Abstract
An autoimmune disease is a disorder in which the body’s immune system attacks itself. The dysregulation of the immune system associated with systemic autoimmune diseases can affect various organs systems, including the brain. This chapter will review the neuropsychological involvement and the resulting cognitive changes associated with three systemic autoimmune or rheumatic diseases: systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and primary Sjögren’s syndrome (SS). Diagnosis, neuropsychological assessment, and treatment planning are challenging since most of the disease manifestations are nonspecific. Due to the abundant literature on cognitive dysfunction in SLE as compared to the other two diseases, the discussion of cognition is focused mainly in SLE.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Introduction
An autoimmune disease is a disorder in which the body’s immune system attacks itself. The dysregulation of the immune system associated with systemic autoimmune diseases can affect various organs systems, including the brain. This chapter will review the neuropsychological involvement and the resulting cognitive changes associated with three systemic autoimmune or rheumatic diseases: systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and primary Sjögren’s syndrome (SS). Diagnosis, neuropsychological assessment, and treatment planning are challenging since most of the disease manifestations are nonspecific. Due to the abundant literature on cognitive dysfunction in SLE as compared to the other two diseases, the discussion of cognition is focused mainly in SLE.
Systemic Lupus Erythematosus
Definitions and Epidemiology
SLE is an autoimmune disease with predominance among women of child-bearing age. In the United States, SLE is more prevalent among African-Americans, Hispanics, and Asians compared to non-Hispanic Caucasians [1]. This autoimmune disease is characterized by chronic tissue/organ inflammation mediated through autoantibodies, immune complexes, and complement activation that results in multiorgan involvement. Chronic vascular inflammation is a hallmark of SLE. Although the molecular and cellular mechanisms responsible for this condition are largely unknown, the complement system participates in virtually all inflammatory and immune-mediated processes and may also contribute to vascular pathology in SLE.
Neuropsychiatric SLE (NPSLE) is arguably the least understood yet perhaps the most prevalent manifestation of lupus. It occurs in 14 to over 80% of patients with SLE and is associated with increased morbidity and mortality [2,3,4,5,6]. The clinical spectrum of NPSLE is broad and includes severe and acute symptoms such as psychosis, cerebrovascular accident, and myelopathy, in addition to more chronic symptoms such as headache and cognitive dysfunction.
Classification of Neuropsychiatric SLE
The manifestations of NPSLE can be diverse and can occur in the absence of SLE activity or serologic markers. The American College of Rheumatology (ACR) research committee established case definitions for 19 neuropsychiatric syndromes involving the central and peripheral nervous systems as shown in Table 21.1 [7]. Seizure and psychosis, however, are the only two NPSLE manifestations that comprise the neurologic component of the ACR classification criteria for SLE [8, 9]. Cognitive dysfunction is one of the case definitions for NPSLE. Some studies may include subjects with NPSLE based on the ACR case definitions, whereas other studies may have subjects with SLE who do not have overt symptoms of NPSLE or are termed as “non-NPSLE” but may actually have underlying cognitive dysfunction upon neuropsychological testing during the study. Currently, there is no case definition for neuropsychiatric syndromes in other autoimmune diseases such as RA and SS .
Pathophysiology of Cognitive Dysfunction Is Elusive
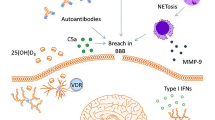
Among the protean manifestations of NPSLE, cognitive dysfunction may be the most difficult to comprehend due to the varying definitions and complexity of its pathophysiology. The prevalence of cognitive dysfunction ranges from 21 to over 80% of patients with SLE, depending on how the condition is defined [2,3,4,5]. Indeed, cognitive impairment can occur without signs of overt structural brain abnormalities. However, in order to treat the varied presentations of NPSLE, one needs to understand the mechanisms of cognitive dysfunction in hopes of identifying therapeutic targets. Using murine models, Diamond and colleagues have demonstrated that both a leaky blood–brain barrier and the presence of DNA antibodies that cross-react with NR2 subunits of the N-methyl-D-aspartate (NMDA) receptors are required to cause neuronal death in the hippocampus with resulting cognitive impairment [10]. The neuronal death was non-inflammatory by histopathologic examination and could be prevented by the administration of memantine, an NMDA receptor antagonist. Similarly, epinephrine, a catecholamine, also breached the blood–brain barrier and caused selective neuronal loss in the lateral amygdala, leading to emotional disorder in the murine model [11]. This study implies that agents such as epinephrine can determine the region of brain that is made vulnerable to neurotoxic autoantibodies. However, clinical findings have been less consistent than this animal model. A recent cross-sectional study of 60 SLE patients demonstrated the association of serum anti-NR2 antibodies with depressive mood but not with cognitive dysfunction [12]. In another study of 93 SLE patients, no association was found between serum anti-NR2 antibodies and cognitive dysfunction, depressive symptoms, or anxiety [13]. Similarly, in a study of 65 SLE female patients by Hanly and colleagues it was found that the prevalence of anti-NR2 antibodies was 35% and the presence of this antibody was not associated with cognitive dysfunction or change in cognitive function over time [14]. The negative findings in these clinical studies, all of which included well-recognized neuropsychological assessments, may be due to the small sample size and the lack of assessment of the breach in blood–brain barrier in these patients.
There have been several other notable studies to support a potential mechanism for central nervous system (CNS) changes associated with NPSLE that involve antiphospholipid (aPL) antibodies , platelets, complement activation, and thrombosis. First, a longitudinal study that followed 123 SLE patients for at least 3 years identified the presence of aPL antibodies as a predictor of cognitive dysfunction [15]. Furthermore, in this study, aspirin, an anti-platelet agent, appeared to be protective in the older age group of 42–69 years. A cross-sectional study showed that the presence of aPL antibodies along with hypertension, cumulative organ damage due to SLE, and brain lesions identified by magnetic resonance imaging (MRI) was independently associated with severity of cognitive impairment in SLE [16]. Second, cognitive dysfunction was frequently found in patients with aPL syndrome, independent of history of CNS involvement [17]. Third, studies have demonstrated aPL-mediated direct neuronal injury in the absence of ischemia [18,19,20,21]. Fourth, the presence of aPL antibodies have been associated with vascular occlusive events, particularly stroke in non-lupus patients [22, 23]. Finally, the capacity of aPL to bind to platelets provides further evidence that aPL, platelets, and complement activation may participate in a pathogenic thrombotic and/or vasculopathic mechanism in SLE.
Risk Factors for Cognitive Dysfunction
The risk factors for patients having CNS involvement are poorly defined. Various risk factors have been proposed but are difficult to delineate. For instance, while there does seem to be a role for aPL antibodies in cognitive dysfunction, most studies have failed to show an association between neuroradiologic findings and cognitive deficits or a clear correlation between aPL positivity and specific neuroradiologic lesions [16]. It is also unclear whether cardiovascular risk factors or Raynaud’s phenomenon via cerebral vasospasm contribute to the risk of CNS disease. Tomietto and colleagues studied 52 consecutive SLE patients to determine the presence and severity of cognitive impairment, in addition to the assessment of risk factors associated with neuropsychological deficits and cardiovascular disease [16]. They also studied patients with RA as controls since they were likely to have a similar background of prolonged disease and chronic corticosteroid use. Study subjects had a variety of testing including neuropsychological assessment, psychiatric evaluation, serologic tests including aPL antibodies, neuroradiographic testing, as well as historical evidence regarding presence of Raynaud’s phenomenon and cardiovascular risk factors. Several risk factors including hypertension, obesity, and age all played a substantial role in patients with SLE as compared to patients with RA. In addition to these risk factors, both Raynaud’s phenomenon and aPL antibodies are also independent risk factors for cognitive dysfunction. Raynaud’s phenomenon is vasospasm of small vessels causing tricolor changes in the hands and feet that is frequently seen in SLE and sometimes RA. Antiphospholipid antibodies, also seen frequently in SLE, are often associated with a predisposition for hypercoagulability and patients may manifest with venous and/or arterial thrombosis. The association of Raynaud’s phenomenon may be related to cerebral vasospasm. Ferraccioli and colleagues note that cerebral vasospasm is more frequent in individuals with both SLE and peripheral Raynaud’s compared to those without SLE [24]. In addition, cerebral vasospasm is related to more frequent headaches.
