Abstract
Risk factors for kidney stones include genetic and environmental factors. An underlying genetic condition should be suspected in those diagnosed at a young age or with recurrent or bilateral disease. Most genetic defects increase production or excretion of lithogenic components, while others alter urinary pH. Genetic alterations cause hypercalciuria mostly by decreasing calcium resorption, thereby increasing the risk of calcium-based stones. Hyperoxaluria can be caused by several genetic factors related to increased oxalate production and excretion. There are two major genes responsible for the development of cystinuria and genotype is now used for classification. Adenine phosphoribosyltransferase (APRT) deficiency is an autosomal recessive disease caused by deficiency in the enzyme responsible for adenine metabolism that is associated with both kidney stones and renal failure.
Environmental factors such as warmer climate, type of work, socioeconomic class, and geography may cooperate with underlying genetic factors to contribute to the development of kidney stones.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Genetics
- Environmental factors
- Adenine phosphoribosyltransferase (APRT) deficiency
- Cystinuria
- Hypercalciuria
- Hyperoxaluria
-
Genetic factors may result in the overproduction of lithogenic solutes, their over-excretion, or alterations in urinary pH to increase the risk of nephrolithiasis.
-
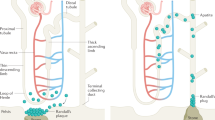
Genetic causes of hypercalciuria can be divided along the nephron segment classically affected.
-
With a central role in the fine regulation of calcium reabsorption and excretion, the loop of Henle is the target of several known mutations resulting in hypercalciuria, including those of the calcium-sensing receptor and its downstream targets of the claudins.
-
Primary hyperoxaluria has three known distinct forms resulting in marked elevations of urinary oxalate, with an increased risk of nephrolithiasis and loss of kidney function.
-
Cystinuria is a genetic disease involving an amino acid transporter in the proximal nephron that results in a distinct nephrolithiasis phenotype.
-
Environmental factors including warmer temperatures and professions associated with restricted access to fluids have been linked to an increased risk of kidney stone formation.
Introduction
Kidney stones affect approximately 9% of the US population [1]. When specific risk factors for kidney stones can be identified, dietary habits are most frequently implicated. However, an underlying genetic cause for nephrolithiasis may occasionally be encountered, including several well-described syndromes. Genetic causes should be suspected in patients diagnosed at a young age, with recurrent or bilateral disease or with concurrent renal failure. Genetic disorders may result in loss of enzyme or transporter function or alteration of metabolic pathways. Known genetic diseases typically result in overproduction or over-excretion of lithogenic components or more rarely alteration of the urine pH to favor crystal precipitation. Although the total number of genes involved is not clear, one study showed at least 15% of stones can be related to 14 monogenic genes [2]. Despite this, a single identifiable genetic cause for increased stone risk is not identified in the majority of patients with kidney stones, even those with a strong family history, suggesting that most cases of nephrolithiasis may ultimately be polygenic in nature. Additionally, environmental factors may contribute to an increased risk of stone formation, and these are addressed at the end of the chapter.
Genetic Causes
Genetic Causes of Hypercalciuria
Hypercalciuria may result from any process that leads to increased renal filtration of calcium or decreased calcium reabsorption by the kidney. The defect may be in the kidneys themselves or from increased calcium liberation from the bone or absorption from the gastrointestinal tract.
Renal Causes
Each segment of the nephron has a role in the handling of calcium. Genetic defects affecting a variety of these pathways have been identified and are known to lead to hypercalciuria and thereby an increased risk of calcium-based nephrolithiasis [3, 4]. Although a detailed description of these individual mutations and polymorphisms is beyond the scope of this chapter, it is helpful to approach these diseases with representative examples of defects at each segment, with a focus on the more commonly encountered disorders (Fig. 3.1).
