Abstract
Since its early development in 1866 arthroscopic surgery has progressed to allow the diagnosis and treatment of a plethora of conditions of the upper and lower limb. The indications for its use include management of bony and soft-tissue complaints with the advantage of minimal invasive surgery leading to a shorter recovery time. This chapter outlines the background of arthroscopy, patient setup, and portal placement to allow arthroscopy assessment and treatment of the hip, knee, foot ankle, shoulder, elbow, and wrist.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Endoscopic surgery developed as a less invasive means to access body cavities. The first documented use of the endoscope was of the bladder in 1806. As technology and light sources progressed the use of endoscopes to investigate and treat joint problems was explored [1].
Globally the earliest arthroscopists include Severin Nordentoft (1866–1922), a surgeon from Denmark who presented his work on endoscopy of the knee using optics and illumination. At a similar time Keji Takagin (1888–1963) in Tokyo reported the use of a knee arthroscope for the diagnosis of TB. Eugen Bircher of Switzerland (1882–1956) published his work on the arthroendoscope for the diagnosis of conditions of the knee. Phillip Heinrich Kreuscher (1883–1943) was the pioneering arthroscopist of the USA publishing on the diagnosis of cartilage injury in sport by means of the arthroscope. Further development of the arthroscope was carried out by Masaki Watanabe (1911–1994). He continued the work of Takagin. He introduced a supplemental light source and separate portal and in 1970 he introduced the first fiber optic and the concept of triangulation, the use of which is common practise today [1, 2].
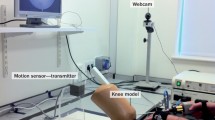
The common current setup of the arthroscopy system is an arthroscopic stack (Fig. 33.1) on the opposite side of the patient to the operating surgeon and a back table with the surgical equipment as seen in Fig. 33.2.
The arthroscopic system includes the arthroscope, light source, and an irrigation system. The rigid arthroscope is a classic thin lens, a rod-lens system, or a graded index lens system. The design of the arthroscope and its given field of view vary. This is dependent on the diameter of the arthroscope (these range from 2.7 to 7.5 mm) and the angle of inclination of the distal arthroscopic lens. The angle of inclination is measured between the axis of the arthroscope and a line perpendicular to the surface of the lens (this varies from 10° to 120°) [3]. Most commonly used are 25–30° (70–90° may be used in more specific situations for viewing around corners). The field of view is increased by rotating the arthroscope; however a blind spot will be present; its size will depend on the angle of inclination of the arthroscope.
For routine procedures the basic arthroscopy set contains the arthroscope with a 30–70° arthroscope, a probe, scissors, grasping forceps, punch forceps, arthroscopic knives, a motorized meniscal cutter and shaver, electrosurgical, laser, and radiofrequency instruments (Fig. 33.3). Procedure-specific instruments have also been developed and variations exist depending on manufacturers and surgeon preferences.
Irrigation systems are vital to allow distension of the joint in arthroscopy. These generally flow along the arthroscopic sheath. The solution used is commonly Hartmann’s solution supplied by 2.5 L bags joined by a y connector placed high above the level of the joint allowing a pressure of approximately 66–88 mmHg.
Advantages of arthroscopy include the following:
-
Reduced postoperative morbidity
-
Smaller incisions
-
Less intense inflammatory response
-
Improved visualization
-
Reduced length of stay
-
Reduced complication rate
-
Permit easier “second look” surgery
-
Allow access to perform some procedures not possible through arthrotomy
Disadvantages of arthroscopy include the following:
-
Technically challenging
-
Learning curve
We now look at arthroscopy as relevant to different joints in the body.
