Abstract
Snakes are a diverse group of squamate reptiles characterized by a unique feeding system and other traits associated with elongation and limblessness. Despite the description of transitional fossil forms, the evolution of the snake feeding system remains poorly understood, partly because only a few snakes have been studied thus far. The idea that the feeding system in most snakes is adapted for consuming relatively large prey is supported by studies on anatomy and functional morphology. Moreover, because snakes are considered to be gape-limited predators, studies of head size and shape have shed light on feeding adaptations. Studies using traditional metrics have shown differences in head size and shape between males and females in many species that are linked to differences in diet. Research that has coupled robust phylogenies with detailed morphology and morphometrics has further demonstrated the adaptive nature of head shape in snakes and revealed striking evolutionary convergences in some clades. Recent studies of snake strikes have begun to reveal surprising capacities that warrant further research. Venoms, venom glands, and venom delivery systems are proving to be more widespread and complex than previously recognized. Some venomous and many nonvenomous snakes constrict prey. Recent studies of constriction have shown previously unexpected responsiveness, strength, and the complex and diverse mechanisms that incapacitate or kill prey. Mechanisms of drinking have proven difficult to resolve, although a new mechanism was proposed recently. Finally, although considerable research has focused on the energetics of digestion, much less is known about the energetics of striking and handling prey. A wide range of research on these and other topics has shown that snakes are a rich group for studying form, function, behavior, ecology, and evolution.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Snake Origins and Evolution
Recent advances, including the description of new fossils and the redescription of known fossils (Scanlon 2012; Longrich et al. 2012), have enhanced our understanding of the origin of snakes. A recent phylogenetic analysis based on genetic as well anatomical data (Hsiang et al. 2015) showed that snakes likely originated in the early Cretaceous (~128.5 Ma), as suggested by the fossil Coniophis, which is considered to be the earliest known true snake. Coniophis is important because it shows features of the skull that are intermediate between those of lizards and other snakes. The animal had hooked teeth and an intramandibular joint, which suggests that it fed on relatively large, soft-bodied prey (Longrich et al. 2012). Yet the maxilla was still firmly attached to the rest of the skull (Longrich et al. 2012). Mosasaurs have also been thought to possess a flexible lower jaw with a well-developed intramandibular joint, suggesting that this feature may have evolved in the ancestor of both mosasaurs and snakes (Lee et al. 1999). The crown group of snakes probably evolved 20 million years later, with the diversification of caenophidian snakes taking place after the Cretaceous–Paleogene mass extinction (Hsiang et al. 2015). One of the ongoing debates concerning the origin of snakes has been whether they derive from a burrowing (Conrad 2008; Gauthier et al. 2012; Martill et al. 2015; Hsiang et al. 2015) or marine (Lee et al. 1999) ancestor. Recent studies on the morphology and evolution of the visual system and inner ear of lizards and snakes suggest that snakes most likely had a terrestrial and fossorial origin (Simoes et al. 2015; Yi and Norell 2015). Indeed, not only does an analysis of visual opsins suggest that the visual system in stem snakes was reduced, but also the vestibule of the inner ear in the stem snake Dinilysia was strikingly similar to that of burrowing lizards, suggesting that Dinilysia was specialized for detecting ground-borne vibrations. In a recent integrative study of skull evolution in snakes, Da Silva et al. (2018) concluded that all snakes and their sister group evolved from a surface-dwelling terrestrial ancestor with non-fossorial behavior. Yet the most recent common ancestor of crown snakes had a skull shape adapted for fossoriality. Although the debate on the origin of snakes is far from resolved, a consensus is now emerging that the earliest snakes, or at least the stem snakes leading to the crown group of snakes, were fossorial or semi-fossorial animals that had a functional intramandibular joint and ate relatively large prey (Hsiang et al. 2015; Martill et al. 2015; Simoes et al. 2015; Yi and Norell 2015). The unusual jaws and feeding mechanics observed in the earliest branching lineages of snakes (Scolecophidia; Haas 1973; Kley 2001, 2006; Kley and Brainerd 1999; Rieppel et al. 2009) must thus be considered derived secondary specializations associated with an obligate underground lifestyle that went hand in hand with the loss of additional visual pigments (Simoes et al. 2015). The jaw elements became elongated and more highly kinetic in some later lineages of snakes, and these snakes have been called macrostomate snakes in reference to their enlarged gapes and abilities to ingest relatively large prey. In phylogenies based on morphological data or combined morphological and molecular data, macrostomate snakes form a monophyletic group. The macrostomate condition likely did not evolve before the late Cretaceous (Hsiang et al. 2015). Although the position of the Tropidophiidae remains debated, recent molecular phylogenies place this taxon as the sister group to Anilius (Pyron et al. 2013; Figueroa et al. 2016), suggesting that the macrostomate condition evolved or was lost more than once within snakes. As data sets and phylogenetic analyses become more comprehensive, the evolution of macrostomate morphology may be resolved with more confidence. For this review, we refer to the macrostomate condition and its consequences for feeding, without implying the monophyly of macrostomate snakes.
Most lizards have relatively akinetic skulls and eat small prey, although some can eat prey as large as 35% of their own body mass (Shine and Thomas 2005). Lizards typically rely on high bite forces to reduce prey into smaller parts, a strategy rarely observed in snakes (but see Jayne et al. 2002). In contrast, snakes typically rely on cranial kinesis to ingest prey whole. Although the early branching lineages of alethinophidian snakes (all extant snakes except for blind snakes) retained relatively akinetic skulls, the macrostomate morphology of later lineages is characterized by a greater degree of cranial kinesis than in lizards, allowing them to swallow relatively large prey. The maximum size of prey that a snake can ingest is limited by the maximum size of its open mouth, which is often referred to as gape limitation (Greene 1983). Macrostomate snakes have lengthened several cranial elements including the palatomaxillary arch, the suspensorium (quadrate and supratemporal), and the mandible, which allow the mouth to open to a greater extent than in earlier lineages of snakes. The evolution of increased gapes was associated with diverse changes in the morphology, mechanics, physiology, behavior, and ecology of feeding. In this review, we highlight recent research on many of these aspects of snakes, particularly research published after the last major review of feeding in snakes by Cundall and Greene (2000).
2 Morphology
Understanding mechanisms of movement, including those associated with feeding, requires a strong foundation in morphology. The static and dynamic properties of bones, ligaments, muscles, tendons, and other morphological structures are crucial to the diverse functions that the structures support and produce. Excellent reviews on the cranial anatomy of snakes exist (Cundall and Greene 2000; Cundall and Irish 2008; McDowell 2008), and here we briefly review studies that appeared after these reviews or that were not discussed in detail in them.
Recent years have seen the publication of anatomical descriptions of the cranial skeleton in some early branching lineages of snakes that were relatively poorly known, including anatomical descriptions of several blind snakes (Kley 2006; Rieppel et al. 2009), uropeltid snakes (Olori and Bell 2012), and Xenopeltis (Frazzetta 1999), as well as some descriptions of ontogenetic changes in skull morphology (Palci et al. 2016; Scanferla 2016). The homology of the jaw muscles in lizards and snakes was also discussed in a recent paper (Johnston 2014), revising the homology hypothesis proposed by McDowell (1986). Johnston pointed out that the jaw muscles in macrostomate snakes appear adapted for mouth opening rather than powerful closing, and that this change appears to have involved increasing muscle fiber length by the loss of aponeuroses and a modification of the M. levator anguli oris (LAO) that allowed it to act both on the venom gland and as a jaw adductor. The original function of the LAO would then have been taken over by the apomorphic M. neurocostomandibularis.
Two recent papers describing dental specializations in relation to diet were also published (Jackson and Fritts 2004; Britt et al. 2009). Jackson and Fritts (2004) described dental specializations in wolf snakes, including enlarged maxillary teeth, an arched maxilla with a large diastema, and ungrooved posterior maxillary fangs that allow them to hold on to and slice through hard or protected prey such as skinks. Britt et al. (2009) examined differences in the teeth of thamnophiine snakes that eat slugs and those that eat fish or are more generalist feeders. Interestingly, slug eaters showed pronounced posterior ridges on the posterior maxillary teeth. In some snail-eating snakes, directional asymmetries in tooth number and lower jaw shape have been documented that are related to the predominance of dextrality (clockwise turning) in snails (Hoso et al. 2007; dos Santos et al. 2017). The snakes can extract snails from their shells faster when feeding on dextral than sinistral snails, suggesting that these asymmetries are adaptive (Hoso et al. 2007). These papers, along with many discussed below, nicely demonstrate how understanding morphology is crucial to understanding function.
