Abstract
Naturally occurring compounds can have protective effects towards mutagens and carcinogens, as shown by numerous studies. Several lichen species have been given much attention by researchers because their extracts and compounds have been used in traditional medicine to cure diseases such as ulcer, arthritis, tuberculosis, and cancer throughout the ages. Although a wide variety of scientific investigations on the biological activities of lichen extracts and their constituents have been performed, there is rather less research on their genotoxicity and antigenotoxic activity. To date, most results for the genotoxic/antigenotoxic activities of lichens have been obtained for lichen extracts and their constituents using the Ames/Salmonella and the E. coli WP2 microsome, chromosome aberration, micronucleus, sister chromatid exchange, single-cell gel electrophoresis, 8-OH-dG activity, and wing somatic mutation and recombination assays. In the present chapter, findings on antigenotoxic/genotoxic activities and its mechanisms are evaluated. By using the most common bacterial and nonbacterial assays, extracts of various lichen species have been shown to have promising antigenotoxic activity with somewhat less genotoxic activity. Lichen extracts may have a possible therapeutic potential. Therefore, these extracts must be further investigated by other multiple in vitro bioassays for development of therapeutic agents.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
Naturally occurring organic compounds from a variety of organisms, including medicinal plants, are very important. Development of these compounds for new therapeutic agents has become a popular research objective. Investigation of the biological potential of several classes of natural agents, dietary constituents, hormones, and vitamins using in vitro and in vivo bioassays have shown that they can act as genotoxicity inhibitors as well as protectors against cytostasis or environmental carcinogens (Okai et al. 1996; Scarpato et al. 1998; Ipek et al. 2003, 2005; Mersch-Sundermann et al. 2004; Jayaprakasha et al. 2007; Zeytinoglu et al. 2008; Hoshina and Marin-Morales 2014; Delebassée et al. 2017; Makhuvele et al. 2018; Kaura et al. 2018; Kuete et al. 2018). Also, investigation of the possible genotoxicity of such agents captures the attention of researchers because of their use in folk medicine or potential applications. Most of the medicinal plants used traditionally have never been subjected to toxicological tests such as that required for modern pharmaceutical compounds. However, research has shown that quite a few plants that are used in traditional medicine or similar applications may have genotoxic or carcinogenic properties (Santos et al. 2009; Nieminen et al. 2002; Sponchiado et al. 2016; Quadros et al. 2017; Kahaliw et al. 2018; Prinsloo et al. 2018). Recently, development of an in silico method by using different computer models for genotoxicity and carcinogenicity prediction of secondary plant metabolites suggested that these potentials can be a first step for prioritization (Glück et al. 2018). Therefore, it becomes very important to search extracts derived from plants that contain a variety of compounds for their nontoxic, antigenotoxic, or genotoxic properties.
Lichen species have attracted much of the attention of researchers because their extracts and compounds have been used in traditional medicine in Europe, Asia, and North America (Richardson 1988; Cabrera 1996; Tilford 1997). Although lichen extracts and their compounds have been subjected to many scientific investigations for their biological activities such as immunostimulation, analgesic, anti-ulcerogenic, antipyretic, antimicrobial, antioxidative, and antitumor (Kumar and Müller 1999; Ingolfsdottir et al. 2000; Ingolfsdottir 2002; Türk et al. 2003; Tay et al. 2004; Yılmaz et al. 2004; Halici et al. 2005; Karunaratne et al. 2005; Behera et al. 2006; Zeytinoglu et al. 2008; Yeash et al. 2017; Tomović et al. 2017; reviewed in Prashith Kekuda et al. 2018), there is rather less research on their genotoxic or antigenotoxic activity. Scientific investigation of the genotoxic and antigenotoxic properties of lichens includes in vitro and in vivo studies, mostly using the aqueous, methanol, acetone, or n-hexane extracts. In the recent past few years, the genotoxicity and antigenotoxicity of isolated compounds from lichen species has attracted attention. In the present chapter, findings on the antigenotoxic and genotoxic activity of lichen extracts or second metabolites and the relevant mechanisms are evaluated.
6.2 Bioassays for the Antigenotoxicity/Genotoxicity of Lichens
The Organization for Economic Co-operation and Development (OECD 2012) and the European Centre for the Validation of Alternative Methods (ECVAM 2012) have widely investigated the validation of mutagenicity tests. A set of assays is recommended to determine the genotoxicity of a test agent. The methods most frequently used for the assessment of genotoxic/antigenotoxic activity of lichen extracts or components based on bacterial short-term assays and mammalian test system are recommended by the OECD and the ECVAM. The Ames/Salmonella/microsome (Ames) and the Escherichia coli WP2/microsome (WP2) reverse mutation assays are the most common bacterial systems, and micronucleus (MN), chromosome aberration (CA), sister chromatid exchange (SCE), single-cell gel electrophoresis (COMET), and wing somatic mutation and recombination test (SMART) the most common nonbacterial systems, that have been used to date.
6.2.1 Bacterial Short-Term Assays
The Ames and the WP2 assays are short-term bacterial reverse mutation assays specifically designed to detect a wide range of chemicals or other agents which can produce genetic damage. The combination of Salmonella typhimurium and Eschericha coli strains in the tests recommended by OECD (1997) are given in Table 6.1, including their characteristics such as mutation types, plasmids, reversion events, and the DNA target of mutations (Maron and Ames 1983; reviewed by Mortelmans and Zeiger 2000). These assays provide a very sensitive study of potentially mutagenic pathways for the metabolism of compounds in both absence and presence of a rat liver microsomal system (S9 mix). The Ames assay employs several histidine-dependent Salmonella strains, each carrying different mutations in various genes in the histidine operon, pointing to different mutagenic mechanisms. When the Salmonella strains carrying mutations in the his gene are grown on a minimal media agar plate with a trace of histidine, only those bacteria that revert to histidine independence are able to form colonies (Fig. 6.1). When a mutagen is added to the plate, the number of revertant colonies per plate is increased. Base-pair substitution (A:T to G:C or G:C to A:T) and frameshift mutations (deletions) in S. typhimurium strains are represented to identify both types of mutation caused by a test compound. Therefore, differences in the activity of a test compound acting in these strains may yield some insight into how the compounds interact with the DNA of bacteria. Additionally, some genetic markers have been developed to make the strains more sensitive to certain types of mutagens.
Ames test plates of TA100 strain of Salmonella typhimurium. (a) Control: spontaneous revertants. (b) A mutagenic dose response to sodium azide. (From Mortelmans and Zeiger 2000)
The Escherichia coli WP2 assay detects trp (−) to trp (+) reversion at a site blocking a step in the biosynthesis of tryptophan before the formation of anthranilic acid. The different auxotrophic WP2 strains all carry the same A:T base pair at the critical mutation site within the trpE gene (see Table 6.1). The most widely used E. coli WP2 strains, each carrying the trpE mutation, are WP2 (wild type for DNA repair), WP2 (pKM101), WP2 uvrA, and WP2 uvrA (pKM101) (Mortelmans and Riccio 2000; Sugiyama et al. 2016). The assay is currently used by many researchers in conjunction with the Ames assay for screening chemicals for their mutagenicity. The Ames assay procedures are the same as for the WP2 assay with the exception that limited histidine instead of limited tryptophan is used. International guidelines have been established for performing these mutagenicity assays. These assays are used worldwide as an initial screen to determine the mutagenic/antimutagenic potential of new chemicals, drugs, or natural products from plants or animals (Sponchiado et al. 2016).
