterium Oxalobacter formigenes. Appl Environ Microbiol. 1996;62:2494–500.
Knight J, Deora R, Assimos DG, Holmes RP. The genetic composition of Oxalobacter formigenes and its relationship to colonization and calcium oxalate stone disease. Urolithiasis. 2013;41:187–96.
Ellis ME, Mobley JA, Holmes RP, Knight J. Proteome dynamics of the specialist oxalate degrader. J Proteomics Bioinform. 2016;9:19–24.
Hatch M, Allison MJ, Yu F, Farmerie W. Genome sequence of Oxalobacter formigenes strain OXCC13. Genome Announc. 2017;5(28):pii: e00534-17.
Hatch M, Allison MJ, Yu F, Farmerie W. Genome sequence of Oxalobacter formigenes strain HC-1. Genome Announc. 2017;5(27):pii: e00533-17.
Cornick NA, Allison MJ. Assimilation of oxalate, acetate, and CO2 by Oxalobacter formigenes. Can J Microbiol. 1996;42:1081–6.
Cornick NA, Allison MJ. Anabolic incorporation of oxalate by Oxalobacter formigenes. Appl Environ Microbiol. 1996;62:3011–3.
Arvans D, Jung YC, Antonopoulos D, Koval J, Granja I, Bashir M, Karrar E, Roy-Chowdhury J, Musch M, Asplin J, Chang E, Hassan H. Oxalobacter formigenes-derived bioactive factors stimulate oxalate transport by intestinal epithelial cells. J Am Soc Nephrol. 2017;28:876–87.
Whittamore JM, Hatch M. The role of intestinal oxalate transport in hyperoxaluria and the formation of kidney stones in animals and man. Urolithiasis. 2017;45:89–108.
Jiang J, Knight J, Easter LH, Neiberg R, Holmes RP, Assimos DG. Impact of dietary calcium and oxalate, and Oxalobacter formigenes colonization on urinary oxalate excretion. J Urol. 2011;186:135–9.
Freter R, Brickner H, Fekete J, Vickerman MM, Carey KE. Survival and implantation of Escherichia coli in the intestinal tract. Infect Immun. 1983;39:686–703.
Mogna L, Pane M, Nicola S, Raiteri E. Screening of different probiotic strains for their in vitro ability to metabolise oxalates: any prospective use in humans? J Clin Gastroenterol. 2014;48(Suppl 1):S91–5.
Lieske JC. Probiotics for prevention of urinary stones. Ann Trans Med. 2017;5:29.
Sidhu H, Enatska L, Ogden S, Williams WN, Allison MJ, Peck AB. Evaluating children in the Ukraine for colonization with the intestinal bacterium Oxalobacter formigenes, using a polymerase chain reaction-based detection system. Mol Diagn. 1997;2:89–97.
Cornelius JG, Peck AB. Colonization of the neonatal rat intestinal tract from environmental exposure to the anaerobic bacterium Oxalobacter formigenes. J Med Microbiol. 2004;53:249–54.
Kaufman DW, Kelly JP, Curhan GC, Anderson TE, Dretler SP, Preminger GM, Cave DR. Oxalobacter formigenes may reduce the risk of calcium oxalate kidney stones. J Am Soc Nephrol. 2008;19:1197–203.
Kelly JP, Curhan GC, Cave DR, Anderson TE, Kaufman DW. Factors related to colonization with Oxalobacter formigenes in U.S. adults. J Endourol. 2011;25:673–9.
Liu M, Koh H, Kurtz ZD, Battaglia T, PeBenito A, Li H, Nazzal L, Blaser MJ. Oxalobacter formigenes-associated host features and microbial community structures examined using the American Gut Project. Microbiome. 2017;5:108.
Barnett C, Nazzal L, Goldfarb DS, Blaser MJ. The presence of Oxalobacter formigenes in the microbiome of healthy young adults. J Urol. 2016;195:499–506.
Kharlamb V, Schelker J, Francois F, Jiang J, Holmes RP, Goldfarb DS. Oral antibiotic treatment of Helicobacter pylori leads to persistently reduced intestinal colonization rates with Oxalobacter formigenes. J Endourol. 2011;25:1781–5.
Lange JN, Wood KD, Wong H, Otto R, Mufarrij PW, Knight J, Akpinar H, Holmes RP, Assimos DG. Sensitivity of human strains of Oxalobacter formigenes to commonly prescribed antibiotics. Urology. 2012;79:1286–9.
Sidhu H, Hoppe B, Hesse A, Tenbrock K, Bromme S, Rietschel E, Peck AB. Absence of Oxalobacter formigenes in cystic fibrosis patients: a risk factor for hyperoxaluria. Lancet (London, England). 1998;352:1026–9.