2.1 Head Size and Shape
Head shape is remarkably variable in snakes and has been suggested to be adaptive, with relative head width, in particular, being related to the maximal prey size that can be consumed in a wide sample of snakes (Vincent et al. 2006a). In some cases, such as egg-eating snakes in the genus Dasypeltis, adaptations for consuming extremely large food items may limit the ability to eat some kinds of prey (Gans 1952, 1974; Gartner and Greene 2008). Indeed, the specializations for egg eating have resulted in the loss of most teeth, effectively restricting these animals to eating only eggs. Although head shape in snakes is typically investigated because of its expected importance to feeding, snake heads serve many functions in addition to prey capture and transport. For example, head size and shape may be important in anti-predator mechanisms. Specifically, head triangulation, the ability of a snake to flatten its head and make it more triangular, has been suggested to reduce predator attacks based on studies with clay models (Valkonen et al. 2011; Dell’Aglio et al. 2012). In addition, measurements of head shape may be informative in phylogenetic and systematic analyses (Mangiacotti et al. 2014; Ruane 2015).
Head size and shape are not static, however, and significant plasticity in head shape has been documented (Forsman 1996; Queral-Regil and King 1998; Bonnet et al. 2001; Aubret et al. 2004; Smith 2014). Pioneering work by Forsman (1996) first demonstrated that adders that were fed more frequently developed relatively larger heads than those subject to food restriction. Moreover, Bonnet et al. (2001) showed that feeding frequency also impacted relative head and fang proportions, with snakes that were fed more frequently having relatively wider heads and relatively longer fangs. Subsequent studies manipulated feeding frequency and prey size (Queral-Regil and King 1998; Aubret et al. 2004; Smith 2014). These studies showed that feeding larger prey to snakes resulted in relatively longer jaws (Queral-Regil and King 1998; Aubret et al. 2004) or broader heads (Smith 2014). Interestingly, Aubret and Shine (2009) demonstrated that the plasticity in head shape diminished with the time after colonization of a new island. The ability to respond plastically may consequently depend on the environmental variability encountered by a species. Most of these studies quantified head shape externally using either linear measures or geometric morphometric approaches. In contrast, one study that used radiographs to quantify the effects of prey size on the sizes of cranial skeletal elements did not find a treatment effect (Schuett et al. 2005), suggesting that the observed effects on head shape in other studies may reflect differences in musculature rather than differences in the skeletal elements.
Given the importance of these features to feeding mechanisms and diet, many studies have investigated the growth of the head in snakes (see review in Cundall and Greene 2000; Vincent et al. 2007; Borczyk 2015; Andjelkovic et al. 2016a; Palci et al. 2016). Generally, studies have found a negative allometry of head size, with the possible exception of head width, which in some species grows with positive allometry (Borczyk 2015). Furthermore, allometry generally explains a significant proportion of the shape differences observed during growth (Andjelkovic et al. 2016a; Murta-Fonseca and Fernandes 2016). Adult snakes typically have more strongly developed muscle attachment sites (Palci et al. 2016), greater physiological cross-sectional areas of the cranial muscles (Vincent et al. 2007), and relatively longer quadrates (Palci et al. 2016; Scanferla 2016). Given that juvenile snakes have relatively large heads for their body sizes, some studies have investigated whether juveniles show increased levels of performance despite their smaller absolute sizes (Vincent et al. 2006b; Hampton 2014; Hampton and Kalmus 2014). These studies have shown that although gape size increases with negative allometry, juveniles do not have a relatively better performance than adults in terms of transport time or the number of jaw movements needed to ingest prey. The skeletal elements that best predict gape size also appear to differ between species (Hampton 2014; Hampton and Kalmus 2014). In some species, the observed differences in head shape have been linked to variation in diet (Vincent et al. 2004; Meik et al. 2012; Natusch and Lyons 2014; see Vincent and Herrel 2007 for a review). Furthermore, the morphological differences between juveniles and adults may also explain the observed ontogenetic changes in diet in many species (Vincent et al. 2004; Natusch and Lyons 2012; Lopez et al. 2013; Scanferla 2016). Prey size typically increases with head size in most snakes (Arnold 1993). However, in some cases, changes in head size and shape have been suggested to be linked to a shift from ectothermic to endothermic prey (Natusch and Lyons 2012; Scanferla 2016). In addition to differing between juveniles and adults, head shape often also differs between males and females of the same species, often in response to the sexual differences in diet (Vincent et al. 2004; Krause and Burghardt 2007; Meik et al. 2012; Henao-Duque and Ceballos 2013; Andjelkovic et al. 2016a, b).
However, beyond the variation between adults and juveniles or between the sexes, different species or populations of snakes also often vary considerably in head shape (Voris and Voris 1983; Grudzien et al. 1992; Dwyer and Kaiser 1997; Forsman and Shine 1997; Mori and Vincent 2008; Brecko et al. 2011; Hampton 2011; Henderson et al. 2013; Natusch and Lyons 2014; Fabre et al. 2016; Segall et al. 2016; Fig. 14.1). This variation has been suggested to be adaptive, as it allows the consumption of different types and sizes of prey (Dwyer and Kaiser 1997), the occupation of different microhabitats (Fabre et al. 2016), the capture of elusive prey underwater (Herrel et al. 2008; Segall et al. 2016), or a combination of these. Among populations of garter snakes and adders, for example, nonadaptive hypotheses for the observed inter-population divergence in relative head size were rejected, suggesting that geographically heterogeneous selection optimizes prey-handling ability (Grudzien et al. 1992; Forsman and Shine 1997). However, in some cases, no differences in diet were observed despite considerable variation in head size and shape among populations (Natusch and Lyons 2014). Across species, head shape is often associated with variation in diet, especially when it involves specialization for handling food items such as hard-shelled prey that impose specific functional demands (Dwyer and Kaiser 1997; Fabre et al. 2016). In many cases, subtle differences have also been observed between snakes that eat bulky prey such as frogs and those that eat more streamlined prey such as fish or lizards (Mori and Vincent 2008; Hampton 2011). These differences become more pronounced for snakes that capture prey under water, where the wide heads of snakes that eat bulky prey could interfere with the ability to capture streamlined, elusive aquatic prey (Hibbitts and Fitzgerald 2005; Herrel et al. 2008; Segall et al. 2016). This trade-off is especially likely to occur in frontally striking species, as wide heads tend to generate bow waves that alert prey sooner to the presence of the predator, and may even push prey away from the line of attack of the predator (Hibbitts and Fitzgerald 2005; Van Wassenbergh et al. 2010). Because of these strong constraints, strongly convergent head shapes are generally observed in aquatic snakes (Hibbitts and Fitzgerald 2005; Herrel et al. 2008; Vincent et al. 2009; Segall et al. 2016).
The advent of comprehensive phylogenies for many snake lineages has permitted analyses of these radiations and the roles that head size and shape have played in them. A recent study of Indo-Australian sea snakes demonstrated the presence of two distinct ecomorphs that differ in head shape (Sanders et al. 2013). The microcephalic ecomorph is smaller, has a narrow head and body, and specializes on eels that are captured in burrows. The macrocephalic ecomorph, on the other hand, has a large head and feeds on crevice dwelling eels and gobies. Interestingly, both ecomorphs have evolved independently at least twice, suggesting that differences in head shape may be the basis of the rapid divergence of species within the clade (Sanders et al. 2013). That head size can rapidly respond to strong selective pressures is nicely shown by the decrease in relative head size in Australian snakes that eat toads (Phillips and Shine 2004). Yet, a recent study using a geometric morphometric approach showed strong convergence in head shape in boas and pythons occupying similar habitats, suggesting that habitat use, in addition to diet, may drive the evolution of head shape (Esquerré and Keogh 2016; but see Henderson et al. 2013 for an example of diet-driven convergence in tree boas of the genus Corallus). A study on the convergence between oxyuranine elapids from Australia and a distantly related assemblage of snakes from North America even suggested that diet did not drive the phenotypic convergence between these groups, despite the fact that head measurements were analyzed in the data set. Thus, recent analyses have not always supported the idea that the head should reflect the type and size of prey eaten in gape-limited predators such as snakes (Gans 1961), although in many case, this is likely to be so (e.g., Fabre et al. 2016; Klaczko et al. 2016). Rather than solely reflecting adaptations to diet, head size and shape clearly respond to multiple selective factors, including both diet and habitat use. When physical constraints on head shape are strong (as with underwater prey capture; see Herrel et al. 2008; Segall et al. 2016), convergence is to be expected regardless of the selective drivers (diet or locomotion).
3 Function
3.1 Prey Detection
Searching for prey typically involves at least some locomotion, which is integral to feeding biology but usually studied separately from feeding (Higham 2007). Foraging involves morphological, physiological, mechanical, and behavioral traits, and has immediate consequences for feeding success as well as longer term consequences for life history, reproduction, and fitness (McLaughlin 1989; Beaupre and Montgomery 2007). Although different foraging modes are broadly associated with distinct sets of traits, variation, and flexibility among snakes defy simple characterization of foraging modes into active and ambush foragers (Greene 1997; Beaupre and Montgomery 2007).