Due to the multiple confounding factors including disease state and morbidity associated with therapeutic medications used to treat these conditions including prednisone (i.e., glucocorticoid) – often at high doses, it is difficult to clearly define the risk factors that contribute to cognitive dysfunction seen in autoimmune disease. SLE disease activity has not been associated with cognitive dysfunction in cross-sectional and longitudinal studies [25, 26]. Furthermore, cognitive impairment appears to be a stable symptom of NPSLE. Carlomagno and colleagues conducted a longitudinal study of SLE patients (10 with NPSLE and 5 non-NPSLE) with cognitive impairment based on the Mental Deterioration Battery and the Mini-Mental State Examination, cognitive deficits persisted in all patients except for one non-NPSLE patient at mean follow-up of 21.5 months [27]. The Mental Deterioration Battery [28] evaluates for verbal abilities (Verbal Fluency and Phrase Construction tests), short- and long-term verbal memory (Rey Auditory Verbal Learning Test), immediate visual–spatial memory, visual–spatial reasoning (Raven Colored Progressive Matrices), and visuoconstructive abilities (Simple Copy and Copy with Landmarks tests).
Glucocorticoids are commonly used to treat SLE, RA, and SS. These patients, especially SLE patients, may be exposed to acute or short-term high dosages of glucocorticoids and then long-term lower maintenance dosages. Long-term glucocorticoid exposure may cause cognitive impairment from cumulative and long-lasting influences on hippocampal function and volume [29,30,31]. Acute effects of glucocorticoids can also impair memory retrieval [31, 32]. However, most studies did not find a relationship between glucocorticoid use and cognitive impairment [33,34,35,36,37].
Patients with cognitive impairment may also have co-existing mood disorder (i.e., depression) and fatigue that can further exacerbate the impairment [38,39,40]. In fact, depression has been reported to be present frequently in SLE patients with and without overt neuropsychiatric manifestations. In a study of 52 SLE patients without neuropsychiatric manifestations (non-NPSLE), 23 NPSLE patients and 27 healthy controls, Monastero and colleagues showed that depression levels significantly and independently predicted cognitive performance in SLE patients [41]. Both SLE groups demonstrated significant impairment compared with controls on tasks that assess verbal and non-verbal long-term memory and visuoconstructional abilities. Interestingly, NPSLE patients were more likely to be anxious and depressed compared to the other two groups. In a recent study of 67 non-NPSLE patients and 29 healthy controls by Kozora and colleagues, patients without overt NPSLE or neurologic dysfunction defined by standardized neurologic examination (the Scripps Neurologic Rating Scale) showed greater depressive symptoms on the Beck Depression Inventory-II and perceived cognitive difficulties compared with controls [42] . Furthermore, another study by Kozora and colleagues on 13 depressed SLE patients, 10 depressed control subjects, and 25 healthy controls showed a moderate agreement (86.4%) between the comprehensive neuropsychological battery and the American College of Rheumatology (ACR)-SLE battery of cognitive impairment in the depressed SLE patients [43]. In addition, depressed SLE patients performed worse than the depressed controls and healthy controls on the cognitive impairment index, a global score of cognitive functioning generated from the ACR-SLE battery . However, cognitive impairment in depressed SLE patients was not explained by depression alone. Other investigators have found that daily stress, but not depression or anxiety, was associated with impairments in visual memory, fluency, and attention in patients with SLE [44].
Risk factors for development of cognitive dysfunction are numerous, which can be related to the autoimmune disease, its treatment, and the associated comorbidities including cardiovascular disease, depression, and daily stress. However, SLE disease activity has not been associated with cognitive dysfunction. Furthermore, cognitive dysfunction persists and appears to be stable in a small longitudinal study of SLE patients.
The Role of Neuropsychological Testing in the Diagnosis of Cognitive Dysfunction in SLE
There is no single laboratory test that can confirm either the diagnosis of NPSLE or the associated cognitive impairment. Autoantibodies to ribosomal P protein are highly specific for SLE in serum and cerebral spinal fluid and have been found to be associated with psychosis and/or depression in some studies [45, 46]. In a larger series of 149 SLE patients using the ACR nomenclature for NPSLE, there was no association between anti-ribosomal P antibodies and cognitive dysfunction [47]. Table 21.2 provides descriptions and neuropsychological domains assessed for SLE studies that we were able to identify from the current literature. In general, most studies found neuropsychological impairments to be more prevalent in the SLE group than in healthy controls. Some, but not all, investigations report higher prevalence or severity of impairment in SLE compared to RA. Several studies discussed below have linked neuropsychological results to neuroimaging findings and/or hormonal and autoantibody status. In SLE, domains of impairment varied across studies, with deficits found in verbal fluency, visuospatial skills, memory, attention, and executive function. The myriad of cognitive changes associated with NPSLE have led to attempts to develop relatively brief neuropsychological test batteries that would be sensitive to the types of cognitive deficits associated with SLE.
ACR Neuropsychological Test Battery. The ACR Ad Hoc Committee on Neuropsychiatric Lupus Nomenclature defined cognitive dysfunction as documented impairment in any or all of the following cognitive domains: simple or complex attention, reasoning or problem solving, executive skills (e.g., planning, organizing, and sequencing), memory (e.g., learning and recall), visual–spatial processing, language (e.g., verbal fluency), and psychomotor speed. This research committee also proposed a standard 1-h battery of neuropsychological tests for use in patients with SLE as outlined in Table 21.3. Kozora and colleagues found that the validity and reliability of this ACR battery to be acceptable in a study of 31 patients with history of NPSLE, 22 non-NPSLE patients, and 25 healthy controls [48]. Findings for this study also indicate that the 1-h ACR battery for SLE patients has good sensitivity and specificity as compared to a 4-h comprehensive battery in patients without NPSLE as compared to controls. However, a problem with the brief battery becomes apparent in patients with NPSLE. Due to the wide variety of presentations seen in these patients, overall agreement between the 1- and 4-h battery decreases. The 1-h battery may be adequate to detect global impairment; however, a comprehensive traditional neuropsychological evaluation is recommended to identify specific deficits in NPSLE.
Automated Neuropsychological Assessment Metrics (ANAM) Testing. ANAM is a repeatable computerized cognitive battery that was initially developed by the United States military to monitor performance changes in healthy individuals undergoing environmental challenges [49, 50]. It is used to assess the effects of chemical agents, extreme environments, and fatigue on cognitive function and includes complex attention, cognitive processing speed, and cognitive efficiency. Since its development, this test has been used for measurement in various disease states including multiple sclerosis and SLE. ANAM tests typically used in SLE studies include Simple Reaction Time, Continuous Performance, Code Substitution, Immediate and Delayed Memory, Simultaneous Spatial Processing, Sternberg Task (i.e., sustained attention/working memory), Digit Span, and Matching to Sample and Mathematical Processing [51]. Various studies have attempted to evaluate the validity of ANAM testing in SLE [52, 53]. A 5-year longitudinal study of neuropsychiatric disease in SLE conducted by Holliday and colleagues in the San Antonio Study of Lupus Neuropsychiatric Disease (SALUD) compared both the traditional neuropsychological battery and the ANAM [52]. Sixty-seven patients with SLE and predominantly Hispanic/Latino (54%) completed the ANAM and the battery of traditional neuropsychological tests. ANAM testing was able to replicate the high prevalence (80%) of cognitive deficits in SLE and may be useful for assessment of cognitive impairment in the mixed-ethnic population with Hispanic patients. ANAM testing was also found to moderately correlate with the traditional neuropsychological test battery. The Hispanic SLE patients were younger, had less education, and had more current SLE disease activity. Hispanic and younger patients were found to be more impaired on the traditional tests, whereas ANAM test was not affected by Hispanic ethnicity or education. It appears that ANAM testing may less likely be influenced by confounding factors including effects of education, English language proficiency, and ethnic differences as compared to a traditional neuropsychological battery. Furthermore, Roebuck-Spencer and colleagues showed that ANAM is an efficient tool for screening and monitoring of cognitive functioning and emotional distress in SLE [53]. Sixty patients with SLE and without NPSLE were administered a 2-h battery of traditional neuropsychological tests and the Beck Depression Inventory-II. ANAM cognitive subtests were significantly correlated with many traditional neuropsychological tests (i.e., psychomotor processing speed and executive functioning using WAIS-III Digit Symbol and Part B of the Trail Making Test). After controlling for premorbid levels of cognitive ability, ANAM cognitive subtests also predicted SLE patients who had probable cognitive impairment versus no impairment with sensitivity of 76.2% and specificity of 82.8%.