Proximal Convoluted Tubule
Most of the filtered calcium is reclaimed by the proximal nephron. Therefore, it is not surprising that genetic defects at this location may result in hypercalciuria. Dent’s disease is a rare X-linked recessive mutation of the ClC5 chloride channel, with 250 affected families reported. This channel colocalizes with albumin-containing endocytic vesicles of the proximal tubule, and its absence is believed to result in a generalized trafficking dysfunction and impaired sodium-coupled transport that normally occurs at this site [5]. This leads to diminished calcium reabsorption, and when distal calcium reclamation is unable to fully compensate, hypercalciuria may result. Studies in ClC5 knockout mice showed that other sodium-coupled transporters are also affected, resulting in the Fanconi syndrome with glycosuria, amino aciduria, bicarbonaturia, and phosphaturia [6]. As this cell type is also the location of 1-alpha hydroxylation of 25-OH vitamin D, diminished calcitriol production is seen and may result in bone mineral disease and rickets. Patients may present with kidney stones in childhood or early adulthood and may show progression to renal failure over time. Treatment of the hypercalciuria is centered on thiazide diuretics, and preclinical research has shown that a high citrate diet may delay the progression of renal disease.
Loop of Henle
In the kidney, the calcium-sensing receptor (CaSR) is primarily located on the basolateral membrane of the cell of the thick ascending limb of the loop of Henle. In states of calcium surfeit, the receptor is activated, triggering a signaling cascade that inactivates potassium flux on the luminal membrane, effectively shutting down the activity of the Na-K-2Cl transporter (loop diuretic-like effect). Loss of the luminal positive charge results in less paracellular reclamation of divalent cations (Ca++ and Mg++), which appear in the urine [7].
Therefore, an activating mutation of the CaSR would likely result in constitutive renal calcium loss, increasing the risk of calcium-based stones. Such a situation is present in the disease autosomal dominant hypocalcemia, whose name indicates its mode of inheritance as well as its major clinical feature of low serum calcium. In the parathyroid gland, the mutated receptor behaves as it would in states of calcium excess, and the gland is unable to sense the prevailing hypocalcemia; parathyroid hormone (PTH) levels remain low [8]. A mutation in the CaSR has also been implicated in type 5 Bartter’s syndrome, with a similar clinical presentation [9]. In fact, any variant of Bartter’s syndrome may result in hypercalciuria, given that a reduction in Na-K-2Cl transporter activity ultimately results in the same loss of the luminal positive charge and diminished calcium reabsorption.
The mechanism of this paracellular calcium transport has also been implicated in another family of mutations that may lead to hypercalciuria and increased nephrolithiasis risk. Calcium (and magnesium) transport occurs through the tight junctions via Claudin-16 and Claudin-19 channels. A disorder called familial hypomagnesemia with hypercalciuria and nephrocalcinosis (FHHNC) has been associated with a mutation in Claudin-16 [10]. Claudin-14, another member of the family, may serve as a link between the CaSR and Claudin-16/Claudin-19 activity. Claudin-14 is inhibitory of Claudin-16 activity and is itself stimulated by excess calcium through the CaSR. Polymorphisms of Claudin-14 with increased activity have been described in families in the Netherlands and Iceland with known susceptibility to calcium-based kidney stones [11].
Distal Convoluted Tubule
The Na-Cl cotransporter (NCCT) is found at the distal convoluted tubule segment of the nephron. Blockade of this transporter with a thiazide diuretic results in an increased activity of the basolateral Na+/Ca++ exchanger and a calcium sink facilitating luminal entry of calcium through the TRPV5 channel [12]. This is one of the suspected mechanisms by which thiazide diuretics assist with calcium reabsorption making them useful in the treatment of patients with hypercalciuria.
Conversely, overactivity of the NCCT may be expected to result in the opposite effect, with excess calcium in the urine. Indeed, this has been variably observed in patients with Gordon’s syndrome (pseudohypoaldosteronism type 2) where mutations in WNK1 and WNK4 result in a diminished ability to suppress the NCCT. These patients also present with hypertension, hyperkalemia, and a non-anion gap metabolic acidosis [13]. Treatment is typically centered on the administration of thiazide diuretics.
