Abstract
Starting from birth, sex hormonal fluctuations mark the various phases of female life leading from puberty to menopause.
During the aging process, there are physiological, structural, and functional changes of the hypothalamus-hypophysis-gonadal axis that lead over time to reach the definitive menopausal state. At the central level (thalamus, hypothalamus, amygdala, medial prefrontal cortex, and cingulate gyrus), changes in hormone secretion and receptor reassignment determine, in part of the perimenopausal population, neurological symptoms such as mood and sleep disorders, Alzheimer disease, hot flushes, and migraine. The mainly implicated receptors have been identified in ER α and ER β; thanks to the link with sex hormones they lead to the activation of different pathways of response. Moreover, they allow the nervous system to modulate synapsis, neuronal plasticity, cell-cell communication, myelin organization and stability; they protect from oxygen deprivation, stroke, and Aβ- and glutamate-induced toxicities. A further proof could be what happens when women undergo surgical menopause (bilateral oophorectomy) before their natural aging process: neurological symptoms seem to have a sudden and severe onset. Nowadays numerous studies are emerging, able to precisely define the intrinsic causes of these changes, in a way to improve in the near future the postmenopausal quality of life.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
18.1 Introduction
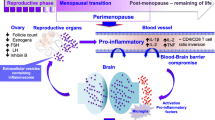
Menopause, defined as cessation of menses after 1 year, represents an important transition in reproductive states in women. It occurs at a median age of 51, preceded by 4–6 years of cycle alterations, and it’s associated with fluctuating hormone levels and emergence of symptoms like sleep disturbances, mood and memory changes, cognitive impairment, hot flushes, and vaginal dryness. Those symptoms occur with different grades of severity, frequency, and duration and can invalidate women’s life. In this scenario, specific brain areas are involved: olfactory bulb, thalamus, hypothalamus, amygdala, mammillary bodies, nucleus accumbens, septum, hippocampal formation, parahippocampal gyrus, insula, orbitofrontal cortex, medial prefrontal cortex, and cingulate gyrus. Increasing evidences suggest that cognitive complaints after menopause may represent an important marker for early neural dysfunction and dementia connected with the physiological postmenopausal loss of estradiol. In fact, even if subjective cognitive decline can be associated with nonmenopause-related conditions (i.e., normal aging; psychiatric, neurologic, and medical disorders; substance use; and drug effects), several studies demonstrate organic neurological changes caused by sex hormone deprivation [1]. This opens new frontiers to an additional understanding of peripheral hormones affecting cognitive and, in general, neurological functions.
18.2 From Reproductive Age to Menopausal Transition
The estrogen and progesterone synthesis from enzymatic modifications of cholesterol takes place primarily in the ovaries but also in the adrenal gland and adipose tissue. The two main active estrogens in nonpregnant women are estrone and estradiol, while pregnant women also produce significant quantities of estriol. Gonadotropins, luteinizing hormone (LH), and follicle-stimulating hormone (FSH), released from the pituitary gland under hypothalamus control, modulate both production and secretion. During the entire life, physiological hormonal fluctuations may determine organic and cognitive changes (Fig. 18.1). During childhood, estrogen and progesterone levels are low but increase at the onset of puberty under the influence of LH and FSH leading to regular menstrual cycling. In this last scenario, it’s possible to identify two phases: follicular phase when serum estrogen levels are high and progesterone low and luteal phase when progesterone level is high compared to the estrogen level. It’s interesting to notice that luteal phase can be associated with several premenstrual symptoms such as abdominal bloating, cramping, weight changes, headaches, decreased concentration, depression, irritability, and anxiety. Specific hormonal changes happen during pregnancy: both estrogen and progesterone increase across the three trimesters and then return to baseline after parturition. During postpartum phase on the one hand, the high prolactin level suppresses the synthesis of sex hormones (GnRH mediated) so that ovulation can’t occur; on the other hand, the relatively low estrogen level detected can cause in some women postpartum depression. Interestingly, another hormonal modification starts during perimenopause when progesterone levels decline more quickly than estrogen. Instead, levels of progesterone and estrogen become permanently low after menopause transition. Specifically, the decline of sex steroid and inhibins leads to a loss of hypothalamic feedback inhibition that stimulates GnRH and gonadotropin production. Moreover, the decrease inhibin production results in a decreased activin receptor inhibition and, together with the increase in bioavailable activin, leads to a further increase in the secretion of GnRH and gonadotropins. As a consequence, without a negative feedback, the elevated secretion of GnRH, LH, and FSH causes ovarian senescence and impairment.
