Abstract
The MAT as well as the RPT provide information about the biological impact of the sample, whereas the BET determines the Endotoxin content (Table 14.1). Consequently the RPT and the MAT are better performed as end-product assays (a failed batch is lost), whereas the BET due to its speed and precision is additionally valuable as in-process control. In line with the Process Analytical Technology (PAT) initiative established by the FDA it offers the possibility to react during the production based on quick test results. Indeed most of BET are performed on starting materials and in-process controls (including process water). The MAT has a small dynamic range, similar to the end-point versions of the BET.
Mankind has experienced fever episodes from their first day on earth, typically driven by diseases or traumata. Various hypotheses about the cause and the purpose of fever had been proposed throughout the millennia, first individual antipyretic treatments such as willow bark had been developed hundreds of years ago. The wish to establish successful treatment for more patients led to the need for standardized drug manufacturing. Indeed, besides the merchants the pharmacists (not the charlatans) belonged to the first advocates of standardization. With increased knowledge and the need to manufacture bigger lots of drugs, the first Pharmacopeias of modern kind were established (USP 1820; British Pharmacopeia 1864).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Fever Related to Drug Application and the RPT
With the beginning of the twentieth century the first commercially available infusion/injection solutions were applied to patients. The broad and successful application of Salvarsan (introduced 1910), the anti-Syphilis drug developed by Nobel laureate Paul Ehrlich, was the starting point of modern chemotherapy. Besides the desired therapeutic effects drug related side effects termed “Injection fever” or “water fever”(associated to the diluent) were described frequently [1]. There was an obvious need for a suitable safety test (pyrogen test), rabbits turned out immediately (1912!) as predictive fever model. The urgent need for Large Volume Parenterals (LVP) in World War II led to the implementation of the Rabbit Pyrogen Test (RPT) in the United States Pharmacopeia in 1942. Since then both the predictive value of RPT and improved pharmaceutical manufacturing have contributed to the safety level achieved today.
The design of the RPT (Intravenous sample application, 180 minutes observation time) has never been changed substantially, and reflects a worst case scenario: the intravenous application of drugs contaminated with Endotoxin. For drugs with other application routes and pyrogens beyond Endotoxin this test design might be less predictive [2].
The RPT is mainly designed to detect pyrogenic batches of typically apyrogenic drugs [3]. Frequently the RPT has been replaced by the Bacterial Endotoxin test (BET). New in-vitro Alternatives to the RPT as the Monocyte Activation Test (MAT) combine the advantages of the RPT (assessment of pyrogenicity beyond Gram-negative Endotoxin) with the benefits of an in-vitro method (non-animal, high-throughput, easy to modify). Several drugs (including modern vaccines containing detoxified Endotoxin as adjuvant) will benefit from these new options. The replacement of animal experiments by validated (and available) alternatives is statutory according to European law.
In a worst-case scenario pyrogenic contaminations in Parenterals (especially i.v. or intrathecal application) induce a fatal systemic response of the recipients innate immune system. Symptoms range dose- and patient-dependent from fever to septic shock like symptoms. Fever as readout in the RPT is the alarm sign, but the events to be strictly avoided are the shock like symptoms. The RPT is a true pyrogen test, after intravenous application of the drug into the outer ear veins of 3–5 rabbits the body temperature is recorded for 180 minutes. The drug complies if the sum of the temperature increase (…fever) is within defined temperature ranges. Surprisingly the RPT has never been harmonized, nevertheless the different setups from EP, USP and JP obviously generate the same level of safety [4]. The test has proven over decades to be a safe and predictive pyrogen test. By its design it’s a qualitative (pass/fail) safety test for typically non-pyrogenic drugs [3]. Products with intrinsic pyrogenicity do not perfectly fit into the current RPT design, recently this problem occurred with modern vaccine formulations containing Outer membrane vesicles (OMV) [5] or modified endotoxins [6].