During foraging, snakes may detect prey chemically, visually, or mechanically. Tongue flicking is a particularly important sensory behavior in snakes that involves the transport of stimuli from the external environment to the vomeronasal organ (also known as the Jacobson’s organ) in the roof of the mouth. Tongue flicking is brought about by a hydrostatic mechanism involving elongation of the posterior part of the tongue (de Groot et al. 2004). Recent studies have shown that oscillatory tongue flicks are most likely used to collect odorants (Daghfous et al. 2012), such as during foraging. In contrast, simple downward extension when the tongue touches an object or the substrate likely serves to sample nonvolatile chemical stimuli (Daghfous et al. 2012). After tongue extension, the tongue is retracted into the oral cavity and chemicals are transferred to the Jacobson’s organ. However, the exact mechanism of transfer remains poorly understood. For lizards with unforked tongues, the transfer from the tongue to the vomeronasal organ may take place through a hydraulic mechanism, with the anterior tongue acting as a piston (Filoramo and Schwenk 2009). Whether a similar mechanism operates in snakes remains unknown. Although it had been suggested that a forked tongue may allow snakes to compare paired stimuli (Schwenk 1994), recent studies that employed unilateral transections of the vomeronasal nerve indicated that this may not be the case, as rattlesnakes were able to trail prey even after unilateral nerve transection (Parker et al. 2008). Thus, the deeply forked tongue may serve only to increase the odor sampling area (Parker et al. 2008). In some aquatic species, the tongue may also be used to lure fish by keeping it rigidly extended with only the very tips touching the water (Welsh and Lind 2000).
In addition to using chemical and visual cues, sand vipers (Cerastes; Young and Morain 2002) and probably other snakes can use ground-borne vibrations to localize prey (Randall and Matocq 1997; Friedel et al. 2008). Aquatic snakes also possess specialized mechanoreceptors (scale sensillae) that may detect water motion (Povel and van de Kooij 1997; Westhoff et al. 2005; Catania et al. 2010; Crowe-Riddell et al. 2016), especially in snakes that forage in low-visibility environments (e.g., Hart et al. 2012; Crowe-Riddell et al. 2016). Yellow anacondas (Eunectes notaeus) can detect water-borne vibrations in laboratory experiments, although the extent to which they do so in the wild or for predation remains unknown (Young 2007). Snakes can also detect airborne sounds and respond with consistent predatory or defensive behaviors, which suggests that auditory stimuli may be more important to snakes than is currently recognized (Young and Aguiar 2002; Young 2003).
Pit vipers and some boas and pythons can detect infrared light (heat) using pit organs on the face. Pit organs may be used for predation, defense, thermoregulation, or other functions. To date, pit organs have been studied more thoroughly in pit vipers than in boas and pythons. Recent work has shown that infrared detection involves heat-sensitive ion channels in the pit organs (Gracheva et al. 2010). Eye and pit sizes are negatively correlated in crotaline snakes, suggesting a trade-off in functions and perhaps selective pressures between eyes and pits (Liu et al. 2016). However, the results of heat-transfer analyses suggesting that images formed by pit organs are poorly focused and of low contrast (Bakken and Krochmal 2007) seem to imply a limit to how much the trade-off between eyes and pits could favor the pits. Nevertheless, pit organs are highly sensitive and can be used in prey acquisition. For example, rattlesnakes (Crotalus atrox) can detect infrared stimuli that simulate a rodent at distances up to 100 cm (Ebert and Westhoff 2006). Ball pythons (Python regius) can detect moving infrared stimuli at distances up to 30 cm, and assess their direction and distance independently of visual cues (Ebert et al. 2007). Respiratory evaporative cooling of the snout in rattlesnakes can enhance the temperature difference between the pit organs and endothermic prey, thus enhancing pit organ function in predation (Cadena et al. 2013). Pit organs may also be important to other functions besides predation. Krochmal and Bakken (2003) found that rattlesnakes can use their pit organs in thermoregulation; they further suggested use in thermoregulation as a possible alternative hypothesis to prey acquisition for the evolution of pit organs. In a broader comparative study, Krochmal et al. (2004) showed that diverse pit vipers can rely on facial pits for thermoregulatory movements, suggesting that the use of pit organs for thermoregulatory behaviors may represent an ancestral trait among pit vipers. It seems plausible that the evolution of pit organs involved simultaneous benefits to predation, defense, and thermoregulation; however, it may be difficult to test these hypotheses separately from one another.
3.2 Prey Capture
Snakes capture prey using movements of the head, jaws, and some length of the anterior trunk; the same or similar movements are often used in defensive contexts. These movements are usually called “strikes,” although other terms have been used (lunge, lateral sweeping, slow-capture techniques, and fast-capture techniques) based on the use and speed of the body and head (Cundall and Greene 2000; Alfaro 2003; LaDuc 2002). Of any behavior displayed by snakes, striking is one of the most widely known, yet least studied and understood behaviors. Confounding the dearth of knowledge is inconsistency in the variables used to characterize strikes and ambiguity in the literature about differences between predatory and defensive strikes. It is difficult to draw generalizations when comparing results from different methods, variables, species, and types of strikes. Nevertheless, here we review some key themes from research on snake strikes and note directions of future research that are likely to be productive. Cundall and Greene (2000) equated prey capture with ingestion. However, prey capture often involves distinct movements from ingestion. Below we discuss the distinct movements involved in prey-capture mechanisms such as striking and biting, prey-handling mechanisms such as constriction and pinioning, and ingestion mechanisms such as mandibular or maxillary raking, snout shifting, and pterygoid walking movements. Once a prey animal has been detected, it must be captured. Capture can involve simple biting or seizing of prey, lateral sweeping movements with open jaws to capture prey (particularly in aquatic feeding), or lunging or striking to make contact with prey from some distance.
3.2.1 Biting and Simple Seizing
Small prey, regardless of type, are often simply grasped with the jaws and quickly swallowed alive (Cundall and Greene 2000). This simple-seizing method of prey capture appears to be the only prey-capture mechanism used by scolecophidian snakes, although scolecophidians also use unique intraoral transport mechanisms (Kley and Brainerd 1999; Kley 2001; discussed further below) and sometimes further processing (i.e., decapitating termites; Mizuno and Kojima 2015). Many alethinophidian snakes also employ simple biting or seizing behaviors, although they have not yet been well studied (Cundall and Greene 2000). Some alethinophidians appear to use biting or simple seizing exclusively, whereas others modulate their prey-handling behaviors in response to cues from the prey (de Queiroz 1984; Cundall and Greene 2000; Bealor and Saviola 2007; Fig. 14.2). Even some larger prey that are neonatal or harmless will be ingested without further use of more complex prey-handling behaviors (de Queiroz 1984). In a separate section below, we discuss ingestion mechanisms employed once a prey item has been captured.
a Pinion method of prey handling used by the bullsnake, Pituophis melanoleucus, b pinion plus nonoverlapping loop, c fully encircling coils (reproduced from de Queiroz 1984)
Upon prey capture, many nonvenomous snakes must remain in contact with the prey, regardless of its type or size, until it is consumed or subdued. Snakes may use their jaws or portions of their body to hold onto prey, and in some cases will release the prey from the jaws after initial contact. The high degree of cranial kinesis in snakes is thought to reduce bite forces (Jayne et al. 2002). Some theoretical models have predicted bite capacity in snakes based on skull morphology (Mori and Vincent 2008), but unfortunately bite forces in snakes are largely unexplored. The strong bites required to hold onto and sometimes directly subdue large and strong prey indicates that quantifying the jaw forces used in biting and ingestion in snakes could provide interesting and surprising results. However, to our knowledge, bite force has been quantified in only one species of snake thus far (Lampropeltis getula; Penning 2017a). Penning (2017a) found that bite forces in kingsnakes (Lampropeltis getula) were within the range of bite forces in lizards, but lower for a given head size than in lizards. Interestingly, when kingsnakes experienced simulated prey struggling (via manual movement of the bite-force sensor), they responded by momentarily increasing their bite forces, although the forces never reached the initial peak performance. Bite force and constriction pressure were positively correlated with one another, which is expected because snakes often use their jaws to capture and hold onto prey while they constrict it (Penning 2017a). Given the great diversity of diets among snakes, it seems likely that bite forces also vary widely among species and may be surprisingly high in large snakes and those that subdue vigorous prey using only their jaws. For example, snakes in the genera Drymarchon and Masticophis are noted for having powerful jaws (Werler and Dixon 2000; Ernst and Ernst 2003; Gibbons and Dorcas 2015) and have been documented to subdue large prey such as rats, cats, rabbits, and opossums using only their jaws (reviewed in Ernst and Ernst 2003; Stevenson et al. 2010). These snakes appear to deliver strong bites and may forcefully thrash prey. Thrashing appears to quickly incapacitate prey but is well tolerated by the snakes (Cundall and Greene 2000). These snakes are also noted for swallowing live vertebrates that are still mobile and can be seen moving within the esophagus (Lillywhite 2014). Potential differences between feeding and defensive bites in all snakes remain to be determined.