18.3 Estrogen Receptor Alpha and Beta
Estrogen receptors are members of the nuclear receptor superfamily and DNA-binding transcription factors localized in synaptic terminals, dendritic spines and shafts, axons, mitochondria, and glial cell. It’s possible to identify two different isoforms, ER α and ER β, differing in expression and actions [2]. Both are able to heterodimerize, suggesting that different responses are modulated by their coexistence [3]. During aging, changes in the ratio ER α/ER β regulate estrogen-mediated gene transcription and control memory, cognition, attention, sensory integration, mood, emotion, and motivation [4]. In this scenario estrogens act both via traditional and nontraditional mechanisms, on the one hand binding ER α or ER β in the nucleus and on the other hand cooperating in association with lipid rafts and receptors like G protein-coupled estrogen receptor (GPER) [5]. In the classical mechanism, after the dimerization between receptors, a binding to a DNA estrogen receptor (ERE) occurs; thus transcription of estrogen-sensitive genes and proteins can initiate. It’s interesting to notice that there is a higher affinity between ER α and ERE than the one with ER β that may reflect in a more successful transcription. In nonclassical mechanism, estrogens bind to membrane-bound receptors, including GPER, which activates second messenger systems, causing the resulting response. Evidence suggests a combination of both membrane-initiated and genomic actions to affect transcription.
Evidences have demonstrated that ERs may be integrated into the plasma membrane: it’s reasonable that they may be localized in lipid rafts microstructures and they would take part in cell survival with other molecules which may modulate their physiological activities. The list includes GPI-anchored receptors, G protein-coupled receptors (adrenergic receptors, adenosine receptors, and cannabinoid receptors), glutamate receptors (AMPA, NMDA, mGluR), neurotrophin receptors (tyrosine kinase receptors, TrkA, TrkB, ephrin receptor, Eph, c-Ret, ErbB), Src family receptors (c-Src, Lyn, Fyn), cell adhesion molecules (NCAMs, TAG-1, Thy-1), and proteins associated with myelin glycosynapse (LINGO1, p75, NgR1, myelin-associated glycoprotein). What is certain is that numerous studies demonstrate the lipid raft involvement in nervous system functioning like modulating synapsis, neuronal plasticity, cell-cell communication, myelin organization and stability, autophagy, neuronal survival, and neurodegeneration and protection from oxygen deprivation, stroke, and Aβ- and glutamate-induced toxicities [6].
18.4 ER α
It’s primarily expressed as a nuclear receptor in prefrontal cortex, in hippocampus, and especially in amygdala and hypothalamus, regions that mediate memory and emotional process; however, an extranuclear form is found in both cytoplasmic and membrane locations including within dendritic spines and at the synapse (Fig. 18.2). Gonzalez et al. [7] demonstrated that expression of ER α in the nuclei of pyramidal cell of the hippocampus could occur from 15 gestational week to adulthood where it was maintained with the association of the cytoplasmatic isoform. Moreover, during the transition period to menopause, we assist to an increased expression of ERα-splice variant (MB1) as an indicator of its potential role during aging.
Estradiol regulates spine synapses on hippocampal pyramidal neurons through ER α. In the diestrus phase, when estradiol levels are low, spine densities are also low. During proestrus, when ovulation occurs, both estrogen levels and spine densities increase in parallel. In the estrus phase, the day after proestrus, the system prepares for the next cycle, and spine densities return to baseline
Supporting the importance of ER α in neuronal process, the study by Wang AC et al. “Synaptic estrogen receptor-alpha levels in prefrontal cortex in female rhesus monkeys and their correlation with cognitive performance” asserts that in ovariectomized female monkeys, estradiol treatment with the presence of postsynaptic ER α improves cognitive performance.
Moreover ER α nonneuronal expression may contribute to estrogen actions, including injury-sensitive expression of ER α in glial cells.