Collecting Duct
The collecting duct does not play a major direct role in calcium handling by the kidney. However, as a site of urinary acidification, it may indirectly be implicated in a sequence of events that increases the risk of calcium-based kidney stone formation. Decreased hydrogen ion secretion leads to an inability to maximally acidify the urine (distal renal tubular acidosis) with the development of metabolic acidosis. Genetic mutations in α-intercalated cell transporters including those encoding H+ ATPase, anion exchange (AE1), and carbonic anhydrase II have been found to decrease distal acidification [14]. Mutations in H+ ATPase, where ability to secrete H+ is impaired, have an autosomal recessive pattern as do carbonic anhydrase II mutations, where the enzyme catalyzing CO2 to HCO3– is affected. AE1 proteins have a role in bicarbonate exchange with mutations tending to follow an autosomal dominant pattern [15].
The resulting systemic acidosis triggers bone resorption releasing calcium and phosphorus which are eventually filtered by the kidneys. Additionally, the systemic acidosis leads to increased citrate reabsorption at the proximal nephron, which, while helpful from an acid-base standpoint, may increase the risk of nephrolithiasis due to the resultant hypocitraturia. Thus, patients would present with hypercalciuria, hypocitraturia, and an alkaline urine pH, all contributing to calcium phosphate stone formation [16].
Specific genetic mutations of the proton pump have been identified, but these are rare. A more common disorder is medullary sponge kidney . It is considered a developmental disorder, rather than a genetic disease, with no identified mode of inheritance. Dilation of the distal nephron gives it the classic sponge-like appearance when cut in cross section, and urinary stasis in these cyst-like segments increases the risk of urinary tract infections and stone disease [17].
Other Genetic Causes
Other genetic mutations have also been described that can cause hypercalciuria. These include mutations in the diacylglycerol kinase gene , which is important in transplasmalemmal calcium regulation and was found to be associated with hypercalciuria [15]. Those with mutations in the genes encoding NPT2a, which is responsible for most of the phosphate reabsorption in the proximal kidney, develop hypophosphatemia with increased 1,25-(OH)2 vitamin D production and hypercalciuria [4]. The transient receptor potential vanilloid member protein (TRPV5) is found in the distal convoluted tubule, and its mutation has also been found to be associated with hypercalciuria [15].
Non-renal Causes
Genetic diseases can result in hypercalciuria without involving the kidneys themselves, either through excessive bone turnover or gastrointestinal absorption of calcium. Multiple endocrine neoplasia type 1 (MEN1) occurs in 1 in 30,000 people and is associated with overactivity of the parathyroids, pancreas, and pituitary gland. The hyperparathyroidism leads to increased bone turnover, a loss of the well-knit bone matrix, and in increased risk of fracture [18]. Although PTH typically has a calcium-reabsorptive effect on the kidneys, the ensuing hypercalcemia ultimately results in calcium spillage into the urine and an increased stone risk.
Osteogenesis imperfecta type 1 is the most common and the mildest form of osteogenesis imperfecta affecting approximately 1 in 15,000 people in an autosomal dominant fashion. Patients exhibit a deficiency in type 1 collagen that results in abnormal bone formation and calcium homeostasis [19]. Approximately 20% of patients develop kidney stones. Treatment should be centered on avoiding excessive calcium supplements and maintaining a balanced dietary calcium intake.
Genetic disorders resulting in increased gastrointestinal calcium absorption are rare and not well-defined. An animal model has been identified where increased intestinal vitamin D receptor activity results in hyperabsorptive hypercalciuria despite normal active vitamin D levels, but whether human correlates exist is unknown. It should be noted that moderate amounts of dietary calcium (700–800 mg elemental calcium per day) are not generally associated with hypercalciuria, and may actually decrease the risk of stone formation.