Conversely, the anti-mutagenicity of a compound against a selected positive mutagen can be investigated when the two chemicals are co-administered to the bacteria in both test systems. Using known mutagenic compounds as “positive controls,” it is possible to study whether tested components can reduce DNA damage.
6.2.2 Nonbacterial Short-Term Assays
At present, several antigenotoxicity/genotoxicity assays, which include the chromosome aberration (CA), micronucleus (MN), somatic mutation and recombination test (SMART), sister chromatid exchange (SCE), single-cell gel electrophoresis (SCGE), or COMET assays, are available, and they are recommended to use as a set for investigations.
According to the literature, the antigenotoxic/genotoxic potential of lichens has been evaluated, commonly by MN, SCE, CA, COMET, 8-oxo-2-deoxyguanosine (8-oxo-dG) activity assays in mammalian cells and the mitotic index (MI) assay in plant cells. The purpose of the MN test is to examine the structural and numerical chromosomal damage which formed small membrane-bound DNA fragments or micronuclei in the cytoplasm of interphase cells caused by a tested agent or by clastogens and aneugens. Micronuclei can be formed by chromosome fragments lacking a centromere or whole chromosomes that are unable to migrate during cell division. The MN test can be conducted in the presence or in the absence of cytochalasin B, which is used to block cell division and generate binucleated cells (Fig. 6.2a). The cytokinesis-block micronucleus assay is a sensitive, comprehensive and simple methodology for measuring DNA damage, cytostasis, and cytotoxicity which can be scored easily in a variety of systems 3 and in vivo (Fenech 2007; Kirsch-Volders et al. 2011). The assay is being applied successfully for biomonitoring in vivo genotoxin exposure, for in vitro genotoxicity testing, and in diverse research fields such as nutrigenomics and pharmacogenomics.
Photomicrographs for nonbacterial genotoxicity assays. (a) A mitogen-stimulated cytokinesis-blocked lymphocyte containing one micronucleus (MN). (b) Giemsa staining of BrdU-incorporated chromosomes in human lymphocytes for sister chromatic exchange (SCE); arrowheads show chromosome breaks and sister union. (c) Chromosome aberration (CA) shows sister chromatids stained at different densities (photograph kindly provided by Dr. B. Ayaz Tuylu). (d) COMET tails of chromosomes visualized by an epifluorescence microscope (photograph kindly provided by Dr. A.T. Koparal)
The SCE assay is another short-term test useful for the detection of reciprocal exchanges of DNA between two sister chromatids of a duplicating chromosome in mammalian and also nonmammalian cells. Various cytomolecular protocols have been used to perform the SCE assay (Bakkali et al. 2008). SCEs result from the interchange of DNA replication products and involve DNA breakage and reunion (Wilson and Thompson 2007). Detection of SCEs requires the differential staining of sister chromatids, which usually can be achieved by the incorporation of bromodeoxyuridine (BrdU) into chromosomal DNA for two cell cycles (Fig. 6.2b). After labeling, treatment of cells with a spindle inhibitor such as colchicine is required to accumulate cells in a metaphase-like stage of mitosis (Perry and Evans 1975; Ipek et al. 2003).
The short-term in vitro mammalian cell chromosome aberration (CA) test measures the frequency of asymmetrical structural chromosome aberrations after exposure to test chemicals or mutagens. The in vitro CA test may employ cultures of established cell lines or primary cell cultures. Human peripheral blood lymphocytes (HPL) are stimulated to divide by cyclophosphamide in whole blood cultures. Cells in metaphase are analyzed for the presence of chromosomal aberrations (Fig. 6.2c) (Clare 2012).
The COMET assay is used to detect the DNA-strand breaks in eukaryotic cells and named by the shape of the observed DNA distribution, which bears resemblance to a celestial comet. This well-established, highly sensitive, rapid, and simple genotoxicity test is based on the lysing of cells embedded in agarose on a microscope slide to form nucleoids containing supercoiled loops of DNA linked to the nuclear matrix. Then, electrophoresis at high pH results in structures resembling comets, as observed by epifluorescence microscopy (Fig. 6.2d). The intensity of the comet tail relative to the head reflects the number of DNA breaks (Singh et al. 1988; Collins 2004; Speit et al. 2009). Depending on experimental conditions, the migrating DNA reflects the number of single- or double-strand breaks, and alkali-labile sites, including incomplete excision repair sites, but also DNA–DNA and DNA–protein crosslinks (Santos et al. 2009; Verschaeve et al. 2010). A broad spectrum of DNA damage can then be detected either by visual classification of comet morphologies or from morphological parameters obtained by image analysis.
Level of 8-oxo-2′-deoxyguanosine (8-oxo-dG) is a frequently used biomarker of oxidative DNA damage caused by free radicals and other reactive species constantly generated in vivo. 8-Oxo-dG is later removed from DNA by the base excision repair pathway and subsequently transported into body fluids such as saliva, urine, and plasma. Such oxidative damage to DNA is probably the contributor of the age-related development of diseases such as cancer. Agents that decrease oxidative DNA damage should thus decrease the risk of cancer development. Thus, the measurement of 8-oxo-dG is the most common method of assessing DNA damage (Halliwell 2000; Türkez et al. 2012a). An assay for the measurement of 8-oxo-dG has been developed by using a monoclonal antibody specific to 8-oxo-dG (N45.1), and the enzyme-linked immunosorbent assay (ELISA) has been well established (Toyokuni et al. 1997).
The mitotic index (MI) as a parameter for the evaluation of cytotoxic agents is the ratio of the number of cells undergoing mitosis to the number of cells not undergoing mitosis in a cell population. Mutagens can be detected cytologically by cellular inhibition, disruption in metaphase, induction of chromosomal aberrations and chromosomal fragmentation, and disorganization of the mitotic spindle and consequently of all subsequent dependent mitotic phases. MI is used as an indicator of adequate cell proliferation that can be measured by various plant test systems. Cytotoxicity tests using plant test systems in vivo, such as Allium cepa and Zea mays, are validated by several researchers who performed these tests jointly with other organism testing for genotoxicity (Agar et al. 2010; Gökbayrak and Sivas 2011; Aslan et al. 2012b).
