Abstract
Many researches have been conducted to investigate the alternative source of energy from renewable sources and utilization of waste materials. Among which, microalgae, having wide range of environmental adaptability, represents a diverse group of microorganisms; hence, its application in biotechnology is among the scholars’ interest. Microalgae have the ability to store lipids and preserve the nutrients presented in the wastewater, which help in wastewater treatments. Besides, the algal biofuel production gained research interest in recent years. Among few important factors for the microalgal growth, proper presence of light and sufficient nutrients such as nitrogen and phosphorus are essential. Developing algae production would help to mitigate greenhouse gas emissions by capturing CO2, and producing an alternative option for fossil fuels which could be valuable and sustainable. Hence, with growing interest and several analyses, this review discusses the key points in developing microalgal biotechnology toward sustainable development.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Algae are one among the foremost photosynthetic living groups beside plants and bacteria. Algae are composed of eukaryote cell. They are usually found in marine and fresh water with the size extending from a couple of micrometers to a couple of many micrometers. Green growth called algae have an excellent part in nourishment and agribusiness and in misusing microbial exercises for creating significant human items, producing vitality, and tidying up the earth. Green growth can be additionally separated into microalgae and macroalgae in light of their size. Those with diameters below 50 μm are called microalgae and are usually monocellular organisms, while algae with larger sizes are macroalgae. Macroalgae or “seaweeds” are multicellular plants growing in salt or fresh water. They are often fast growing and can reach sizes of up to 60 m in length (McHugh 2003).
Microalgae are minute unicellular species, prokaryotic or eukaryotic, photosynthetic microorganisms that exist in both aquatic and fresh water. Microalgae are among the quickest developing photosynthetic creatures on earth. Their photosynthetic system is like land-based plants; however because of a straightforward cell structure, and submerged in a watery situation where they have proficient access to water, CO2, and different supplements, they are for the most part more productive in changing over sun-based vitality into biomass. They can be totally presented to light, so every cell can lead photosynthesis at full speed and twofold inside a few hours. The photosynthetic productivity of microalgae is normally more than ten times that of the higher plants and macroalgae (Hemschemeier et al. 2009; Kamyab et al. 2016a).
The most frequently used microalgae are Cyanophyceae (blue-green algae), Chlorophyceae (green algae), Bacillariophyceae (including the diatoms), and Chrysophyceae (including golden algae). All cells require wellsprings of carbon and energy for growth. Chemoorganotrophs, chemolithotrophs, and phototrophs utilize natural chemicals, inorganic chemicals, or light, respectively, as their wellspring of energy. Autotrophs utilize CO2 as their carbon source, while heterotrophs utilize natural compounds (Xu et al. 2006). Numerous microalgae species could change from phototrophic to heterotrophic growth. As heterotrophs, the green algae growth depends on glucose or other utilizable carbon sources for carbon digestion and energy. Limited algae can also grow mixotrophically.
Microalgae have been the focus and interest of researchers, government, and industries because of (i) their growth rate and simple structure; (ii) cellular components such as carbohydrate, proteins, and lipids which can be utilized for biotechnological applications like creating biofuel and product of nutritional, pharmaceutical, and cosmetic value; and (iii) the shift toward the development of green processes. Microalgae represent very interesting natural sources of bioactive compounds such as carotenoids, fatty acids, vitamins, and sterols (Plaza et al. 2009; Chacón-Lee and González-Mariño 2010). They have been generally utilized for different applications such as biofuels, animal feed, food supplements, cosmetics, pharmaceuticals, nutraceuticals, CO2 capture, etc. (Nesamma et al. 2015; Kamyab et al. 2017). In addition, microalgae are additionally utilized as a part of wastewater treatment for toxin and supplement expulsion. That is on the grounds that microalgae developments are subject to supplement focus and in addition light, temperature, saltiness, and pH (Venkatesan et al. 2015). Microalgae-construct wastewater treatment depends with respect to the capacity of phototrophic microorganisms to supply oxygen to vigorous natural toxin degraders and upgrade the evacuation of supplements and pathogens. It is generally known that microalgae plays an important role in self-purification of natural waters and therefore offers an alternative means as a tertiary treatment of organic wastewater (Venkatesan et al. 2015).
Microalgae are recognized as one of the oldest living microorganisms in earth (Lam and Lee 2011). They are prokaryotic or eukaryotic photosynthetic microorganisms that can develop quickly and live in brutal conditions (Mata et al. 2010; Resdi et al. 2016). Biologists have categorized microalgae in a variety of classes, mainly distinguished by their pigmentation, life cycle, and basic cellular structure (Khan et al. 2009). Microalgae are available in all current earth biological systems, not just aquatic but also terrestrial, instead of a major assortment of groups living in an extensive variety of ecological conditions, such as lakes, rivers, ponds, wetlands, and deserts, and even living in the North and South Poles (Mata et al. 2010). It is estimated that more than 50,000 species exist, but only a limited number of around 30,000 have been studied and analyzed (Mata et al. 2010). Like plants, microalgae require basically three parts to develop: daylight, carbon dioxide, and water. Photosynthesis is an imperative bio-compound process in which plants, microalgae, and a few microorganisms change over the vitality of daylight to synthetic energy. Among others, microalgae consist of lipids and unsaturated fats as membrane segments, stockpiling items, metabolites, and wellsprings of energy. Lipids are characterized as any of a gathering of natural compounds that are insoluble in water but dissolvable in natural solvents. The synthetic highlights are introduced in an expansive scope of molecules, for example, unsaturated fats, phospholipids, sterols, sphingolipids, terpenes, and others (Fogg 1959; Fahy et al. 2011).