Genetic Causes of Hyperoxaluria
Excessive excretion of oxalate in the urine can result from a number of processes. Most commonly, hyperoxaluria is related to dietary intake or gastrointestinal absorption. However, genetic conditions may result in increased oxalate production and urinary excretion. There are several distinct forms of primary hyperoxaluria, characterized by the conversion of endogenous metabolic precursors to oxalate, resulting in massive elevations in urinary oxalate, tissue deposition, stone formation at an early age, and loss of renal function [20].
Primary Hyperoxaluria Type 1
A rare autosomal recessive disorder, primary hyperoxaluria type 1 (PH1) occurs in 1–3 per 1,000,000 live births. The defect is in the AGXT gene that encodes for the hepatic alanine glyoxylate aminotransferase, an enzyme that converts glyoxylate to glycine. The reduced ability to metabolize the glyoxylate results in its accumulation and ultimate conversion to oxalate.
The fate of the oxalate determines the clinical manifestations. Urinary excretion of the oxalate results in profound hyperoxaluria (>135 mg/d, normal <40 mg/d) with an increased risk of calcium oxalate nephrolithiasis in over 80% of affected individuals. The oxalate which is not excreted can also complex with calcium and lead to tissue deposition in the heart, bone, eyes, and kidneys [21]. Renal oxalosis with nephrocalcinosis results in diminished kidney function often in late childhood and leads to end-stage renal disease, with 50% requiring renal replacement therapy by the age of 25.
Diagnostic testing typically begins with the finding of severely elevated urinary oxalate levels. The additional finding of an elevated urine glycolate is suggestive of the disease. As kidney function declines, urinary levels of oxalate and glycolate may become less reliable, and blood levels of oxalate can be measured instead. In the past, liver biopsy showing decreased alanine glyoxylate aminotransferase enzyme activity has been used for confirmation, though this is being replaced by genetic testing of the AGXT gene.
Treatment is centered on dietary oxalate restriction, avoidance of vitamin C, administration of calcium-based binders with meals, and increased fluid intake. Traditional stone risks should be addressed and managed. Additionally, pyridoxine (vitamin B6) has been used in the treatment of PH1 but is only effective in lowering the oxalate excretion in approximately one-third of patients with the disease. The mechanism of action may be related to the improved peroxisomal targeting of a specific mutant form (Gly170Arg) and thus may explain why not all patients show a benefit with therapy [22]. All patients should be offered treatment with pyridoxine 5 mg/kg/d increasing to 20 mg/kg/d over 3 months.
As many patients with PH1 progress to end-stage kidney disease, renal transplantation is frequently considered. However, isolated kidney transplant would be ineffective long term given that deposition will recur in the allograft. Instead, combined liver-kidney or sequential liver-kidney transplantation is recommended, as the liver allograft would provide the missing enzyme and normalize glyoxylate metabolism [23]. For patients on hemodialysis, intensive daily dialysis may help reduce the risk of systemic manifestations of oxalate accumulation.
Primary Hyperoxaluria Type 2
Primary hyperoxaluria type 2 (PH2) is also an autosomal recessive genetic disorder with a frequency much lower than that of PH1 accounting for approximately 10% of cases of primary hyperoxaluria. It is caused by a defect in the enzyme glyoxylate reductase/hydroxypyruvate reductase (GRHPR) that normally metabolizes glyoxylate. As with PH1 this excess glyoxylate is converted to oxalate which can be deposited in tissues and increase the risk of kidney stone formation.
While nephrolithiasis and nephrocalcinosis are seen in over 80% of individuals with PH2, progression to end-stage kidney disease is less common than in PH1 in less than one-third of patients [24]. Diagnosis may rely on measuring increased urinary oxalate and l-glycerate (as the D-isomer is deficient); confirmation can be through reduced glyoxylate reductase activity on liver biopsy or by molecular testing of the GRHPR gene [25].
Dietary treatment measures are similar as with PH1. Given the rarity of this disease, pyridoxine has not been studied specifically in PH2, although multivitamins may allow for better glyoxylate metabolism. Isolated kidney transplantation has been performed in patients with PH2 with varying success, which may be accounted for by the milder phenotype as compared with PH1.
