Abstract
Urinary stone disease (USD) is a common and increasingly burdensome disease affecting one out of ten individuals at some point during their lifetime. Historically, the role of bacteria has been limited to struvite stones and their association with urease-producing bacteria. However, evidence has been accumulating supporting association of bacteria with non-infection stones. For example, urinary tract infection often presents concurrently with USD, bacteria can be grown from calcium containing stones, and 16S rRNA sequencing has detected bacteria in stones of all compositions. The precise role of bacteria in non-infection USD pathogenesis is not well understood, but bacteria may contribute to urinary modification or crystal aggregation or be merely coincidental to the process. To distinguish between these possibilities, future analysis of bacteria isolated from urinary stones will be necessary.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Nephrolithiasis
- Urinary stones
- Urinary stone disease
- 16S rRNA sequencing
- Urine culture
- Microbiome
- Microbiota
- Crystal aggregation
Introduction
Urinary stone disease (USD) is frequently encountered in United States healthcare with an estimated lifetime prevalence of 10.6% in men and 7.1% in women [1]. The prevalence of urinary stone disease has doubled over the last 15 years, with increases more pronounced in historically less affected groups, such as children and women [1, 2]. Moreover, the risk of recurrence is significant with 39% of first time stone formers having a second episode within 15 years of follow up [3]. The economic burden of USD in the United States is immense, resulting in over 600,000 emergency room visits and $2 billion in annual expenditures [4].
The role of bacteria in USD has historically been limited to the association between urease-splitting organisms and magnesium-ammonium-phosphate (struvite) stones, as discussed in previous chapters. However, infection stones make up only 4% of stones with calcium-based stones (calcium oxalate (CaOx) and calcium phosphate (CaPhos)) constituting the majority. Other more common stones include uric acid and mixed composition stones [5]. The role of bacteria in non-infection stone disease has not been well defined, but mounting evidence indicates that bacteria may play an important role.
Evidence of Bacteria in Non-infection Urinary Stone Disease
Urinary Tract Infection and Urinary Stone Disease
The concurrent presentation of urinary tract infections with USD for both obstructing and non-obstructing stones is a common clinical occurrence. In a cohort of 1325 Scandinavian patients with USD, 28% had a positive standard urine culture. Of the 535 patients with calculi available for analysis, 31% had a positive standard urine culture at the time of presentation, regardless of stone composition [6]. This association was true in Japanese patients as well; 7% of stone forming patients had a positive standard urine culture within 1 month of surgical intervention [7]. In the pediatric population of Taiwan with newly diagnosed USD, the most commonly associated condition was a history of urinary tract infection at 34.1% (23.5% of males, 43.9% of females) [8].
Outside of clinical presentation and association, many patients with recurrent UTIs have resolution of bacteriuria on standard urine culture after stone removal. In an analysis of 120 patients with recurrent UTI and asymptomatic, non-obstructing renal calculi, Omar and colleagues found that 48% of patients were infection free at an average follow up of 14 months. The majority of stone compositions were calcium-based, while only 6 (5%) patients had struvite stones [9]. Oliver and colleagues looked at a similar cohort of 103 patients with positive preoperative standard urine culture (79%) or recurrent UTIs with negative preoperative standard urine culture (21%). Following ureteroscopy, 70.7% of patients were infection-free at 12 month with most stones (74%) being composed of calcium oxalate. Moreover, 80% of patients with stone recurrence also had recurrent infections, suggesting an association between the recurrent infection and the recurrent stone [10].
Infectious complications are a known hazard of USD management for all stone compositions. The American Urological Association and European Association of Urology recommend the routine use of preoperative standard urine culture and prophylactic antibiotics prior to any surgical stone manipulation [11, 12]. Despite this practice, sepsis occurs in 4.7% of patients undergoing percutaneous nephrolithotomy (PCNL) [13]. In reviews from the Endourological Society, 8.8% of patients with negative preoperative standard urine culture undergoing PCNL developed fever [14]. Though infectious complications are more commonly associated with infection stones [15], the development of systemic inflammatory response syndrome (SIRS) has been demonstrated in up to 5.3% of patients with non-infection stones [16]. Similarly, Rivera and colleagues reviewed their experience in 227 patients undergoing PCNL for management of USD; infectious complications (UTI/SIRS/Fever/Sepsis) occurred in 37 patients (16%). Overall, 73% of patients experiencing infectious complications had non-struvite stone composition [17].