2 Classification of Microalgae
According to Fogg (1959), microorganisms are classified according to their morphological properties, the nature of their life cycle, the chemical nature reserve photosynthetic products (intracellular accumulation product), and pigmentation. Various algae species can be found growing in lakes, oceans, rocks, and soil. There are basically two groups of microalgae, prokaryotic and eukaryotic. Prokaryotes microalgae are unicellular organisms that lack a membrane-bound nucleus (karyon), mitochondria, or any other membrane-bound organelles and rarely have cellular organelles. Under its categories is Cyanophyceae. Cyanophyceae is also called cyanobacteria. It is photosynthetic bacteria that are found in fresh and salt water and have chlorophyll a and phycobilins. Cyanophyceae are thought to be blue-green microalgae which have a high tolerance to extreme temperatures (Pulz and Gross 2004). The best known species are Spirulina (Arthrospira) platensis, Nostoc commune, and Aphanizomenon flos-aquae. Eukaryotes are uni- or multicellular organisms which have a complex structure comprising a core surrounded by a membrane and many intracellular organelles. These microorganisms are additionally inexhaustible in fresh water. A few types can likewise live in terrestrial area, developing on wet soils, wet rocks, and soggy wood. They can show up as single cells or as colonies. Eukaryotes are ordered into an assortment of classes for the most part characterized by their pigmentation, life cycle, and fundamental cell structure (Khan et al. 2009). The most vital classes are green growth algae (Chlorophyta), red algae (Rhodophyta), and diatoms (Bacillariophyta). These microalgae usually contain three main types of pigments which are chlorophylls, carotenoids, and phycobiliproteins. Microalgae signify to an exceptionally intriguing asset as normal wellsprings of bioactive compounds, for example, carotenoids, unsaturated fats, vitamins, and sterols (Plaza et al. 2009; Chacón-Lee and González-Mariño 2010). To sum up the classes of microalgae, there are 11 different divisions of microalgae, which are Cyanophyta, Prochlorophyta, Glaucophyta, Rhodophyta, Heterokonta, Haptophyta, Cryptophyta, Chlorarachniophyta, Dinophyta, Euglenophyta, and Chlorophyta. The largest groups are Chlorophyceae (green algae), Bacillariophyceae (diatoms), Phaeophyceae (brown algae), Chrysophyceae (golden-grown algae), Pyrrophyceae (dinoflagellates), and Rhodophyceae (red algae) (Milano et al. 2016). Green growth algae are the transformative progenitors of current plants, yet dissimilar to plants, they are basically aquatic. These microorganisms have the filamentous forms. The best known species are Chlorella, Chlamydomonas, Dunaliella, and Haematococcus (Pulz and Gross 2004). The main storage compound of these algae is starch, although oils can also be produced.
3 Culture Parameters
3.1 Temperature
Most of the microalgae are able to survive at a temperature range between 16 and 27 °C. Chlorella sorokiniana had an upper growth limit at 38–42 °C (Kessler 1985). For a multi-specific microalgal biomass, as studied by Golueke et al. (1957), the rate of microalgae biodegradability from 5% to 10% can be enhanced by a temperature increase from 35 to 50 °C. However, maximal methane productivity at 40 °C that was found by Chen et al. (1996), confirms most mesophilic temperatures considered as optimal conditions for methane production.
3.2 Nitrogen Concentration
Nitrogen-limiting conditions were in fact reported to significantly increase the lipid fraction of many microalgae (Illman et al. 2000). Nitrogen is vital for peptides, proteins, enzymes, chlorophylls, ATP, DNA, and other cellular constituents’ synthesis. The increment of lipid fraction occurred due to the reduction of nitrate in growth medium, although of an almost constant growth rate, thus doubling the productivity of the oil (Converti et al. 2009).
3.3 Light Intensity
The limiting factor for microalgal growth is light intensity. Consequently, a suitable light intensity for microalgal cultivation needs about 1/10 of amount of light from direct sunlight. This photosynthetic microorganism produces microalgal biomass by utilizing the sunlight, water, and carbon dioxide. Twenty-five percent consumption of the biomass produced during daylight might be consumed during the night to sustain the cells until sunrise (Chisti 2007). Some microalgae are fit for heterotrophic development on monosaccharide or natural acids. This method of development offers the likelihood of extraordinarily enhancing the profitability of microalgal culture using fed-batch and high cell-density strategies, which can’t be connected to photosynthetic frameworks. These strategies are routinely connected to bacterial and yeast cultures to product cell densities in the request of 150–200 g 1 dry weight (Suzuki et al. 1985).
3.4 pH
Acid or basic condition is the suitable pH for microalgal growth. Carbon dioxide consumption may lead to the increasing of pH in the medium. Therefore, CO2 sparging maintains the pH of the medium. Culture pH was not affecting Chlorella sorokiniana when the value was higher than pH 4.0, and the growth rate was inhibited drastically at pH 3.0 (Morita et al. 2001).
3.5 Culture Parameters
The most critical parameters controlling algal growth by methods for photosynthesis are ecological conditions, for example, light power, pH, temperature, turbulence, saltiness, and supplements (Kayombo et al. 2003). The ideal range and the tolerable scope of working conditions depends on different species, which could associate with different components.
3.6 Nutrients
Microalgae production requires high concentrations of essential nutrients (C, N, P, S, K, Fe, etc.). The main focus is on the three most significant nutrients, i.e., carbon, nitrogen, and phosphorus; supplements are normally taken up in the inorganic shape; however a few natural types of them are additionally assimilable. A few supplements don’t show any restraint impacts on microalgal development, while others, for example, NO2 or NH3, have inconvenient impacts when exhibited in high focuses. Supplements in the vaporous frame, for example, CO2 and NO, face a noteworthy impediment which is connected basically to their mass exchange from the vaporous to the fluid state (Markou et al. 2014).
3.7 Carbon Sources
According to the mode of cell growth (heterotrophic, autotrophic, or mixotrophic), microalgae can utilize organic and/or inorganic carbon sources for cell growth (Feng et al. 2011). From the perspective of microalgae cultivation, the most common organic carbon sources for heterotrophic and mixotrophic cultivation of microalgae are glucose, sucrose, lactase, lactose, acetate, glycerol, ethanol (Liang et al. 2009; Perez-Garcia et al. 2011), and other sugars derived from starch, sugar cane, lignocellulosic biomass, and other sugar sources (Perez-Garcia et al. 2011).