Primary Hyperoxaluria Type 3
Most recently, a third form of primary hyperoxaluria (PH3) has been described, with an activating mutation in the HOGA1 gene that encodes for the mitochondrial 4-hydroxy-2-oxoglutarate aldolase enzyme [26]. This results in an overproduction of glyoxylate and eventual conversion to oxalate. Less is known about the natural history of PH3, although it is believed to follow a more benign course, with no known reports of progression to end-stage kidney disease yet available. Patients present with calcium oxalate nephrolithiasis early in life and may also show concomitant hypercalciuria, with treatment focused on managing traditional stone risk factors.
Cystinuria
Cystinuria is a genetic disease that causes 1% of adult and 6–8% of pediatric stone disease [27, 28]. It results from a mutation in the genes encoding the amino acid transporter in the proximal nephron responsible for reabsorption of cystine, ornithine, lysine, and arginine. Cystine, however, is the only one of these amino acids insoluble enough in urinary pH to cause kidney stones [29].
Two major genes are responsible for the development of this disease, SLC3A1 and SLC7A9, which are responsible for the heavy subunit rBAT and light subunit b0,+AT, respectively, composing the amino acid transporter [29]. Disease severity is not related to genotype [28, 30]. The traditional classification of this disease was based on the quantification of urinary cystine, however, a more recent classification was developed that is based on genotype. Type A is associated with a mutation of SLCA1 and type B involves a mutation of SLC7A9 [30]. The disease is generally thought to have an autosomal recessive inheritance pattern, however, heterozygotes may have a variable degree of penetrance, with type B mutations more commonly expressing a hyperexcretor phenotype [31]. Type AB includes mutations of each gene; further evidence shows that digenic inheritance is rare and that compound heterozygotes are unlikely to form cystine stones [31, 32].
Diagnosis should be suspected in those with recurrent or bilateral disease, those with young age at onset, or those with a family history [27]. Those with the disease are at significant risk for developing chronic kidney disease, with approximately 70% developing a measurable decrement in renal function [27]. Historically, patients with cystinuria had an increased serum creatinine as compared to patients with calcium oxalate nephrolithiasis, although part of this risk may have been driven by the increased performance of open surgical stone removal and nephrectomy [33]. Progression to end-stage renal disease is not common in cystinuria.
Adenine Phosphoribosyltransferase Deficiency
Adenine phosphoribosyltransferase (APRT) deficiency is an autosomal recessive disease that is a rare cause of uric acid kidney stones and chronic kidney disease.
APRT is important in the metabolism of adenine. When deficient, adenine is catabolized to 2,8-dihydroxyadenine (DHA) by xanthine oxidase [34]. DHA is insoluble in urine and can lead to kidney stones with a significant number of patients developing chronic kidney disease and end-stage kidney disease [35] even if no stones are detected [34, 36]. Given similarity to standard uric acid stone disease, this disorder may remain undiagnosed or only diagnosed in adulthood [34, 35]. The use of allopurinol is thought to reduce the risk of renal disease progression [36].
Environmental Factors
The prevalence of kidney stone disease is significantly increasing with time [1, 37]. This may be due to a combination of genetic, behavioral, and environmental factors. Climate, profession, socioeconomic class, and geography have all been found to be associated with the development of kidney stones.
Many studies have shown a positive association between higher temperature and the development of kidney stones [38, 39]. With increasing temperature, insensible losses increase with subsequent development of intravascular depletion and lower urinary output [38]. This will lead to precipitation of salts into crystals and the creation of stones [38]. Urine studies have shown higher urine calcium, supersaturation of calcium oxalate and phosphate, and decreasing urine sodium [40] in higher ambient temperature environments.
For each 1° increase in temperature, there is an approximate 10% increase in stone incidence [41]. In spinal cord injury patients, average temperature explained variability of stones by 21% within the first year of injury and up to 71% in those after year one [41]. With global temperatures expected to continue to rise due to climate change, the annual cost of treating kidney stones is projected to rise by $0.9–1.3 billion [42].