3.2.2 Open-Mouthed Sweeping
Many aquatic and semiaquatic snakes capture prey by sweeping the head and anterior trunk from side to side with an open mouth (e.g., reviewed by Cundall and Greene 2000, and later quantified by Alfaro 2003). Nerodia rhombifer and Thamnophis elegans both sometimes forage using relatively slow lateral sweeping movements of the anterior trunk and head with the mouth open (Alfaro 2003). These sweeping movements may occur from a stationary position, during forward swimming, or after an unsuccessful forward strike. However, the faster lateral and forward strikes also differed kinematically among N. rhombifer, T. elegans, and T. couchii. Hence, the term “sideways sweeping” is inadequate for characterizing the diversity of foraging movements both within and among species (Alfaro 2003). The similarities in lateral sweeping movements among distantly related species suggested convergent evolution of this prey-capture mechanism (Cundall and Greene 2000). However, upon testing for convergence in European and North American natricine snakes, Bilcke et al. (2006) found that aquatic prey-capture strategy and strike velocity were significantly correlated with prey density, not with diet. Additional research on the mechanisms and evolution of aquatic feeding in snakes is likely to reveal yet more diversity.
3.2.3 Striking
Snakes that feed on wary, highly mobile, or large prey often capture it using lunges or strikes from some distance. Both classic (Klauber 1972; Parker and Grandison 1977) and recent work (LaDuc 2002) have described a strike as a lunge. Cundall and Greene (2000) noted the distinction between short and slow lunges and long and fast strikes. This dichotomy is qualitative and probably represents an oversimplification of a performance continuum (Cundall et al. 2007), although the dichotomy seems to apply at least broadly to the thamnophiine snake studied by Alfaro (2002, 2003). Given our limited data and the variable nature of striking in snakes (Smith et al. 2002; Alfaro 2002, 2003; Cundall et al. 2007; Penning et al. 2016), as well as the likelihood of future advances in our understanding of snake strikes, we feel that it is premature to try to classify and apply standardized terms to the types of strikes. Similarly, Higham et al. (2017) felt that strikes must be quantified in nature before we can fully assess strike performance. In this review, we define a strike broadly as a distinct movement of the head toward a target that may be a threat or prey; such a strike may be forward or lateral, and may be slow, intermediate, or fast.
Whether scolecophidian snakes can even strike remains to be seen. Given their burrowing lifestyle and slow-moving prey, it is unlikely that predatory striking behavior is needed or could even be employed in their subterranean environments (Cundall et al. 2007). To date, what we know about striking derives from alethinophidians, and particularly from booid (Frazzetta 1966; Cundall and Deufel 1999; Deufel and Cundall 1999; Cundall et al. 2007) and colubroid snakes (Van Riper 1954; Greenwald 1974, 1978; Janoo and Gasc 1992; Kardong and Bels 1998; LaDuc 2002; Herrel et al. 2011; Penning et al. 2016). Most studies have addressed forward strikes in a terrestrial environment over a relatively narrow range of temperatures (ca. 25–30 °C). A few studies have addressed strikes that involve lateral or undulatory movements (e.g., Kardong and Bels 1998; Smith et al. 2002; Alfaro 2003; Catania 2009, 2010), and even fewer studies have addressed terrestrial-to-aquatic strikes (e.g., Vincent et al. 2005), or arboreal strikes (Herrel et al. 2011). There is no typical pattern that represents these diverse strikes. Future studies are likely to discover considerable variation in strikes related to size, temperature, behavioral context, environment, species, lineages, and other variables. Both prestrike behaviors and strikes differ in feeding and defensive contexts (see Young et al. 2001b; LaDuc 2002), and the outcomes of strikes can have major fitness consequences. The roles of feeding strikes are clear: to make contact with prey so that the next stages of feeding can occur (such as constriction, envenomation, ingestion, etc.). The overall role of defensive strikes is also fairly clear: to deter a potential threat from a predator. However, the specific goals of defensive strikes are not well known, and may include maintaining or enlarging the gap between the snake and the threat (i.e., no contact), making contact to deter the threat directly by bluffing, startling it, causing pain, or perhaps other functions. It is important to be careful when making inferences about one kind of strike from another because predatory strike performance may not be a reliable predictor of defensive strike performance and vice versa. Offensive and defensive strike metrics are often used interchangeably because the behaviors appear qualitatively similar; however, they can be quantitatively distinct and should be treated as separate performance metrics.
Much of the previous work on strike performance has focused on the kinematics of the head and jaws during strikes (Cundall and Deufel 1999; Deufel and Cundall 1999; Cundall and Greene 2000; Cundall et al. 2007). Fewer studies have quantified axial kinematics during striking. The limited evidence available, which is mainly from heavy-bodied vipers, indicates that prestrike posture does not appear to affect strike kinematics (Kardong and Bels 1998; Young 2010). Whether this applies to non-viperid snakes warrants testing. Different patterns of axial movement can be used to push the head forward. In feeding strikes, Kardong and Bels (1998) described a “gate model” of straightening in which accordion-like axial bends in the anterior trunk straighten out, and a “tractor-tread” model in which the body flows through a postural curve, with limited straightening. Alfaro (2003) also noted feeding strikes matching both the open-gate model and the tractor-tread model in thamnophiine snakes. For his study species with the fastest strikes, Thamnophis couchii, the open-gate mechanism produced the highest speeds by enabling the snake to recruit a larger proportion of the body. Indeed, more axial bends should sum to a greater resultant velocity. Alfaro (2003) also noted that the tractor-tread model applied more clearly to the posterior parts of the trunk than anterior ones in T. elegans, and that by exerting forces posteriorly against the water, the posterior part of the body probably contributes to sideways sweeping and may contribute to the motion of forward strikes as well. The similarity of the tractor-tread model to undulatory locomotion was clear (Kardong and Bels 1998), and makes us wonder whether the patterns of axial muscle activation are similar to those of locomotion, and whether tractor-tread strikes are slower than open-gate strikes and involve more continuous motor control rather than ballistic control that cannot be adjusted via feedback once initiated. Such questions remain to be addressed in future research.
As with axial movements during striking, little work has addressed the mechanisms that produce and control strikes. Young (2010) showed that several muscles are electrically active in Bitis arietans before defensive strike movements begin, suggesting that the strikes are ballistic and powered by an elastic recoil mechanism. However, both the ballistic nature and possible elastic mechanisms of strikes remain uncertain. Some snake strikes may be ballistic, with the rapid movements involved and functional limitations of sensory processing precluding mid-strike adjustments (Cundall et al. 2007). Kardong and Bels implied ballistic motion in feeding strikes by stating that adjustments to strike trajectory or in response to prey evasion are made after contact, not during forward movement. However, Frazzetta (1966) discussed a snake changing course during a strike. Some indirect evidence suggests that strikes may not be ballistic. Many ballistic movements powered by elastic recoil mechanisms are independent of temperature across diverse organisms (Anderson and Deban 2010; Deban and Lappin 2011; Deban and Richardson 2011; Deban and Scales 2016), whereas the few relevant studies thus far have shown significant temperature dependence in strike performance in snakes (Greenwald 1974, 1978; Shine et al. 2002). Young (2010) suggested that some snakes may use elastic mechanisms to power striking, whereas others may use different mechanisms. Additional research on strike mechanisms is clearly needed to resolve these issues, and may have broader implications for our understanding of vertebrate muscle function and its evolution.
Three other important aspects of snake strike performance are beginning to be studied: How body size, environment, and predator–prey interactions affect strike performance. In general, larger snakes can strike over longer absolute distances than smaller snakes if they use the same proportion of their body. Due to correlations among variables in strikes (duration, distance, velocity, and acceleration) and the fact that some snakes continue to accelerate through both feeding and defensive strikes (Young et al. 2001a; LaDuc 2002; Vincent et al. 2005; Herrel et al. 2011), longer strikes typically produce higher velocities before prey contact. Herrel et al. (2011) showed that adult Trimeresurus albolabris striking defensively from arboreal perches cover the same absolute strike distance as juveniles, leading to similar strike velocities across a range of body sizes. This result also means that the strike distances of juveniles are longer relative to their body size than in adults, suggesting selective pressures on juvenile strike performance. However, LaDuc (2002) showed that Crotalus atrox, a terrestrial pit viper, will strike over twice as far at a potential threat than at prey. Whether or not arboreal feeding strikes differ from the defensive strikes studied by Herrel et al. (2011) remains to be tested in future research.