3.8 Phosphorus
Phosphorus is another component that has huge pertinence to the cell development and metabolism of microalgae. It is one of the basic components containing DNA, RNA, ATP and cell layer materials, and so on. It is significant that, as a constituent component of ATP, phosphorus is fundamental to the cell forms identified with vitality exchange (e.g., photophosphorylation). On another pertinent idea, photosynthesis requires a lot of proteins, and the proteins are orchestrated by phosphorus-rich ribosomes (Ågren 2004). Phosphorus-containing ATP/ADP are fundamental for photophosphorylation. As a result, confinement of development by phosphate starvation may severely affect different parts of microalgal digestion, including photosynthesis and lipid aggregation. Phosphorus is especially absorbed as inorganic phosphates as H2PO4 - and HPO42− (Martinez et al. 1999).
3.9 Other Elements
Magnesium, sulfur, iron, and different components are additionally key for the development of microalgae (Zhang et al. 2000). Magnesium is required for nitrogenase movement utilizing a creatine phosphate−/kinase-/ATP-producing framework as one of its parts in cell digestion (Roden et al. 1996). Sulfur is a fundamental part of cysteine and methionine. Without sulfur, protein biosynthesis is hindered, and the photosynthetic framework PSII repair cycle is blocked (Zhang et al. 2000). Iron is associated with electron spill out of H2O to NADP+ (Roden et al. 1996). According to Raven et al (1999), some metals could enter in (noncyclic) photosynthetic electron transport systems.
3.10 Factors Limiting Microalgae Growth
A few natural parameters, for example, light’s source and intensity, photoperiod, temperature, saltiness, pH, blending, and so forth, impact the development of microalgae (Atta et al. 2013; Singh and Singh 2015). Therefore, it is suggested to adjust and keep up these parameters amid the cultivation time frame. Table 1 indicates parameters and their ideal ranges.
4 Culture of Microalgae
The growth qualities and arrangement of microalgae are known to essentially rely upon the cultivation conditions (Chen et al. 1996). There are four noteworthy sorts of development conditions for microalgae: photoautotrophic, heterotrophic, mixotrophic, and photoheterotrophic cultivation (Chen et al. 1996). Microalgae, however, developed under pressure conditions, for example, supplement starvation, high saltiness, high temperature, and so forth, aggregate extensive sums (up to 60–65% of dry weight) of lipids or sugars alongside a few auxiliary metabolites (Markou et al. 2014).
4.1 Phototrophic Cultivation
Phototrophic development happens when the microalgae utilize light, for example, daylight, as the energy source, and inorganic carbon (e.g., carbon dioxide) as the carbon source to shape of chemical energy (e.g., polysaccharides, proteins, lipids, and hydrocarbons) through photosynthesis (Chen et al. 1996; Huang et al. 2010). This species is the most regularly utilized development condition for microalgae growth (Gouveia and Oliveira 2009; Yoo et al. 2010). Rather, both lipid substance and biomass generation should be considered simultaneously. Thus, lipid profitability, considering the consolidated impacts of oil substance and biomass creation, is a more reasonable execution file to show the capacity of a microalga with respect to oil generation (Chen et al. 1996). The real favorable advantage of utilizing autotrophic cultivation contrasted with different kinds of development; the defilement issue is less serious when utilizing autotrophic development. In this way, outside scale-up microalgae development systems (e.g., open ponds and raceway ponds) are normally worked under phototrophic development conditions (Mata et al. 2010).
4.2 Heterotrophic Cultivation
Heterotrophic development is the condition when microalgae utilize natural carbon as both the energy and carbon source. This sort of development offers a few points of interest over phototrophic development as far as end of light prerequisite, great control of the development procedure, and ease for collecting the biomass (Chen et al. 1996). Some microalgae species demonstrate higher lipid content amid heterotrophic development, and a 40% expansion in lipid content was gotten in Chlorella protothecoides by changing the development condition from phototrophic to heterotrophic (Xu et al. 2006; Chen et al. 1996). Zheng et al., (2013) studied on the biomass and lipid productivities of heterotrophic refined microalgae Chlorella sorokiniana. The authors investigated the impact of temperature and medium variables such as carbon source, nitrogen source, and their underlying fixations. Authors reported the most extreme lipid substance of 31.5% was accomplished in 96 h, and the lipid profitability was 2.9 g L−1 d−1. Carbon sources are the most imperative component for heterotrophic culture of microalgae in the generation of lipids. Microalgae can absorb an assortment of natural carbon sources, for example, glucose, acetic acid derivation, glycerol, fructose, sucrose, lactose, galactose, and mannose relying upon microalgae species utilized for development (Liang et al. 2009). For example, (Chen et al. 1996) analyzed three carbon sources like acetic acid derivation, glucose, and glycerol for creating significantly higher biomass and in addition lipid content in cells by Chlorella protothecoides performance. A few investigations have along these lines concentrated on finding less expensive natural carbon sources, for example, corn powder hydrolysate (CPH) rather than sugars, bringing about high biomass (2 g/L/day) and lipid (932 mg/L/day) productivities (Plaza et al. 2009). Once more, heterotrophic development gives considerably higher lipid efficiency, as the most noteworthy lipid profitability from heterotrophic development is almost 20 times higher than that got under phototrophic development. In any case, the sugar-based heterotrophic framework much of the time experiences issues with tainting (Chen et al. 1996).
4.3 Mixotrophic Cultivation
Mixotrophic cultivation happens when microalgae experience photosynthesis and utilize both natural mixes and inorganic carbon (CO2) as a carbon source for growth. This mentions that microalgae are able to live under either phototrophic or heterotrophic conditions or both (Chen et al. 1996). Natural mixes and CO2 as a carbon source absorbed by microalgae, at that point microalgae discharge CO2 by means of breath, will be caught and reused under phototrophic growth (Mata et al. 2010). Contrasted and phototrophic and heterotrophic cultivation, mixotrophic development is once in a while utilized as a part of microalgal oil generation (Chen et al. 1996). For instance, the development and lipid efficiency of a separated microalga Chlorella vulgaris ESP-31 were explored under various media and developmental conditions, including phototrophic (NaHCO3 or CO2, with light), heterotrophic (glucose, without light), photoheterotrophic (glucose, with light), and mixotrophic (glucose and CO2, with light). The results demonstrated the higher lipid efficiency (67–144 mg/l/d) were obtained under mixotrophic growth along with the utilized media (Yeh and Chang 2012).