For many products the RPT was replaced by the Bacterial Endotoxin Test (BET; Limulus Amebocyte Lysate Assay (LAL)) after successful validation. The BET is specific for Endotoxin (Lipopolysaccharide, LPS), the main constituent of the outer cell wall of Gram-negative bacteria. LPS is the best known pyrogen, and due to its chemical robustness and outstanding biological activity a highly relevant threat for drug and patient safety. The BET is much more sensitive for Endotoxin than the RPT, and is typically performed as a quantitative assay with a wide dynamic range (kinetic versions). The Endotoxin content enumerated allows both a safety estimation (for the given drug batch) as well as a consistency estimation (between different batches of the same drug). Though replacing the RPT for many drugs, the BET is a specific assay for Endotoxin, not for pyrogenicity.
2 MAT Overview
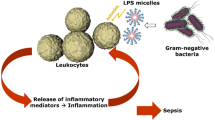
The MAT as well as the RPT provide information about the biological impact of the sample, whereas the BET determines the Endotoxin content (Table 14.1). Consequently the RPT and the MAT are better performed as end-product assays (a failed batch is lost), whereas the BET due to its speed and precision is additionally valuable as in-process control. In line with the Process Analytical Technology (PAT) initiative established by the FDA it offers the possibility to react during the production based on quick test results. Indeed most of BET are performed on starting materials and in-process controls (including process water). The MAT has a small dynamic range, similar to the end-point versions of the BET. The MAT is an unspecific test, detecting various Pathogen associated molecular patterns by several receptors (Figs. 14.1 and 14.2) [7].
The detection of relevant or unusual Endotoxin levels in a sample is a sign of danger, but presence or absence of endotoxin can’t be directly converted into pyrogenicity/absence of pyrogenicity. The BET-activity of various Endotoxins compared to the E. coli Standard varies by a factor of up to 1000 [8], different Endotoxins exhibit different levels of pyrogenicity in the RPT [9] or MAT [8, 10]. The susceptibility of different Endotoxins to Low Endotoxin recovery or Endotoxin masking [11] too is depending on structural differences between various Endotoxins (additionally depending on sample matrix), highlighting again the diversity of Endotoxins.
The Endotoxin regions required for Factor C activation (first reaction step of BET) and membrane receptor binding are different, thus BET in contrast to MAT or RPT can’t distinguish chemically detoxified Endotoxin (non-pyrogenic, e.g. MPL derived adjuvants)) from pyrogenic endotoxin [12]. Pyrogenicity elicited by Non-Endotoxin pyrogens (NEP) [13] and conflicting BET/RPT/MAT results have been reported [14, 15].
3 Monocyte Activation Test Performance
The examination of the febrile response in rabbits soon led to the concept of exogenous and endogenous pyrogens. Exogenous pyrogens (like LPS) induce the release of endogenous pyrogens in vertebrates, which finally are the elicitors of fever. This was revealed decades before the first cytokines were described and denominated. After the first description of Interleukin-1ß (the leadoff member of the still growing cytokine network) IL-6 and TNFα were characterized subsequently. IL-1ß, IL-6 and TNFα are endogenous pyrogens, if their release exceeds a threshold limit this signal (via the blood-brain barrier and Prostaglandins as mediators) finally induces a shift in the temperature regulation center of the hypothalamus. As soon as suitable reagents for cytokine assays were available, the first approaches to perform in-vitro pyrogen tests were developed [16]. These in-vitro pyrogen tests (IVPT) or monocyte activation tests (MAT) follow a similar approach. Human monocytes (whole blood, peripheral blood mononuclear cells (PBMC) or monocytic cell lines) are incubated together with the sample of interest under pyrogen-free conditions (all consumables cell culture grade or above). At the end of the incubation the samples are screened for endogenous pyrogens (mostly IL-6 or IL-1ß), the response is compared to the response elicited by an Endotoxin standard curve (or a reference batch of the drug) within the given experiment. The critical Endotoxin concentrations are known for the international Endotoxin Standard derived from E. coli from animal data [4, 17] and tests with volunteers [18,19,20]. The derived threshold limits are based on the assumption that Endotoxin is the only proinflammatory contamination in the test sample. This has to be taken into account for drugs with a pro-inflammatory mode of action or NEP-contamination, as synergistic effects with subfebrile LPS might occur. In contrast to the specific BET, the MAT is able to detect synergistic effects between subfebrile amounts of several contaminations (or a proinflammatory drug) [21, 22].