A multicenter study by Petri and colleagues assessed 111 patients with recently diagnosed SLE (within 9 months of enrollment) and 79 healthy controls [51]. The SLE patients were more likely to be female, African-American, and Asian-American compared to the control group. After adjusting for age, sex, ethnicity, and education, the SLE patients performed significantly worse than normal controls on four of the nine ANAM cognitive subtests that require sustained attention/vigilance (continuous performance subtest) and sustained attention/working memory (Sternberg subtest), visual–spatial perception/working memory (matching to sample subtest), and non-verbal memory (code substitution immediate recall subtest). In the SLE patients, those with greater cumulative organ damage related to SLE or its treatment had worse performance on the spatial recognition test and the continuous performance test. The SLE patients with higher Calgary depression scale scores also had worse performance in the spatial recognition test. SLE medications and laboratory measures that include autoantibodies were not significantly associated with cognitive dysfunction.
Neuroimaging Modalities in Studies of Cognitive Dysfunction
Neuroimaging provides noninvasive assessment of brain pathology in NPSLE. Magnetic resonance imaging (MRI) is commonly used to review anatomical lesions in the brain tissue of patients with NPSLE; however, these lesions can be nonspecific and not reflective of the activity of NPSLE. Other neuroimaging modalities that have been used to study NPSLE include proton magnetic resonance spectroscopy (1HMRS), functional MRI (fMRI) , single photon emission computed tomography (SPECT), and positron emission tomography (PET). The majority of these studies are pilot investigations using small sample sizes.
MRI. Conventional MRI of the brain evaluates volume and findings varying from ischemic lesions to nonspecific small hyperintense deep white matter lesions. Lesions detected by MRI have been shown to correlate with cognitive impairment measured by neuropsychological testing in 72% of SLE patients (Kappa statistics for agreement = 0.42, p = 0.005) [16]. MRI abnormalities, such as T1- and T2-weighted lesions and cerebral atrophy, are more commonly detected in patients with SLE related to NPSLE compared to sex- and age-matched controls from the general population [54]. In SLE patients, cerebral atrophy was associated with cognitive dysfunction, seizures, and cerebrovascular disease, whereas T1- and T2-weighted lesions were more specifically associated with seizures and cognitive dysfunction, respectively.
1H-MRS. 1H-MRS has identified abnormal levels of neurometabolites as markers of neuronal function in areas that appear normal on anatomical MRI in SLE patients with cognitive dysfunction or active disease [12, 55, 56]. N-Acetylaspartate (NAA), choline (Cho), and creatine (Cr) are the neurometabolites most frequently measured in patients with SLE. NAA is a marker of neuronal and axonal integrity, and Cho appears to reflect cell membrane metabolism. A decrease in NAA peak in MR spectrum may represent neuronal or axonal dysfunction or loss and an increased in Cho peak may represent a heightened state of cell membrane turnover seen in demyelination, remyelination, or inflammation [57]. In SLE patients, progressive increase in Cho/Cr has been associated with an increased number of T2-weighted white matter hyperintense lesions in the 1H-MRS region of interest during follow-up [58]. SLE patients with moderate or severe cognitive dysfunction also had significantly higher Cho/Cr than those with mild or no cognitive dysfunction [12]. SLE patients with active disease, independent of CNS manifestations, had decreased NAA/Cr that returned to normal range after disease remission [56]. Conversely, patients who had active SLE during follow-up developed significant reduction in NAA/Cr. These findings suggest evidence of reversible neuronal dysfunction during periods of inactive SLE.
SPECT and PET. SPECT with technetium-99m hexamethylpropylene amine oxime has been used to assess regional cerebral blood flow. PET scan using glucose metabolism with fluorine-18 2-fluoro-2-deoxy-D-glucose (FDG-PET) can identify changes in regional cerebral metabolism in patients with NPSLE even without obvious structural lesions on conventional MRI. However, due to its expense and availability, PET is not suitable for routine clinical use. Abnormal FDG-PET can be found in SLE patients without obvious NPSLE or with normal MRI findings [59, 60]. Several studies have showed reduced cerebral blood flow in SPECT but intact glucose metabolism in PET in patients with NPSLE, suggesting a cerebrovascular disorder rather than a neuronal tissue disorder [59, 61]. Furthermore, in SLE patients with normal conventional MRI, glucose hypometabolism by PET along with decrease in cerebral blood flow by SPECT is associated with major NPSLE presentation such as confusion, and psychosis, whereas normal PET with decreases in cerebral blood flow by SPECT may be found in patients with or without NPSLE [60].
fMRI. fMRI is a promising functional neuroimaging technique, currently used in research applications, that evaluates brain activation patterns associated with specific cognitive tasks and may elucidate mechanisms involved in the development of cognitive dysfunction in SLE . Deoxyhemoglobin acts as an endogenous contrast agent to identify areas of increased perfusion in blood oxygen level-dependent fMRI or BOLD-fMRI. Contrast between images obtained during active and control task periods of paradigms reflects changes in regional brain activity. One fMRI study of 14 right-handed NPSLE patients and 14 sex- and age-matched right-handed healthy controls has shown an altered brain pattern of cortical activation in NPSLE patients when compared to healthy controls during simple motor task performance using the maximum finger-tapping frequency rate and the nine-hole peg test [62]. There were no neuropsychological testings performed in this study. Strong correlations were found between activation of sensorimotor areas and the extent and severity of brain lesions detected by conventional MRI. The findings suggest that cortical reorganization may contribute to the maintenance of normal function capacities in patients with NPSLE. Similarly, another fMRI study of nine NPSLE patients, nine RA patients, and nine healthy controls showed a greater frontoparietal activation during a working memory task (i.e., N-Back task) in NPSLE patients compared to RA patients and controls [63] but no between-group differences on the activation task. According to the SLE Disease Activity Index (SLEDAI) [64], none of the patients had neuropsychiatric symptoms at the time of fMRI scan. The CNS manifestations of the NPSLE patients varied and included cognitive deficits, seizure, brain stem lesions, mood disorder, psychosis, and stroke. This study suggests a need to recruit extra-cortical pathways as a compensatory mechanism in patients with NPSLE to achieve the same level of function as controls. In a small study of 10 female patients with childhood-onset SLE (i.e., age of onset <16 years) and 10 healthy controls, fMRI findings reveal widespread differences and imbalances of brain activation in the SLE patients compared with healthy controls [65]. They underwent formal neuropsychological testing and fMRI using three paradigms: a continuous performance task to evaluate attention, an N-Back task to assess working memory, and verbal generation to evaluate language processing. Composite Z maps were generated to summarize the brain activation patterns for each fMRI paradigm in the SLE patients and compared the patterns in the healthy controls. Cognitive dysfunction was found in 6 of the 10 SLE patients using the formal neuropsychological testing. None of these SLE patients had any active CNS manifestations as defined by the SLEDAI [64] or damage in the neuropsychological category of the Systemic Lupus International Collaborating Clinics Damage Index [66]. In the absence of an active stimulus, the SLE patients showed more baseline activity in the cingulate gyrus, an inhibitory brain region, during times of paradigm control tasks. These findings implied damage or malfunction of the underlying neural network connectivity in these SLE patients. In other words, more effort is needed to perform a task in SLE patients, whereas less effort is applied to inhibit task action during control periods.