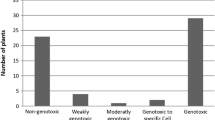
Somatic mutation and recombination tests (SMART) is a fast and inexpensive mutagenicity detection test using a higher eukaryotic organism in vivo. Genetic damages induced by compounds that generate reactive oxygen species through different action mechanisms such as point mutations, deletions, and somatic recombination can be detected in cells of the wing imaginal disks of Drosophila melanogaster in this assay (Fig. 6.3). In particular, the w/w + eye and the mwh/flr wing variants of D. melanogaster have been extensively used to investigate the genotoxicity of a great number of chemicals, including plant extracts and pure compounds (Graf et al. 1984; Sponchiado et al. 2016; Teixeira Zafred et al. 2016).
Wings of Drosophila melanogaster illustrated by scanning electronic microscopy after a SMART mutagenicity assay: wild (a); multiple wing hair (mwh) mutation (b); and flare (flr3) mutation (c). (From Teixeira Zafred et al. 2016)
Overall these assays are those most used worldwide to determine the genotoxic/antigenotoxic potential of chemicals or natural products (as reviewed by Sponchiado et al. 2016). Thus, it is obvious that it is very important to always include bacterial and mammalian tests for genotoxicity/antigenotoxicity screening of compounds. Antigenotoxicity analysis of plant products is of valuable interest because they are a potential source of therapeutic agents, and thus safety assessments are crucial to validate their use in various areas, not only in phototherapy.
6.3 Antigenotoxic or Genotoxic Potential of Lichen Extracts
The first report on the genotoxicity of lichens using the Ames mutagenicity assay was on a secondary metabolite, physodic acid isolated from Hypogymnia enteromorpha (Ach.) Nyl. by Shibamoto and Wei (1984); the second one was on usnic acid by Al-Bekairi et al. (1991). Then, after 2007, biological activities of lichen compounds have been received more attention and several studies have been performed on the genotoxicity/antigenotoxicty of lichens in about the past 10 years. The studies up to date are summarized in two separate tables according to their activities. In Table 6.2, lichen species tested for only their antigenotoxicity or both genotoxicity and antigenotoxicity are listed. The lichen species which were tested for only their genotoxicity are listed in Table 6.3.
The first report describing the therapeutic potential of lichens against drug genotoxicity was from Geyikoglu et al. (2007) (Table 6.2). Aqueous extracts of four common lichen species collected from Giresun Province in Turkey, namely, Dermatocarpon intestiniforme, Pseudevernia furfuracea, Parmelia pulla, Ramalina capitata, and Rhizoplaca melanophthalma were tested for their genotoxic and antigenotoxic potentials. Dermotocarpon intestiniforme, Pseudevernia furfuracea, Parmelia pulla, and Ramalina capitata were found to be antigenotoxic at 5–10 μg/ml against colloidal bismuth subcitrate (CBS)-induced SCE and MN formation in human peripheral lymphocytes (HPL) in vitro. However, one other species, Rhizoplaca melanophthalma, was not antigenotoxic. The order of antigenotoxicity efficacy against CBS was Pseudevernia furfuracea, Dermotocarpon intestiniforme, Ramalina capitata, and Parmelia pulla. On the other hand, all lichen extracts tested were not genotoxic alone (Table 6.2).
After this work, a fresh aqueous extract of Cetraria aculeatea (Schreb.) Fr., one of the common species in Turkey, was studied for its genotoxic/antigenotoxic activities in both Ames and mammalian cell systems (Zeytinoglu et al. 2008). The extract (at 0.1–500 μg/ml) exhibited strong antigenotoxic activity against three known mutagenic agents, 4-nitrophenylenediamine (4-NPD), 2-aminoflourene (2-AF), and sodium azide (NaN3), in TA98 and TA100 strains of Salmonella typhimurium in the presence and absence of metabolic activation, without any mutagenic activity (Table 6.2). Preincubation of bacteria with the extract prevented the mutagenic activity of 4-NPD in the higher concentration range in both strains grown without metabolic activation than those grown with metabolic activation. It was suggested that the antigenotoxic potential of the extract was higher in the absence of the metabolic system and in inhibiting frameshift mutations. Result indicates a direct and specific activation of the extracts. However, in a further investigation, the extract of Cetraria aculeata (Schreb.) Fr. did not have antigenotoxic activity against mitomycin C (MMC) in term of MN formation in HPL. The extract was not also genotoxic alone in the mammalian system. As reported very recently by Ceker et al. (2018), non-genotoxicity of methanol extracts of C. aculeata has been confirmed by Ames, WP2, and SCE assays. The extract at 5–20 μg/ml also had antigenotoxic activity, interestingly including a mammalian system. Furthermore, methanol extracts of two other species of Cetraria, C. islandica and C. olivetorum, showed activities similar to the activities of C. aculetea (Kotan et al. 2011; Aslan et al. 2012b; Ceker et al. 2018). According to the overall results, the extract of C. aculeata is significantly antigenotoxic in a bacterial system whereas it is not capable of inhibiting MN formation in MMC-induced human peripheral blood cells, which suggests variation in the effects of different solvent extracts and bioassay systems.
Recently, more investigation has been performed with an aqueous extract of Dermatocarpon intestiniforme in cultured HPL (Table 6.2). The extract at 25 and 50 ppm concentration conferred protection against cadmium chloride (CdCl2) (30 ppm)-induced MN formation despite its non-genotoxicity in the cells (Guner et al. 2012). It was also shown that the SCE and MN rates induced by mercury chloride (HgCl2) were alleviated in the cells treated with 50 μg/ml of the extract (Türkez and Dirican 2012). The extract was also antigenotoxic against imazalil (IMA)-induced CA and MN formation in cultured HPL. The lymphocytes were treated in vitro with varying concentrations of the lichen extract (25–100 μg/ml), and tested in combination with IMA (336 μg/ml). The extract alone was not genotoxic, and when combined with IMA treatment, it reduced the frequency of CAs and the rate of MNs (Türkez et al. 2012b). According to overall results of the MN, CA, and SCE assays, the extract of Dermatocarpon intestiniforme (5–100 μg/ml) is quite antigenotoxic against different types of clastogens or aneugens, which causes structural and numerical chromosomal damage.
One other aqueous extract of the lichen species Peltigera rufescens and Xanthoria elegans (25–100 μg/ml) has been assessed by four genotoxicity endpoints including CA, MN, SCE, and 8-oxo-dG assays in HPL (Türkez et al. 2012a, d; Aydin and Türkez 2011b). IMA- and MMC-induced frequencies of four genotoxic indices were diminished by the extract, indicating its inhibitory effect on oxidative DNA damage of reactive agents besides the structural and numerical chromosomal damages. The extract and its secondary metabolites may have potential to decrease the risk of cancer development.