There has also been geographic variation in the incidence of stone formation. In the United States, rates tend to increase from north to south and west to east, with prevalence almost twice as high in the Southeast as compared to the Northwest [37, 41, 43]. This is thought to be secondary to differences in temperature and sunlight; it could also be due to an enriched gene pool [43]. In men, sunlight index was responsible for more variation than beverage intake or temperature, although they equally contributed to the geographic effect in women [43].
Profession may also affect the risk of kidney stone formation, especially as it pertains to access to fluid. Studies have shown that those exposed to higher temperatures, such as steel workers, and those with restricted access to fluid, such as physicians working in the operating room, have a significantly higher prevalence of stone formation as compared to others in the same institution [44, 45].
Lower socioeconomic status has also been associated with the formation of kidney stones [1], with worsening poverty associated with higher urine calcium and supersaturation of calcium oxalate and phosphate [46].
Summary
Kidney stones affect about 9% of the US population with prevalence increasing over time. There are several genetic and environmental factors that are thought to contribute to this risk. Genetic factors can increase the risk by increasing the production of specific lithogenic solutions or their secretion. They are also responsible for changing the pH to favor precipitation of crystals that form the stones. The formation of calcium stones, the most commonly encountered, can develop from hypercalciuria. There are several diseases that are associated with this form of kidney stones depending on the location of the nephron. Hyperoxaluria has three distinct entities as well where there is development of increased urinary oxalate depending on specific genetic defects.
References
Scales CD Jr, Smith AC, Hanley JM, Saigal CS. Prevalence of kidney stones in the United States. Eur Urol. 2012;62(1):160–5.
Rumsby G. Genetic defects underlying renal stone disease. Int J Surg. 2016;36(Pt D):590–5.
Moe OW, Bonny O. Genetic hypercalciuria. J Am Soc Nephrol. 2005;16(3):729–45.
Arcidiacono T, Mingione A, Macrina L, Pivari F, Soldati L, Vezzoli G. Idiopathic calcium nephrolithiasis: a review of pathogenic mechanisms in the light of genetic studies. Am J Nephrol. 2014;40(6):499–506.
Wang SS, Devuyst O, Courtoy PJ, Wang XT, Wang H, Wang Y, et al. Mice lacking renal chloride channel, CLC-5, are a model for Dent’s disease, a nephrolithiasis disorder associated with defective receptor-mediated endocytosis. Hum Mol Genet. 2000;9(20):2937–45.
Devuyst O, Jouret F, Auzanneau C, Courtoy PJ. Chloride channels and endocytosis: new insights from Dent’s disease and ClC-5 knockout mice. Nephron Physiol. 2005;99(3):69–73.
Gamba G, Friedman PA. Thick ascending limb: the Na (+):K (+):2Cl (−) co-transporter, NKCC2, and the calcium-sensing receptor, CaSR. Pflugers Arch. 2009;458(1):61–76.
Pearce SH, Williamson C, Kifor O, Bai M, Coulthard MG, Davies M, et al. A familial syndrome of hypocalcemia with hypercalciuria due to mutations in the calcium-sensing receptor. N Engl J Med. 1996;335(15):1115–22.
Vezzoli G, Arcidiacono T, Paloschi V, Terranegra A, Biasion R, Weber G, et al. Autosomal dominant hypocalcemia with mild type 5 Bartter syndrome. J Nephrol. 2006;19(4):525–8.
Weber S, Schneider L, Peters M, Misselwitz J, Ronnefarth G, Boswald M, et al. Novel paracellin-1 mutations in 25 families with familial hypomagnesemia with hypercalciuria and nephrocalcinosis. J Am Soc Nephrol. 2001;12(9):1872–81.
Thorleifsson G, Holm H, Edvardsson V, Walters GB, Styrkarsdottir U, Gudbjartsson DF, et al. Sequence variants in the CLDN14 gene associate with kidney stones and bone mineral density. Nat Genet. 2009;41(8):926–30.