Mittal RD, Kumar R, Bid HK, Mittal B. Effect of antibiotics on Oxalobacter formigenes colonization of human gastrointestinal tract. J Endourol. 2005;19:102–6.
Sidhu H, Schmidt ME, CorneliusT JG, Thamiselvam S, Khan SR, Hesse A, Peck AB. Direct correlation between hyperoxaluria/oxalate stone disease and the absence of the gastrointestinal tract dwelling bacterium Oxalobacter formigenes: possible prevention by gut recolonization or enzyme replacement therapy. J Am Soc Nephrol. 1999;10:S334–40.
Ticinesi A, Milani C, Guerra A, Allegri F, Lauretani F, Nouvenne A, Mancabelli L, Lugli GA, Turroni F, Duranti S, Mangifesta M, Viappiani A, Ferrario C, Dodi R, Dall’Asta M, Del Rio D, Ventura M, Meschi T. Understanding the gut-kidney axis in nephrolithiasis: an analysis of the gut microbiota composition and functionality of stone formers. Gut. 2018;67:2097.
Duncan SH, Richardson AJ, Kaul P, Holmes RP, Allison MJ, Stewart CS. Oxalobacter formigenes and its potential role in human health. Appl Environ Microbiol. 2002;68:3841–7.
Hoppe B, Beck B, Gatter N, von Unruh G, Tischer A, Hesse A, Laube N, Kaul P, Sidhu H. Oxalobacter formigenes: a potential tool for the treatment of primary hyperoxaluria type 1. Kidney Int. 2006;70:1305–11.
Hoppe B, von Unruh G, Laube N, Hesse A, Sidhu H. Oxalate degrading bacteria: new treatment option for patients with primary and secondary hyperoxaluria? Urol Res. 2005;33:372–5.
Batislam E, Yilmaz E, Yuvanc E, Kisa O, Kisa U. Quantitative analysis of colonization with real-time PCR to identify the role of Oxalobacter formigenes in calcium oxalate urolithiasis. Urol Res. 2012;40:455–60.
Troxel SA, Sidhu H, Kaul P, Low RK. Intestinal Oxalobacter formigenes colonization in calcium oxalate stone formers and its relation to urinary oxalate. J Endourol. 2003;17:173.
Siener R, Bangen U, Sidhu H, Honow R, von Unruh G, Hesse A. The role of Oxalobacter formigenes colonization in calcium oxalate stone disease. Kidney Int. 2013;83:1144–9.
Canales BK, Hatch M. Kidney stone incidence and metabolic urinary changes after modern bariatric surgery: review of clinical studies, experimental models, and prevention strategies. Surg Obes Relat Dis. 2014;10:734–42.
Duffey BG, Miyaoka R, Holmes R, Assimos D, Hinck B, Korman E, Kieley F, Ikramuddin S, Kellogg T, Moeding A, Monga M. Oxalobacter colonization in the morbidly obese and correlation with urinary stone risk. Urology. 2011;78:531.
Froeder L, Arasaki CH, Malheiros CA, Baxmann AC, Heilberg IP. Response to dietary oxalate after bariatric surgery. Clin J Am Soc Nephrol. 2012;7:2033–40.
Hatch M, Canales BK. The mechanistic basis of hyperoxaluria following gastric bypass in obese rats. Urolithiasis. 2016;44:221–30.
Hatch M, Cornelius J, Allison M, Sidhu H, Peck A, Freel RW. Oxalobacter sp. reduces urinary oxalate excretion by promoting enteric oxalate secretion. Kidney Int. 2006;69:691–8.
Hatch M, Freel RW. A human strain of Oxalobacter (HC-1) promotes enteric oxalate secretion in the small intestine of mice and reduces urinary oxalate excretion. Urolithiasis. 2013;41:379–84.
Hatch M, Gjymishka A, Salido EC, Allison MJ, Freel RW. Enteric oxalate elimination is induced and oxalate is normalized in a mouse model of primary hyperoxaluria following intestinal colonization with Oxalobacter. Am J Physiol Gastrointest Liver Physiol. 2011;300:G461–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Knight, J., Holmes, R.P. (2019). Role of Oxalobacter formigenes Colonization in Calcium Oxalate Kidney Stone Disease. In: Lange, D., Scotland, K. (eds) The Role of Bacteria in Urology. Springer, Cham. https://doi.org/10.1007/978-3-030-17542-9_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-17542-9_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-17541-2
Online ISBN: 978-3-030-17542-9
eBook Packages: MedicineMedicine (R0)