Bacteria Can Be Cultured from Non-infection Stones
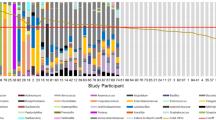
In addition to the association with UTI and infectious complications, multiple studies have demonstrated the ability to culture bacteria from urinary stones (Table 16.1) [18,19,20,21,22,23,24,25]. Depending on the study, bacteria have been isolated from urinary stones in 7% to 75% of stone cultures. When limiting to non-infection stones, positive culture results have been obtained in 5–33% of stones. Pure calcium stones (CaOx or CaPhos) are culture positive in up to 44% of cases. Moreover, these stone cultures contain bacterial isolates of both non-urease splitting and urease splitting organisms. Figure 16.1 [18, 21, 26, 27] demonstrates the frequency of primary isolates from 455 positive urinary stone cultures. Commonly implicated urease splitting bacterial genera include Staphylococcus, Proteus, Klebsiella, Pseudomonas, and Providencia. However, non-urease splitting bacteria also have been isolated, including known uropathogens Escherichia coli and Enterococcus spp.
Enhanced Culture Techniques and 16S rRNA Sequencing of Urinary Stones
The previous studies relied on culture protocols similar to techniques popularized by Stamey and colleagues in the 1970s [25]. These culture protocols involve washing the stones in saline and crushing, prior to plating on standard culture media. However, these protocols are not designed to isolate the slow growing, fastidious organisms that make up a majority of urinary biomass [28]. The recent use of enhanced culture-based methods, such as enhanced quantitative urine culture (EQUC), and culture-independent methods, such as 16S rRNA gene sequencing, have demonstrated the existence of resident microbes in the urinary bladder (called the urinary microbiome or urobiome) and debunked the historical view that the bladders of women and men are sterile [28,29,30,31]. EQUC utilizes increased urine volumes, longer incubation time, multiple media types, and a variety of atmospheric conditions to isolate slower growing bacteria [29]. 16S rRNA sequencing allows for the identification of bacteria that cannot be cultured (e.g., those exposed to antibiotics prior to collection). In initial experiments utilizing 16S rRNA sequencing on five kidney stones from calcium oxalate stone-formers, our group identified members of several bacterial taxa, including Pseudomonas, Gardnerella, Lactobacillus, Enterobacteriaceae, Bradyrhizobium, Phyllobacterium, and Brucella. As stones represent a low biomass medium, available in limited quantities, strategies to determine which sequenced bacteria are truly stone associated are ongoing. The incorporation of EQC, a stone-relevant derivative of EQUC, allowed for the isolation of live Pseudomonas and E. coli strains in two of the stones collected. In each case, this was concordant with the dominant organism identified by 16S sequencing [32]. Analysis of 52 additional kidney stones obtained via ureteroscopy identified 29 (55.7%) sequence positive stones (Fig. 16.2). 16/29 (55%) were composed entirely of non-infection stone compositions, while only one stone contained elements of struvite. Furthermore, EQC was able to isolate bacteria from 11/29 (37.9%) of sequenced stones [33]. These results reflect the idea that live bacteria are associated with non-infection stones , regardless of antibiotic exposure.
Contribution of Bacteria to Urinary Stone Disease
It is now clear that bacteria are associated with stones of all compositions. Patients with USD often have concomitant UTI or infectious complications following intervention, and bacteria are readily sequenced and cultured from stone samples. Though the presence of bacteria in non-infection stones is apparent, it is unclear whether a causal relationship exists.
Supersaturation and Bacteria as a Modifier of Urinary Composition
Supersaturation of urinary solutes has long been recognized as a major pathophysiologic factor in USD. As the concentration of urinary components, mainly calcium and oxalate, reach their limits of solubility, crystallization can occur resulting in stone formation [34, 35]. Historically, the understanding of bacterial contribution to urinary solutes has focused on the intestinal microbiota; specifically, the role of Oxalobacter formigenes, which metabolizes dietary oxalate, reducing oxalate concentration of the urine, and providing a protective effect for recurrent CaOx stone formation [36, 37]. The role of O. formigenes in CaOx stone formation is discussed in depth in other chapters; notably, however, its role as a probiotic to protect against CaOx stone formation has been of limited success [38,39,40]. Oxalobacter does not commonly inhabit the genitourinary tract. However, other oxalate-degrading bacteria have been identified in the mammalian gut including Lactobacillus, Enterococcus, Bifidobacterium, and Streptococcus; microbiota that are more routinely isolated from the genitourinary tract [41]. The role of these bacteria in the urine is not currently understood.