These studies illustrate the importance of not only using a well-defined sample in studies of SLE patients but also the need to carefully consider the activation task used during fMRI procedures. For example, some tests may not be sensitive enough to activate brain regions of interest and others may lack validity with respect to the construct in question. Recent advances in computerized testing using paradigms, such as N-Back test as described above and the touch screen Cambridge Neuropsychological Test Automated Battery (CANTAB) [67,68,69] which has been used along with fMRI in non-SLE studies, developed by cognitive neuroscientists hold promise for use during imaging procedures.
Rheumatoid Arthritis
Rheumatoid arthritis (RA) is an autoimmune disease that manifests primarily as symmetric inflammation of multiple joints with the development of joint deformities from joint erosion and destruction over the course of many years. Presenting symptoms usually include morning stiffness as well as joint pain and swelling. However, RA can involve extra-articular organs and can be the underlying cause of interstitial lung disease, pericarditis, and premature atherosclerotic cardiovascular disease. Although not as well defined as in SLE, neuropsychiatric manifestations have been described in RA, including difficulties in memory, attention, and executive function [4].
Bartolini and colleagues investigated the hypothesis that CNS alterations in RA could directly affect behavior in 30 inpatients (27 females) with RA in Italy [70]. The mean age of the patients was 55.6 years with average disease duration of 11.8 years. Importantly, RA patients with motor impairment due to joint deformities were excluded from the sample, as were patients with current depression and previous psychiatric or neurological history. The patients received cerebral MRI scans, SPECT, and a 2-h neuropsychological battery that included attention, memory, visual–spatial, and executive function tests. Only two patients performed in the normal range on all tasks. Visuospatial planning ability (Block Design) was impaired in 71% of patients, and visual memory (Rey Complex Figure) was impaired in 50%. Forty-seven percent were impaired on the Wisconsin Card Sort Test, a measure of novel problem solving and higher order reasoning abilities. Phonemic verbal fluency was impaired in 44% but semantic verbal fluency (e.g., animal naming) was impaired in only 6%, suggesting more prominent left frontal involvement. Verbal memory (Rey Auditory Verbal Learning Test) was impaired in 35%. The authors correlated the NP results with the results of clinical evaluations, including swollen joint count, Ritchie articular index , morning stiffness in minutes, erythrocyte sedimentation rate, C-reactive protein, and overall disease severity using the Lee functional index. For the most part, impairment on specific tests was not correlated with the clinical parameters. However, impairment on Block Design was associated with swollen joint count, the articular index, and Lee functional impairment. This finding is not unexpected because this test requires the manual manipulation of blocks under strict time constraints. Mental flexibility on Trails B and WCST was also associated with the Lee scale. In multivariable regression analysis using cognitive scores as dependent variables and age, education, disease duration, and the disease severity indices as independent variables, there was an effect of age on WCST, and an effect of Ritchie and Lee severity indices on executive function overall (Block Design, Phonemic Verbal Fluency, and WCST). On MRI, 35% of patients (11 of 30) showed white matter hyperintensities, and each of these patients had low scores on attentional, executive function, and visuospatial tests. On SPECT, hypoperfusion was evident in the frontal lobes in 85% of patients, and in the parietal lobes in 40% of patients. The authors postulated that motor impairment could be, in part, due to microangiopathy in subcortical and parietal–frontal areas and that joint pain and stiffness could lead to sensory changes that affect motor planning processes. Although the study is notable for its attention to parameters such as depression and hand deformities that might confound NP testing in RA, there were several methodological limitations. These included subjective interpretation of MRI and SPECT images, lack of control group, and unavailability of Italian norms for some neuropsychological tests.
An investigation of cognitive function in systemic-onset juvenile idiopathic arthritis (SJIA) [71] contrasts with the Bartolini study. The 31 children and adolescents with SJIA and a healthy age-matched control group all scored within normal limits on Verbal, Performance, and Full-Scale IQ scores on the WISCR and WAIS-R. No memory deficits were seen on the Auditory Verbal Learning Test in either group, and no deficits were seen on a computerized fine motor performance task. The children and adolescents, who had average disease duration of 6 years and 2 months, also showed no difficulties in social and emotional adjustment on Achenbach’s child behavior checklist.
Dick and colleagues compared attentional abilities in adults with and without chronic pain: 20 RA patients, 20 fibromyalgia syndrome patients, 20 musculoskeletal pain patients, and 20 pain-free community controls age-matched to the RA patients [72]. Those with a history of neurologic disorder or psychiatric illness were excluded. The participants completed the Test of Everyday Attention (TEA) , a standardized neuropsychological battery with ecological validity. The TEA provides a composite score as well as age-referenced domains scores for selective attention, sustained attention, attention switching, and auditory–verbal working memory. RA patients had lower scores compared to the pain-free controls on TEA composite as well as on three of the four test domains: selective attention, sustained attention, and working memory. The between-group differences using analysis of variance remained significant after controlling for age, depressive symptoms, anxiety, and pain catastrophizing. Scores on attention switching did not differ among the four groups, and there were no significant differences between the three different pain groups on the attention tasks. This study may not have included a large enough sample size to detect differences among the pain groups. The authors did not report the numbers of patients in each diagnostic group who scored in the clinically impaired range. However, they did report that 60% of patients scored in the clinically impaired range on at least one TEA subtest, compared to 20% of healthy controls. Moreover, 38% of patients and 5% of healthy controls had more than one subtest in the clinically impaired range. The study suggests that having a history of chronic pain, whether due to RA, fibromyalgia, or other musculoskeletal origin, is associated with greater attentional difficulties on everyday tasks relative to pain-free controls.
In a controlled study by DeLuca and colleagues [73] designed to investigate working memory and speed of information processing in chronic fatigue syndrome (CFS) patients, 18 RA patients were included as a medically ill control group. The RA patients were without history of psychiatric or neurologic disorder. A series of computerized tasks adapted from the Paced Auditory Serial Addition Test (PASAT) were administered to assess speed of information processing and working memory. A set of simple auditory and visual reaction time tasks and choice auditory and visual reaction time tasks were also administered. CFS participants who were without comorbid psychiatric disorder (CFS-no-psych) had slower choice auditory reaction time and simple visual reaction time than RA patients. The RA patients did not differ significantly from a healthy control group of 29 individuals on any of the tasks of information processing speed or memory. This finding contrasts with the study by Dick and colleagues [72], in which RA performed more poorly compared to the pain-free controls in the areas of working memory and attention. The inconsistency in results could be attributed to differences in task, sample selection, and demographics. For example, in the study by Dick and colleagues, 75% of RA patients in the pain group were hospitalized and predominantly female, whereas the majority of pain-free controls were male. In the DeLuca study, the RA patients were recruited from rheumatology outpatient offices.
Brown and colleagues [74] highlight the importance of pain and depression as possible contributors to cognitive problems in autoimmune disease. These authors used structural equation modeling to determine whether depression mediates the association between pain and cognitive function. The participants consisted of 100 women and 21 men with RA from a larger medication adherence study. The average RA disease duration was 3.8 years (range 34–84 years). The majority of the patients (80%) rated their RA disease as moderate or severe. In a single study visit, participant completed the Arthritis Impact Measurement Scales-2 Pain scale and another pain scale devised for the adherence study, the Depressive Affect subscale of the Center for Epidemiologic Studies Depression Scale, and the Depression subscale of the Multiple Affect Adjective Checklist – Revised. Participants completed assessments of processing speed, inductive reasoning, working memory, and long-term episodic memory. However, the specific tests were not those typically used by clinical neuropsychologists, limiting a comparison of results with those of other studies. Pain and depression were associated with worse performance on the set of cognitive measures. Depression was a mediator of the pain–cognitive function relationship, in that the effect of pain on cognition was no longer significant after controlling for depression. These authors also found that older age had a negative effect on cognitive functioning that was largely independent of pain and depression, not a surprising finding considering recent work regarding mild cognitive impairment [75]. The cross-sectional design is a limitation of the study, as are the lack of control group and use of relatively infrequently used cognitive tasks. No conclusions can be drawn regarding the prevalence and severity of cognitive dysfunction in RA based on this study. However, it suggests that treatment addressing pain and depression may have positive effects on cognitive performance in RA.