18.5 ER β
This receptor is transcribed from a different gene than ER α, and for this reason, despite similar properties, it has both ligand-dependent and ligand-independent actions. It’s expressed as a nuclear receptor in the hippocampus, neocortex, claustrum, and thalamus, participating to preservation of neuronal integrity. ER β is also found in the mitochondria, protecting from the oxidative and metabolic stress implicated in Alzheimer and Parkinson disease. Its first detection appears at 15 gestational weeks in proliferative zones, and then, at 25 gestational weeks, ER β is detected in cortical neurons, both in the nuclei and in the cytoplasm. This condition changes during life; in fact in adulthood much of the nuclear isoform is lost, while cytoplasmatic ER β receptors become predominant; however, the total percentage of synapse connected to ER β decreases.
18.6 Neuroendocrine Changes Across the Menopausal Transition
Hypothalamic-pituitary-adrenal axis activity regulates several physiological and neurological functions by monoamine neurotransmitter and glucocorticoids. Given the early change of GNRH and FHS in middle-aged women before ovarian decline, the hypothesis outlined is that HPA undergoes independent functional modifications during its physiological aging. The dysregulation of this axis causes loss of coordination with ovarian hormonal secretion. In particular the loss of estrogenic sensitivity at the hypothalamic level determines an increased production of gonadotropins that, with a concomitant lower ovarian response to FSH and LH, leads to anovulatory cycles.
Furthermore, the general evidence is that in areas such as the prefrontal cortex, limbic system, and hypothalamus, high levels of ERs are expressed so that, thanks to its interactions with ER alpha and beta estradiol, these can modulate the synthesis, release, and metabolism of peptides like dopamine, serotonin, acetylcholine, β-endorphin, and neurosteroids, such as allopregnanolone and dehydroepiandrosterone (DHEA) (Fig. 18.3) [8]. In fact, during climacterium and menopause, the fluctuation of steroid hormones induces a dysregulation of neurotransmitter pathways that concomitant with the persistently high levels of FSH and LH leads to neurologic, mood, memory, sleep, and thermoregulation disturbances. Moreover, the decreases in both DHEA/cortisol ratio and androgens may have implication in the lack of energy, sexual arousal and satisfaction, and learning and memory processes [9]. Finally, a study by Farrag et al. [10] suggested that surgical menopause obtained with oophorectomy before the natural onset of menopause may result in a more negative symptom manifestation when compared to oophorectomy after the menopause transition is completed.
18.7 Symptoms and Consequence
18.7.1 Mood Disorders and Depression
Mood disorders are the second leading cause of disability in developed countries, affecting over 350 million people worldwide without distinction of sex, age, and social status.
Epidemiologic data suggest two different incidence and prevalence peaks of depressive symptoms in women: the first at 40s and the second between 55 and 64 years, following the trend of menopause transition. During this period, the risk of developing mood disorders is greater the more the woman is exposed to sex hormone fluctuations rather than to their decline. Since perimenopause can last for several years, the risk of developing depression increases over time as the transition is longer [11]. Furthermore the Mayo Clinic Cohort Study of Oophorectomy and Aging by Rocca et al. asserts that mono- or bilateral oophorectomy before the onset of menopause can cause a major risk of anxiety symptoms, standing for a risk factor for depression.
Underlying all these modifications are some emerging hypotheses such as the reduction of neurosteroids and the expression of high serum MKP-1 levels.
Low circulating allopregnanolone levels (caused by ovarian failure) could be responsible for depressive symptoms in vulnerable women since they determine a consequent reduced synthesis of GABA-A receptor in specific brain areas deputed to mood and emotional control [12].
Ling-yun et al. [13] find a correlation between high serum MKP-1 (a protein that inactivate mitogen-activated protein kinase MAKP-1), low serum levels of brain neurotrophic derived factor (BDNF), and depression. However more studies need to be elaborated.
Moreover vasomotor symptoms represent an index of increased prevalence of mood disorders that can be studied in the late reproductive years. Indeed, Boulant et al. [14] confirm this association describing an increased number of hot flushes episodes in women with severe depressive symptoms rather than in those without mood alterations. In addition, general data support the fact that high levels of anxiety correlate with more severe and frequent vasomotor symptoms.
Finally as glucose is fundamental for brain metabolism, a decrease in its blood concentration and consumption in the hippocampus, parahippocampal gyrus, and temporal lobe, shown by 18F-FDG_PET images, may have a plausible role in mood disorders [15].