To resemble the most sensitive RPT (injection of 10 ml sample per kg bodyweight of the rabbit), a MAT must at least detect 50 pg LPS/ml sample (fever threshold of rabbits is 500 pg/kg body weight; for 10 ml injection volume this resembles 50 pg/LPS per ml) [4]. Depending on the test setup this minimal sensitivity of 50 pg LPS/ml sample can be increased to approximately 3 pg/ml. As for the RPT or BET, all data on sensitivity or concentrations deal with the concentration of the standard or the analyte(s) in the tested sample, not in the final reaction mix.
Endotoxin is the major exogenous pyrogen, the pro-inflammatory cytokines IL-1ß, IL-6 and TNFα as endogenous pyrogens are the executors of the endotoxic impact. The comparison of the resulting cytokine levels induced by the samples to the cytokine levels induced by known amounts of standard endotoxin (or a reference batch of the drug) provides an estimation about the pyrogenic potential of the sample.
Six variations of MAT had been validated successfully in Europe from 2000 to 2003 [23], an update with pooled cells (to overcome donor variability) and cryopreserved cells primary cells (to faciliate access to suitable cells) was conducted in 2004 [24]. In 2006 these validations were reviewed independently by the Scientific steering committee of ECVAM (European Centre for the Validation of Alternative Methods; structure of the European Union) and rated positively. A MAT Expert group of the European Pharmacopeia started the creation of the MAT Chapter 2.6.30., which came into force in 2010. The wish for improvements of 2.6.30. and the need to comply with EU Directive 2010/63/EC led to a revision of 2.6.30. (additionally 5.1.10. (Guidelines for using the test for bacterial endoitoxins) and 2.6.8. (Pyrogens)) had been updated 2015), which was finished in 2016. The new chapter 2.6.30. will come into force in 2017, driven by the clear statement that the RPT is to be replaced by MAT or BET now (e.g. 2019 in Germany).
The phrasing of the MAT Chapter 2.6.30. was intentionally deduced from the BET Chapter. The MAT-calculations are based on the resulting readout levels induced by either Endotoxin (standard), the test sample, and the combination of both (interference test). In the MAT Chapter 2.6.30. the Endotoxin Equivalent unit (EEU; sometimes EE (Endotoxin Equivalent)) was established, 1 EEU equals the amount of readout which is induced by 1 EU (100 pg/ml) Endotoxin within the given experiment. As 1 EEU = 1 EU, the Endotoxin Limit Concentration (ELC) of drugs equals the Contaminant Limit Concentration (CLC) of the drug for the MAT. ELC (or CLC) are calculated by dividing the K-value (depending on the route of administration; see Table 14.2) by the maximum recommended dose of product per kilogram of body mass (or square metre body surface). If no K-value is defined for the intended application, the Endotoxin/Contaminant Limit is determined on results from the development phase.
The typical sensitivity of the chosen MAT (above the Lower Limit of Quantification (LLOQ), not LOD as currently mentioned in 2.6.30.) for Endotoxin resembles the Lysate sensitivity (λ) of the BET. If the CLC (equals the ELC) of a product is known, appropriate MAT-versions can be selected by their stated sensitivity. In general, the sensitivity of the MAT can be increased by expanding the sample volume (or the concentration of the sample).
The Maximal valid dilution (MVD) is derived by dividing the CLC by the test sensitivity.
Methods A (quantitative Assay) and B (semiquantitative; may also be performed as limit test) are only possible if a MVD can be calculated (calculation depends on a special K-value for different application routes of drugs or deduced K-value), and valid results (in terms of interference) can be obtained within this MVD. In A and B the product-induced readout level is compared to the readout generated by the Endotoxin standard curve. The amount of pyrogens can not be quantified by the unspecific MAT, but the pyrogenic impact of the sample can be enumerated if the reaction of the sample is within the small dynamic range of the MAT. Methods A and B might be fused, ending up in a version of semi-quantitative or limit test where linearity of the standard curve and parallelism of the sample dilutions to the standard curve are skipped.