Knee
The knee was the primary joint in the development of arthroscopy [1, 2]. Arthroscopy of the knee remains a common clinical practise today [4, 5]. The indications for knee arthroscopy include assessment and repair of the meniscus, anterior cruciate ligament, posterior cruciate ligament, synovectomy for rheumatoid arthritis, management of osteochondral injuries, chondroplasty, microfracture, and in some select cases early osteoarthritis. It can also be used as a complementary method in the fixation of tibial plateau fractures and in the management of septic arthritis [1, 5]. There are few contraindications to knee arthroscopy; they include superficial soft-tissue infection due to the risk of introducing infection into the joint. A thorough preoperative assessment including history examination and imaging is necessary to ensure that unnecessary arthroscopies are not undertaken [4].
Patient setup for knee arthroscopy is commonly supine on the operating table with a circumferential tourniquet to the upper thigh. A surgical leg holder or side support is placed at the upper thigh (the side support is usually placed approximately 5 cm superior to the upper pole of the patella); this allows an unassisted surgeon to stand between the affected leg and the operating table and apply a valgus strain across the knee during the procedure, thus visualizing the medial compartment.
A systematic approach is taken to the assessment of the knee most commonly through two portals (anteromedial and anterolateral). Surface anatomy marking of the patella, tibial tubercle, patella tendon, fibula head, and medial and lateral joint lines can be easily identified. Portals can be made horizontally or vertically (horizontal being more cosmetic, vertical allowing more freedom of movement of the scope). The lateral joint line is slightly higher than the medial.
The lateral portal is created first in the soft spot on the anterolateral 1 cm above the joint line next to the patella tendon. The anteromedial portal is identified by placing a spinal needle through the soft spot 1 cm above the joint line on the medial side 1 cm medial to the patella tendon; the portal placement is confirmed with the arthroscope entering from the lateral portal [5].
Through movement of the arthroscope within the portals and angling the light source visualization of the suprapatellar pouch, medial gutter, lateral gutter, medial compartment, lateral compartment, intercondylar notch, and posteromedial and posterolateral compartments can be carried out. A standardized and systematic approach to this is essential to ensure that no pathology is missed [6].
Hip
The use of the arthroscope in the hip has been less popular than in the knee. Potential reasons for this are the complexity of the procedure, steep learning curve, or requirement of specialist equipment. It requires larger and more flexible instruments than arthroscopy of the knee, use of traction, and fluoroscopy. It does however allow excellent visualization of the articular surfaces of the hip joint as well as the peritrochanteric surfaces and extra-articular space [7, 8].
Its used has been described in the management of septic hip joints, removal of loose bodies, and synovial abnormalities. The most common indication for hip arthroscopy is for lesions of the acetabular labrum for which debridement of repair can be undertaken arthroscopically. It has also been used in the management of the causative factors of femoroacetabular impingement to normalize joint mechanics. Extra-articular indications for hip arthroscopy include refractory cases of snapping hip which have failed conservative management and thermal capsular shrinkage for problematic ligamentous laxity [7].
The patient is placed in the lateral or supine position with traction applied to the operated leg. The joint is distracted under fluoroscopic guidance. The joint is infiltrated with fluid for further distension of the capsule.
Superficial landmarks of the greater trochanter, femoral head, and sciatic nerve are marked out [8]. Typically three portals are used: a direct lateral paratrochanteric portal, a second anterolateral paratrochanteric portal, and an anterosuperior portal. The latter can be used to visualize the peripheral compartment, for this traction must be released [7]. Arthroscopy allows visualization of femoral head and acetabular pathology, soft tissue such as the ligamentum teres, acetabular labrum synovial folds, and synovium.
Complications of hip arthroscopy are rare and are reported to include bleeding, infection neuropraxia to the sciatic femoral or pudendal nerves secondary to traction and to the lateral femoral cutaneous nerve due to portal placement [9]. Late complications include trochanteric bursitis, osteonecrosis, dislocation of the femoral head and fluid extravasation to the abdomen [7, 10].