18.8 Alzheimer Disease
Alzheimer disease, one of the major neurodegenerative causes of dementia, mostly affects women (16.7% versus 9.1% for men). This is confirmed by several evidences which show the influence of sex hormone fluctuation on brain changes during women aging. The transition from pre-menopause to menopause may represent the crucial time frame in which the metabolically active and healthy status of the female brain switches to a hypometabolic and oxidative state.
Short et al. [16], studying gonadotrophin levels in Alzheimer postmenopausal women, prove the relation between increased production of amyloid-β and high levels of FSH and LH. Indeed, the use of leuprolide acetate reduces the plaque formation blocking FSH and LH release.
Long et al. [17], testing neuronal mitochondria, suggest that the reduction of ER β expression in the mitochondria associated with a consequent increase in oxidative stress may play an important role in Alzheimer disease in women.
Many other studies on apolipoprotein E (APOE), a protein responsible for the transport of lipids in the blood, show that its expression is stimulated in specific brain areas dedicated to learning and memory including the hippocampus and cortex by interactions between 17β-estradiol and ERs. In particular, increasing evidence indicates that the link between APOE4 and Alzheimer disease is far more prominent in women, suggesting that female sex hormones play a role in modulating the effect of this protein in the development of this neurodegenerative disorder.
Finally, data like the one by Brinton et al. [18] demonstrate a significantly reduced risk for the development of Alzheimer in women who start estrogen therapy immediately after menopause, while this benefit disappears and turns into adverse effect if therapy is taken years later after menopause.
18.9 Sleep Disruption
Sleep disturbances are one of the major complaints of women during climacterium because of their impact on the quality of life, mood, productivity, and general physical health. Specifically, as for brain functions, multiple studies show the correlation between sleep deprivation and reduced hippocampus and parietal gray matter volume, causing decreased memory, cognitive impairment, and increased risk of β-amyloid accumulation [19]. The biological mechanism underlying sleep difficulties is still unclear; however, the suprachiasmatic nucleus may have a relevant role, thanks to its ER β expressions which follows a diurnal rhythm [20]. Furthermore lower levels of inhibin B, which represent a marker for early menopausal transition, can be used to predict poor sleep quality. Instead, the same marker role in late menopausal transition and in postmenopause is described by high urinary FSH levels. As confirmed, the FSH increase is associated with longer sleep duration, indicative of less restful and non-REM sleep [21]. Moreover, the Penn Ovarian Aging Study 8 asserts that the decrease in sex hormone levels is associated with worse sleep quality and more severe sleep problems such as obstructive sleep apnea (considering the respiratory-stimulating properties of progesterone).
18.10 Thermoregulation
Hot flushes are one of the characteristic symptoms of perimenopause, affecting about 80% of women, with an average duration of about 7 years. They are described as sudden increases in body temperature associated with profuse sweating and redness mainly of the upper body, followed by dissipation within seconds to minutes. The frequency and severity of such episodes can affect the woman’s lifestyle, leading in some cases to stress, anxiety, and drowsiness [22]. Behind this there is the thermoregulatory center, located in the hypothalamus and more specifically in the anterior nucleus and the adjacent preoptic area regions. As the temperature varies from the baseline, endocrine production initiates control mechanisms to increase or decrease energy production/dissipation as needed to return to the initial temperature. Thermoregulatory dysfunctions can be associated with alteration of the noradrenergic and serotoninergic pathways that occur during estrogen level fluctuations. As a matter of fact, the progressive reduction of vasomotor system during menopausal transition indicates a readjustment of the brain to the different sex hormone concentrations. Despite the certain role of the hypothalamus, new theories have been proposed. A hypothesis deriving from RMN studies of brain functions suggests a control of thermoregulation also at subcortical level, showing an activation of the insula and anterior cingulate cortex during hot flushes [23]. Another finding is the association between thermoregulatory dysfunction and low brain glucose metabolism highlighted by 18F-FDG PET. As confirmed, in the SWAN study, the rising of hot flushes frequency is related to a simultaneous increase in fasting blood glucose and HOMA index. Finally other factors play a role in this scenario: low levels of adiponectin, high levels of leptin, and cytokine monocyte chemotactic protein 1 indicating a strong association with impaired glucose homeostasis in perimenopausal women [14].