The antigenotoxic effects of methanol extracts of Rhizoplaca melanophthalma and Rhizoplaca chrysoleuca against known mutagens have been evaluated in two different organisms, a plant and bacteria, using different assays (Agar et al. 2010). The extract (5–40 μg/plate) prevented NaN3-induced mitotic index partially in Zea mays seeds. Furthermore, it was antimutagenic against 9-aminoacridine (9-AA)-induced mutation in the TA1537 strain at all tested concentrations (0.5–5 μg/plate) in the Ames test. The inhibition rates ranged from 70.73% to 85.71%. The extract was also found to have antigenotoxic activity against AFB1-induced genetic damage (Alpsoy et al. 2011).
Several investigators have focused on the possible antigenotoxic potential of lichens against the well-known mutagen aflatoxin B1 (AFB1). Türkez et al. (2010) reported the antigenotoxic activity of another lichen species, Pseudevernia furfuracea, using its methanol, acetone and n-hexane extracts. None of these lichen extracts induced a significant number of SCEs and MN in cytokinesis-blocked HPL. Moreover, the results indicated that AFB1-induced SCEs were inhibited by the application of 50 μM methanol or acetone extracts. The positive effect of methanol, acetone, and ether extracts in decreasing the incidence of MN in comparison with an unprotected level was attained when cultures were treated simultaneously with AFB1 and the extracts. Agar et al. (2011) reported that methanol extracts obtained from Usnea longissima suppress the mutagenic effects of AFB1 in HPL examined by the SCE and MN tests. Kotan et al. (2011, 2013) also found that AFB1-induced genotoxicity has been suppressed by the methanol extract of other lichen species, Cetraria islandica and Cladonia rangiformis. The results showed that the frequencies of SCE and MN level decreased when 5 and 10 mg/ml of extract was added to AFB1-treated cultures. The methanol extracts of Rhizoplaca chrysoleuca and Lecanora muralis, at 5 and 10 μg/ml (Alpsoy et al. 2011), and Umbilicaria vellea and Xanthoparmelia somloensis (Aslan et al. 2012a) were antigenotoxic against AFB1-induced SCE and MN formation in HPL in vitro.
The methanol extract of Evernia prunastri (Huds.) Willd. was a strong antimutagenic on TA1537 and WP2 strains, with 37.70% and 69.70% inhibition rates against methylnitronitrosoguanidine (MNNG) and acridine-induced mutagenicity, respectively. Co-treatments of HPL with the extract and AFB1 decreased the frequencies of SCE (Alpsoy et al. 2013).
The genotoxic and antigenotoxic effects of the methanol extract of Cladonia foliacea (Huds.) Willd., Cladonia chlorophaea, and Cetraria olivetorum were studied using WP2, Ames (TA1535 and TA1537), and SCE test systems (Anar et al. 2013; Ceker et al. 2018). According to their results, a 5 μM concentration of AFB1 changed the frequencies of SCE. When 5 and 10 μg/ml concentrations of extract were added to AFB1, the frequencies of SCE were decreased. The extracts of Cladonia chlorophaea and Cetrelia olivetorum had further antigenotoxic activity against a series of known mutagens, MNNG, NaN3, and 9-AA besides AFB1 (Ceker et al. 2018). On the other hand, the extracts of both species were not mutagenic in tested organisms although each has antimutagenic activity.
Pseudevernia furfuracea and Cetraria islandica were tested using their methanol extracts for both genotoxic and antigenotoxic activities. The extracts of these two species were not mutagenic in Ames and Zea mays mitotic index test systems. Furthermore, some extracts showed significant antimutagenic activity against 9-AA in the Ames test. Inhibition rates for 9-AA mutagenicity ranged from 25.51% (Pseudevernia furfuracea, 0.05 μg/plate) to 66.14% (Cetraria islandica, 0.05 μg/plate). In addition, all the extracts were significantly antimutagenic against NaN3, increasing the MI values of Zea mays (Aslan et al. 2012b). Gormez et al. (2013) showed that the methanol extract of Peltigera canina posses an antigenotoxic potential in Ames and WP2 tests.
Another seven lichen species were collected from Erzurum and Artvin provinces in Turkey. Aspicilia calcarea, Bryoria capillaris, Cetraria chlorophylla, Hypnogymnia physodes, Peltigera rufescens, Physcia aipolia, Ramalina polymorpha, and Usnea florida have been tested only for genotoxicity of the water extracts in cultured HPL (Table 6.3) (Aydin and Türkez 2011a, b; Türkez et al. 2012c). All tested lichen extracts up to 500 or 1000 mg/l concentration had no genotoxic effects on the cells by the application of CA and MN assays; however, antioxidant properties were shown. The methanol extract of Hypogymnia physodes (L.) Nyl. was also studied for its genotoxicity using CA and MN tests. Relatively higher concentrations are required for its genotoxic activity (Ari et al. 2012).
6.4 Antigenotoxic/Genotoxic Potential of Lichen Secondary Metabolites
Lichen secondary metabolites exert various biological actions such as antitumor, antimicrobial, antiinflammatory, apoptotic, and cytotoxic activities (Ingolfsdottir et al. 1997; Vijayakumar et al. 2000; Huneck 2001; Tay et al. 2004; Yılmaz et al. 2004; Karunaratne et al. 2005; Mayer et al. 2005; Einarsdottir et al. 2010; Mitrovic et al. 2011; Molnar and Farkas 2010). Usnic acid is one of the most abundant lichen secondary metabolites studied for its biological activities. It has been used widely in the pharmaceutical and cosmetic industry because it has high antimicrobial activity (Ingolfsdottir 2002). Furthermore, usnic acid exhibited an antiproliferative effect on human leukemia (K562) and endometrial carcinoma (Ishikawa, HEC-50) cells (Cardarelli et al. 1997; Kristmundsdóttir et al. 2002).
A few findings present the genotoxic/antigenotoxic activities of lichen secondary metabolites. The earliest genotoxicity reports for the secondary metabolites of lichens come from Shibamoto and Wei (1984). They have tested physodic and physodalic acids isolated from Hypogymnia enteromorpha (Ach.) Nyl. for their mutagenicity in the Ames assay (Table 6.3). Among them only physodalic acid exhibited significant genotoxicity against Salmonella typhimurium strain TA 100 with or without S9 mix in both plate-incorporation and preincubation assays. Later, after several studies, usnic acid was shown to possess genotoxic activity. In a study by Al-Bekairi et al. (1991), mice were treated orally with aqueous suspensions of (+)-usnic acid enantiomers in a single dose of either 100 or 200 mg/kg. A slight increase in the micronucleated polychromatic erythrocytes (MNPCEs) without affecting DNA synthesis was reported and an effect of usnic acid on spindle apparatus was suggested. (+)-Usnic acid and (−)-usnic acid enantiomers isolated from Ramalina farinacea and Cladonia foliacea, respectively, have been found to be non-genotoxic because of the absence of MN induction in HPL (Koparal et al. 2006). Polat et al. (2013) also showed the nonmutagenicity of usnic acid by CA and MN assays.