Outside of oxalate-degrading bacteria , the gut microbiome appears to have a complex relationship with urinary solute concentration. Stern and colleagues, investigated the gut microbiome of 11 stone formers and their 24 h urine collection. They found an inverse relationship between Escherichia and urinary citrate; as well as an inverse relationship between Eubacterium and urinary oxalate [42].
Though the relationship is complex, the gut microbiome may have a protective role in USD formation. However, as previously discussed, urinary bacteria appear to be strongly associated with urinary stone formation. Hypocitraturia is a known risk factor for calcium stone formation. Urinary citrate is a strong inhibitor of stone formation as it binds to free calcium, reducing urinary calcium concentration, making urinary calcium less available to complex with oxalate [34]. Urinary bacteria may contribute to stone formation by metabolizing citrate, lowering urinary citrate concentration, thereby promoting calcium oxalate supersaturation and urinary crystal formation. In a study of idiopathic calcium stone formers, De Ferrari and colleagues noted significantly decreased urinary citrate concentrations in 17 urine culture positive patients compared to standard urine culture negative patients. These standard urine cultures grew Escherichia, Streptococcus, Staphylococcus, Pseudomonas and Citrobacter species [43]. Moreover, using in vitro urinary models, E. coli, both pathogenic and non-pathogenic, decreases urine citrate levels, promoting crystallization [44, 45].
Bacteria as Crystal Aggregator
Bacterial modification of urinary solute concentrations potentiates known risk factors of nephrolithiasis . However, supersaturation of urinary solutes alone does not always result in USD, as there is considerable variation in urine chemistries between stone formers and non-stone formers [46, 47]. As such, bacteria may play a role in crystal adherence, acting as a nidus and promoting crystal deposition in patients with stone solutes. Using an in vivo murine model, our group induced a CaOx nephropathy and uropathogenic E. coli (UPEC) pyelonephritis in ten mice. There was a significantly higher number of CaOx deposits in the CaOx and UPEC inoculated mice compared to CaOx or UPEC inoculated mice alone [32]. Similarly, crystals have been shown to aggregate on both Gram-negative and Gram-positive bacteria. Chutipongtanate and colleagues analyzed strains of E. coli, K. pneumoniae, S. aureus, and S. pneumoniae, finding increased CaOx crystal growth and aggregation with all four bacteria compared to controls [48].
There also is the possibility that bacteria may play an indirect role in crystal aggregation. In addition to crystals, urinary stones contain a protein matrix that frequently contains innate immune proteins [49]. Urinary stones create an inflammatory response that results in the release of inflammatory proteins and cytokines. This promotes the growth and adhesion of CaOx and uric acid crystals [50,51,52,53]. In our study, mice inoculated with CaOx and UPEC had an increased expression of inflammatory and stone matrix protein genes compared to inoculation with either substance alone [32]. Therefore, it is reasonable to believe that the presence of bacteria may work synergistically with CaOx to potentiate stone formation and aggregation. This is similar to findings in vascular calcifications in which bacteria potentiate atherosclerotic plaque formation [54,55,56].
Conclusion
The role of bacteria in non-infection USD is complex and poorly understood relative to that of infection stones, but the association of bacteria with all stone compositions is undeniable. Patients with non-infection stones present with UTIs, experience infectious complications after stone procedures despite negative standard urine culture, and have positive stone cultures. The use of 16S rRNA sequencing and enhanced culture techniques has expanded our knowledge of stone-associated bacteria and allowed for isolation of these bacteria. The mechanism by which bacteria promote stone formation in non-infection stone formation is an area of active research. Going forward, it is important that we further investigate this association by sequencing and isolating a larger number of stone bacteria from a diverse patient population, evaluating the genomic and proteomic capacities of these bacterial isolates, and determining their effect on stone contribution via in vitro and in vivo models.