4.4 Photoheterotrophic Cultivation
Photoheterotrophic cultivation takes place when the microalgae require light when utilizing natural mixes as the carbon source. The principal distinction among mixotrophic and photoheterotrophic cultivation is that the last requires light as the energy source, while mixotrophic cultivation can utilize natural mixes to fill this need. Along these lines, photoheterotrophic development needs the two sugars and light in the meantime. Despite the fact that the creation of some light-directed valuable metabolites can be improved by utilizing photoheterotrophic cultivation, utilizing this way to deal with the delivery of biodiesel is exceptionally uncommon, similar to the case with mixotrophic cultivation (Chen et al. 1996). Algal–bacterial framework that productively debases thiocyanate (SCN−), a lethal contaminant, and displays high lipid efficiency was created. A consortium of blended microscopic organisms (activated sludge) and microalgae was consecutively cultivated under photoautotrophic and photoheterotrophic modes. The development mode was changed to photoheterotrophic conditions in a consecutive way. Algal–bacterial culture containing Chlorella protothecoides and Ettlia sp. yielded essentially higher lipid efficiency under photoheterotrophic conditions contrasted with photoautotrophic conditions showing 28.7 and 17.3 higher productivity (Ryu et al. 2014).
5 Microalgae Harvesting Methods
Choice of harvesting technique is subject to attributes of microalgae, e.g., size, thickness, and e-value of the target products (Olaizola 2003). Generally, microalgae harvesting is a two-phase process, including (1) bulk gathering—aimed at detachment of biomass from the mass suspension. The focus factors for this activity are generally 100–800 times to reach 2–7% total solid matter. This will rely upon the initial biomass fixation and advances utilized, including flocculation, flotation, or gravity sedimentation. (2) Thickening—the point is to concentrate on the slurry through methods, for example, centrifugation, filtration, and ultrasonic accumulation, subsequently—is for the most part a more energy concentrated advancement than mass harvesting (Olaizola 2003).
5.1 Gravity and Centrifugal Sedimentation
Gravity and centrifugal sedimentation techniques depend on Stokes’ law (Schenk et al. 2008), i.e., settling characteristics of suspended solids are determined by thickness and range of algae growth cells (Stoke’s sweep) and sedimentation velocity. Gravity sedimentation is the most well-known harvesting method for algae growth biomass in wastewater treatment due to the extensive volumes treated and the low scale of the biomass produced (Nurdogan and Oswald 1996). However, the strategy is reasonable for expansive (ca. >70 mm) microalgae, for example, Spirulina (Munoz and Guieysse 2006). Centrifugation recovery (CR) is favored for gathering of high-value metabolites and expanded time frame of realistic usability concentrates for incubators in aquaculture (Heasman et al. 2000). The procedure is quick and energy intensive; biomass recuperation relies upon the settling qualities of the cells, slurry residence time in the centrifuge, and settling depth (Grima et al. 2003). The drawback point of the procedure incorporates high energy costs and potentially higher support requirements because of freely moving parts (Bosma et al. 2003). Harvesting productivity of>95% [50] and increment in slurry focus by up to 150 times for 15% aggregate suspended solids are actually feasible (Mohn 1980).
6 Growth Cycle of Microalgae
In the batch cultures, the growth bend of algae growth, as with most microbial frameworks, can be represented by the accompanying stages: (a) lag phase, (b) exponential growth stage, (c) deceleration growth stage, (d) stationary stage, and (e) demise stage (Shuler and Kargi 2002) as described in Fig. 1. The cellular structure and metabolic way can be differed amid each stage because of supplement levels inside the batch vessel (Shuler and Kargi 2002).
6.1 Lag Phase
Lag stage is a time of cell adjustment in accordance with the new condition, which occurs instantly after inoculation. In this stage, cells encounter a supplement-rich condition, however altogether not the same as that of the seed culture from which the cell was exchanged. The growth occurs in the new framework; adaption happens by incorporating enzymes and co-proteins essential for cell multiplication (Shuler and Kargi 2002). Length of the lag stage depends on the measure of cells exchanged to the new growth medium, the supplement levels present, and culture age. The time duration which the inoculum has been cultured strongly affects the length of lag stage in a batch culture. Typically, the lag time frame increments with the age of the inoculums (Shuler and Kargi 2002).
6.2 Exponential Growth Phase
At this level, also called as the logarithmic growth phase , the cells have changed in accordance with their new environment. At the point when there is indication of growth, the development curve is moving from the lag stage into the exponential growth stage or logarithmic growth stage. After cells are adjusted to the new condition and new protein/compounds are orchestrated, cells begin to develop exponentially quickly (Shuler and Kargi 2002). Nevertheless, exponential development not just occurred after the lag stage yet additionally can occur after stationary stage (Monod 1949). This is a period-adjusted growth, in which all parts of the cells grow at a similar rate, because the normal organization of a single cell remains roughly steady during exponential period of development. During this adjusted growth, the net particular growth rate determined from either cell number or cell mass would be the same. Since the supplement focuses are vast in this stage, the development rate is free of supplement concentration. The exponential development rate is first requested (Shuler and Kargi 2002):
where integration X and X0 are cell concentrations at time t and t = 0.
The time required to double the microbial mass is given by Eq. 2. The exponential growth is characterized by a straight line on a semilogarithmic plot of ln X versus time:
where τd is the doubling time of cell mass.
Similarly, a doubling time based on cell numbers and the net-specific rate of replication may be calculated:
where τ′d is the doubling time based on the replication rate. During balanced growth, τd will equal τ′d, since the average cell composition and size will not change with time.
6.3 Deceleration Growth Phase
Supplements are expended, and poisons are discharged into nature, in this stage. Space or volume required for cell multiplication may wind up restricted. Assumptions in the exponential stage are never again substantial. As such, expanding lethality and diminishing supplement level decrease the growth rate of cell. Additionally the cell morphology and physiology may change (i.e., size, cell structure, and metabolic pathways) (Shuler and Kargi 2002). Additionally, this stage takes place as quickly as changing conditions results in unbalanced development, in which τd, and τ′d will not be equivalent. This stage is called deceleration development stage before the net growth rate achieves zero. The model created on the exponential growth stage cannot be utilized to foresee precise biomass fixation without redesigning the condition (Shuler and Kargi 2002).