Many snakes are aquatic or semiaquatic, and experience stronger environmental constraints on fast movements such as striking due to the higher drag experienced when moving in water than in air. The fully aquatic tentacled snake, Erpeton tentaculatus, reaches one of the highest strike accelerations and shortest durations yet determined in any snake (Smith et al. 2002). Tentacled snakes also exploit the stereotyped C-start maneuver of fish to capture them effectively (Catania 2009, 2010). They do so by feinting with their body to elicit a C-start in a nearby fish, which then moves toward the snake’s advancing jaws or toward a position the snake anticipates and strikes toward (Catania 2009, 2010). The prey-capture mechanism of tentacled snakes appears unique. However, some snakes can use rapid forward strikes to capture aquatic prey, in addition to the lateral sweeps described above, despite apparent hydrodynamic constraints. Alfaro (2002, 2003) found that Thamnophis couchii, T. elegans, and Nerodia rhombifer all can use rapid forward strikes in addition to the slower lateral sweeps. Thamnophis couchii achieves the highest forward strike performance by recruiting and straightening nearly its entire body (Alfaro 2003). Researchers have hypothesized that underwater strikes may be hindered by drag and may generate bow waves that displace prey and make capturing it more difficult (Young 1991; Vincent et al. 2005). However, recent hydrodynamic modeling has shown that the effects of drag and displacement of prey during aquatic strikes is probably less important than previously thought (Van Wassenbergh et al. 2010). The effects of gape appear to be particularly important in aquatic strikes (Van Wassenbergh et al. 2010), yet largely remain to be studied experimentally.
Studies of strike kinematics have begun to illustrate differences in strike performance as well. However, which aspects of strikes are good indicators of performance is not yet clear and may differ with circumstances. Variables that may be good indicators of performance include strike acceleration, velocity, duration, success rate, and perhaps other variables. In predatory strikes, which often involve only short distances (LaDuc 2002; Clark et al. 2012; Higham et al. 2017), acceleration may be more important than velocity because strikes typically do not involve a chase, and rapid acceleration is necessary to help a snake close the gap between itself and the prey before the prey can evade the strike (Penning et al. 2016). In defensive strikes, acceleration may be the crucial variable if contact is important, because high acceleration is required to make contact before the target evades. However, if contact is not a goal of defensive strikes, then neither acceleration nor velocity may be important, except perhaps to the extent that they contribute to an effective bluff or startle effect. Vipers have often been assumed to have the highest strike performance (e.g., Van Riper 1954; Klauber 1972; Janoo and Gasc 1992; Whitaker et al. 2000). However, Penning et al. (2016) showed that at least one nonvenomous colubrid snake (Pantherophis obsoletus) can strike defensively with similar levels of performance to vipers (Agkistrodon piscivorus and Crotalus atrox; Fig. 14.3). Although the performance of feeding strikes in these snakes is still under study, we predict that feeding strikes will also involve similar and high levels of performance. These snakes often feed on the same or similar prey (e.g., small rodents), which presumably imposes similar demands on strike performance, regardless of which snake is involved. To catch a rodent, any snake needs to strike fast enough to make contact before the rodent escapes. What happens next, whether simple biting, constriction, or injection of venom, is a separate stage of the feeding process that could not happen if the snake did not make contact with the prey. More generally, we suspect that snake strike performance is driven largely by the response times of predators and prey, largely independent of phylogeny.
Video images of defensive strikes by Pantherophis obsoletus (top) and Crotalus atrox (bottom) recorded at 250 frames s−1 with a Keyence camera (Itasca, IL, USA) (reproduced from Penning et al. 2016)
Historically, field observations of snake strike performance were rare. We know so little about strikes in natural environments that lab tests may not reflect the actual conditions in which snakes use this behavior, although a few studies have observed that wild rattlesnakes may strike with similar kinematics to captive snakes (Cundall and Beaupre 2001; Clark et al. 2012; Higham et al. 2017; Fig. 14.4). These studies have begun to offer important insights into strike performance in nature. During feeding strikes in the wild, rattlesnakes (Crotalus spp.) took averages of 0.07–0.2 s to reach their targets (Cundall and Beaupre 2001; Clark et al. 2012; Higham et al. 2017); the fastest strikes overlapped with the 0.05–0.08 s average strike durations measured under laboratory conditions, but strikes in the wild often took much longer than those in the lab (LaDuc 2002; Penning et al. 2016). Strike durations in both the lab and the wild can be so short that snakes may avoid sensory detection by their prey, reaching them before the prey are even aware of the threat (Penning et al. 2016). However, field recordings of rattlesnake strikes show that some prey can detect imminent strikes and rapidly evade them or retaliate if evasion is unsuccessful (Cundall and Beaupre 2001; Clark et al. 2012; Higham et al. 2017). Clark et al. (2012) found that strike success (prey capture) in the field was significantly related to strike distance (Fig. 14.5), which supports laboratory results of snakes striking only when prey move close enough (LaDuc 2002). In 49% of field strikes, prey initiated evasive maneuvers, significantly increasing their chances of avoiding predation (Clark et al. 2012); similarly, Higham et al. (2017) recorded four successful and four unsuccessful strikes by C. scutulatus in the wild. Selection for high strike performance may be influenced by the prey’s sensory and response capacities (Penning et al. 2016; Higham et al. 2017). Recent field recordings have shown that both rattlesnakes and their prey are capable of high accelerations (ca. 500–600 m s−2) during striking and evasion over very short durations (Higham et al. 2017). More analyses of strikes in nature and of prey response times and behaviors are needed to better understand the dynamics and evolution of this crucial component of snake predation.
The predictive framework for escape maneuvers of kangaroo rats in response to strikes from rattlesnakes. This sequence of events is expected during natural interactions, and was observed in multiple interactions. Amy Cheu provided these illustrations (reproduced from Higham et al. 2017)
Trajectory of prey movement was categorized depending on the relative angle between the anteroposterior axis of the snake (ap) and the movement vector of the prey (pv). PV was calculated by connecting the position of the prey 1 s before strike initiation (P1) with the position of the prey when the strike was initiated (P2). If angle θ was greater than 45°, prey was categorized as moving laterally; if θ < 45°, prey was categorized as moving anteroposteriorly. The case shown would be categorized as lateral retreat, because θ > 45° and the prey item had crossed the AP axis before the strike was initiated (reproduced from Clark et al. 2012)
As a snake makes contact with prey, it must quickly accomplish the next stage, such as anchoring the jaws onto the prey, injecting venom and retracting, or forming a constriction coil or equivalent posture, before the prey can escape or defend itself. Whatever the subsequent predation mechanism, the snake’s jaws become the interface between the two organisms. Small or harmless prey is often ingested alive, with the snake remaining in contact with the prey from the moment of capture onward. Larger and more dangerous prey is usually handled differently. Upon contact with prey, jaw closing marks the end of a strike (Cundall and Greene 2000). The mechanism of tooth engagement with prey is not well known and may involve snaring (Deufel and Cundall 1999), downward or rearward stabbing (Frazzetta 1966; LaDuc 2002; but see Deufel and Cundall 1999), or other movements by both snake and prey. For many venomous snakes, venom is quickly delivered and the head is retracted away from the prey after brief contact for venom delivery (reviewed in Cundall and Greene 2000; Lillywhite 2014), although some snakes will remain in contact with prey based on prey size and habitat complexity (Lillywhite 2014). Strikes likely produce enough kinetic energy to drive fangs through tissue without the need for additional contractile forces from the jaws themselves (Anderson et al. 2016). Although strong bite forces are probably not required for fang penetration, pressure at the point of fang penetration may be extremely high because the force of impact of the snake’s head is applied to extremely small areas of the fang tips.
3.2.4 Venom Delivery
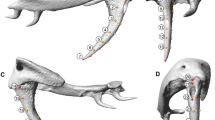
Venom delivery systems in snakes typically consist of a pair of venom glands, their associated muscles and enlarged teeth (Jackson 2003; Fig. 14.6). Recent years have seen significant advances in our understanding of the regulation of venom expulsion in rattlesnakes. In a series of studies, Young and collaborators describe how the gland musculature in rattlesnakes is functionally subdivided, allowing the regulation of venom flow (Young et al. 2000). Furthermore, the fang sheath is important in allowing venom expulsion by displacing the inner fang membrane from the entrance orifice of the fang, thus allowing venom flow (Young et al. 2001a; Young and Kardong 2007). The duration of venom flow, flow rate, and total volume of venom delivered were lower in predatory than defensive strikes (Young and Zahn 2001). Surprisingly, although it is difficult to observe without high-speed video recordings, vipers rapidly reposition fangs after contact with prey in more than one-third of their strikes (Cundall 2009). In a comparative study by Cundall and Deufel (2006), there were no significant differences in ingestion performance between colubrids and viperids despite major differences in cranial morphology, perhaps because the venom delivery system of viperids has been subject to little selection pressure for intraoral prey transport or because there are trade-offs between intraoral prey transport and strike performance in vipers. In many vipers and some elapids, venom has complex proteolytic and necrotizing effects, which may enhance the digestion of large prey (Thomas and Pough 1979; Nicholson et al. 2006), although such effects were not found in several recent studies (McCue 2007; Chu et al. 2009; LaBonte et al. 2011). The relationship between snake venoms and diets, and whether or not venom composition is subject to selection, has been difficult to resolve and may vary among species (e.g., Daltry et al. 1996; Sasa 1999a, b; Wüster et al. 1999; Barlow et al. 2009). The wide variation in venom composition, prey types and sizes, and temperature effects on enzyme and digestive function, all indicate the need for more research on the relationship between venom composition and diet, and the functional significance of venom (Mackessy 2010). Significant advances in our understanding of the evolution of the venom itself have come from several recent papers and books (e.g., Chippaux and Huchzermeyer 2006; Mackessy 2009; Fry et al. 2013; Fry 2015; Mackessy and Saviola 2016). Unexpectedly, it is also the venom (specifically the venom disintegrins) that provides the cues used by viperid snakes to relocate their prey after prey release (Saviola et al. 2013).