Jang HR, Kim S, Heo NJ, Lee JH, Kim HS, Nielsen S, et al. Effects of thiazide on the expression of TRPV5, calbindin-D28K, and sodium transporters in hypercalciuric rats. J Korean Med Sci. 2009;24(Suppl):S161–9.
Mayan H, Munter G, Shaharabany M, Mouallem M, Pauzner R, Holtzman EJ, et al. Hypercalciuria in familial hyperkalemia and hypertension accompanies hyperkalemia and precedes hypertension: description of a large family with the Q565E WNK4 mutation. J Clin Endocrinol Metab. 2004;89(8):4025–30.
Gambaro G, Vezzoli G, Casari G, Rampoldi L, D’Angelo A, Borghi L. Genetics of hypercalciuria and calcium nephrolithiasis: from the rare monogenic to the common polygenic forms. Am J Kidney Dis. 2004;44(6):963–86.
Vasudevan V, Samson P, Smith AD, Okeke Z. The genetic framework for development of nephrolithiasis. Asian J Urol. 2017;4(1):18–26.
Zuckerman JM, Assimos DG. Hypocitraturia: pathophysiology and medical management. Rev Urol. 2009;11(3):134–44.
Higashihara E, Nutahara K, Tago K, Ueno A, Niijima T. Medullary sponge kidney and renal acidification defect. Kidney Int. 1984;25(2):453–9.
Falchetti A, Marini F, Luzi E, Giusti F, Cavalli L, Cavalli T, et al. Multiple endocrine neoplasia type 1 (MEN1): not only inherited endocrine tumors. Genet Med. 2009;11(12):825–35.
Chines A, Petersen DJ, Schranck FW, Whyte MP. Hypercalciuria in children severely affected with osteogenesis imperfecta. J Pediatr. 1991;119(1 Pt 1):51–7.
Mohebbi N, Ferraro PM, Gambaro G, Unwin R. Tubular and genetic disorders associated with kidney stones. Urolithiasis. 2017;45(1):127–37.
Hoppe B. An update on primary hyperoxaluria. Nat Rev Nephrol. 2012;8(8):467–75.
Hoyer-Kuhn H, Kohbrok S, Volland R, Franklin J, Hero B, Beck BB, et al. Vitamin B6 in primary hyperoxaluria I: first prospective trial after 40 years of practice. Clin J Am Soc Nephrol. 2014;9(3):468–77.
Bergstralh EJ, Monico CG, Lieske JC, Herges RM, Langman CB, Hoppe B, et al. Transplantation outcomes in primary hyperoxaluria. Am J Transplant. 2010;10(11):2493–501.
Chlebeck PT, Milliner DS, Smith LH. Long-term prognosis in primary hyperoxaluria type II (L-glyceric aciduria). Am J Kidney Dis. 1994;23(2):255–9.
Cramer SD, Ferree PM, Lin K, Milliner DS, Holmes RP. The gene encoding hydroxypyruvate reductase (GRHPR) is mutated in patients with primary hyperoxaluria type II. Hum Mol Genet. 1999;8(11):2063–9.
Williams EL, Bockenhauer D, van’t Hoff WG, Johri N, Laing C, Sinha MD, et al. The enzyme 4-hydroxy-2-oxoglutarate aldolase is deficient in primary hyperoxaluria type 3. Nephrol Dial Transplant. 2012;27(8):3191–5.
Thomas K, Wong K, Withington J, Bultitude M, Doherty A. Cystinuria-a urologist’s perspective. Nat Rev Urol. 2014;11(5):270–7.
Pereira DJ, Schoolwerth AC, Pais VM. Cystinuria: current concepts and future directions. Clin Nephrol. 2015;83(3):138–46.
Saravakos P, Kokkinou V, Giannatos E. Cystinuria: current diagnosis and management. Urology. 2014;83(4):693–9.
Dello SL, Pras E, Pontesilli C, Beccia E, Ricci-Barbini V, de Sanctis L, et al. Comparison between SLC3A1 and SLC7A9 cystinuria patients and carriers: a need for a new classification. J Am Soc Nephrol. 2002;13(10):2547–53.