6.4 Stationary Phase
Stationary stage resulted in light of the fact that microorganism might not grow uncertainly, in alternate words that the net growth rate achieves zero. The growth bend enters the stationary stage from deceleration growth stage. The metabolic pathway is changed from essential metabolites to auxiliary metabolites (Shuler and Kargi 2002). A few preparations can be framed in this stage, for example, aggregation of lipid (Gouveia and Oliveira 2009). In this stage, the aggregate cell fixation may remain consistent, but total practical cells may diminish. This leads the consistent decline of the net particular growth rate into the demise stage. Another exponential stage might be seen after the stationary stage with a lower net particular growth rate (in contrast with the illustration during the initial exponential growth stage). This could be because of the cell lysis, where the lysed cells could be utilized for growth (Shuler and Kargi 2002).
6.5 Death Phase
Death stage is the decay rate of the microbial populations which is higher than the growth rate. This stage occurs after the stationary stage. In any case, the outline between late stationary and early demise stage might be hard to characterize with accuracy. In the death stage, the net particular stage is negative because of reducing quantities of reasonable cells, supplement consumption, and presence of lethal pressure (Shuler and Kargi 2002).
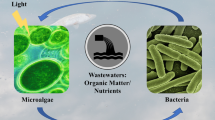
7 Microalgae Versus Wastewater
Numerous types of microalgae can viably exist in wastewater conditions through their capacity to use copious natural carbon and inorganic N and P in the wastewater (Kamyab et al. 2014;Kamyab et al. 2015). Furthermore, the utilization of microalgae in the wastewater manufacture is still genuinely constrained. Algae growth is utilized all through the world for wastewater treatment but on a generally minor scale. This is either using regular oxidation (adjustment) ponds or the more created suspended algal lake frameworks, for example, high-rate algal lakes which are shallow raceway-type oxidation ponds with mechanical blending and have been observed to be exceedingly successful for wastewater treatment (Pittman et al. 2011). A noteworthy necessity of wastewater treatment is the need to expel high convergences of supplements specifically N and P, which generally can prompt dangers of eutrophication if these supplements aggregate in river and ponds (Pittman et al. 2011). Microalgae are proficient in evacuating N, P, and poisonous metals from wastewater (Wang et al. 2010) and in this manner can possibly assume a critical remediation part especially during the last (tertiary) treatment period of wastewater (Pittman et al. 2011). The noteworthy preferred standpoint of algal procedures in wastewater treatment over the regular substance-based treatment strategies is the potential cost sparing and the lower-level innovation (Mallick 2002; Wang et al. 2010). An extensive variety of studies have broken down the development of microalgae under an assortment of wastewater condition (Pittman et al. 2011).
Regular municipal sewage treatment comprises of an essential treatment stage for the sedimentation of solid materials, a secondary treatment stage in which suspended and dissolved natural materials are expelled, and a tertiary treatment stage in which last treatment of the water is performed before release into condition. During tertiary treatment stage, a considerable decrease in inorganic compounds occures, for example, N and P absorbs by microalgae (Pittman et al. 2011). Some unicellular green microalgae species are especially tolerant to sewage effluent condition (Ruiz-Marin et al. 2010; Pittman et al. 2011). For instance, different types of Chlorella and Scenedesmus can expel up to >80% alkali, nitrate, and aggregate P from auxiliary-treated wastewater (Ruiz-Marin et al. 2010; Zheng et al. 2013). Moreover, microalgae additionally appeared to growth and effectively expel supplements from essential settled sewage wastewater. This showing the capability of microalgae for developing and expelling of supplement is not essentially subject to treatment level (Mallick 2002; Pittman et al. 2011).
In contrast with municipal local sewage-based wastewater, agricultural wastewater, which is frequently resulted from compost, containing high N and P (Pittman et al. 2011). In spite of high supplement fixations, previous scholar investigated that effective development of microalgae on rural waste and additionally municipal wastewater, microalgae are proficient at reducing N and P from wastewater (Pittman et al. 2011). Investigations of algal-intervened supplement recuperation from dairy manure have surveyed the capability of benthic freshwater green growth (algae) instead of planktonic (suspended) algae growth because of the potential higher supplement take-up rates in a few types of benthic algae. These species incorporate Microspora willeana, Ulothrix sp., and also Rhizoclonium hierglyphicum. Different studies on using a semicontinuous cultivation technique where the benthic algal growth was developed in reusing wastewater with new fertilizer has been increased by the scholars. For instance, algal cultivation rates and supplement take-up were observed to be high and identical to values from algae growth cultivation on municipal wastewater (Pittman et al. 2011).
A few investigations have analyzed microalgae cultivation and supplement expulsion characteristics utilizing artificial wastewater (Aslan and Kapdan 2006;Pittman et al. 2011). Usage of a simulated medium has advantages, for example, usability for starting research facility-based examinations. It additionally takes into account a streamlined examination of the significant segments in a wastewater medium without one expecting to consider obscure factors, for example, biotic parts. Most manufactured wastewater media are made out of inorganic constituents including high concentrations of particular supplements and will lack solid natural material and other potential poisons. Hence, there might be a few disadvantages in utilizing manufactured wastewater to survey conditions in actual wastewater. Real examinations of artificial wastewater with municipal wastewater have discovered that albeit supplement expulsion rates are comparable, microalgae growth rates are higher in simulated wastewater (Ruiz-Marin et al. 2010; Pittman et al. 2011). This is likely because of expanded poisonous quality of the actual wastewaters, inhibitory or aggressive impacts of indigenous microorganisms and protozoa, and by the distinctive synthetic organization of the wastewaters (Pittman et al. 2011).
There is critical enthusiasm for the utilization of microalgae for remediation of mechanically determined wastewaters, principally for the evacuation of substantial metal contaminants (cadmium, chromium, zinc, etc.) and natural compound poisons (hydrocarbons, biocides, and surfactants), instead of N and P. Because of, for the most part, the low N and P focus and high poison fixations, algal development rates are brought down in numerous modern wastewaters. Therefore, there is less potential for using modern wastewaters for expansive scale age of microalgae biomass (Pittman et al. 2011). The wastewater incorporates process chemicals and shades utilized as a part of the mills, plus a range of inorganic components including low concentrations of metals and generally low concentrations of aggregates P and N. This wastewater was appeared to be sufficiently low in poisons and had enough P and N to help microalgae growth, with two freshwater microalgae Botryococcus braunii and Chlorella saccharophila and a marine alga Pleurochrysis carterae, ready to become especially well on the untreated wastewater (Chinnasamy et al. 2010). With the huge measure of wastewater accessible from this industry, a lot of biomass and possibly likewise biodiesel could be produced from this asset (Pittman et al. 2011).