18.11 Migraine
Headache is one of the relatively common symptoms that occurs during menopause, increasing in peri- and decreasing in postmenopausal period. It tends to be more frequent for menopausal women who experienced premenstrual migraine during fertile years; however, its prevalence is stable at between 10 and 29% [24]. Migraine in women is usually caused by the abrupt decline in estrogen levels that occurs both before menses and during menopausal transition. In fact, several findings confirm the importance of areas such as hippocampus and prefrontal cortex rich in ERs, in influencing electrical excitability and neurotransmission, thanks to the release of neurotransmitters and neuropeptides. Emerging data suggest that the typical pulsing pain reported could be exacerbated by changes in estradiol levels in the brain that induce an inflammatory neurogenic state and determine vasodilatation and plasma extravasation [25].
In this scenario, the age at the onset of menopause represents a critic parameter that impacts differently cognitive outcomes, influencing hormone therapy efficacy, and for this reason it has to be examined in depth in future researches.
18.12 Imaging of the Menopause
Increasing evidence for hormone-dependent modification of function and behavior during the menstrual cycle leads to research of the corresponding data from neuroimaging. The study conducted by Hagemann et al. [26], using MRI to investigate brain volume changes connected to estrogens in fertile women, asserts that there is an increase in gray matter volume and a decrease in cerebrospinal fluid between the luteal and periovulatory phases, while no changes are detected in white matter volume. The hippocampus, parahippocampal gyrus, fusiform gyrus, cingulate cortex, insula, middle frontal gyrus, thalamus, and cerebellum are areas found to have a hormone structural plasticity. In these regions, estrogen seems to have trophic effects.
Goto et al. [27], using the same technique and comparing neurological volume changes in premenopausal vs. postmenopausal women, show a significant decline in hippocampal volume in the second group of studies rather than the first.
Thanks to MRI and PET, Kantarci et al. [28] studied the effects of oral conjugated equine estrogen (oCEE, Premarin 0.45 mg/dL), transdermal 17β-estradiol (tE2, Climara 50 μg/day), and placebo therapy within 3–36 months after the onset of menopause. This study leads to a major finding: no difference is detected in global brain volumes and cognitive function between mHT group (tE2 or oCEE) and placebo group 3 years after the exposure to mHT. However, during those first 3 years, white matter hyperintensity volume increases in the oCEE group more than in placebo and dorsolateral prefrontal cortex volumes are preserved (and correlated with lower global cortical β-amyloid deposition) in the tE2 group more than in the placebo. Furthermore, PET images show a correlation between the lower PiB uptake (i.e., β-amyloid deposition) and the preservation of the dorsolateral prefrontal cortex volume in the tE2 group (but not in the oCEE group). The subsequent normalization of changes in brain structure after the cessation of mHT confirms the transient mHT effects and the difference in all formulations.
18.13 Hormonal Treatment and the Brain
Studies on the effects of postmenopausal hormone treatment on cognitive functions are still not conclusive; however, new evidences suggest that cognitive effects are influenced by specific hormone formulations [29]. The KEEPS Cog study analyzing the efficacy of oral conjugated equine estrogen and transdermal estradiol suggests the second formulation to have a more effective action on cognitive function in peri- and early menopausal women. In general, more positive cognitive effects have been identified with the single estrogen therapy rather than the one combined with progestin. For example, the combination of equine estrogen with medroxyprogesterone acetate (MPA) may not have the same positive effects of estrogen alone because of the MPA neutralizing activity. In addition, the new common trend leads to think that progestins and progesterone have no the same influence on neurobiological mechanisms of cognitive function and that progesterone is probably more beneficial and less dangerous than its synthetic counterparts. As confirmed, verbal ability appears to be negatively influenced by progestins, while those formulations with less antiestrogenic effects seem to have neutral or positive impact on benefits conferred by estradiol treatment. Moreover neurotrophic and neuroprotective progesterone actions are well known in animal models (i.e., modifying hippocampus response, reducing cell inflammation, protecting from traumatic brain injury and cerebral ischemia) so that it gives the input to examine in depth its potential effects on women postmenopausal cognition.