For products without MVD (e.g. no K-value prescribed or deducable), or products where interference can’t be overcome within the MVD or products with profound intrinsic pyrogenicity Method C (Reference Lot comparison Test) is the correct choice. In Method C, the batch under investigation is compared to a reference batch (which might be a pyrogenic (bad) batch or a nonpyrogenic (representative)) batch.
The purpose of this Article is to explain the MAT and its application and thereby support the implementation of the MAT.
3.1 Methodology
According to the revised Monograph 2.6.30. cells intended for pyrogen testing have to be qualified initially for the detection of at least two different NEP. Suitable NEP sources have been described [15]. NEP have to exhibit (if at all) an endotoxin contamination far below the detection limit of the chosen MAT. The sensitivity for the Endotoxin standard is determined. Typically these QC assurances (including tests for absence of specific blood borne diseases (as for transfusion purposes)) are delivered by the supplier of the cells. If pooled cells are used, the averaging effect should be considered (e.g. stricter safety limits). If the cells are prepared by the user itself, the local legal and ethical requirements as well as safety precautions have to be taken into account.
The methodology has been described extensively [15, 23,24,25,26,27]. During the Incubation phase (37 °C, 5% CO2, cell culture breeder) all consumables and media have to be pyrogen free (typically low endotoxin, as tested by BET), at least far below the detection limit of the MAT chosen. Incubation times range from 8 to 24 h [23, 24, 26]. All samples (including Endotoxin standard curve and negative control) are incubated in the MAT in 4 separate wells (true replicates). This is performed to balance the inherent variability of the bioassay, and to facilitate outlier procedures if necessary. The spike concentration is around the middle of the standard curve for Methods A and B. For products with intrinsic activity you might need to spike below the middle of the standard curve. The detection is performed by ELISA or other suitable techniques, the consumables don’t need to be pyrogen free for the detection step. During the product specific validation, the pure sample (without MAT incubation) should be tested on the ELISA too, to exclude interference with the detection system or the presence of the analyte in the drug. Some vaccines derived from human diploid cells are known to contain human cytokines.
Dilutions within the MVD with a spike recovery between 50% and 200% are identified. From these, the product dilution for the batch release is assigned. Three dilutions (including the release dilution) are used in routine testing (Methods A and B). In the future Method B might be performed with a single dilution (release dilution). For Methods A and B, the samples are incubated with (interference) and without Endotoxin, the resulting analyte release is compared to the levels induced by the endotoxin standard curve. In Method C, the samples are compared to reference samples. The sensitivity has to be confirmed within 50–100%, spike recovery has to be between 50% and 200%.
3.2 Ethics Statement
Donors must be informed about the medical procedure (venipuncture) and its risks, the intended use of their blood (test reagent instead of transfusion; handling of screening results for blood borne diseases; blinding procedure) and the intended time frame for using the derived cells (Shelf life) until proper disposal.
Acknowledgment of informed consent for medical procedure and sample/data treatment must be obtained from the volunteer blood donors before venipuncture.
3.3 Example: Human Serum Albumin 50 ml
A manufacturer of 20% HSA detected a pyrogenic 50 ml batch in the RPT. Instead of instantly discarding the batch without notice, the manufacturer kindly offered us several bottles of this uncommon batch for MAT development as a true pyrogenic sample.
Procedure:
A human serum albumin preparation (50 ml) is to be tested. The sensitivity of the MAT chosen is stated to be 50 pg LPS/ml. If 50 ml are chosen (of course you can choose the maximum dosage of HSA too, the daily dose should not exceed 2 g of Albumin (Human) 20% per kg of body weight) as the maximal intravenous human dose for an average adult (70 kg body weight), the CLC is:
For children or cachecticFootnote 1 patients lower mean body weights are to be applied (or dosage information per m2 body surface, see Table 14.1). The samples (incriminated batch and marketed batch) were tested in the MAT according to Method B (semi-quantitative or Limit test). A Cryoblood pool of four donors (stored since 150 days at −80 °C) was used, the chosen readout was IL-ß.