Font-Llitjos M, Jimenez-Vidal M, Bisceglia L, Di Perna M, de Sanctis L, Rousaud F, et al. New insights into cystinuria: 40 new mutations, genotype-phenotype correlation, and digenic inheritance causing partial phenotype. J Med Genet. 2005;42(1):58–68.
Chillaron J, Font-Llitjos M, Fort J, Zorzano A, Goldfarb DS, Nunes V, et al. Pathophysiology and treatment of cystinuria. Nat Rev Nephrol. 2010;6(7):424–34.
Assimos DG, Leslie SW, Ng C, Streem SB, Hart LJ. The impact of cystinuria on renal function. J Urol. 2002;168(1):27–30.
Nasr SH, Sethi S, Cornell LD, Milliner DS, Boelkins M, Broviac J, et al. Crystalline nephropathy due to 2,8-dihydroxyadeninuria: an under-recognized cause of irreversible renal failure. Nephrol Dial Transplant. 2010;25(6):1909–15.
Bollee G, Dollinger C, Boutaud L, Guillemot D, Bensman A, Harambat J, et al. Phenotype and genotype characterization of adenine phosphoribosyltransferase deficiency. J Am Soc Nephrol. 2010;21(4):679–88.
Runolfsdottir HL, Palsson R, Agustsdottir IM, Indridason OS, Edvardsson VO. Kidney disease in adenine phosphoribosyltransferase deficiency. Am J Kidney Dis. 2016;67(3):431–8.
Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int. 2003;63(5):1817–23.
Fakheri RJ, Goldfarb DS. Ambient temperature as a contributor to kidney stone formation: implications of global warming. Kidney Int. 2011;79(11):1178–85.
Geraghty RM, Proietti S, Traxer O, Archer M, Somani BK. Worldwide impact of warmer seasons on the incidence of renal colic and kidney stone disease: evidence from a systematic review of literature. J Endourol. 2017;31(8):729–35.
Eisner BH, Sheth S, Herrick B, Pais VM Jr, Sawyer M, Miller N, et al. The effects of ambient temperature, humidity and season of year on urine composition in patients with nephrolithiasis. BJU Int. 2012;110(11 Pt C):E1014–7.
Chen YY, Roseman JM, DeVivo MJ, Huang CT. Geographic variation and environmental risk factors for the incidence of initial kidney stones in patients with spinal cord injury. J Urol. 2000;164(1):21–6.
Brikowski TH, Lotan Y, Pearle MS. Climate-related increase in the prevalence of urolithiasis in the United States. Proc Natl Acad Sci U S A. 2008;105(28):9841–6.
Soucie JM, Coates RJ, McClellan W, Austin H, Thun M. Relation between geographic variability in kidney stones prevalence and risk factors for stones. Am J Epidemiol. 1996;143(5):487–95.
Linder BJ, Rangel LJ, Krambeck AE. The effect of work location on urolithiasis in health care professionals. Urolithiasis. 2013;41(4):327–31.
Atan L, Andreoni C, Ortiz V, Silva EK, Pitta R, Atan F, et al. High kidney stone risk in men working in steel industry at hot temperatures. Urology. 2005;65(5):858–61.
Eisner BH, Sheth S, Dretler SP, Herrick B, Pais VM Jr. Effect of socioeconomic status on 24-hour urine composition in patients with nephrolithiasis. Urology. 2012;80(1):43–7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Yamout, H., Goldberg, S. (2019). Genetic and Environmental Risk Factors for Kidney Stones. In: Han, H., Mutter, W., Nasser, S. (eds) Nutritional and Medical Management of Kidney Stones. Nutrition and Health. Humana, Cham. https://doi.org/10.1007/978-3-030-15534-6_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-15534-6_3
Published:
Publisher Name: Humana, Cham
Print ISBN: 978-3-030-15533-9
Online ISBN: 978-3-030-15534-6
eBook Packages: MedicineMedicine (R0)