The first findings of genotoxic and antigenotoxic potentials of (+)-usnic acid against methylmethanesulfonate (MMS)-induced chromosomal and genome damage in mammalian cells in vitro and in vivo have been shown by Leandro et al. (2013). Usnic acid alone induced DNA damage at concentrations of 60 and 120 g/ml determined by COMET assay. However, it did not induce MN formation in V79 cells at the concentrations tested, and no genotoxic effects were observed in vivo. The combined administration of usnic acid and MMS significantly reduced the frequencies of MN and DNA damage in vitro and in vivo when compared to treatment with MMS alone (Table 6.2). Recently, more results about genotoxic/antigenotoxic activities of usnic acid enantiomers isolated from two different lichen species, Cladonia arbuscula and Cladonia stellaris, have been reported by Prokopiev et al. (2017), Prokop’ev et al. (2018). Both (+)- and (−)-usnic acids at 0.01–1.00 μM were inhibited by genotoxicity around 37–70%, induced by DN and MMS in HPL cells, as indicated by COMET assay. On the other hand, both enantiomers exhibited genotoxic effects at concentrations of 40–300 μM, but (−)-usnic acid induced 2.5–3.5 times more genotoxicity in COMET than (+)-usnic acid. Usnic acid triggered oxidative stress and disruption of the normal metabolic processes of breast cancer cell line MCF7 and lung cancer cell line H1299 (null for p53); however, it was not involved in DNA damage. It was suggested that the property of usnic acid as a non-genotoxic anticancer agent that works in a p53-independent manner makes it a potential candidate for novel cancer therapy (Mayer et al. 2005).
Increasing amounts information on the genotoxicity and antigenotoxicity of other secondary metabolites of lichens are appearing. Lately, different species of lichen isolated by hypostictic acid, protocetraric acid, salazinic acid, and psoromic acid have been found to act as inhibitors of DXR-induced mutagenicity in the somatic cells of Drosophila melanogaster (Guterres et al. 2017). Tested concentrations of the compounds at 6 mM were not genotoxic. However, psoromic acid isolated from a different species, Rhizoplaca melanophthalma, and other two compounds as phyosodic acid and olivetoric acid were genotoxic by inducing DNA damage at different concentrations from each other in HPL, PRCC, and U87MG cells (Emsen et al. 2016, 2018). There were notable differences among the 8-OH-dG levels caused by olivetoric acid and phyosodic acid (5–40 mg/l) showing the highest genotoxic effects. Therefore, it was suggested that olivetoric acid and then phyosodic acid may have high potential in the treatment of glioblastoma multiform. On the other hand, phyosodic acid can be used as a natural antioxidant because of its low genotoxic but high antioxidant capacity.
6.5 Conclusion
The methods most frequently used for the assessment of genotoxic and antigenotoxic activities of lichen extracts and products in vitro and in vivo are described here. These methods are not meant to be comprehensive of all existing methods, but more must be in consideration for further investigation of the genotoxicity for their safety assessment or antigenotoxicity, especially of secondary metabolites alone or in combination for their synergetic activities. Positive results of in vitro/in vivo testing indicate that the tested substance is genotoxic or antigenotoxic, and negative results indicate that the test substance is not genotoxic under the conditions of the assay performed. Genotoxicity and antigenotoxicity of lichens have appeared to be evaluated using several type of assays by detecting direct or indirect base substitution and frameshift mutagenicity (Ames and WP2), clastogenicity (chromosome breakage), and aneugenicity (chromosome lagging from dysfunction of mitotic apparatus) (MN), numerical and structural DNA damage (CA), DNA-strand breaks (COMET), oxidative DNA damage (8-oxo-dG), and somatic mutation and recombination (SMART).
Accumulated data from short-term in vitro and in vivo studies have shown that lichen extracts could possess antigenotoxic effects. There are a small number of results for extracts which do not have antigenotoxic effects and some for those having genotoxic effects. The tests generally used for this purpose were common bacterial tests such as Ames and WP2 and human lymphocytes tests such as MN and SCE. However, there are gaps in the lichen genotoxicity/antigenotoxicity data because some groups studied only mutagenicity and others antigenotoxicity without genotoxicity. Most findings are extremely promising in that lichens may have therapeutic potential, at least for cancer, because of their antigenotoxic activities without genotoxic activity. The extracts of 13 species of the 20 lichen species tested, namely, Cetraria aculeata, Cetraria islandica, Cetralia olivetorum, Cladonia chlorophaea, Cladonia foliacea, Cladonia rangiformis, Dermatocarpon intestiniforme, Parmelia pulla, Peltigera rufescens, Peltigera canina, Pseudevernia furfuracea, Ramalina capitata, and Xanthoria elegans, have antigenotoxic activities but they are not genotoxic (Table 6.2). The extracts of 6 species, that is, Evernia prunastri, Lecanora muralis, Rhizoplaca chrysoleuca, Usnea longissima, Umbilicaria vellea, and Xanthoparmelia somloensis are antigenotoxic, but not tested for their genotoxic activities. On the other side, the aqueous extracts of Cetraria aculeatea and Rhizoplaca melanophthalma are neither genotoxic nor antigenotoxic for E. coli WP2 and human peripheral blood lymphocytes, respectively. The aqueous extracts of the other eight lichen species tested are not also genotoxic, except for the methanol extract of Hypogymnia physodes (Table 6.2).
There is minor evidence about the genotoxic and antigenotoxic activities of the secondary metabolites of lichens. Hypostictic acid, protocetraric acid, psoromic acid, and salazinic acid are antigenotoxic without genotoxic activity in Drosophila somatic cells. However, psoromic acid induced oxidative DNA damage in mammalian cells. Although phyosodic acid is nonmutagenic in the Salmonella assay, it induces oxidative DNA damage in mammalian cells.
Interestingly, secondary metabolites of the lichens tested show variation in their effects because each is either genotoxic or antigenotoxic according to the type of assays and laboratories where the work was done. Also, variation in the effective doses of the extract on different cells or test systems suggests the necessity of more in vitro and in vivo antigenotoxicity studies to know the exact potential of the extract. It may then find application for treatment. Further investigation to complete the gap and more data for other lichen species will be very useful for possible therapeutic applications.
The mechanism of antigenotoxic action of all these lichen extracts is not completely known but appears to be the antioxidative potential of their secondary metabolites, as described in Chap. 1. Because most of the extracts have been investigated for their antigenotoxicity and antioxidant activities, quite strong antioxidative activity is also indicated (Aydin and Türkez 2011a, b; Kotan et al. 2011; Polat et al. 2013; Kekuda et al. 2018). The chemoprevention potential of several lichen extracts or secondary metabolites against DNA damage induced by the known compounds such as AFB1, MNNG, MMS, IMA, and CBS strongly indicates that lichens can be a resource of new therapeutics.