Foot and Ankle
Smaller arthroscopes and instrumentation have allowed arthroscopy to be useful in smaller joints. Indications for ankle arthroscopy include impingement syndrome, osteochondral lesions, instability and fracture [11], posttraumatic arthritis, adhesions, locking [12] loose bodies, arthrofibrosis, and synovitis [11].
For ankle arthroscopy the patient is typically positioned supine. The ankle is either dorsiflexed or distracted to allow visualization of the articular surface and ligaments [13]. Distraction can be manual or mechanical, and invasive or noninvasive.
Prior to portal placement landmarks of important structures are located; these include both malleoli, the anterior joint line, tibialis anterior tendon, peroneus tertius tendon, and Achilles tendon, great saphenous vein, and superficial peroneal nerve.
Numerous portals for ankle arthroscopy have been described due to the anatomic region being covered by an extensive network of neurovascular structures. They include anterior, posterior, transmalleolar, and transtalar. The most frequently used are the anteromedial and anterolateral ones. The anteromedial is medial to the tibialis anterior tendon at the anterior joint line. A visible and palpable soft spot can be found here when the ankle is dorsiflexed. The saphenous nerve and veins are at risk of injury from this portal. The anterolateral portal is identified on the anterior joint line lateral to peroneus tertius tendon, taking care not to injure the superficial peroneal nerve [14]. Posterior portals are more technically challenging and provide increased surgical risk.
Complications have been reported to include reflex sympathetic dystrophy , fibular fracture [12], superficial peroneal nerve damage, persistent drainage through portal sites, and infection [15].
Arthroscopy has also been used in other joints of the foot, namely the subtalar and first metatarsophalangeal joints. Arthroscopy of the hallux requires small instruments which are delicate and vulnerable to damage [13].
Shoulder
Shoulder arthroscopy remains a commonly performed procedure with an estimated 21,000 subacromial decompressions alone carried out in England in 2009/2010 [16]. Its use for this indication has become more controversial recently with the publication of the results of a pragmatic multicenter randomized controlled trial [17]. However, it is effective for a wide number of conditions ranging from diagnostic surgery, soft-tissue procedures (including cuff repairs, labral/SLAP repair, glenohumeral joint stabilization, synovectomy, and capsular release), bony procedures (AC joint excision and acromioplasty), and even suprascapular nerve release.
Patients can be positioned in either the beach chair or the lateral decubitus position depending on surgeon preference. The beach chair position [18] offers the advantages of an anatomical orientation and the use of stand-alone regional anesthesia but may present technical difficulties in terms of mechanical blocks when using the scope via a posterior portal. Via traction applied to the arm, the lateral decubitus position provides excellent joint space visualization albeit in a nonanatomical orientation. The potential for nerve injury is also higher with this approach due to a combination of traction and increased risk when placing the anteroinferior portal to the axillary and musculocutaneous nerves [19].
A number of options for primary and secondary portals exist and combinations are often used depending on the procedure being performed. The posterior portal is the first established for most procedures. It is located 2 cm inferior and 1 cm medial to the posterolateral corner of the acromion with the trochar inserted anteriorly towards the tip of the coracoid, often after the insertion of a spinal needle into the joint with or without infiltration of saline [20, 21]. This portal also allows access to the subacromial space via the same skin incision. The anterior portal is then usually established under direct vision by exploiting the rotator interval ensuring that a skin incision is made lateral to the coracoid process [21].
Secondary portals can be made along a line moving anterior to posterior using the borders of the acromion as a landmark. A superolateral portal placed 1 cm lateral to the anterolateral corner of the acromion allows access to the rotator cuff and anterior glenoid labrum. The lateral subacromial portal, the workhorse of most arthroscopic shoulder procedures, is sited inferior to the midpoint of the acromion. It allows access to the majority of intra- and extra-articular structures of the shoulder. Finally, the posterolateral portal, usually sited 1 cm anteroinferiorly to the posterolateral corner of the acromion, can prove useful when access to the posterior cuff and labrum is required [21].