Overall, there are few studies of cognitive function in RA, and fewer still that include healthy comparison groups or imaging studies. Although the different methodologies and tests used in the RA investigations make cross-study comparisons problematic, several studies found greater cognitive impairment in RA than healthy controls [37, 72] and less cognitive impairment in RA than SLE [16, 17, 76]. This latter finding suggests that disease mechanisms specific to SLE may contribute to the more prevalent cognitive dysfunction in that disorder as compared to RA, another autoimmune disease with involvement of inflammation and pain. Joint pain, joint stiffness, and RA-related factors may impact cognition function in RA.
Sjögren′s Syndrome
Another rheumatic disease that can manifest as neurologic dysfunction is Sjögren’s syndrome (SS). Primary SS is a chronic autoimmune disorder that targets exocrine glands resulting in dry eyes and dry mouth as the main symptoms. However, there may also be extra-glandular manifestations, including CNS symptoms, and patients with SS may have memory disorders and impaired intellectual performance. Other neurologic manifestations have been reported in patients with SS, including central nervous system (e.g., transverse myelitis), cranial neuropathies (e.g., optic neuritis), myopathy, and peripheral neuropathies. Secondary SS can be commonly associated with the presence of other systemic autoimmune diseases, such as SLE, RA, and systemic sclerosis. This overlap makes it difficult to attribute CNS manifestations to SS alone.
In primary SS, there is evidence that cerebral anti-muscarinic acetylcholine receptor (mAChR) autoantibodies may have a pathogenic role in immune-mediated neuroinflammation and on cognitive dysfunction. In a study of 15 women with primary SS who had frontal lobe syndrome-related disorder (defined as slowness, shifting capacity disorder, incapacity to resist cognitive conflict, programming capacity disorder, and decrease verbal fluency) and 15 age-matched controls, the circulating antibodies from the primary SS patients interacted with rat cerebral frontal cortex by activating the mAChR [77, 78]. These antibodies also have agonistic activity that promotes proinflammatory/cytotoxic prostaglandin E2 production and nitric oxide synthase (NOS) activity. The proposed downstream effect is the progressive loss of cerebral muscarinic receptor expression and activity, leading to cognitive dysfunction that involves synaptic plasticity and memory.
Few studies have systematically evaluated cognitive function using neuropsychological testing in SS patients; and none included large sample sizes. An investigation by Belin and colleagues [79] provides support for prevalent CNS involvement in SS. This study included 14 women with SS who were under 60 years old and not being treated with pain or antidepressant medications. They completed neurological examination, brain MRI, brain HMPAO-SPECT, and a battery of neuropsychological tests. Half of the patients had primary SS, and the other half had SS secondary to diseases that are not known to involve thrombosis or brain vasculitis (RA, progressive systemic scleroderma , and chronic hepatitis). Specific neuropsychological tests included are as follows: Rey Complex Figure Test with 5 min delay, semantic and phonemic verbal fluency, object and face recognition tasks, Trail Making Test, Stroop Color–Word Test, Wisconsin Card Sorting Test, digit span forward and backward, a block tapping task to assess immediate recall, and Wechsler Memory Scale. Only one patient had signs of CNS involvement on neurological exam. MRI revealed multiple areas of hyperintensity in half of the patients, six of whom were without any neurological history. All patients had abnormality on SPECT , with mild or moderate hypoperfusion in the periventricular white matter and/or subcortical rim. Likewise, abnormalities on neuropsychological testing were seen in all patients. Executive function was mildly or moderately impaired in all patients, compared to age and gender norms. Memory was impaired in 10/14 patients, primarily on the delayed memory task from the Rey Complex Figure. The authors concluded that cognitive evaluation using neuropsychological tests is the most sensitive method to diagnose CNS involvement in SS.
In a German descriptive study [78], 16/20 patients with primary SS were administered a vocabulary test to estimate Full-Scale IQ, the Benton Visual Memory Test, and the Zahlen-Verbindungs-Test, a test of perceptual speed similar to Trails A. Only 1 patient had an estimated IQ that was below average, but 4 patients (25%) showed below average visual memory; and 11 (70%) had deficits in perceptual speed. In contrast to the high rate of cognitive impairment on neuropsychological testing, only 4 of 20 patients showed cortical atrophy on head CT.
In a sample of 40 patients with SS, Malinow and colleagues [80] administered the Wechsler Memory Scale and an abbreviated Wechsler Adult Intelligence Scale – Revised to 16 patients with suspected cognitive impairments. The authors found 7/16 (46.6%) had mild to moderate memory and concentration difficulties. Unfortunately, comprehensive neuropsychological testing and neurologic evaluations were not performed in all patients, so the study is not informative regarding overall prevalence of cognitive dysfunction in SS. Other investigations report up to 25% prevalence of clinical manifestations of CNS involvement in SS, but without systematic neuropsychological evaluation [81]. A recent population study of 68 SLE patients and 72 primary SS patients by Harboe and colleagues showed common and comparable frequency of cognitive dysfunction, headache, and mood disorders in these diseases [82]. However, cerebrovascular disease was more prevalent in SLE, whereas peripheral neuropathies were more common in SS. The few SS studies that included neuropsychological testing indicate that cognitive dysfunction is prevalent in SS, particularly in areas of memory and executive function, even in the absence of neurological signs or MRI abnormalities.
Family and Social Issues
Neurocognitive changes increase the psychosocial burden of SLE, RA, and SS for both the patients and their families. SLE is typically characterized by flare-ups or fluctuations in tissue and organ inflammation that may last for weeks or months and are often associated with cognitive changes. The unpredictable flares so often associated with SLE disrupt family caregiving roles, and the disease is associated with work disability in 15–48% of patients [83,84,85,86]. When the flare involves the CNS, the associated acute cognitive disturbance may further compromise social, work, and family roles. In a survey of 829 SLE patients, reports of CNS involvement, cognitive difficulties, greater fatigue, and higher rating of SLE activity were associated with disability in valued life activities in a multivariable model [85]. Over 91% of patients reported disability in at least one valued life activity. Problems with family care were reported by over 50% of patients, and social activities were affected in 39–48% of patients. A study of work disability in 143 SLE patients revealed that 42.7% reported formal work disability due to their SLE. Cumulative damage due to SLE, severity of fatigue, African-American race, and global pain score was associated with formal work disability in a multivariable logistic regression model [87]. The same research team also reported on presence of neuropsychiatric dysfunction by neuropsychological testing in 50 work-disabled and non-disabled SLE patients [87]. Visual memory (Rey Complex Figure Test), processing speed and attention (Trail Making Test, Stroop Color–Word Test, and Symbol Digit Modalities Test) differed significantly between the 16 patients reporting formal work disability and 26 non-disabled patients. Verbal memory, verbal fluency, and motor speed did not differ between these groups. In a multivariable logistic regression model examining the effects of demographic and clinical variables on disability status, only cognitive impairment and cumulative organ damage due to SLE remained independently predictive of work disability. A recent survey study of 741 SLE patients found that severe memory impairment on the Hopkins Verbal Learning Test – Revised was associated with self-reported work disability in SLE [88]. Although these studies found somewhat different cognitive domains to be associated with work dysfunction in SLE, the role of cognitive impairment as an independent predictor of work role changes is noteworthy. In addition to flares, the majority of SLE patients experienced fluctuations in pain and fatigue. These symptoms, and associated depressive symptoms, can have a profound impact on the patient’s ability to plan and carry out activities and can also contribute to poorer cognitive performance in domains such as attention and memory [15, 89].