References
Scales CD Jr, Smith AC, Hanley JM, Saigal CS, Urologic Diseases in America Project. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160–5.
Tasian GE, Ross ME, Song L, Sas DJ, Keren R, Denburg MR, Chu DI, Copelovitch L, Saigal CS, Furth SL. Annual incidence of nephrolithiasis among children and adults in South Carolina from 1997 to 2012. Clin J Am Soc Nephrol. 2016;11:488–96.
Rule AD, Lieske JC, Li X, Melton LJ 3rd, Krambeck AE, Bergstralh EJ. The ROKS nomogram for predicting a second symptomatic stone episode. J Am Soc Nephrol. 2014;25:2878–86.
Pearle MS, Calhoun EA, Curhan GC, Urologic Diseases of America Project. Urologic diseases in America project: urolithiasis. J Urol. 2005;173:848–57.
Pak CYC, Poindexter JR, Adams-Huet B, Pearle MS. Predictive value of kidney stone composition in the detection of metabolic abnormalities. Am J Med. 2003;115:26–32.
Holmgren K, Danielson BG, Fellström B, Ljunghall S, Niklasson F, Wikström B. The relation between urinary tract infections and stone composition in renal stone formers. Scand J Urol Nephrol. 1989;23:131–6.
Ohkawa M, Tokunaga S, Nakashima T, Yamaguchi K, Orito M, Hisazumi H. Composition of urinary calculi related to urinary tract infection. J Urol. 1992;148:995–7.
Huang W-Y, Chen Y-F, Chen S-C, Lee Y-J, Lan C-F, Huang K-H. Pediatric urolithiasis in Taiwan: a nationwide study, 1997–2006. Urology. 2012;79:1355–9.
Omar M, Abdulwahab-Ahmed A, Chaparala H, Monga M. Does stone removal help patients with recurrent urinary tract infections? J Urol. 2015;194:997–1001.
Oliver R, Ghosh A, Geraghty R, Moore S, Somani BK. Successful ureteroscopy for kidney stone disease leads to resolution of urinary tract infections: prospective outcomes with a 12-month follow-up. Cent Eur J Urol. 2017;70:418–23.
Assimos D, Krambeck A, Miller NL, et al. Surgical management of stones: American Urological Association/Endourological Society Guideline, PART I. J Urol. 2016;196:1153–60.
Türk C, Petřík A, Sarica K, Seitz C, Skolarikos A, Straub M, Knoll T. EAU guidelines on interventional treatment for urolithiasis. Eur Urol. 2016;69:475–82.
Michel MS, Trojan L, Rassweiler JJ. Complications in percutaneous nephrolithotomy. Eur Urol. 2007;51:899–906.
Gutierrez J, On behalf of the CROES PCNL Study Group, Smith A, Geavlete P, Shah H, Kural AR, de Sio M, Amón Sesmero JH, Hoznek A, de la Rosette J. Urinary tract infections and post-operative fever in percutaneous nephrolithotomy. World J Urol. 2012;31:1135–40.
Korets R, Graversen JA, Kates M, Mues AC, Gupta M. Post-percutaneous nephrolithotomy systemic inflammatory response: a prospective analysis of preoperative urine, renal pelvic urine and stone cultures. J Urol. 2011;186:1899–903.
Zhong W, Leto G, Wang L, Zeng G. Systemic inflammatory response syndrome after flexible ureteroscopic lithotripsy: a study of risk factors. J Endourol. 2015;29:25–8.
Rivera M, Viers B, Cockerill P, Agarwal D, Mehta R, Krambeck A. Pre- and postoperative predictors of infection-related complications in patients undergoing percutaneous nephrolithotomy. J Endourol. 2016;30:982–6.
Paonessa JE, Gnessin E, Bhojani N, Williams JC Jr, Lingeman JE. Preoperative bladder urine culture as a predictor of intraoperative stone culture results: clinical implications and relationship to stone composition. J Urol. 2016;196:769–74.
Wang X, Krambeck AE, Williams JC Jr, et al. Distinguishing characteristics of idiopathic calcium oxalate kidney stone formers with low amounts of Randall’s plaque. Clin J Am Soc Nephrol. 2014;9:1757–63.