As with all arthroscopic procedures a systematic approach is required to identify the biceps tendon, supraspinatus, infraspinatus, rotator interval, middle and inferior glenohumeral ligaments, subscapular recess, anterior labrum, and glenohumeral joint [22].
Complications associated with shoulder arthroscopy relate predominantly to the neurological structures around the shoulder. The axillary and suprascapular nerves are at risk with a poorly placed posterior portal while the musculocutaneous nerve is at risk with a misplaced anterior portal [23].
Elbow
Elbow joint arthroscopy is a rapidly evolving area of elbow surgery thanks largely to advances in technology and a better understanding of the complex anatomy of the elbow joint [24]. Its use has moved far from merely the purpose of diagnosis and is now indicated for a number of conditions ranging from removal of loose bodies to the treatment of osteo and septic arthritis.
Patients can be positioned in either a supine, prone, or lateral decubitus position. It is of vital importance to accurately mark out surface anatomy prior to distension of the joint to minimize the risk to the numerous neurovascular structures sited around the joint [25, 26]. The epicondyles of the humerus, olecranon process, and radiocapitellar joint should be identified and marked. When the patient’s body habitus allows, ideally the course of the ulnar nerve should also be determined [25].
Similar to the shoulder a number of potential portals are described about the elbow. Prior to portal creation the elbow joint is usually distended via infiltration of the joint using saline via the lateral “soft spot.” The choice of portal used depends on the procedure being performed.
Broadly speaking there are three lateral, three medial, and three posterior portals that may be utilized [24]. Lateral portals include the mid-anterolateral (most commonly used), distal anterolateral, and proximal anterolateral. The radial nerve is at risk with all three of these portals, the highest risk of injury lying with the distal anterolateral portal which has fallen out of favor [24]. The medial portals mirror their lateral counterparts in terms of their positions. The most commonly sited medial portal is the proximal anteromedial portal (often performed under direct vision once a lateral portal has been established) with the anteromedial portal being the second most commonly used. The mid-anteromedial portal is often seen as redundant due to its close proximity to the other two medial portals [24]. The posterior aspect of the elbow joint can be accessed via a direct posterior or “transtricipital” portal which splits the tendinous portion of the triceps allowing visualization of the olecranon fossa as well as the lateral and medial gutters [24] (Camp 23). Direct lateral and distal ulna portals are also described that facilitate access to the radiocapitellar joint.
Elbow arthroscopy continues to develop and is technically demanding. The biggest risk is to the nerves surrounding the elbow which can be damaged during portal placement. The most commonly injured is the ulna nerve followed by the radial nerve with the risk increased in patients with underlying contractures or rheumatoid arthritis, or those who have undergone previous ulna nerve transposition [27]. There have been reports also of heterotropic ossification though the reported rates are less than those seen in open surgery [27].
Wrist
Like elbow arthroscopy, the indications for wrist arthroscopy have increased over the last few decades. It is now used to treat or assess a wide range of conditions including TFCC injuries, chondral lesions of the carpus, dynamic assessment of carpal instability, soft-tissue pathologies such as ganglions or carpal tunnel syndrome, and even in assisting fracture reductions [28, 29].
Patients undergoing wrist arthroscopy are placed supine with the arm in approximately 7–10 lbs of in-line traction via finger traps. This can be performed either with the elbow flexed at 90° and the forearm vertical or with the arm extended on an arm table [30]. Portals for wrist arthroscopy are named after their relation to the extensor compartments of the wrist. Surface landmarks that need to be identified to ensure accurate placement are Lister’s tubercle, scaphoid and lunate, DRUJ, and ECU.
Broadly speaking, portals can be divided into radiocarpal portals (primarily used for viewing and TFCC repairs), midcarpal portals (used for visualizing the carpal bones and allowing for evaluation of wrist instability), and portals based around the first extensor compartment that allow for arthroscopic debridement of thumb base osteoarthritis [30, 31]. The 3-4 portal is usually the first established portal in wrist arthroscopy.