8 Sustainable Algal Biomass Production with Wastewater
Algae grow naturally in a wide range of environments. Typical requirements for phototrophic algae include sunlight, CO2, temperatures between 20 and 30 °C, water, and nutrients (primarily N, P, and K). Algae have been grown on an industrial scale for different purposes such as treatment of organic residues, nutrient recovery for animal feed and fertilizer, human food, and production of biofuels. In industrial algae production, the ideal conditions may be provided, such as artificial light with the appropriate photoperiod and wavelength, consistent CO2 supply, optimal temperature, and essential nutrients like nitrogen (N) and phosphorous (P). Providing optimal conditions improves the algae growth rate and potentially improves the composition (oil, starch, protein) of the algae, although it increases the costs of the production. Table 2 presents a recent comparison between open and close cultivation system in terms of environmental impact, biological issues, process issues, and costs. Measuring the algae concentration and growth rate during cultivation is a critical parameter for evaluating the feasibility of algae production. Carbon sources are essential for microalgae growth. Photoautotrophic organisms are the organisms that derive their energy for food synthesis from light and are capable of using carbon dioxide as their principal source of carbon. Hence, photoautotrophic cultivation implies that inorganic types of carbon (CO2 or bicarbonates) are provided to the cultures, while light energy is changed into compound energy through photosynthesis (Ren et al. 2014). Other microalgae strains can utilize natural carbon as both energy and carbon source (heterotrophic cultivation); this cultivation framework is however practiced for the creation of high-value items as it were. Mixotrophic nutrition mode is the mix of both autotrophic and heterotrophic. Table 3 shows a comparison between photoautotrophic, heterotrophic, mixotrophic, and photoheterotrophic cultivation conditions. The following section provides extra details on each metabolism system.
8.1 Application of Microalgae in Biomass Production and CO2 Sequestration
Microalgae have been broadly utilized for different applications, for example, biofuels, animal feed, nourishment supplements, beautifiers, pharmaceuticals, nutraceuticals, CO2 capture, and so on (Nesamma et al. 2015; Kamyab et al. 2016c). In addition, microalgae are likewise utilized as a part of wastewater treatment for contamination and supplement evacuation. That is due to microalgae growths which are subject to supplement focus and in addition light, temperature, saltiness, and pH (Venkatesan et al. 2015). The use of microalgae mainly in wastewater treatment depends on light capacity of phototrophic microorganisms to supply oxygen to vigorous natural contamination which could degrade and improve the absorption of supplements and pathogens (Kamyab et al. 2016b).
Major greenhouse gas is CO2 leading to global warming. Most of CO2 emission is from electrical power plants, automobiles, and industrial sources such as cement processing. Using algae-based CO2 sequestration to reduce CO2 is a sustainable solution to reduce global carbon footprint. CO2 sequestration is a notion based on capturing the total CO2 emitted from various sources by growing microalgae which are used to make biofuel (Eloka-Eboka and Inambao 2017). Many algal species such as Chlorella vulgaris, Nannochloropsis sp., Scenedesmus quadricauda, Chlamydomonas reinhardtii, and Nannochloris sp. have been studied to sequester CO2. One of the most attractive features of microalgae is to trap gaseous CO2 in ponds or photobiorectors and have higher photosynthetic efficiencies than terrestrial plants. CO2 dissolves in water and exists in the form of CO2, HCO3 ̄, H2CO3 ̄, and CO32 ̄. Among them, microalgae transport CO2 and HCO3 ̄ into cell for photosynthesis (Zhao and Su 2014). Features of microalgae such as high protein, lipid, and carbohydrate contents make them attractive feedstock for biofuel production (Pavlik et al. 2017). The algal lipids can be extracted and converted to biodiesel to be used as biofuel (Mondal et al. 2017). The potential benefit of making biofuel from algae is it reduces lifecycle of greenhouse gases (GHG), as algae biomass converts CO2 through photosynthesis into bio-plant material which is eventually released back to the atmosphere via microorganisms when used as a fuel, via engine tail-pipe emissions (Shirvani 2012).