Abbreviations
- 2-AF:
-
2-Aminoflourene
- 4-NPD:
-
4-Nitrophenylenediamine
- 8-oxo-dG:
-
8-Oxo-2′-deoxyguanosine, 8-hydroxy-2′-deoxyguanosine
- 9-AA:
-
9-Aminoacridine
- AFB1:
-
Aflatoxin B1
- BrdU:
-
Bromodeoxyuridine
- CA:
-
Chromosome aberration
- CBS:
-
Colloidal bismuth subcitrate
- COMET:
-
Single-cell gel electrophoresis
- DN:
-
Dioxidine
- DXR:
-
Doxorubicin
- HPL:
-
Human peripheral blood lymphocytes
- IMA:
-
Imazalil
- MI:
-
Mitotic index
- MMC:
-
Mitomycin C
- MMS:
-
Methyl methanesulfonate
- MN:
-
Micronucleus
- MNNG:
-
Methylnitronitrosoguanidine
- SCE:
-
Sister chromatid exchange
- SCGE:
-
Single-cell gel electrophoresis
- SMART:
-
Wing somatic mutation and recombination test
References
Agar G, Gulluce M, Aslan A et al (2010) Mutation preventive and antigenotoxic potential of methanol extracts of two natural lichen. J Med Plants Res 4(20):2132–2137
Agar G, Aslan A, Sarioglu EK et al (2011) Protective activity of the methanol extract of Usnea longissima against oxidative damage and genotoxicity caused by aflatoxin B(1) in vitro. Turk J Med Sci 41(6):1043–1049
Al-Bekairi AM, Qureshi S, Chaudhry MA et al (1991) Mitodepressive, clastogenic and biochemical effects of (+)-usnic acid in mice. J Ethnopharmacol 33(3):217–220
Alpsoy L, Aslan A, Kotan E et al (2011) Protective role of two lichens in human lymphocytes in vitro. Fresenius Environ Bull 20(7):1661–1666
Alpsoy L, Orhan F, Nardemir G et al (2013) Antigenotoxic potencies of a lichen species, Evernia prunastri. Toxicol Ind Health 31:153. https://doi.org/10.1177/0748233712469655
Anar M, Orhan F, Alpsoy L et al (2013) The antioxidant and antigenotoxic potential of methanol extract of Cladonia foliacea (Huds.) Willd. Toxicol Ind health 32:721–729. https://doi.org/10.1177/0748233713504805
Ari F, Celikler S, Oran S et al (2012) Genotoxic, cytotoxic, and apoptotic effects of Hypogymnia physodes (L.) Nyl. On breast cancer cells. Environ Toxicol 29(7):804–813. https://doi.org/10.1002/tox.21809
Aslan A, Agar G, Alpsoy L et al (2012a) Protective role of methanol extracts of two lichens on oxidative and genotoxic damage caused by AFB(1) in human lymphocytes in vitro. Toxicol Ind Health 28(6):505–512
Aslan A, Gulluce M, Agar G et al (2012b) Mutagenic and antimutagenic properties of some lichen species grown in the eastern Anatolia region of Turkey. Cytol Genet 46(5):291–296
Aydin E, Türkez H (2011a) Antioxidant and genotoxicity screening of aqueous extracts of four lichens collected from North East Anatolia. Fresenius Environ Bull 20(8A):2085–2091
Aydin E, Türkez H (2011b) Effects of lichenic extracts (Bryoria capillaris, Peltigera rufescens and Xanthoria elegans) on human blood cells: a cytogenetic and biochemical study. Fresenius Environ Bull 20(11A):2992–2998
Bakkali F, Averbeck S, Averbeck D et al (2008) Biological effects of essential oils-a review. Food Chem Toxicol 46:446–475
Behera BC, Verma N, Sonone A, Makhija U (2006) Determination of antioxidative potential of lichen Usnea ghattensis in vitro. LWT 39:80–85
Cabrera C (1996) Materia medica: Usnea spp. Eur J Herbal Med 2:11–13
Cardarelli M, Serino G, Campanella L et al (1997) Antimitotic effects of usnic acid on different biological systems. Cell Mol Life Sci 53(8):667–672
Ceker S, Orhan F, Sezen S et al (2018) Anti-mutagenic and anti-oxidant potencies of Cetraria aculeata (Schreb.) Fr., Cladonia chlorophaea (Flörke ex Sommerf.) Spreng. and Cetrelia olivetorum (Nyl.) W.L. Culb. & C.F. Culb.) Iran J Pharm Res 17(1):326–335
Clare G (2012) The in vitro mammalian chromosome aberration test. Methods Mol Biol 817:69–91
Collins AR (2004) The comet assay for DNA damage and repair: principles, applications, and limitations. Mol Biotechnol 26(3):249–261
Delebassée S, Mambu L, Pinault E et al (2017) Cytochalasin E in the lichen Pleurosticta acetabulum. Anti-proliferative activity against human HT-29 colorectal cancer cells and quantitative variability. Fitoterapia 121:146–151
ECVAM (2012, Jan). http://ecvam.jrc.it/consulted
Einarsdottir E, Groeneweg J, Björnsdottir GG et al (2010) Cellular mechanisms of the anticancer effects of the lichen compound usnic acid. Planta Med 76:969–974
Emsen B, Aslan A, Togar B et al (2016) In vitro antitumor activities of the lichen compounds olivetoric, physodic and psoromic acid in rat neuron and glioblastoma cells. Pharm Biol 54(9):1748–1762
Emsen B, Togar B, Türkez H et al (2018) Effects of two lichen acids isolated from Pseudevernia furfuracea (L.) Zopf in cultured human lymphocytes. Z Naturforsch 73(7–8C):303–312
Fenech M (2007) Cytokinesis-block micronucleus cytome assay. Nat Protoc 2:1084–1104
Geyikoglu F, Türkez H, Aslan A (2007) The protective roles of some lichen species on colloidal bismuth subcitrate genotoxicity. Toxicol Ind Health 23(8):487–492
Glück J, Buhrke T, Frenzel F et al (2018) In silico genotoxicity and carcinogenicity prediction for food-relevant secondary plant metabolites. Food Chem Toxicol 116:298–306
Gökbayrak S, Sivas H (2011) Investigation of cytotoxic effects of pyridine in root meristem cells of Allium cepa. BioDiCon 4(2):92–98
Gormez A, Karadayi M, Güllüce M et al (2013) Determination of genotoxic and antigenotoxic effects of Peltigera canica by the bacterial reverse mutation assays. Curr Opin Biotechnol 24S:S112
Graf U, Würgler FE, Katz AJ et al (1984) Somatic mutation and recombination test in Drosophila melanogaster. Environ Mol Mutagen 6:153–188
Guner A, Türkez H, Aslan A (2012) The in vitro effects of Dermotocarpon intestiniforme (a lichen) extracts against cadmium induced genetic and oxidative damage. Ekoloji 21(84):38–46
Guterres ZR, Honda NK, Coelho RG et al (2017) Antigenotoxicity of depsidones isolated from Brazilian lichens. Orbital: Electron J Chem 9(1):50–54
Halici M, Odabasoglu F, Suleyman H et al (2005) Effects of water extract of Usnea longissima on antioxidant enzyme activity and mucosal damage caused by indomethacin in rats. Phytomedicine 12:656–662