The samples were tested undiluted, 1:7 and 1:14 (MVD), the corresponding CLC’s are 7, 1 and 0.5 EEU/ml. According to the sensitivity of 50 pg/ml the samples were spiked with 100 pg/ml (2× sensitivity). The incriminated batch was clearly exceeding the respective CLC at the 1:7 dilution and 1:14 (MVD), thus being assigned “PYROGENIC”. The undiluted sample had more than 2 EEU/ml, but as this is in the upper plateau of this experiment its unclear if its below the CLC of 7 EEU/ml. Spike recovery was very good for all dilutions.
If Methods A or B are performed with a sensitivity of 50 pg/ml (resembling sensitive rabbits and 10 ml injection volume), a result at the MVD below the CLC is as safe as the most sensitive rabbit test. If Dilution A or B are used for release, the lower concentrations (B and C or C) have to be below their CLC too. If desired, a higher concentration of the test item might be used for release testing. Test sensitivity can be increased by enlarging the sample volume, thus enlarging the MVD too.
4 Discussion
The MAT was developed to replace the RPT. Mainly not for safety reasons, but to fulfil the legal requirements of animal protection. By choosing a minimal sensitivity for Endotoxin of 50 pg/ml, the MAT is at least as sensitive as the most sensitive RPT. There is no mandatory need to perform the MAT more sensitive than the RPT for the same product. Nevertheless, for large volume parenterals the sensitive MAT-setups (sensitivity 3–6 pg/ml possible) offer the opportunity for pyrogen testing, where the RPT was not sensitive enough.
In contrast to the RPT and the MAT (biological impact of the sample), the BET determines specifically the amount of Endotoxin. This is of special interest during the production of a drug (trending; process analytical technologies). Consequently, much more BET-assays are performed during the production (API, drug substance, process water) of a drug than compared to the final product testing. The RPT in contrast is a typical end product safety test. The MAT is a compendial method in Europe, but not in the United States until now. The USP has announced to create a MAT-Chapter in their current 5 year plan, they already published a revision proposal of Chapter <151> Pyrogens in which the use of validated equivalent in-vitro pyrogen tests is mentioned. The FDA has mentioned both the MAT and the rFc in their 2012 “Guidance for Industry Pyrogen and Endotoxins Testing: Questions and Answers”. The NIFDC (China) did a lot of work on MAT, and is planning to propose it for the Chinese Pharmacopoeia [27]. The Indian Pharmacopeia is working on a MAT-Chapter too. Various agencies have practical experience with the MAT (Table 14.3).
A compelling way to replace the RPT would be to implement the MAT on the end product testing, but to keep all BET on the process steps (and end product), thus combining the advantages of the MAT (in-vitro pyrogen test) with the known advantages of the BET (specific, fast, trending). The MAT is a necessary replacement for the RPT, and a predictive and versatile tool for special applications [e.g. drugs with intrinsic pyrogenicity (the European Pharmacopeia is working on a MAT-Chapter 2.6.40. on vaccines with intrinsic pyrogenicity); vaccines, adjuvants, detoxified LPS, maybe Low Endotoxin recovery (LER) …]. The introduction of the MAT as replacement for the RPT will hopefully initiate the further exploration of its capabilities [e.g. Masking/Demasking of Endotoxin; selection of representative donor pools (age, gender, genetical background) for various applications, material mediated pyrogenicity [28]]. If manufacturers decide to switch from the RPT to the BET, the presence of Non Endotoxin pyrogens (NEP) has to be excluded by the MAT (as it has been done in the past by the RPT). If the RPT data are there from the past (indicating no pyrogenic problems apart from Endotoxin), no additional animal experiments are required. Variations in the production scheme initiate a further comparison of MAT vs. BET (typically three production batches).