Fluctuations in pain and fatigue level are also a hallmark of RA and other rheumatic diseases. These symptoms can have similar effects on family roles and social and work functioning. In a cohort of 210 employed patients with recently diagnosed RA and other inflammatory conditions, 75% of sick leave periods were due to their joint conditions [90]. In multivariable analyses, high levels of pain, poorer physical function, and passive behavioral coping with pain were independently associated with increased sick leave. A telephone survey study of subclinical disability in 508 RA patients’ valued life activities revealed that over 75% reported disability in at least one valued life activity [91]. Difficulty with and need for accommodations in child care was reported by 39.5% of respondents, whereas difficulty with preparation of meals was experienced in 44.7%. Leisure activities, such as socializing, were problematic in approximately a third of patients. Patients who reported disabilities at baseline were more likely to report greater functional limitations at follow-up 2 years later (OR 1.14, 95% CI 10.6–1.23). No studies have directly evaluated the effects of cognitive difficulties on social functioning and family roles in patients with RA or SS. Nonetheless, the musculoskeletal pain, general fatigue, psychological distress, and cognitive difficulties are likely contributors of psychosocial burden in these autoimmune diseases.
Treatment
The recognition and treatment of cognitive dysfunction in patients with SLE, RA, or primary SS continue to be a major diagnostic and therapeutic challenge. Treating the underlying rheumatic disease may not be effective in the management of cognitive deficits since several studies, specifically in SLE, have not demonstrated the relationship between disease activity and cognitive dysfunction [27, 92]. However, the regular use of aspirin in older SLE patients with diabetes especially is associated with improved cognitive function in the SALUD study [15]. On the other hand, consistent glucocorticoid use, which may be a surrogate of more active or severe disease, is associated with decline in cognitive function.
Cognitive rehabilitation programs may teach patients the ways to adapt to their cognitive impairment and improve the ability to perform daily activities. A pilot study of 8-week psychoeducational group intervention for 17 female SLE patients with reported cognitive dysfunction showed improvement of metamemory and memory self-efficacy after participation [93]. The heterogeneity of the neuropsychological manifestations and the affected cognitive domains has led to a paucity of controlled clinical trials for cognitive rehabilitation of SLE patients. Thus, the current therapeutic approach is empirical and based on clinical experience and small clinical studies.
Summary and Conclusions
Cognitive dysfunction can occur in SLE patients with or without overt neuropsychological manifestations with varying prevalence depending on the definitions. In SLE, cognitive impairment commonly appears in attention and information processing, learning, memory, and executive/reasoning skills. However, there is no specific pattern of cognitive deficits. Although not as extensively studied as in SLE, patients with other autoimmune diseases such as RA and SS can also exhibit cognitive changes, particularly in the areas of attention, memory, and executive function. Since the batteries of NP tests and definition of cognitive dysfunction have varied in different studies, some recent studies began to use the brief ACR Neuropsychological Test Battery with established validity and reliability. While studies have attempted to identify the potential risk factors and mechanisms of cognitive dysfunction that would shed light on this challenging area, neuroimaging modalities, particularly fMRI, coupled with highly specialized, computer-administered tests based on experimental paradigms adopted from cognitive neuroscience hold the most promise to improve our understanding of the biological involvement in the brain of patients with autoimmune diseases. Thus, a multidisciplinary approach is needed to improve our understanding of the mechanisms of CNS involvement in autoimmune disease and to identify and treat these patients with cognitive deficits.
The widespread scientific interest in applying neuropsychological assessment and neuroimaging to evaluate neuropsychiatric involvement in systemic autoimmune and rheumatic diseases is a relatively recent phenomenon. Understandably, the field has been subject to certain growing pains. For example, small cross-sectional studies using diverse test batteries and case definition have been conducted in the past, leading to conflicting or inconclusive results. In order to have a better understanding of cognitive dysfunction, including possible mechanisms and risk factors, it is crucial to conduct multicenter longitudinal studies with a large sample size using the same definition of cognitive dysfunction and methodology in neuropsychological assessment and other data collection. The growing acceptance of the 1-h ACR Neuropsychological Test Battery and ANAM computerized testing battery along with ever improving neuroimaging methods should lead to advances in detection and classification of cognitive dysfunction in SLE. As the field advances, treatment to reduce the suffering of patients with neurocognitive dysfunction can also be addressed systematically.
References
Danchenko N, Satia J, Anthony M. Epidemiology of systemic lupus erythematosus: a comparison of worldwide disease burden. Lupus. 2006;15(5):308–18.
Ainiala H, Loukkola J, Peltola J, et al. The prevalence of neuropsychiatric syndromes in systemic lupus erythematosus. Neurology. 2001;57:496–99.
Brey RL, Holliday SL, Saklad AR, et al. Neuropsychiatric syndromes in lupus: prevalence using standardized definitions. Neurology. 2002;58(8):1214–20.
Hanly J, Fisk J, McCurdy G, et al. Neuropsychiatric syndromes in patients with systemic lupus erythematosus and rheumatoid arthritis. J Rheumatol. 2005;32(8):1459–1456.
Hanly JG, Urowitz MB, Sanchez-Guerrero J, et al. Neuropsychiatric events at the time of diagnosis of systemic lupus erythematosus: an international inception cohort study. Arthritis Rheum. 2007;56(1):265–73.
Kovacs J, Urowitz MB, Gladman D. Dilemmas in neuropsychiatric lupus. Rheum Dis Clin North Am. 1993;19:795–819.
ACR Ad Hoc Committee on Neuropsychiatric Lupus Nomenclature. The American College of Rheumatology nomenclature and case definitions for neuropsychiatric lupus syndromes. Arthritis Rheum. 1999;42:599–608.
Tan E, Cohen A, Fries J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–77.
Hochberg M. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725.
Kowal C, DeGiorgio LA, Nakaoka T, et al. Cognition and immunity: antibody impairs memory. Immunity. 2004;21(2):179–88.
Huerta PT, Kowal C, DeGiorgio LA, et al. Immunity and behavior: antibodies alter emotion. Proc Natl Acad Sci USA. 2006;103(3):678–83.
Lapteva L, Nowak M, Yarboro CH, et al. Anti-N-methyl-D-aspartate receptor antibodies, cognitive dysfunction, and depression in systemic lupus erythematosus. Arthritis Rheum. 2006;54(8):2505–14.
Harrison MJ, Ravdin LD, Lockshin MD. Relationship between serum NR2a antibodies and cognitive dysfunction in systemic lupus erythematosus. Arthritis Rheum. 2006;54(8):2515–22.
Hanly J, Robichaud J, Fisk J. Anti-NR2 glutamate receptor antibodies and cognitive function in systemic lupus erythematosus. J Rheumatol. 2006;33(8):1553–8.
McLaurin EY, Holliday SL, Williams P, et al. Predictors of cognitive dysfunction in patients with systemic lupus erythematosus. Neurology. 2005;64(2):297–303.
Tomietto P, Annese V, D‘agostini S, et al. General and specific factors associated with severity of cognitive impairment in systemic lupus erythematosus. Arthritis Rheum. 2007;57(8):1461–72.
Tektonidou MG, Varsou N, Kotoulas G, et al. Cognitive deficits in patients with antiphospholipid syndrome: association with clinical, laboratory, and brain magnetic resonance imaging findings. Arch Intern Med. 2006;166(20):2278–84.
Kent M, Alvarez F, Vogt E, et al. Monoclonal antiphosphatidylserine antibodies react directly with feline and murine central nervous system. J Rheumatol. 1997;24(9):1725–33.
Shoenfeld Y, Nahum A, Korczyn A, et al. Neuronal-binding antibodies from patients with antiphospholipid syndrome induce cognitive deficits following intrathecal passive transfer. Lupus. 2003;12(6):436–42.
Caronti B, Pittoni V, Palladini G, et al. Anti-beta 2-glycoprotein I antibodies bind to central nervous system. J Neuro Sci. 1998;156(2):211–19.