References
Ågren GI (2004) The C: N: P stoichiometry of autotrophs–theory and observations. Ecol Lett 7(3):185–191
Aslan S, Kapdan IK (2006) Batch kinetics of nitrogen and phosphorus removal from synthetic wastewater by algae. Ecol Eng 28(1):64–70
Atta M, Idris A, Bukhari A, Wahidin S (2013) Intensity of blue LED light: a potential stimulus for biomass and lipid content in fresh water microalgae Chlorella vulgaris. Bioresour Technol 148:373–378
Barros AI, Gonçalves AL, Simões M, Pires JC (2015) Harvesting techniques applied to microalgae: a review. Renew Sust Energ Rev 41:1489–1500
Bosma R, van Spronsen WA, Tramper J, Wijffels RH (2003) Ultrasound, a new separation technique to harvest microalgae. J Appl Phycol 15(2–3):143–153
Chacón-Lee TL, González-Mariño GE (2010) Microalgae for “healthy” foods—possibilities and challenges. Compr Rev Food Sci Food Saf 9(6):655–675
Chen F, Zhang Y, Guo S (1996) Growth and phycocyanin formation of Spirulina platensis in photoheterotrophic culture. Biotechnol Lett 18(5):603–608
Chinnasamy S, Bhatnagar A, Hunt RW, Das KC (2010) Microalgae cultivation in a wastewater dominated by carpet mill effluents for biofuel applications. Bioresour Technol 101(9):3097–3105
Chisti Y (2007) Biodiesel from microalgae. Biotechnology Advances 25:294–306. Google Scholar
Converti A, Casazza AA, Ortiz EY, Perego P, Del Borghi M (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Process Process Intensif 48(6):1146–1151
Eloka-Eboka AC, Inambao FL (2017) Effects of CO2 sequestration on lipid and biomass productivity in microalgal biomass production. Appl Energy 195:1100–1111
Fahy E, Cotter D, Sud M, Subramaniam S (2011) Lipid classification, structures and tools. Biochim Biophys Acta 1811(11):637–647
Feng D, Chen Z, Xue S, Zhang W (2011) Increased lipid production of the marine oleaginous microalgae Isochrysis zhangjiangensis (Chrysophyta) by nitrogen supplement. Bioresour Technol 102(12):6710–6716
Fogg GE (1959) Nitrogen nutrition and metabolic patterns in algae. Symp Soc Exp Biol 13:106–125
Golueke CG, Oswald WJ, Gotaas HB (1957) Anaerobic digestion of algae. Appl Microbiol 5(1):47
Gouveia L, Oliveira AC (2009) Microalgae as a raw material for biofuels production. J Ind Microbiol Biotechnol 36(2):269–274
Grima EM, Belarbi EH, Fernández FA, Medina AR, Chisti Y (2003) Recovery of microalgal biomass and metabolites: process options and economics. Biotechnol Adv 20(7–8):491–515
Heasman M, Diemar J, O’connor W, Sushames T, Foulkes L (2000) Development of extended shelf-life microalgae concentrate diets harvested by centrifugation for bivalve molluscs–a summary. Aquac Res 31(8–9):637–659
Hemschemeier A, Melis A, Happe T (2009) Analytical approaches to photobiological hydrogen production in unicellular green algae. Photosynth Res 102(2–3):523–540
Huang G, Chen F, Wei D, Zhang X, Chen G (2010) Biodiesel production by microalgal biotechnology. Appl Energy 87(1):38–46
Illman AM, Scragg AH, Shales SW (2000) Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzym Microb Technol 27(8):631–635
Kamyab H, Tin Lee C, Md Din MF, Ponraj M, Mohamad SE, Sohrabi M (2014) Effects of nitrogen source on enhancing growth conditions of green algae to produce higher lipid. Desalin Water Treat 52(19–21):3579–3584
Kamyab H, Din MFM, Keyvanfar A, Majid MA, Talaiekhozani A, Shafaghat A, Ismail HH (2015) Efficiency of microalgae Chlamydomonas on the removal of pollutants from palm oil mill effluent (POME). Energy Procedia 75:2400–2408
Kamyab H, Din MFM, Hosseini SE, Ghoshal SK, Ashokkumar V, Keyvanfar A, Majid MZA (2016a) Optimum lipid production using agro-industrial wastewater treated microalgae as biofuel substrate. Clean Techn Environ Policy 18(8):2513–2523
Kamyab H, Din MFM, Ghoshal SK, Lee CT, Keyvanfar A, Bavafa AA, Lim JS (2016b) Chlorella pyrenoidosa mediated lipid production using Malaysian agricultural wastewater: effects of photon and carbon. Waste Biomass Valoriz 7(4):779–788
Kamyab H, Md Din MF, Ponraj M, Keyvanfar A, Rezania S, Taib SM, Abd Majid MZ (2016c) Isolation and screening of microalgae from agro-industrial wastewater (POME) for biomass and biodiesel sources. Desalin Water Treat 57(60):29118–29125
Kamyab H, Chelliapan S, Din MFM, Shahbazian-Yassar R, Rezania S, Khademi T, Azimi M (2017) Evaluation of Lemna minor and Chlamydomonas to treat palm oil mill effluent and fertilizer production. J Water Process Eng 17:229–236
Kayombo S, Mbwette TSA, Katima JH, Jorgensen SE (2003) Effects of substrate concentrations on the growth of heterotrophic bacteria and algae in secondary facultative ponds. Water Res 37(12):2937–2943
Kessler JO (1985) Hydrodynamic focusing of motile algal cells. Nature 313(5999):218
Khan SA, Hussain MZ, Prasad S, Banerjee UC (2009) Prospects of biodiesel production from microalgae in India. Renew Sust Energ Rev 13(9):2361–2372
Lam MK, Lee KT (2011) Renewable and sustainable bioenergies production from palm oil mill effluent (POME): win–win strategies toward better environmental protection. Biotechnol Adv 29(1):124–141
Liang Y, Sarkany N, Cui Y (2009) Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol Lett 31(7):1043–1049
Mallick N (2002) Biotechnological potential of immobilized algae for wastewater N, P and metal removal: a review. Biometals 15(4):377–390
Markou G, Vandamme D, Muylaert K (2014) Microalgal and cyanobacterial cultivation: the supply of nutrients. Water Res 65:186–202
Martinez ME, Jimenez JM, El Yousfi F (1999) Influence of phosphorus concentration and temperature on growth and phosphorus uptake by the microalga Scenedesmus obliquus. Bioresour Technol 67(3):233–240
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sust Energ Rev 14(1):217–232
McHugh DJ (2003) A guide to the seaweed industry FAO Fisheries Technical Paper 441. Food and Agriculture Organization of the United Nations, Rome
Milano J, Ong HC, Masjuki HH, Chong WT, Lam MK, Loh PK, Vellayan V (2016) Microalgae biofuels as an alternative to fossil fuel for power generation. Renew Sust Energ Rev 58:180–197
Mohn FH (1980) Experiences and strategies in the recovery of biomass from mass cultures of microalgae. Algae biomass: production and use/[sponsored by the National Council for Research and Development, Israel and the Gesellschaft fur Strahlen-und Umweltforschung (GSF), Munich, Germany]; editors, Gedaliah Shelef, Carl J. Soeder