Halliwell B (2000) Why and how should we measure oxidative DNA damage in nutritional studies? How far have we come? Am J Clin Nutr 72(5):1082–1087
Hoshina MM, Marin-Morales MA (2014) Anti-genotoxicity and anti-mutagenicity of Apis mellifera venom. Mutat Res Genet Toxicol Environ Mutagen 762:43–48
Huneck S (2001) New results on the chemistry of lichen substances. In: Falk H, Kirby GW, Moore RE (eds) Progress in the chemistry and organic natural products, vol 81. Springer, New York, pp 1–276
Ingolfsdottir K (2002) Molecules of interest usnic asid. Phytochemistry 61:729–736
Ingolfsdottir K, Hjalmarsdottir MA, Guojonsdottir GA et al (1997) In vitro susceptibility of Helicobacter pylori to protolichesterinic acid from Cetraria islandica. Antimicrob Agents Chemother 41:215–217
Ingolfsdottir K, Lee SK, Bhat KPL et al (2000) Evaluation of selected lichens from Iceland for cancer chemopreventive and cytotoxic activity. Pharm Biol 38:313–317
Ipek E, Tuylu BA, Zeytinoglu H (2003) Effects of carvacrol on sister chromatid exchanges in human lymphocyte culture. Cytotechnology 43:145–148
Ipek E, Zeytinoglu H, Okay S et al (2005) Genotoxicity and antigenotoxicity of Origanum oil and carvacrol evaluated by Ames Salmonella/microsomal test. Food Chem 93:551–556
Jayaprakasha GK, Negi PS, Jena BS et al (2007) Antioxidant and antimutajenic activities of Cinnamomum zeylanicum fruit extracts. J Food Compos Anal 20:330–336
Kahaliw W, Hellman B, Engidawork E (2018) Genotoxicity study of Ethiopian medicinal plant extracts on HepG2 cells. BMC Comp Altern Med 18:45. https://doi.org/10.1186/s12906-017-2056-x
Karunaratne V, Bombuwela K, Kathirgamanathar S et al (2005) Review lichens: a chemically important biota. J Natl Sci Found 33(3):169–186
Kaura V, Kumarb M, Kumara A et al (2018) Pharmacotherapeutic potential of phytochemicals: implications in cancer chemoprevention and future perspectives. Biomed Pharmacother 97:564–586
Kekuda TRP, Vinayaka KS, Sachin MB (2018) Chemistry, ethnobotanical uses and biological activities of the lichen genus Heterodermia Trevis. (Physciaceae; Lecanorales; Ascomycota): a comprehensive review. J Appl Pharm Sci 8(05):148–155
Kirsch-Volders M, Decordier I, Elhajouji A et al (2011) In vitro genotoxicity testing using the micronucleus assay in cell lines, human lymphocytes and 3D human skin models. Mutagenesis 26(1):177–184
Koparal AT, Tüylü BA, Türk H (2006) In vitro cytotoxic activities of (+)-usnic acid and (−)-usnic acid on V79, A549, and human lymphocyte cells and their non-genotoxicity on human lymphocytes. Nat Prod Res 20(14):1300–1307
Kotan E, Alpsoy L, Anar M et al (2011) Protective role of methanol extract of Cetraria islandica (L.) against oxidative stress and genotoxic effects of AFB(1) in human lymphocytes in vitro. Toxicol Ind Health 27(7):599–605
Kotan E, Agar G, Alpsoy L et al (2013) Anti-genotoxic and anti-oxidative effects of Cladonia extracts against aflatoxin B-1 in vitro. Fresenius Environ Bull 22(4A):1139–1143
Kristmundsdóttir T, Aradóttir HA, Ingólfsdóttir K et al (2002) Solubilization of the lichen metabolite (+)‐usnic acid for testing in tissue culture. J Pharm Pharmacol 54(11):1447–1452
Kuete V, Ngnintedo D, Fotso GW et al (2018) Cytotoxicity of seputhecarpan D, thonningiol and 12 other phytochemicals from African flora towards human carcinoma cells. BMC Complement Altern Med 18:36. https://doi.org/10.1186/s12906-018-2109-9
Kumar SKC, Müller K (1999) Lichen metabolites. 1. Inhibitory action against leukotriene B4 biosynthesis by a non-redox mechanism. J Nat Prod 62:817–820
Leandro LF, Munari CC, Ferreira LS et al (2013) Assessment of the genotoxicity and antigenotoxicity of (+)-usnic acid in V79 cells and Swiss mice by the micronucleus and comet assays. Mutat Res Genet Toxicol Environ Mut 753(2):101–106
Makhuvele R, Matshoga RG, Antonissen R et al (2018) Genotoxicity and antigenotoxicity of selected South African indigenous plants. S Afr J Bot 114:89–99
Maron DM, Ames BN (1983) Revised methods for the Salmonella mutagenicity test. Mutat Res 113:173–215
Mayer M, O’Neill MA, Murray KE et al (2005) Usnic acid: a non-genotoxic compound with anti-cancer properties. Anti-Cancer Drug 16(8):805–809
Mersch-Sundermann V, Kassie F, Böhmer S et al (2004) Extract of Toxicodendron quercifolium caused genotoxicity and antigenotoxicity in bone marrow cells of CD1 mice. Food Chem Toxicol 42:1611–1617
Mitrovic T, Stamenkoviç S, Cvetkovic V et al (2011) Lichens as source of versatile bioactive compounds. Biol Nyssana 2(1):1–6
Molnar K, Farkas E (2010) Current results on biological activities of lichen secondary metabolites: a review. Z Naturforsch C 65:157–173
Mortelmans K, Riccio ES (2000) The bacterial tryptophan reverse mutation assay with Escherichia coli WP2. Mutat Res 455(1–2):61–69
Mortelmans K, Zeiger E (2000) The Ames Salmonella/microsome mutagenicity assay. Mutat Res 455(1–2):29–60
Nieminen SM, Maki-paakkanen J, Hirvonen MR et al (2002) Genotoxicity of gliotoxin, a secondary metabolite of Aspergillus fumigatus, in a battery of short-term test systems. Mutat Res 520:161–170
OECD (1997) Test No. 471: bacterial reverse mutation test, OECD guidelines for the testing of chemicals, section 4. OECD Publishing, Paris. https://doi.org/10.1787/9789264071247-en
OECD (2012) Consulted, 4, Jan 2012. https://www.oecd-ilibrary.org/environment/oecd-guidelines-for-the-testing-of-chemicals-section-4-health-effects_20745788
Okai Y, Higashi-Okai K, Nakamura S et al (1996) Suppressive effects of retinoids, carotenoids and antioxidant vitamins on heterocyclic amine-induced Umu C gene expression in Salmonella typhimurium (TA 1535/Psk 1002). Mutat Res 368:133–140
Perry P, Evans HJ (1975) Cytological detection of mutagen-carcinogen exposure by sister chromatid exchange. Nature 258(5531):121–125