The BET is no animal experiment, but the Lysate is derived from a wildlife stock of animals. Most animals survive this well designed procedure (mortality is estimated between 8% to >15% by different stakeholders), but the demand for Lysate is growing continuously. Consequently the number of animals used for bleeding is growing. All four Horseshoe crab species are listed on the red list of the International union for the conservation of nature (IUCN). The main BET-supplier (Limulus polyphemus; east coast of the USA) is well protected and surveilled, but the situation of the three Asian species is alarming. The use of recombinant Factor C (or other recombinant setups) or BET-Assays with reduced Lysate volume would immediately improve the situation of the animals, and make the industry (and the regulators) less dependent on these fascinating animals. The European Pharmacopeia intends to create a separate Monograph 2.6.32. on recombinant Endotoxin Tests. The first draft was published at the end of 2018, combined with the hope that harmonization will take place soon. In 2018 the first drug tested by rFc was approved by the FDA. It’s time to use the new tools.
Notes
- 1.
Physical wasting with loss of weight and muscle mass due to disease.
References
Hort EC, Penfold WJ. The relation of Salvarsan fever to other forms of injection fever. Proc R Soc Med. 1912;5(Pathol Sect):131–9.
Cartmell T, Mitchell D, Lamond FJD, Laburn HP. Route of administration differentially affects fevers induced by gram-negative and gram-positive pyrogens in rabbits. Exp Physiol. 2002;87(3):391–9. https://doi.org/10.1113/eph8702298.
Williams KL, editor. Endotoxins: pyrogens, LAL testing, and depyrogenation. 2nd ed. New York u.a: Marcel Dekker; 2001.
Hoffmann S, Luderitz-Puchel U, Montag T, Hartung T. Optimisation of pyrogen testing in parenterals according to different pharmacopoeias by probabilistic modelling. J Endotoxin Res. 2005;11(1):25–31.
Vipond C, Findlay L, Feavers I, Care R. Limitations of the rabbit pyrogen test for assessing meningococcal OMV based vaccines. ALTEX. 2016;33(1):47–53. https://doi.org/10.14573/altex.1509291.
Gerke C, et al. Production of a Shigella sonnei vaccine based on generalized modules for membrane antigens (GMMA), 1790GAHB. PLoS One. 2015;10(8):e0134478. https://doi.org/10.1371/journal.pone.0134478.
Hasiwa N, et al. Evidence for the detection of non-endotoxin pyrogens by the whole blood monocyte activation test. ALTEX. 2013;30(2):169–208.
Dehus O, Hartung T, Hermann C. Endotoxin evaluation of eleven lipopolysaccharides by whole blood assay does not always correlate with Limulus amebocyte lysate assay. J Endotoxin Res. 2006;12(3):171–80.
Greisman SE, Hornick RB. Comparative pyrogenic reactivity of rabbit and man to bacterial endotoxin. Proc Soc Exp Biol Med. 1969;131(4):1154–8.
Bache C, et al. Bordetella Pertussis Toxin does not induce the release of pro-inflammatory cytokines in human whole blood. Med Microbiol Immunol. 2012;201(3):327–35. https://doi.org/10.1007/s00430-012-0238-1.
Reich J, Lang P, Grallert H, Motschmann H. Masking of endotoxin in surfactant samples: effects on Limulus-based detection systems. Biologicals. 2016;44(5):417–22. https://doi.org/10.1016/j.biologicals.2016.04.012.
Brandenburg K, Howe J, Gutsman T, Garidel P. The expression of endotoxic activity in the Limulus test as compared to cytokine production in immune cells. Curr Med Chem. 2009;16(21):2653–60.
Huang LY, Dumontelle JL, Zolodz M, Deora A, Mozier NM, Golding B. Use of toll-like receptor assays to detect and identify microbial contaminants in biological products. J Clin Microbiol. 2009;47(11):3427–34. https://doi.org/10.1128/JCM.00373-09.
Perdomo-Morales R, Pardo-Ruiz Z, Spreitzer I, Lagarto A, Montag T. Monocyte Activation Test (MAT) reliably detects pyrogens in parenteral formulations of human serum albumin. ALTEX. 2011;28(3):227–35.