Complications of wrist arthroscopy (like all other upper limb arthroscopies) are predominantly due to nerve injuries affecting the dorsal sensory branch of the ulnar nerve as well as the superficial branch of the radial nerve. Less commonly the extensor tendons may become damaged or an iatrogenic osteochondral defect may occur, more often than not due to a lack of sufficient space [32].
Sternoclavicular Joint
A technique has been described for arthroscopy of the sternoclavicular joint which allows for diagnostic evaluation as well as treatment of degenerative conditions of the medial end of the clavicle [33].
Patients are positioned supine with a sandbag between their scapulae and 2.9 mm instruments are used [34]. The main concerns for most with relation to performing this procedure would be the proximity of important mediastinal structures. The authors describe an inferior portal, made 1 cm directly inferior to the joint line after palpation of the sternum and medial end of clavicle, followed by a more superior portal inserted under direct vision [34]. This relatively new procedure has the potential benefits of better joint visualization, less risk of compromising SCJ stability, and infection but would not be appropriately carried out by an inexperienced surgeon in a low-volume center [34].
References
Jackson RW. A history of arthroscopy. Arthroscopy. 2010;26(1):91–103.
Treuting R. Minimally invasive orthopedic surgery: arthroscopy. Ochsner J. 2000;2(3):158–63.
Ogilvie-Harris DJ. Operative arthroscopy. 2nd ed. Philadelphia, PA: Lippincott–Raven Publishers; 1996.
Onyema C, Oragui E, White J, Khan WS. Evidence-based practice in arthroscopic knee surgery. J Perioper Pract. 2011;21(4):128–34.
Ward BD, Lubowitz JH. Basic knee arthroscopy part 2: surface anatomy and portal placement. Arthrosc Tech. 2013;2(4):e501–2.
Ward BD, Lubowitz JH. Basic knee arthroscopy part 3: diagnostic arthroscopy. Arthrosc Tech. 2013;2(4):e503–5.
Griffiths EJ, Khanduja V. Hip arthroscopy: evolution, current practice and future developments. Int Orthop. 2012;36(6):1115–21.
Glick JM, Sampson TG, Gordon RB, Behr JT, Schmidt E. Hip arthroscopy by the lateral approach. Arthroscopy. 1987;3(1):4–12.
Griffin DR, Villar RN. Complications of arthroscopy of the hip. J Bone Joint Surg Br. 1999;81(4):604–6.
Matsuda DK. Acute iatrogenic dislocation following hip impingement arthroscopic surgery. Arthroscopy. 2009;25(4):400–4.
Cavallo M, Natali S, Ruffilli A, Buda R, Vannini F, Castagnini F, et al. Ankle surgery: focus on arthroscopy. Musculoskelet Surg. 2013;97(3):237–45.
Feder KS, Schonholtz GJ. Ankle arthroscopy: review and long-term results. Foot Ankle. 1992;13(7):382–5.
de Leeuw PA, Golanó P, Clavero JA, van Dijk CN. Anterior ankle arthroscopy, distraction or dorsiflexion? Knee Surg Sports Traumatol Arthrosc. 2010;18(5):594–600.
Golano P, Vega J, Perez-Carro L, Gotzens V. Ankle anatomy for the arthroscopist. Part I: the portals. Foot Ankle Clin. 2006;11(2):253–73.. v
Blazquez Martin T, Iglesias Duran E, San Miguel Campos M. Complications after ankle and hindfoot arthroscopy. Rev Esp Cir Ortop Traumatol. 2016;60(6):387–93.
Judge A, Murphy RJ, Maxwell R, Arden NK, Carr AJ. Temporal trends and geographical variation in the use of subacromial decompression and rotator cuff repair of the shoulder in England. Bone Joint J. 2014;96-B(1):70–4.