Polat Z, Aydın E, Türkez H et al (2013) In vitro risk assessment of usnic acid compound. Toxicol Ind Health 32:1–8. https://doi.org/10.1177/0748233713504811
Prashith Kekuda TR, Vinayaka KS, Sachin MB (2018) Chemistry, ethnobotanical uses and biological activities of the lichen genus Heterodermia Trevis. (Physciaceae; Lecanorales; Ascomycota): a comprehensive review. J Appl Pharm Sci 8(05):148–155
Prinsloo G, Nogemane N, Street R (2018) The use of plants containing genotoxic carcinogens as foods and medicine. Food Chem Toxicol 116(part B):27–39
Prokop’ev IA, Filippov EV, Filippova GV et al (2018) Pro/antigenotoxic activity of usnic acid enantiomers in vitro. Bull Exp Biol Med Pharm Toxicol 164(9):289–293
Prokopiev A, Filippov EV, Filippova GV et al (2017) Genotoxicity of usnic-acid enantiomers in vitro in human peripheral-blood lymphocytes. Cell Tissue Biol 11(2):141–146
Quadros APO, Mazzeo DEC, Marin-Morales MA et al (2017) Fruit extract of the medicinal plant Crataegus oxyacantha exerts genotoxic and mutagenic effects in cultured cells. J Toxicol Environ Health Part A 80(3):161–170
Richardson DHS (1988) Medicinal and other economic aspects of lichens. In: Galun M (ed) CRC handbook of lichenology, vol 3. CRC Press, Boca Raton, pp 93–108
Santos DB, Schiar VP, Ribeiro MC et al (2009) Genotoxicity of organo selenium compounds in human leukocytes in vitro. Mutat Res 676:21–26
Scarpato R, Bertoli A, Naccarati A et al (1998) Different effects of newly isolated saponins on the mutagenicity and cytotoxicity of the anticancer drugs mitomycin C and bleomycin in human lymphocytes. Mutat Res 420:49–54
Shibamoto T, Wei CI (1984) Mutagenicity of lichen constituents. Environ Mutagen 6(5):757–762
Singh NP, McCoy MT, Tice RR et al (1988) A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res 175:184–191
Speit G, Vasquez M, Hartmann A (2009) The comet assay as an indicator test for germ cell genotoxicity. Mutat Res 681:3–12
Sponchiado G, Adam ML, Silva CD et al (2016) Quantitative genotoxicity assays for analysis of medicinal plants: a systematic review. J Ethnopharmacol 178(3):289–296
Sugiyama K, Yamada M, Awogi T et al (2016) The strains recommended for use in the bacterial reverse mutation test (OECD guideline 471) can be certified as non-genetically modified organisms. Genes Environ 38:2
Tay T, Türk AÖ, Yılmaz M et al (2004) Evaluation of the antimicrobial activity of the acetone extract of the lichen Ramalina farinacea and its (+)-usnic acid, norstictic acid and protocetraric acid constituents. Z Naturforsch 59C:384–388
Teixeira Zafred RR, Spano MA, Martins GR et al (2016) Pro-oxidant activity and genotoxicity of the Astronium fraxinifolium using wing SMART and Allium cepa test. Res J Med Plants 10:276–285
Tilford GL (1997) Edible and medicinal plants of the west. Mountain Press, Missoula
Tomović J, Kosanić M, Ristić S et al (2017) Chemical composition and bioactive properties of the lichen, Pleurosticta acetabulum. Trop J Pharm Res 16(12):2977–2984
Toyokuni S, Tanaka T, Hattori Y et al (1997) Quantitative immunohistochemical determination of 8-hydroxy-2′-deoxyguanosine by a monoclonal antibody N45.1: its application to ferric nitrilotriacetate-induced renal carcinogenesis model. Lab Investig 76:365–374
Türk AÖ, Yılmaz M, Kıvanç M et al (2003) The antimicrobial activity of extracts of the lichen Cetraria aculeata and its protolichesterinic acid constituent. Z Naturforsch 58C:850–854
Türkez H, Dirican E (2012) A modulator against mercury chloride-induced genotoxic damage: Dermatocarpon intestiniforme (L.). Toxicol Ind Health 28(1):58–63
Türkez H, Geyikoglu F, Aslan A et al (2010) Antimutagenic effects of lichen Pseudovernia furfuracea (L.) Zoph. extracts against the mutagenicity of aflatoxin B-1 in vitro. Toxicol Ind Health 26(9):625–631
Türkez H, Aydin E, Aslan A (2012a) Xanthoria elegans (Link) (lichen) extract counteracts DNA damage and oxidative stress of mitomycin C in human lymphocytes. Cytotechnology 64(6):679–686
Türkez H, Aydin E, Aslan A (2012b) An antidote for imazalil-induced genotoxicity in vitro: the lichen Dermatocarpon intestiniforme (Korber) Hasse. Acta Biol Hung 63(3):354–361
Türkez H, Aydin E, Aslan A (2012c) Effects of lichenic extracts (Hypogymnia physodes, Ramalina polymorpha and Usnea florida) on human blood cells: cytogenetic and biochemical study. Iran J Pharm Res 11(3):889–896
Türkez H, Aydin E, Sisman T et al (2012d) Role of Peltigera rufescens (Weis) Humb. (a lichen) on imazalil-induced genotoxicity analysis of micronucleus and chromosome aberrations in vitro. Toxicol Ind Health 28(6):492–498
Verschaeve L, Juutilainen J, Lagroye I et al (2010) In vitro and in vivo genotoxicity of radiofrequency fields. Mutat Res 705:252–268
Vijayakumar CS, Viswanathan S, Reddy MK et al (2000) Anti-inflammatory activity of (+)-usnic acid. Fitoterapia 71:564–566
Wilson DM III, Thompson LH (2007) Molecular mechanisms of sister-chromatid exchange. Mutat Res-Fund Mol M 616(1–2):11–23
Yeash EA, Letwin L, Malek L et al (2017) Biological activities of undescribed north American lichen species. J Sci Food Agric 97:4721–4726
Yılmaz M, Türk AÖ, Tay T et al (2004) The antimicrobial activity of extracts of the lichen Cladonia foliacea and its (−)-usnic acid, atranorin, and fumarprotocetraric acid constituents. Z Naturforsch 59C:249–254
Zeytinoglu H, Incesu Z, Tuylu Ayaz B et al (2008) Determination of genotoxic, antigenotoxic and cytotoxic potential of the extract from lichen Cetraria aculeata (Schreb.) Fr. in vitro. Phytother Res 22(1):118–123
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sivas, H. (2019). Antigenotoxic Effect of Some Lichen Metabolites. In: Ranković, B. (eds) Lichen Secondary Metabolites. Springer, Cham. https://doi.org/10.1007/978-3-030-16814-8_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-16814-8_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-16813-1
Online ISBN: 978-3-030-16814-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)