Head muscles of a Python regius, showing an unspecialized pattern of external adductor muscles; b the viperid, Vipera aspis; c the elapid, Elapsoidea sundevalli, and d the atractaspidid, Atractaspis dahomeyensis. The adductor externus muscles are shown in color: red, adductor externus superficialis and derived fibers; yellow, adductor externus medialis and derived fibers; blue, adductor externus profundus and derived fibers. Snakes not drawn to the same scale (reproduced from Jackson 2003)
Whereas the anatomy and function of the venom delivery system in viperid and elapid snakes have been relatively well described in the past (Kochva 1978; Underwood 1997; Jackson 2003), recent studies have shed additional light on the morphology of the venom gland (Duvernoy’s gland) in rear-fanged colubrids (de Oliveira et al. 2016). The role of Duvernoy’s gland in piscivorous species appears to be associated with incapacitating prey to facilitate prey handling and transport (Mori 1998; de Oliveira et al. 2016). Other gland types, including labial glands (de Oliveira et al. 2014, 2017) in goo-eating snakes (i.e., species eating earthworms, snails, and slugs) have also been described recently. In addition, a novel protein-secreting delivery system has been described in goo eaters (Zaher et al. 2014). The unusual part of this system is that it opens into the oral epithelium rather than being associated with the teeth (Zaher et al. 2014). Toxin glands in snakes are not restricted to oral glands, however, as some Asian snakes of the genus Rhabdophis have nuchal defensive glands that sequester the toxins from toad prey (Hutchinson et al. 2007, 2013). Moreover, these toxins can be passed on to the offspring from mothers containing high levels of toxins (Hutchinson et al. 2007). Our increased understanding of venoms and venom delivery systems now allows for integrative studies linking venom composition with anatomical traits such as head shape and fang length (Margres et al. 2015), showing that there is covariation between anatomical traits (fang length) and venom-related traits (i.e., myotoxin concentration; see Margres et al. 2015).
3.2.5 Prey Restraint and Subjugation
As noted above, many snakes remain in contact with the prey after striking and biting. Many nonvenomous and some venomous snakes use portions of the body to subjugate or kill prey prior to ingestion. Critical functions of prey handling after a strike or bite are to prevent the prey animal from escaping and to subdue it so that it can be ingested. Prey-restraint mechanisms include using jaws and teeth to damage prey mechanically, injecting venoms to incapacitate prey, and constricting to incapacitate prey (Cundall and Greene 2000). Five general methods of prey handling have been described in the literature: simple seizing, pinioning, applying a hairpin loop, constriction, and envenomation. Here, we briefly discuss several of these mechanisms, and particularly highlight recent work on venom delivery and constriction.
For many venomous snakes, envenomation is the exclusive prey-handling behavior and precludes the need for other behaviors. Other venomous snakes will remain anchored to the prey during envenomation, but may release it if they experience potentially dangerous struggling or defensive movements from the prey. The control and mechanics of venom injection are not well known. Some evidence indicates that vipers can control or meter the amount of venom injected, which has been referred to as the venom-metering hypothesis (reviewed by Hayes et al. 2002) and may be advantageous because venom production appears to be energetically expensive (McCue 2006a). However, the amount of venom injected could also be affected by the pressures in both the snake’s venom-injecting system and prey tissues, which has been called the pressure-balance hypothesis (Young et al. 2001a). Venom-metering and pressure-balance mechanisms are not entirely mutually exclusive, and both can be affected by the highly dynamic movements involved in predator–prey interactions during striking and biting. The extent to which metering and pressure-balance mechanisms determine the amount of venom injected in vipers and other venomous snakes remains poorly understood and in need of further study. We know much less about feeding mechanisms and essentially nothing about factors that control venom in elapids and venomous colubrids, although the basic physics of the pressure-balance mechanism would certainly apply. Elapid snakes, such as the coral snake Micrurus nigrocinctus, appear to hold onto prey after biting until paralysis occurs (Urdaneta et al. 2004), as do several other elapids (Radcliffe and Chiszar 1980; Kardong 1982; Greene 1984). If paralysis does not occur, such as after venom removal by manual milking, then the snakes change prey-handling behavior by moving their initial bites to the head of the prey and then holding onto the head to immobilize the prey by mechanical means (Urdaneta et al. 2004).
As we noted above, prey that are small or harmless are often captured with simple seizing and then quickly swallowed alive (Cundall and Greene 2000). Many alethinophidian snakes can also modulate their prey-handling behaviors in response to cues from prey (de Queiroz 1984; Cundall and Greene 2000; Bealor and Saviola 2007), using more complex prey-handling behaviors for more active and potentially more dangerous prey (de Queiroz 1984; Cundall and Greene 2000; Bealor and Saviola 2007). If prey struggle or are large, they may be thrashed from side to side while held in the jaws, which appears to subdue them effectively (Cundall and Greene 2000). Alternatively, prey may be further subjugated by pinioning, in which the snake presses the prey against the substrate or the wall of a tunnel (Hisaw and Gloyd 1926; Willard 1977; Greenwald 1978; de Queiroz 1984; Rudolph et al. 2002). Often, pinioning is used to restrain a prey item while it is being consumed (de Queiroz 1984). Pinioning behaviors are not well known, particularly as used in confined spaces that are difficult to observe and record, and need further study. At least two alethinophidian snakes are capable of biting pieces off or out of prey by using a combination of body and jaw movements (Jayne et al. 2002).
Many snakes apply loops of the body to prey and squeeze it in a constriction coil (e.g., Greene and Burghardt 1978). Constriction is a behavioral pattern that immobilizes prey with pressure exerted from two or more points along the body (Greene and Burghardt 1978). However, this definition includes both constriction and hairpin loops, which are considered to be distinct in complexity (Bealor and Saviola 2007; Mehta 2009; Penning and Cairns 2016). Constriction behavior evolved early in snakes and represents an ancient behavioral homology across many families of snakes (Greene 1994), although constriction behaviors vary among taxa in ways that we have only begun to characterize (e.g., Mehta and Burghardt 2008). Constriction behavior has been lost in several lineages of snakes, yet was retained or possibly evolved independently in diverse lineages of venomous snakes (Shine and Schwaner 1985). Constriction behavior serves both to restrain and kill prey (Cundall and Greene 2000). Constriction pressure is a good measure of prey-handling performance in constrictors because pressure is one of the key mechanisms that kills prey (Hardy 1994; Moon 2000a; Boback et al. 2015). Constriction pressure is generated by contractions of the axial muscles (Moon 2000a), which have variable scaling relationships among species (Jayne and Riley 2007; Herrel et al. 2011; Penning and Moon 2017).
Constriction performance increases with body size both among and within species (Moon and Mehta 2007; Penning et al. 2015; Penning and Dartez 2016; Penning 2017a), although these studies found different maximum pressures and scaling exponents that warrant further study (Figs. 14.7 and 14.8). Constriction performance is reduced due to lower muscle cross-sectional areas after fasting during pregnancy (Lourdais et al. 2004), but can be restored within weeks after feeding resumes (Lourdais et al. 2005). Much of the previous work on constriction performance focused on how snake morphology and body condition affect constriction performance. Recent work has also shown differences in constriction performance related to differences in prey characteristics and diet. Prey size does not affect the constriction performance of kingsnakes (Lampropeltis getula) feeding on prey of 5–15% relative prey mass, but prey size plays an important role during sequential encounters with prey (Penning 2017b). Multiple feeding events on large prey led to a reduction in constriction performance not seen in snakes feeding on multiple small prey. Kingsnakes also generate higher constriction pressures than their intraguild competitors (rat snakes, Pantherophis spp.; Penning and Moon 2017) and are capable of killing these other constrictors with constriction (Jackson et al. 2004). Currently, the only distinguishable difference between these snakes is their coil posture during constriction, which suggests that coil posture affects the pressure exertion (Penning and Moon 2017). Kingsnakes reliably use spring-like coils, whereas rat snakes use less regular and highly variable coil postures. Additional research on possible differences in muscle anatomy and function between kingsnakes and rat snakes is currently underway and may help explain the mechanisms of successful intraguild predation by kingsnakes on other constrictors.