Caronti B, Calderaro C, Alessandri C, et al. Serum anti-beta2-glycoprotein I antibodies from patients with antiphospholipid antibody syndrome bind central nervous system cells. J Autoimmun. 1998;11(5):425–29.
Terashi H, Uchiyama S, Hashimoto S, et al. Clinical characteristics of stroke patients with antiphospholipid antibodies. Cerebrovasc Dis. 2005;19(6):384–90.
Nencini P, Baruffi M, Abbate R, et al. Lupus anticoagulant and anticardiolipin antibodies in young adults with cerebral ischemia. Stroke. 1992;23(2):189–93.
Ferraccioli G, Poi ED, Gregorio FD, et al. Changes in regional cerebral blood flow after a cold hand test in systemic lupus erythematosus patients with Raynaud’s syndrome. Lancet. 1999;354(9196):2135–2136.
Carbotte R, Denburg S, Denburg J. Cognitive dysfunction in systemic lupus erythematosus is independent of active disease. J Rheumatol. 1995;22(5):863–67.
Hanly J, Fisk J, Sherwood G, et al. Clinical course of cognitive dysfunction in systemic lupus erythematosus. J Rheumatol. 1994;21(10):1825–31.
Carlomagno S, Migliaresi S, Ambrosone L, et al. Cognitive impairment in systemic lupus erythematosus: a follow-up study. J Neurol. 2000;247:273–79.
Caltagirone C, Gainotti G, Masullo C, et al. Validity of some neuropsychological tests in the assessment of mental deterioration. Acta Psychiatr Scand. 1979;60(1):50–56.
de Quervain DJF, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394(6695):787–90.
Keenan PA, Jacobson MW, Soleymani RM, et al. The effect on memory of chronic prednisone treatment in patients with systemic disease. Neurology. 1996;47(6):1396–402.
Coluccia D, Wolf OT, Kollias S, et al. Glucocorticoid therapy-induced memory deficits: acute versus chronic effects. J Neurosci. 2008;28(13):3474–78.
de Quervain DJF, Roozendaal B, Nitsch RM, et al. Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nat Neurosci. 2000;3(4):313–314.
Holliday SL, Navarete G, Escalante A, et al. Demographic, symptomatic, and serologic predictors of cognitive function in systemic lupus erythematosus: preliminary results from SALUD. Abstract. J Int Neuropsychol Soc. 2000;6:231–232.
Gladman DD, Urowitz MB, Slonim D, et al. Evaluation of predictive factors for neurocognitive dysfunction in patients with inactive systemic lupus erythematosus. J Rheumatol. 2000;27:2367–71.
Glanz BI, Slonim D, Urowitz MB, et al. Pattern of neuropsychologic dysfunction in inactive systemic lupus erythematosus. Neuropsychiatry Neuropsychol Behav Neurol. 1997;10:232–38.
Hay EM, Black D, Huddy A, et al. Psychiatric disorder and cognitive impairment in systemic lupus erythematosus. Arthritis Rheum. 1992;35:411–16.
Kozora E, Thompson LL, West SG, et al. Analysis of cognitive and psychological deficits in systemic lupus erythematosus patients without overt central nervous system disease. Arthritis Rheum. 1996;39(12):2035–45.
Kozora E, Ellison MC, West S. Depression, fatigue, and pain in systemic lupus erythematosus (SLE): relationship to the American College of Rheumatology SLE neuropsychological battery. Arthritis Rheum. 2006;55:628–35.
Carbotte RM, Denburg SD, Denburg JA. Prevalence of cognitive impairment in systemic lupus erythematosus. J Nerv Ment Dis. 1986;174:357–64.
Wekking EM, Nossent JC, van Dam AP, et al. Cognitive and emotional disturbances in systemic lupus erythematosus. Psychother Psychosom. 1991;55:126–31.
Monastero R, Bettini P, Del Zotto E, et al. Prevalence and pattern of cognitive impairment in systemic lupus erythematosus patients with and without overt neuropsychiatric manifestations. J Neurol Sci. 2001;184(1):33–39.
Kozora E, Arciniegas DB, Filley CM, et al. Cognitive and neurologic status in patients with systemic lupus erythematosus without major neuropsychiatric syndromes. Arthritis Rheum. 2008;59(11):1639–46.
Kozora E, Arciniegas D, Zhang L, et al. Neuropsychological patterns in systemic lupus erythematosus patients with depression. Arthritis Res Ther. 2007;9(3):R48.
Peralta-Ramirez MI, Coin-Mejias MA, Jimenez-Alonso J, et al. Stress as a predictor of cognitive functioning in lupus. Lupus. 2006;15(12):858–64.
Isshi K, Hirohata S. Differential roles of the anti-ribosomal P antibody and antineuronal antibody in the pathogenesis of central nervous system involvement in systemic lupus erythematosus. Arthritis Rheum. 1998;41(10):1819–27.
Abdel-Nasser A, Ghaleb R, Mahmoud J, et al. Association of anti-ribosomal P protein antibodies with neuropsychiatric and other manifestations of systemic lupus erythematosus. Clin Rheumatol. 2008;27(11):1377–85.
Gerli R, Caponi L, Tincani A, et al. Clinical and serological associations of ribosomal P autoantibodies in systemic lupus erythematosus: prospective evaluation in a large cohort of Italian patients. Rheumatology. 2002;41(12):1357–66.
Kozora E, Ellison MC, West S. Reliability and validity of the proposed American College of Rheumatology neuropsychological battery for systemic lupus erythematosus. Arthritis Rheum. 2004;51(5):810–8.
Reeves D, Bleiberg J, Spector J. Validation of the ANAM battery in multi-center head injury studies [abstract]. Arch Clin Neuropsychol. 1993;8:356.
Reeves D, Kane R, Winter K. Automated neuropsychological assessment metrics (ANAM V3.11a/96). User’s manual: clinical and neurotoxicology subset. San Diego, CA: National Cognitive Foundation; 1996.
Petri M, Naqibuddin M, Carson K, et al. Cognitive function in a systemic lupus erythematosus inception cohort. J Rheumatol. 2008;35(9):1776–81.
Holliday S, Navarrete M, Hermosillo-Romo D, et al. Validating a computerized neuropsychological test battery for mixed ethnic lupus patients. Lupus. 2003;12(9):697–703.
Roebuck-Spencer TM, Yarboro C, Nowak M, et al. Use of computerized assessment to predict neuropsychological functioning and emotional distress in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;55(3):434–41.
Ainiala H, Dastidar P, Loukkola J, et al. Cerebral MRI abnormalities and their association with neuropsychiatric manifestations in SLE: a population-based study. Scand J Rheumatol. 2005;34(5):376–82.
Kozora E, Arciniegas DB, Filley CM, et al. Cognition, MRS neurometabolites, and MRI volumetrics in non-neuropsychiatric systemic lupus erythematosus: preliminary data. Cogn Behav Neurol. 2005;18(3):159–62.
Appenzeller S, Li LM, Costallat LTL, et al. Evidence of reversible axonal dysfunction in systemic lupus erythematosus: a proton MRS study. Brain. 2005;128(12):2933–40.
Narayana P. Magnetic resonance spectroscopy in the monitoring of multiple sclerosis. J Neuroimaging. 2005;15(4 Suppl):46S–57S.
Appenzeller S, Li LM, Costallat LTL, et al. Neurometabolic changes in normal white matter may predict appearance of hyperintense lesions in systemic lupus erythematosus. Lupus. 2007;16(12):963–71.
Kao C-H, Lan J-L, ChangLai S-P, et al. The role of FDG-PET, HMPAO-SPET and MRI in the detection of brain involvement in patients with systemic lupus erythematosus. Eur J Nucl Med Mol Imaging. 1999;26(2):129–34.
Kao C-H, Ho Y-J, Lan J-L, et al. Discrepancy between regional cerebral blood flow and glucose metabolism of the brain in systemic lupus erythematosus patients with normal brain magnetic resonance imaging findings. Arthritis Rheum. 1999;42(1):61–68.