Shafi H, Shahandeh Z, Heidari B, Sedigiani F, Ramaji AA, Pasha YR, Kassaeian AA, Pasha AA, Mir MM. Bacteriological study and structural composition of staghorn stones removed by the anatrophic nephrolithotomic procedure. Saudi J Kidney Dis Transpl. 2013;24:418–23.
Tavichakorntrakool R, Prasongwattana V, Sungkeeree S, Saisud P, Sribenjalux P, Pimratana C, Bovornpadungkitti S, Sriboonlue P, Thongboonkerd V. Extensive characterizations of bacteria isolated from catheterized urine and stone matrices in patients with nephrolithiasis. Nephrol Dial Transplant. 2012;27:4125–30.
Seema Golechha AS. Bacteriology and chemical composition of renal calculi accompanying urinary tract infection. Indian J Urol. 2001;17:111–7.
Gault MH, Longerich LL, Crane G, Cooper R, Dow D, Best L, Stockall E, Brown W. Bacteriology of urinary tract stones. J Urol. 1995;153:1164–70.
Dajani AM, Shehabi AA. Bacteriology and composition of infected stones. Urology. 1983;21:351–3.
Thompson RB, Stamey TA. Bacteriology of infected stones. Urology. 1973;2:627–33.
Eswara JR, Shariftabrizi A, Sacco D. Positive stone culture is associated with a higher rate of sepsis after endourological procedures. Urolithiasis. 2013;41:411–4.
Osman Y, Elshal AM, Elawdy MM, Omar H, Gaber A, Elsawy E, El-Nahas AR. Stone culture retrieved during percutaneous nephrolithotomy: is it clinically relevant? Urolithiasis. 2016;44:327–32.
Price TK, Dune T, Hilt EE, Thomas-White KJ, Kliethermes S, Brincat C, Brubaker L, Wolfe AJ, Mueller ER, Schreckenberger PC. The clinical urine culture: enhanced techniques improve detection of clinically relevant microorganisms. J Clin Microbiol. 2016;54:1216–22.
Hilt EE, McKinley K, Pearce MM, Rosenfeld AB, Zilliox MJ, Mueller ER, Brubaker L, Gai X, Wolfe AJ, Schreckenberger PC. Urine is not sterile: use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J Clin Microbiol. 2013;52:871–6.
Bajic P, Van Kuiken ME, Burge BK, Kirshenbaum EJ, Joyce CJ, Wolfe AJ, Branch JD, Bresler L, Farooq AV. Male bladder microbiome relates to lower urinary tract symptoms. Eur Urol Focus. 2018; https://doi.org/10.1016/j.euf.2018.08.001.
Pearce MM, Hilt EE, Rosenfeld AB, et al. The female urinary microbiome: a comparison of women with and without urgency urinary incontinence. MBio. 2014;5:e01283-14.
Barr-Beare E, Saxena V, Hilt EE, Thomas-White K, Schober M, Li B, Becknell B, Hains DS, Wolfe AJ, Schwaderer AL. The interaction between Enterobacteriaceae and calcium oxalate deposits. PLoS One. 2015;10:e0139575.
Dornbier RL (2018) 16S sequencing of urinary stones obtained via ureteroscopy. unpublished data.
Miller NL, Evan AP, Lingeman JE. Pathogenesis of renal calculi. Urol Clin N Am. 2007;34:295–313.
Coe FL, Parks JH, Asplin JR. The pathogenesis and treatment of kidney stones. N Engl J Med. 1992;327:1141–52.
Kaufman DW, Kelly JP, Curhan GC, Anderson TE, Dretler SP, Preminger GM, Cave DR. Oxalobacter formigenes may reduce the risk of calcium oxalate kidney stones. J Am Soc Nephrol. 2008;19:1197–203.
Knight J, Deora R, Assimos DG, Holmes RP. The genetic composition of Oxalobacter formigenes and its relationship to colonization and calcium oxalate stone disease. Urolithiasis. 2013;41:187–96.
Milliner D, Hoppe B, Groothoff J. A randomised phase II/III study to evaluate the efficacy and safety of orally administered Oxalobacter formigenes to treat primary hyperoxaluria. Urolithiasis. 2018;46:313–23.
Ellis ML, Shaw KJ, Jackson SB, Daniel SL, Knight J. Analysis of commercial kidney stone probiotic supplements. Urology. 2015;85:517–21.