Solati S, Aarden L, Zeerleder S, Wouters D. An improved monocyte activation test using cryopreserved pooled human mononuclear cells. Innate Immun. 2015;21:677–84. https://doi.org/10.1177/1753425915583365.
Dinarello CA. Cytokines as endogenous pyrogens. J Infect Dis. 1999;179 Suppl 2(0022-1899 (Print)):S294–304.
Spreitzer I, Fischer M, Hartzsch K, Luderitz-Puchel U, Montag T. Comparative study of rabbit pyrogen test and human whole blood assay on human serum albumin. ALTEX. 2002;19(Suppl 1):0946–7785. (Print), 73–5.
Hochstein HD, Fitzgerald EA, McMahon FG, Vargas R. Properties of US Standard Endotoxin (EC-5) in human male volunteers. J Endotoxin Res. 2010;1:52–6.
Suffredini AF, Hochstein HD, McMahon FG. Dose-related inflammatory effects of intravenous endotoxin in humans: evaluation of a new clinical lot of Escherichia coli O:113 endotoxin. J Infect Dis. 1999;179(5):1278–82.
Engler H, Benson S, Wegner A, Spreitzer I, Schedlowski M, Elsenbruch S. Men and women differ in inflammatory and neuroendocrine responses to endotoxin but not in the severity of sickness symptoms. Brain Behav Immun. 52:18–26. https://doi.org/10.1016/j.bbi.2015.08.013.
Pardo-Ruiz Z, Menéndez-Sardiñas DE, Pacios-Michelena A, Gabilondo-Ramírez T, Montero-Alejo V, Perdomo-Morales R. Soluble β-(1,3)-glucans enhance LPS-induced response in the monocyte activation test, but inhibit LPS-mediated febrile response in rabbits: implications for pyrogenicity tests. Eur J Pharm Sci. 2015;81:18–26. https://doi.org/10.1016/j.ejps.2015.09.018.
Kikkert R, Bulder I, de Groot ER, Aarden LA, Finkelman MA. Potentiation of Toll-like receptor-induced cytokine production by (1-->3)-beta-D-glucans: implications for the monocyte activation test. J Endotoxin Res. 2007;13(3):140–9.
Hoffmann S, et al. International validation of novel pyrogen tests based on human monocytoid cells. J Immunol Methods. 2005;298(1–2):161–73.
Schindler S, et al. International validation of pyrogen tests based on cryopreserved human primary blood cells. J Immunol Methods. 2006;316(1–2):42–51.
Daneshian M, von Aulock S, Hartung T. Assessment of pyrogenic contaminations with validated human whole-blood assay. Nat Protoc. 2009;4(12):1709–21. https://doi.org/10.1038/nprot.2009.159.
Koryakina A, Frey E, Bruegger P. Cryopreservation of human monocytes for pharmacopeial monocyte activation test. J Immunol Methods. 2014;405:181–91. https://doi.org/10.1016/j.jim.2014.01.005.
He Q, et al. Analysis of IL-6 and IL-1β release in cryopreserved pooled human whole blood stimulated with endotoxin. Innate Immun. 2018;24(5):316–22. https://doi.org/10.1177/1753425918777596.
Borton LK, Coleman Kelly P. Material-mediated pyrogens in medical devices: applicability of the in vitro monocyte activation Test1. ALTEX. 2018;2018.
Acknowledgements
The author expresses his gratitude to his colleagues of the Paul-Ehrlich-Institut, the German Pharmacopeia, the Members of the EDQM and the Working Party “BET” and various contacts in international agencies and companies for excellent permanent support on the behalf of Endotoxin/Pyrogen testing.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Spreitzer, I. (2019). Evolution and Characteristics of the Monocyte Activation Test (MAT). In: Williams, K. (eds) Endotoxin Detection and Control in Pharma, Limulus, and Mammalian Systems. Springer, Cham. https://doi.org/10.1007/978-3-030-17148-3_14
Download citation
DOI: https://doi.org/10.1007/978-3-030-17148-3_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-17147-6
Online ISBN: 978-3-030-17148-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)