Grunwald F, Schomburg A, Badali A, et al. 18FDG PET and acetazolamide-enhanced 99mTc-HMPAO SPET in systemic lupus erythematosus. Eur J Nucl Med. 1995;22:1073–77.
Rocca MA, Agosta F, Mezzapesa DM, et al. An fMRI study of the motor system in patients with neuropsychiatric systemic lupus erythematosus. NeuroImage. 2006;30(2):478–84.
Fitzgibbon BM, Fairhall SL, Kirk IJ, et al. Functional MRI in NPSLE patients reveals increased parietal and frontal brain activation during a working memory task compared with controls. Rheumatology. 2008;47(1):50–53.
Bombardier C, Gladman D, Urowitz M, et al. Derivation of the SLEDAI: a disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35(6):630–40.
DiFrancesco MW, Holland SK, Ris MD, et al. Functional magnetic resonance imaging assessment of cognitive function in childhood-onset systemic lupus erythematosus: a pilot study. Arthritis Rheum. 2007;56(12):4151–63.
Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the systemic lupus international collaborating clinics/American college of rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996;39(3):363–69.
Robbins T, James M, Owen A, et al. Cambridge neuropsychological test automated battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5(5):266–81.
Robbins T, James M, Owen A, et al. A study of performance on tests from the CANTAB battery sensitive to frontal lobe dysfunction in a large sample of normal volunteers: implications for theories of executive functioning and cognitive aging. Cambridge Neuropsychological Test Automated Battery. J Int Neuropsychol Soc. 1998;4(5):474–90.
Chase HW, Clark L, Sahakian BJ, et al. Dissociable roles of prefrontal subregions in self-ordered working memory performance. Neuropsychologia. 2008;46(11):2650–61.
Bartolini M, Candela M, Brugni M, et al. Are behaviour and motor performances of rheumatoid arthritis patients influenced by subclinical cognitive impairments? A clinical and neuroimaging study. Clin Exp Rheumatol. 2002;20(4):491–97.
Feldmann R, Weglage J, Roth J, et al. Systemic juvenile rheumatoid arthritis: cognitive function and social adjustment. Ann Neurol. 2005;58(4):605–09.
Dick B, Eccleston C, Crombez G. Attentional functioning in fibromyalgia, rheumatoid arthritis, and musculoskeletal pain patients. Arthritis Rheum. 2002;47(6):639–44.
DeLuca J, Christodoulou C, Diamond B, et al. Working memory deficits in chronic fatigue syndrome: differentiating between speed and accuracy of information processing. J Int Neuropsychol Soc. 2004;10(1):101–09.
Brown SC, Glass JM, Park DC. The relationship of pain and depression to cognitive function in rheumatoid arthritis patients. Pain. 2002;96(3):279–84.
Smith G. Bondi MNormal aging, mild cognitive impairment, and Alzheimer’s disease. In: Morgan J, Ricker J, editors. Textbook of clinical neuropsychology. New York, NY: Taylor & Francis; 2008. pp. 762–80.
Kozora E, Laudenslager M, Lemieux A, et al. Inflammatory and hormonal measures predict neuropsychological functioning in systemic lupus erythematosus and rheumatoid arthritis patients. J Int Neuropsychol Soc. 2001;7(6):745–54.
Orman B, Sterin-Borda L, De Couto Pita A, Reina S, Borda E. Anti-brain cholinergic auto antibodies from primary Sjögren syndrome sera modify simultaneously cerebral nitric oxide and prostaglandin biosynthesis. Int Immunopharmacol. 2007;7(12):1535–43.
Mauch E, Volk C, Kratzsch G, et al. Neurological and neuropsychiatric dysfunction in primary Sjogren’s syndrome. Acta Neurol Scand. 1994;89(1):31–35.
Belin C, Moroni C, Caillat-Vigneron N, et al. Central nervous system involvement in Sjogren’s syndrome: evidence from neuropsychological testing and HMPAO-SPECT. Ann Med Interne. 1999;150(8):598–604.
Malinow KL, Molina R, Gordon B, et al. Neuropsychiatric dysfunction in primary Sjogren’s syndrome. Ann Intern Med. 1985;103(3):344–50.
Alexander EL. Neurologic disease in Sjogren’s syndrome: mononuclear inflammatory vasculopathy affecting central/peripheral nervous system and muscle. A clinical review and update of immunopathogenesis. Rheum Dis Clin North Am. 1993;19(4):869–908.
Harboe E, Tjensvoll AB, Maroni S, et al. Neuropsychiatric syndromes in patients with systemic lupus erythematosus and primary Sjogren’s syndrome – A comparative population-based study. Ann Rheum Dis. 2008; ard.2008.098301.
Bertoli A, Fernandez M, Alarcon G, et al. Systemic lupus erythematosus in a multiethnic US cohort LUMINA (XLI): factors predictive of self-reported work disability. Ann Rheum Dis. 2007;66(1):12–7.
Utset TO, Chohan S, Booth SA, et al. Correlates of formal work disability in an urban university systemic lupus erythematosus practice. J Rheumatol. 2008;35:1046–52.
Scofield L, Reinlib L, Alarcon GS, et al. Employment and disability issues in systemic lupus erythematosus: a review. Arthritis Rheum. 2008;59(10):1475–79.
Katz P, Morris A, Trupin L, et al. Disability in valued life activities among individuals with systemic lupus erythematosus. Arthritis Rheum. 2008;59(4):465–73.
Utset TO, Fink J, Doninger NA. Prevalence of neurocognitive dysfunction and other clinical manifestations in disabled patients with systemic lupus erythematosus. J Rheumatol. 2006;33(3):531–38.
Panopalis P, Julian L, Yazdany J, et al. Impact of memory impairment on employment status in persons with systemic lupus erythematosus. Arthritis Rheum. 2007;57(8):1453–60.
Kozora E, West SG, Forrest S, et al. Attention and depression in systemic lupus erythematosus. 59th Annual Meeting of American Psychosomatic Society: 2001 March 7–10 2001.
Geuskens GA, Hazes JMW, Barendregt PJ, et al. Work and sick leave among patients with early inflammatory joint conditions. Arthritis Rheum. 2008;59(10):1458–66.
Katz P, Morris A, Yelin E. Subclinical disability in valued life activities among individuals with rheumatoid arthritis. Arthritis Rheum. 2008;59(10):1416–23.
Kozora E, Thompson LL, West SG, et al. Analysis of cognitive and psychological deficits in systemic lupus erythematosus patients without overt central nervous system disease. Arthritis Rheum. 1996;39(12):2035–45.
Harrison MJ, Morris KA, Horton R, et al. Results of intervention for lupus patients with self-perceived cognitive difficulties. Neurology. 2005;65(8):1325–27.
Stroop J. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–62.
Delis D, Kramer J, Kaplan E, et al. California verbal learning test. 2nd ed. San Antonio, TX: Psychological Corporation; 2000.
Le Osterrieth P. test de copie d’une figure complex. Arch Psychol. 1944;30:286–356.
Wechsler D. Wechsler adult intelligence scale. 3rd ed. San Antonio, TX: Psychological Corporation; 1997.
Denburg S, Carbotte R, Ginsberg J, et al. The relationship of antiphospholipid antibodies to cognitive function in patients with systemic lupus erythematosus. J Int Neuropsychol. 1997;3:377–86.
McLaurin EY, Holliday SL, Williams P, et al. Predictors of cognitive dysfunction in patients with systemic lupus erythematosus. Neurology. 2005;64(2):297–303.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kao, A.H., Greco, C.M., Gharib, S.L., Beers, S.R. (2019). Neurocognitive Function in Systemic Autoimmune and Rheumatic Diseases. In: Armstrong, C., Morrow, L. (eds) Handbook of Medical Neuropsychology. Springer, Cham. https://doi.org/10.1007/978-3-030-14895-9_21
Download citation
DOI: https://doi.org/10.1007/978-3-030-14895-9_21
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-14894-2
Online ISBN: 978-3-030-14895-9
eBook Packages: Behavioral Science and PsychologyBehavioral Science and Psychology (R0)