Peak constriction pressures measured for snakes of different sizes (N = 12 species and 30 individuals). The line indicates a bivariate least-squares linear regression (y = 7.72xl.39, R2 = 0.88). Hollow circles indicate species of Acrantophis, Boa, Charina, Lichanura, and Sanzinia; diamonds indicate species of Morelia; solid circles indicate species of python; squares indicate species of Lampropeltis, Pantherophis, and Pituophis; the star indicates Tropidophis haetianus; and the triangle indicates the predicted pressure for a giant (30 cm in diameter) constrictor (reproduced from Moon and Mehta 2007)
Constricting pythons coil around and squeeze prey animals, which exerts pressure on the prey that scales positively with snake diameter. a A 1081 g juvenile Burmese python (Python molurus bivittatus) constricting a lab rat (Rattus norvegicus) weighing 99 g. b The scaling relationship between peak constriction pressure and snake diameter (reproduced from Penning et al. 2015)
Historically, constriction was thought to kill prey by suffocation, and although an alternative mechanism was proposed almost a century ago (McLees 1928), ventilatory disruption and suffocation remained the most invoked cause of death by constriction in the literature (e.g., Parker and Grandison 1977; Zug 1993; Cundall and Greene 2000). Hardy (1994) reassessed the observations of McLees (1928) that constriction can kill small mammals faster than suffocation alone, and supported McLees’s hypothesis of circulatory arrest as the primary mechanism of death by constriction. Moon (2000a) was the first to measure constriction pressures and showed that Pituophis melanoleucus and Lampropeltis getula could exert pressures well above the systolic blood pressures of mice, which meant that they were high enough to induce circulatory arrest in mice. Furthermore, Moon (2000a) determined that the snakes could detect prey movements as subtle as heartbeats and would respond with increased constriction pressures. Both of these findings were later corroborated by Boback et al. (2012, 2015). Constriction is usually used to kill endothermic prey, whereas restraining constriction may be more common with ectothermic prey, which is then eaten while still alive (Greene and Burghardt 1978; Cundall 1987; Hardy 1994; Cundall and Greene 2000; Boback et al. 2015). However, constriction involves a highly dynamic interaction between predator and prey, and several aspects of this interaction may affect the outcome. Snake and prey sizes are obvious variables that probably affect the mechanism and outcome of constriction (Mehta 2003; Moon and Mehta 2007). Constriction by very small snakes involves low pressures that may kill prey by suffocation (Moon and Mehta 2007). However, the constriction pressures exerted by diverse snakes are often high enough to make circulatory arrest the proximate mechanism of death in mammalian prey (Moon 2000a; Moon and Mehta 2007; Boback et al. 2015; Penning et al. 2015; Penning and Dartez 2016). Constriction may cause internal bleeding and high tissue pressures (Greene 1983; Penning and Dartez 2016), interfere with or damage neural tissue (Penning et al. 2015; Penning and Dartez 2016), and in large snakes perhaps damage the spine of a prey animal (Rivas 2004). The dynamic and variable movements of constrictors and their prey may affect the mechanism and outcome of constriction more than the predator–prey size relationship (Moon and Mehta 2007).
The assumption that constriction restrains rather than kills ectothermic prey appears to be based largely on inferences about ectotherm physiology. Ectothermic prey is thought to be more resistant to constriction than are endotherms (Hardy 1994; Boback et al. 2012, 2015) due to their lower oxygen demands and higher tolerance of hypoxia than endotherms (Pough 1980). Ectotherms are thought to fatigue quickly, but are otherwise eaten alive (Hardy 1994; Cundall and Greene 2000; Boback et al. 2015). When constriction kills ectothermic prey, it is assumed to take much longer than for endotherms (Zug 1993; Boback et al. 2015). However, the hypothesized effects of constriction on ectothermic prey have not been tested and remain speculative. Many questions remain about how constriction affects ectothermic prey. For example, how does the constrictor, an ectotherm, endure the pressures exerted by constriction longer than the prey animal, another ectotherm, if both have similar physiology and similar susceptibility to fatigue? Kingsnake constriction of other snakes has been observed to last over 7 h (Jackson et al. 2004), although we have observed constriction to kill lizards in minutes. Hence, we recommend both caution in perpetuating assumptions about how constriction affects ectothermic prey and more careful research on this issue in the future. The diverse factors that determine constriction performance and how it affects prey clearly need to be studied further.
3.3 Prey Transport
3.3.1 Intraoral Transport
Once a snake has captured a prey item (and subdued it in the species that do so), the snake begins ingestion movements that are collectively called prey-handling, prey-transport, or intraoral transport. Prey-transport mechanisms have been studied in diverse snake lineages, although in relatively few species. Most studies have addressed the morphology and kinematics of jaw function and cranial kinesis, whereas much less research has addressed the forces involved in prey transport and postcranial transport. However, recent studies have advanced our understanding of snake feeding structures and mechanisms in several important ways.
The first detailed functional studies of feeding in scolecophidian snakes (blind snakes or thread snakes) were those of Kley and Brainerd (1999) and Kley (2001). Within the scolecophidians, leptotyphlopids are characterized by having teeth only on the lower jaw and by having toothless upper jaw elements that are firmly attached to the rest of the head. Leptotyphlops dulcis uses bilateral mandibular raking of the highly kinetic lower jaw to ingest very small prey items such as the larvae and pupae of ants and termites (Kley and Brainerd 1999; Kley 2001). These raking movements appear to be powered directly by muscles and are relatively fast, occurring 2–3 times per second, and allow the snakes to ingest many small prey items quickly. As prey is ingested, the anterior part of the trunk is flexed slightly vertically and then straightened in movements that appear to augment ingestion, although axial bending appears not to be involved in postcranial swallowing (Kley and Brainerd 1999). In contrast to the leptotyphlopids, typhlopid snakes are characterized by having teeth only on the upper jaws, which are mobile, and toothless lower jaws that are relatively rigid and akinetic. Typhlops lineolatus and Rhinotyphlops schlegelii have similar cranial morphology and feeding mechanisms that involve rapid (3–5 Hz) bilateral maxillary raking movements to ingest the larvae and pupae of ants and termites (Kley 2001). The fast raking movements appear to be produced indirectly through movements of the pterygoid and palatine bones (Kley 2001); as in the leptotyphlopids, these fast movements allow the snakes to ingest many small prey items quickly.
Both mandibular and maxillary raking function as both prey capture and ingestion mechanisms. In contrast to the distinctive raking mechanisms in blind snakes, alethinophidian snakes all use alternating left and right ratcheting movements of jaws (Kley 2001). Most alethinophidian snakes have long, toothed medial upper jaws (palatopterygoid arches) and toothed mandibles. The tips of the mandibles are connected by a stretchable ligament that allows the left and right mandibles to separate widely during ingestion and to move fore and aft largely independently of one another. The posterior ends are connected by ligaments to the upper jaws, which tends to couple the movements of upper and lower jaws on the same side. Given the unique feeding mechanisms in scolecophidian snakes, Kley (2001) noted that unilateral feeding may have been ancestral for snakes and was subsequently lost in the scolecophidians, or that it may have evolved within the Alethinophidia, which he felt was the more parsimonious hypothesis. In Cylindrophis ruffus, which may represent an early branch of alethinophidian snakes, the palatomaxillary arches are attached fairly firmly to other bones in the snout (Cundall 1995). During intraoral transport, Cylindrophis ruffus uses first lateral movements of the posterior braincase and unilateral movements of the toothed jaws to move its head over prey in a mechanism called “snout-shifting,” and then bilateral movements of the jaws coupled with anterior vertebral bending. The mandibular tips separate only moderately (up to two times their resting separation), which limits their gape, but a mobile intramandibular joint allows the mandibles to conform to variable prey. Upper jaw mobility in Cylindrophis involves the ventral snout and does not result from reduced attachments to the braincase and snout. Cundall (1995) concluded that palatomaxillary kinesis in Cylindrophis is intermediate between the limited degree in lizards and the greater kinesis of advanced snakes, and that evolutionary changes in the snout were critical to diversification of the feeding apparatus in alethinophidian snakes. In some snakes, such as snail-eating snakes, the lower jaws have become more important to feeding than the upper jaws (Hoso et al. 2007; dos Santos et al. 2017).