Lieske JC. Probiotics for prevention of urinary stones. Ann Transl Med. 2017;5:29.
Miller AW, Dearing D. The metabolic and ecological interactions of oxalate-degrading bacteria in the mammalian gut. Pathogens. 2013;2:636–52.
Stern JM, Moazami S, Qiu Y, Kurland I, Chen Z, Agalliu I, Burk R, Davies KP. Evidence for a distinct gut microbiome in kidney stone formers compared to non-stone formers. Urolithiasis. 2016;44:399–407.
De Ferrari ME, Macaluso M, Brunati C, Pozzoli R, Colussi G. Hypocitraturia and Ureaplasma urealyticum urinary tract infection in patients with idiopathic calcium nephrolithiasis. Nephrol Dial Transplant. 1996;11:1185.
Edin-Liljegren A, Hedelin HH, Grenabo L, Pettersson S. Impact of Escherichia coli on urine citrate and urease-induced crystallization. Scanning Microsc. 1995;9:901–5.
Edin-Liljegren A, Rodin L, Grenabo L, Hedelin H. The importance of glucose for the Escherichia coli mediated citrate depletion in synthetic and human urine. Scand J Urol Nephrol. 2001;35:106–11.
Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Twenty-four-hour urine chemistries and the risk of kidney stones among women and men. Kidney Int. 2001;59:2290–8.
Borghi L, Guerra A, Meschi T, Briganti A, Schianchi T, Allegri F, Novarini A. Relationship between supersaturation and calcium oxalate crystallization in normals and idiopathic calcium oxalate stone formers. Kidney Int. 1999;55:1041–50.
Chutipongtanate S, Sutthimethakorn S, Chiangjong W, Thongboonkerd V. Bacteria can promote calcium oxalate crystal growth and aggregation. J Biol Inorg Chem. 2013;18:299–308.
Martelli C, Marzano V, Iavarone F, et al. Characterization of the protein components of matrix stones sheds light on S100-A8 and S100-A9 relevance in the inflammatory pathogenesis of these rare renal calculi. J Urol. 2016;196:911–8.
Suen J-L, Liu C-C, Lin Y-S, Tsai Y-F, Juo S-HH, Chou Y-H. Urinary chemokines/cytokines are elevated in patients with urolithiasis. Urol Res. 2010;38:81–7.
Lu X, Gao B, Wang Y, Liu Z, Yasui T, Liu P, Liu J, Emmanuel N, Zhu Q, Xiao C. Renal tubular epithelial cell injury, apoptosis and inflammation are involved in melamine-related kidney stone formation. Urol Res. 2012;40:717–23.
Mulay SR, Eberhard JN, Desai J, et al. Hyperoxaluria requires TNF receptors to initiate crystal adhesion and kidney stone disease. J Am Soc Nephrol. 2017;28:761–8.
Khan SR. Crystal-induced inflammation of the kidneys: results from human studies, animal models, and tissue-culture studies. Clin Exp Nephrol. 2004;8:75–88.
Clifford A, Hoffman GS. Evidence for a vascular microbiome and its role in vessel health and disease. Curr Opin Rheumatol. 2015;27:397–405.
Lanter BB, Sauer K, Davies DG. Bacteria present in carotid arterial plaques are found as biofilm deposits which may contribute to enhanced risk of plaque rupture. MBio. 2014;5:e01206–14.
Gibson FC 3rd, Hong C, Chou H-H, Yumoto H, Chen J, Lien E, Wong J, Genco CA. Innate immune recognition of invasive bacteria accelerates atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2004;109:2801–6.
Acknowledgments
We acknowledge funding from NIDDK (R01 DK117934 and R01DK106286 to ALS, and R01 DK104718 to AJW).
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Dornbier, R., Bajic, P., Schwaderer, A., Wolfe, A.J. (2019). The Role of Bacteria in Non-infection Stone Formation. In: Lange, D., Scotland, K. (eds) The Role of Bacteria in Urology. Springer, Cham. https://doi.org/10.1007/978-3-030-17542-9_16
Download citation
DOI: https://doi.org/10.1007/978-3-030-17542-9_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-17541-2
Online ISBN: 978-3-030-17542-9
eBook Packages: MedicineMedicine (R0)