Beard DJ, Rees JL, Cook JA, Rombach I, Cooper C, Merritt N, et al. Arthroscopic subacromial decompression for subacromial shoulder pain (CSAW): a multicentre, pragmatic, parallel group, placebo-controlled, three-group, randomised surgical trial. Lancet. 2018;391(10118):329–38.
Mannava S, Jinnah AH, Plate JF, Stone AV, Tuohy CJ, Freehill MT. Basic shoulder arthroscopy: beach chair patient positioning. Arthrosc Tech. 2016;5(4):e731–e5.
Paxton ES, Backus J, Keener J, Brophy RH. Shoulder arthroscopy: basic principles of positioning, anesthesia, and portal anatomy. J Am Acad Orthop Surg. 2013;21(6):332–42.
Farmer KW, Wright TW. Shoulder arthroscopy: the basics. J Hand Surg Am. 2015;40(4):817–21.
Boyle S, Haag M, Limb D, Lafosse L. Shoulder arthroscopy, anatomy and variants—part 1. J Orthop Trauma. 2009;23(4):291–6.
Snyder SJ. Shoulder arthroscopy. Philadelphia, PA: Lippincott Williams & Wilkins; 2003.
Moen TC, Rudolph GH, Caswell K, Espinoza C, Burkhead WZ Jr, Krishnan SG. Complications of shoulder arthroscopy. J Am Acad Orthop Surg. 2014;22(7):410–9.
Camp CL, Degen RM, Sanchez-Sotelo J, Altchek DW, Dines JS. Basics of elbow arthroscopy part I: surface anatomy, portals, and structures at risk. Arthrosc Tech. 2016;5(6):e1339–e43.
Adams JE, King GJ, Steinmann SP, Cohen MS. Elbow arthroscopy: indications, techniques, outcomes, and complications. J Am Acad Orthop Surg. 2014;22(12):810–8.
Stetson WB, Vogeli K, Chung B, Hung NJ, Stevanovic M, Morgan S. Avoiding neurological complications of elbow arthroscopy. Arthrosc Tech. 2018;7(7):e717–e24.
King GJ. In: Bain GI, Safran MR, Pederzini LA, editors. Elbow arthroscopy complications. Berlin Heidelberg: Springer-Verlag; 2013.
Wagner J, Ipaktchi K, Livermore M, Banegas R. Current indications for and the technique of wrist arthroscopy. Orthopedics. 2014;37(4):251–6.
Monaghan BA. Uses and abuses of wrist arthroscopy. Tech Hand Up Extrem Surg. 2006;10(1):37–42.
Parvizi J. Wrist arthroscopy. Philadelphia, PA: WB Saunders; 2010.
Michelotti BF, Chung K. Procedure 20—wrist arthroscopy. 3rd ed. Berlin: Elsevier; 2018.
Leclercq C, Mathoulin C. Complications of wrist arthroscopy: a multicenter study based on 10,107 arthroscopies. J Wrist Surg. 2016;5(4):320–6.
Tytherleigh-Strong G, Rashid A, Lawrence C, Morrissey D. Arthroscopic sternoclavicular joint diskectomy for acute and chronic tears. Arthroscopy. 2017;33(11):1965–70.
Tytherleigh-Strong G, Van Rensburg L. Arthroscopic excision of the sternoclavicular joint. Arthrosc Tech. 2017;6(5):e1697–e702.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Tansey, R.J., Dunne, M.J., Khan, W.S. (2019). Endoscopic Surgery in Orthopedics. In: Iyer, K., Khan, W. (eds) General Principles of Orthopedics and Trauma. Springer, Cham. https://doi.org/10.1007/978-3-030-15089-1_33
Download citation
DOI: https://doi.org/10.1007/978-3-030-15089-1_33
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-15088-4
Online ISBN: 978-3-030-15089-1
eBook Packages: MedicineMedicine (R0)