A study conducted by Berent-Spillson et al. [30] demonstrates the association between both estrogen or progesterone treatments and activation of verbal processing and encoding. In particular these treatments are found to be in association with an increase in number of words remembered, while only progesterone is related to visual memory task. These results are aligned with the general evidence that menopausal estrogen treatment increases prefrontal activation and cognitive processes, thanks to mechanisms like modulation of interactions with neurotransmitter systems, growth factors, and dendritic spine density. Although much more data needs to be collected, the general evidence is that estrogen treatment has positive effect on neurological activity exclusively in the initial period after the cessation of the menstrual cycle or immediately following surgical menopause, while it has deleterious effects in older women.
18.14 Prospective
Having laid the foundations of neurobiological and hormonal changes and their correlation with menopause encourages us to elucidate molecular mechanisms, cognitive pathways, and homeostasis as aging occurs. This will yield insight into how and when the brain responds to endogenous hormone changes as well as to exogenous hormone treatment. Moreover, it will permit to develop new opportunities for menopausal women that not only solve undesirable symptoms but also potentially prevent, attenuate, or postpone cognitive and affective changes. One interesting target would be focusing on receptor dynamics and synaptic regulation rather than on circulating estrogen and progesterone levels, in the way to develop more successful treatments.
Another finding that needs to be more investigated is the differential effect of natural versus surgical menopause. In fact several studies suggest that the abrupt loss of estrogens induced by oophorectomy/ovariectomy in humans produces a more severe consequence in cognitive and synaptic health than what happens with the gradual loss of estrogens in natural menopause. These differences may suggest distinct treatment regimens more efficacious for each condition.
In conclusion, more detailed investigations are required to develop more specific and effective interventions tailoring the treatments to each woman’s need.
References
Vega JN, Zurkovsky L, Albert K, Melo A, Boyd B, Dumas J, et al. Altered brain connectivity in early postmenopausal women with subjective cognitive impairment. Front Neurosci. 2016;10:433.
Foster TC. Role of estrogen receptor alpha and beta expression and signaling on cognitive function during aging. Hippocampus. 2012;22:656–69.
Daniel JM, Witty CF, Rodgers SP. Long-term consequences of estrogens administered in midlife on female cognitive aging. Horm Behav. 2015;74:77–85.
Hara Y, Waters EM, McEwen BS, Morrison JH. Estrogen effects on cognitive and synaptic health over the life course. Physiol Rev. 2015;95(3):785–807.
Gonzalez M, Cabrera-Socorro A, Perez-Garcia CG, Fraser JD, Lopez FJ, Alonso R, et al. Distribution patterns of estrogen receptor alpha and beta in the human cortex and hippocampus during development and adulthood. J Comp Neurol. 2007;503:790–802.
Bomberg J, Schott LL, Kraviz H, Sowers MF, Avis NE, Gold EB, et al. Longitudinal change in reproductive hormones and depressive symptoms across the menopausal transition results from the study of women’s health across the nation (SWAN). Arch Gen Psychiatry. 2010;67(6):598–607.
Giannini A, Gennazzani AR, Simoncini T. Management of symptoms during the menopausal transition. In: Frontiers in gynecological endocrinology. Ovarian function and reproduction—from needs to possibilities. ISGE Series. Vol 3(19). New York: Springer; 2014. p. 161–7.
Farrag AF, Khedr EM, Abdel-Aleem H, Rageh TA. Effects of surgical menopause on cognitive functions. Dement Geritar Cogn Disord. 2002;13:193–8.
Fogle RH, Stanczyk FZ, Zhang X, Paulson RJ. Ovarian androgen production in postmenopausal women. J Clin Endocrinol Metab. 2007;92(8):3040–3.
Avis NE, Brambilla D, McKinlay SM, Vass K. A longitudinal analysis of the association between menopause and depression. Results from the Massachusetts Women’s Health Study. Ann Epidemiol. 1994;4(3):214–20.
Hui L, Wang Y, Zhou F, Ma X, Yan R, Zhang L, et al. Association between MKP-1, BDNF, and gonadal hormones with depression on perimenopausal women. J Womens Health (Larchmt). 2016;25(1):71–7.
Boulant JA. Role of the preoptic-anterior hypothalamus in thermoregulation and fever. Clin Infect Dis. 2000;31(Suppl 5):S157–61.