Mondal M, Goswami S, Ghosh A, Oinam G, Tiwari ON, Das P, Gayen K, Mandal MK, Halder GN (2017) Production of biodiesel from microalgae through biological carbon capture: a review. 3. Biotech 7(2):1–21
Monod J (1949) The growth of bacterial cultures. Annu Rev Microbiol 3(1):371–394
Morita M, Watanabe Y, Okawa T, Saiki H (2001) Photosynthetic productivity of conical helical tubular photobioreactors incorporating Chlorella sp. under various culture medium flow conditions. Biotechnol Bioeng 74(2):136–144
Munoz R, Guieysse B (2006) Algal–bacterial processes for the treatment of hazardous contaminants: a review. Water Res 40(15):2799–2815
Nesamma AA, Shaikh KM, Jutur PP (2015) Genetic engineering of microalgae for production of value-added ingredients. In: Handbook of Marine Microalgae. Elsevier, UK, England, pp 405–414
Nurdogan Y, Oswald WJ (1996) Tube settling of high-rate pond algae. Water Sci Technol 33(7):229–241
Olaizola M (2003) Commercial development of microalgal biotechnology: from the test tube to the marketplace. Biomol Eng 20(4–6):459–466
Pavlik D, Zhong Y, Daiek C, Liao W, Morgan R, Clary W, Liu Y (2017) Microalgae cultivation for carbon dioxide sequestration and protein production using a high-efficiency photobioreactor system. Algal Res 25:413–420
Perez-Garcia O, Escalante FM, de Bashan LE, Bashan Y (2011) Heterotrophic cultures of microalgae: metabolism and potential products. Water Res 45(1):11–36
Pittman JK, Dean AP, Osundeko O (2011) The potential of sustainable algal biofuel production using wastewater resources. Bioresour Technol 102(1):17–25
Plaza M, Herrero M, Cifuentes A, Ibáñez E (2009) Innovative natural functional ingredients from microalgae. J Agric Food Chem 57(16):7159–7170
Pulz O, Gross W (2004) Valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol 65(6):635–648
Raven JA, Evans MC, Korb RE (1999) The role of trace metals in photosynthetic electron transport in O2-evolving organisms. Photosynth Res 60(2–3):111–150
Ren HY, Liu BF, Kong F, Zhao L, Xie GJ, Ren NQ (2014) Enhanced lipid accumulation of green microalga Scenedesmus sp. by metal ions and EDTA addition. Bioresour Technol 169:763–767
Resdi R, Lim JS, Kamyab H, Lee CT, Hashim H, Mohamad N, Ho WS (2016) Review of microalgae growth in palm oil mill effluent for lipid production. Clean Techn Environ Policy 18(8):2347–2361
Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, Shulman GI (1996) Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest 97(12):2859–2865
Ruiz-Marin A, Mendoza-Espinosa LG, Stephenson T (2010) Growth and nutrient removal in free and immobilized green algae in batch and semi-continuous cultures treating real wastewater. Bioresour Technol 101(1):58–64
Ryu BG, Kim EJ, Kim HS, Kim J, Choi YE, Yang JW (2014) Simultaneous treatment of municipal wastewater and biodiesel production by cultivation of Chlorella vulgaris with indigenous wastewater bacteria. Biotechnol Bioprocess Eng 19(2):201–210
Schenk PM, Thomas-Hall SR, Stephens E, Marx UC, Mussgnug JH, Posten C, Hankamer B (2008) Second generation biofuels: high-efficiency microalgae for biodiesel production. Bioenergy Res 1(1):20–43
Shirvani T (2012) The environmental feasibility of algae biodiesel production. Appl Petrochem Res 2:93–95
Shuler ML, Kargi F (2002) How cells grow. In: Bioprocess Engineering Basic Concepts. Prentice Hall, Upper Saddle River, NJ, pp 162–164
Singh SP, Singh P (2015) Effect of temperature and light on the growth of algae species: a review. Renew Sust Energ Rev 50:431–444
Suzuki T, Suzuki M, Furusaki A, Matsumoto T, Kato A, Imanaka Y, Kurosawa E (1985) Teurilene and thyrsiferyl 23-acetate, meso and remarkably cytotoxic compounds from the marine red alga Laurencia obtusa (Hudson) Lamouroux. Tetrahedron Lett 26(10):1329–1332
Venkatesan J, Manivasagan P, Kim SK (2015) Marine microalgae biotechnology: present trends and future advances. In: Handbook of Marine Microalgae. Elsevier, UK, England, pp 1–9
Wang L, Li Y, Chen P, Min M, Chen Y, Zhu J, Ruan RR (2010) Anaerobic digested dairy manure as a nutrient supplement for cultivation of oil-rich green microalgae Chlorella sp. Bioresour Technol 101(8):2623–2628
Xu H, Miao X, Wu Q (2006) High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J Biotechnol 126(4):499–507
Yeh KL, Chang JS (2012) Effects of cultivation conditions and media composition on cell growth and lipid productivity of indigenous microalga Chlorella vulgaris ESP-31. Bioresour Technol 105:120–127
Yoo C, Jun SY, Lee JY, Ahn CY, Oh HM (2010) Selection of microalgae for lipid production under high levels carbon dioxide. Bioresour Technol 101(1):S71–S74
Zhang H, Sun S, Mai K, Liang Y (2000) Advances in the studies on heterotrophic culture of microalgae [J]. Trans Oceanol Limnol 3:010
Zhao BT, Su YX (2014) Process effect of microalgal-carbon dioxide fixation and biomass production: a review. Renew Sustain Energy Rev 31:121–132
Zheng Y, Li T, Yu X, Bates PD, Dong T, Chen S (2013) High-density fed-batch culture of a thermotolerant microalga Chlorella sorokiniana for biofuel production. Appl Energy 108:281–287
Acknowledgments
The authors wish to thank Ministry of Education Malaysia and Universiti Teknologi Malaysia for funding this study under the Fundamental Research Grant Scheme (FRGS) Vote Number R.K130000.7856.5F049. The first author is a researcher of Universiti Teknologi Malaysia (UTM) under the Post-Doctoral Fellowship Scheme (PDRU Grant) for the project: “Enhancing the Lipid Growth in Algae Cultivation for Biodiesel Production” (Vot No. Q. JJJ130000.21A2.03E95).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kamyab, H. et al. (2019). Microalgal Biotechnology Application Towards Environmental Sustainability. In: Gupta, S., Bux, F. (eds) Application of Microalgae in Wastewater Treatment. Springer, Cham. https://doi.org/10.1007/978-3-030-13909-4_19
Download citation
DOI: https://doi.org/10.1007/978-3-030-13909-4_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-13908-7
Online ISBN: 978-3-030-13909-4
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)