Kenna H, et al. Fasting plasma insulin and the default mode network in women at risk for Alzheimer’s disease. Neurobiol Aging. 2013;34:641–9.
Short RA, Bowen RL, O’Brien PC, Graff-Radford NR. Elevated gonadotropin levels in patients with Alzheimer disease. Mayo Clin Proc. 2001;76(9):906–9.
Long J, He P, Shen Y, Li R. New evidence of mitochondria dysfunction in the female Alzheimer’s disease brain: deficiency of estrogen receptor-beta. J Alzheimers Dis. 2012;30:545–58.
Brinton RD. Investigative models for determining hormone therapy-induced outcomes in brain: evidence in support of a healthy cell bias of estrogen action. Ann N Y Acad Sci. 2005;1052:57–74.
Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology-a bidirectional relationship. Nat Rev Neurol. 2014;10:115–9.
Brinton RD, Yao J, Yin F, Mack WJ, Cadenas E. Perimenopause as a neurological transition state. Nat Rev Endocrinol. 2015;11:393–405.
Sowers MF, Zheng H, Kravitz HM, et al. Sex steroid hormone profiles are related to sleep measures from polysomnography and the Pittsburgh Sleep Quality Index. Sleep. 2008;31(10):1339–49.
Avis NE, et al. Change in health-related quality of life over the menopausal transition in a multiethnic cohort of middle-aged women: Study of Women’s Health Across the Nation. Menopause. 2009;16:860–9.
Freedman RR, Benton MD, Genik RJ, Graydon FX. Cortical activation during menopausal hot flashes. Fertil Steril. 2006;85:674–8.
Thurston RC, Chang Y, Mancuso P, Matthews KA. Adipokines, adiposity, and vasomotor symptoms during the menopause transition: findings from the Study of Women’s Health Across the Nation. Fertil Steril. 2013;100:793–800.
Monteleone P, Mascagni G, Giannini A, Genazzani AR, Simoncini T. Symptoms of menopause—global prevalence, physiology and implications. Nat Rev Endocrinol. 2018;14(4):199–215.
Hagemann G, Ugur T, Schleussner E, Mentzel HJ, Fitzek C, Witte OW, Gaser C. Changes in brain size during the menstrual cycle. PLoS One. 2011;6(2):e14655.
Goto M, Abe O, Miyati T, Inano S, Hayashi N, Aoki S, et al. 3 Tesla MRI detects accelerated hippocampal volume reduction in postmenopausal women. J Magn Reson Imaging. 2011;33(1):48–53.
Kantarci K, Tosakulwong N, Lesnick TG, Zuk SM, Lowe VJ, Fields JA, et al. Brain structure and cognition 3 years after the end of an early menopausal hormone therapy trial. Neurology. 2018;90(16):e1404–12.
Tschanz JT, Norton MC, Zandi PP, Lyketsos CG. The cache county study on memory in aging: factors affecting risk of Alzheimer’s disease and its progression after onset. Int Rev Psychiatry. 2013;25(6):673–85.
Gleason CE, Dowling NM, Wharton W, Manson JE, Miller VM, Atwood CS, et al. Effects of hormone therapy on cognition and mood in recently postmenopausal women: findings from the randomized, controlled KEEPS cognitive and affective study. PLoS Med. 2015;12(6):e1001833.
Brinton RD, Nilsen J. Effects of estrogen plus progestin on risk of dementia. J Am Med Assoc. 2003;290:1706.
Berent-Spillson A, Briceno E, Pinsky A, Simmen A, Persad CC, Zubieta J, et al. Distinct cognitive effects of estrogen and progesterone in menopausal women. Psychoneuroendocrinology. 2015;59:25–36.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Antonelli, A., Giannini, A., Caretto, M., Simoncini, T., Genazzani, A.R. (2019). Impact of Menopause on Brain Functions. In: Pérez-López, F. (eds) Postmenopausal Diseases and Disorders. Springer, Cham. https://doi.org/10.1007/978-3-030-13936-0_18
Download citation
DOI: https://doi.org/10.1007/978-3-030-13936-0_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-13935-3
Online ISBN: 978-3-030-13936-0
eBook Packages: MedicineMedicine (R0)