In most alethinophidian snakes, particularly those with large gapes and substantial cranial kinesis, the upper jaws are more loosely connected to the snout and can protract and retract somewhat independently of the rest of the head. During ingestion, the upper and lower jaws protract together on one side and retract on the other side, with lesser movements of the snout and braincase; the protraction and retraction movements alternate on the left and right sides to ingest prey in a mechanism called the pterygoid walk (Boltt and Ewer 1964). The pterygoid walk appears to involve the snake’s head advancing forward over the prey, which often remains stationary relative to the ground (Dullemeijer 1956; Albright and Nelson 1959; Gans 1961; Frazzetta 1966). However, at least a few alethinophidian snakes, such as African mole vipers (Atractaspis spp.), have modified fangs and cranial morphology that make them unable to use pterygoid walking movements (Deufel and Cundall 2003); instead, these snakes ingest prey using cycles of mandibular adduction, anterior trunk compression, ventral flexion of the head, and ultimately extension of the head and anterior trunk over the prey. Few studies have recorded the cranial muscle-activation patterns associated with feeding (e.g., Cundall and Gans 1979; Cundall 1983), and most species remain to be studied. However, recent research has begun to quantify and compare ingestion performance based on the number of pterygoid walking movements (jaw protractions) or time required to ingest prey (e.g., Vincent and Mori 2008; Vincent et al. 2006b, 2009; Hampton 2011, 2014; Pereira et al. 2016).
With large prey items that are a substantial fraction of the snake’s weight, pulling the prey into the mouth probably requires forces higher than the relatively small jaw muscles can exert, whereas advancing the head over the prey may reduce the jaw forces needed and allow some of the axial muscles to contribute to ingestion. However, snakes sometimes lift the prey off the ground at some point before ingestion is complete; we have observed such movements in some boids and colubrids ingesting rodent prey, although how many kinds of snakes use such movements and with what sizes of prey are not yet well known. If the head and prey are tilted upward enough, then snakes may be using gravity to aid in ingestion; alternatively, these movements may indicate that the jaw muscles and bones (and anterior axial muscles associated with the head) exert forces high enough to pull prey into the mouth.
As noted above, an increased gape was important to the evolution of diverse snakes (Cundall and Greene 2000; Rieppel 1988). Surprisingly, despite its importance to the function and evolution of snakes, gape has usually been addressed qualitatively or with indirect quantitative indices. The term “gape” may refer to different aspects of the mouth opening, such as the maximum angle achieved at the jaw joint during mouth opening or maximum cross-sectional area that can be achieved at the posterior end of the oral cavity (Hampton and Moon 2013). Cross-sectional area of the posterior oral cavity and anterior esophagus appear to be critical to gape (Cundall et al. 2014; Fig. 14.9). Among the various skeletal elements that may contribute to gape area, the lengths of the lower jaw and suspensory elements, and the width of the head, have been hypothesized to be particularly important because they could affect the maximum size of the mouth opening (Cundall 1987; Cundall and Greene 2000; Hampton and Moon 2013; King 2002; Miller and Mushinsky 1990). Recently, Hampton and Moon (2013) quantified the maximum gape in western diamond-backed rattlesnakes (Crotalus atrox) as the largest cross-sectional area that could be achieved during forced swallowing in thawed specimens that had been frozen previously but not chemically preserved. They then tested which were the best predictors of gape area among several external and cranial skeletal measurements and two published gape indices from King (2002) and Miller and Mushinsky (1990). They found that body length was the best predictor of maximum gape and when body length was excluded from the analysis, quadrate and mandible lengths were the best predictors of maximum gape. Quadrate length probably contributes to gape area directly; however, the importance of mandible length to gape area was less clear and may relate to its covariation with head width (Vincent et al. 2006a). Two published gape indices did not prove to be better indicators of actual gape than the jaw and quadrate lengths, and the gape values they produced differed significantly from the empirically determined gapes. It would be beneficial to test these results against those from fresh specimens because the properties of soft tissues may be critical in determining maximum gape areas or angles (e.g., Close and Cundall 2014; Close et al. 2014; Cundall et al. 2014). Furthermore, additional research has indicated that the morphological predictors and scaling of gape differ among species and perhaps lineages (Hampton 2014; Hampton and Kalmus 2014).
Lateral diagrammatic view of the head and anterior trunk of a macrostomate snake to show how the floor of the mouth is arranged during ingestion of large prey. The net effect is that the mandibles surround the entry to the esophagus, not the oral cavity, while the palatomaxillary arches rachet the snake’s head over the prey. The tongue is normally not visible during transport, possibly because the tongue sheath is compressed beneath the prey (reproduced from Cundall et al. 2014)
Several aspects of prey items may also limit a snake’s ability to ingest them. For example, prey shape and type may affect ingestion performance (Pough and Groves 1983; Vincent et al. 2006a, b; Wilson and Hopkins 2011; Close and Cundall 2012) in addition to prey mass (Forsman and Lindell 1993). Moreover, prey shape can also affect locomotor performance once the prey has been ingested (Wilson and Hopkins 2011), suggesting that this aspect of diet can have significant fitness consequences. Comparative studies of gape and the effects of prey dimensions and properties on feeding performance could help clarify key functional shifts in the evolution and diversification of snakes.
Two species of snakes are known to circumvent gape limitation by biting pieces out of prey. The homalopsine snakes Gerarda prevostiana and Fordonia leucobalia bite pieces out of freshly molted crabs (Shine and Schwaner 1985; Jayne et al. 2002). This feeding mechanism appears to be unique among snakes, and allows these snakes to feed on prey that would be too large to consume whole. Jayne et al. (2002) argued that the highly kinetic jaws and needle-like teeth of snakes are poorly suited to generating large bite forces, which makes this feeding mechanism particularly surprising. However, as we noted above, it seems likely that some snakes can generate higher bite forces than we currently recognize.
In alethinophidian snakes, several elements associated with the oral cavity—particularly the jaw joint, tongue, hyoid elements, and glottis—are organized differently from those in other vertebrates (Cundall et al. 2014; Fig. 14.10). In particular, the tongue and hyoid elements have shifted ventrally and posteriorly relative to those of other vertebrates. The glottis demarcates the functional beginning of the esophagus in snakes (Cundall et al. 2014). Therefore, Cundall et al. (2014) concluded that mandibular depression shortens the effective length of the oral cavity when snakes swallow large prey, and the upper jaws ratchet prey “almost directly into the esophagus.” During ingestion, a snake’s oral cavity changes shape dynamically, though slowly, as parts of the prey with different shapes move through the oral cavity and anterior esophagus (Cundall et al. 2014). Early studies noted that these changes in shape involve movement of musculoskeletal elements as well as extension and recoil of soft tissues (e.g., Gans 1952, 1961, 1974; Gans and Oshima 1952). The esophagus and stomach must stretch laterally and in some cases longitudinally (e.g., Jackson et al. 2004). However, the functional properties of the soft tissues were quantified only recently. Among the most visible aspects of soft-tissue extension during feeding in snakes are stretching of tissues associated with the lower jaws. Close and Cundall (2014) recently found that in water snakes (Nerodia sipedon), intermandibular separation during ingestion reached over seven times its resting length, which is highly unusual among vertebrate tissues, and involved mainly stretching of the fibrous connective tissues between the scales of the lower jaw. In the epidermis, stretching flattened folds in the skin between the scales, and in the dermis stretching involved collagen realignment and extension of elastin deep in the dermis. Close and Cundall (2014) inferred from histological structure that dermal elastin contributed to passive recovery after stretching. As oral tissues stretch during ingestion of prey, several cranial muscles must not only accommodate the stretch, but maintain contractile function to complete the ingestion. Close et al. (2014) studied the changes in length and function of intermandibular muscles during feeding in snakes, and found that they become highly stretched, with sarcomere lengths more than doubling relative to their resting lengths, yet recover normal function. Such long extension pulls actin and myosin filaments apart beyond overlap, misaligns striations, and distorts the sarcoplasmic reticulum and triad positions (Close et al. 2014). Initial recovery must be passive, because sarcomeres get stretched beyond filament overlap, and probably involves the elastic protein titin; subsequent recovery involves active or passive shortening beyond resting lengths, to a point involving overlap of opposing actin filaments within a sarcomere, eventually followed by a return to resting length (Close et al. 2014). Close et al. (2014) found no apparent damage to sarcomeres during such stretching. The whole muscle stretched even more than the sarcomeres because of stretching in associated connective tissues and slippage of muscle fibers (Close et al. 2014). These recent studies of soft-tissue properties provide a foundation for additional research that could resolve structural and functional changes associated with the evolution and diversification of snakes, particularly the changes associated with the evolution of enlarged gapes. They have also shown that snakes are excellent model organisms for studying mechanisms of extreme tissue deformation without injury.
Organization of the floor of the anterior gut in a representative amniote tetrapod (a) and in a snake (b). Although considerable liberty has been taken with anatomy, in almost all tetrapods except snakes, the trunk-cervical boundary is marked by pectoral elements and skeletal connections (ribs and costal cartilages) between the dorsal and ventral axial components (vertebrae [not shown but in line with the braincase] and sternum). The cranial-cervical boundary is typically marked by the jaw joint, base of the tongue and hyoid, and the glottis. Yellow indicates presumed distribution of epithelial cells of endodermal origin lining the floor of the gut. Abbreviations: jj, jaw joint, and hence location of posterior end of mandible; md, anterior end of mandible; pg, pectoral girdle (reproduced from Cundall et al. 2014)
3.3.2 Postcranial Transport
Once ingested, a meal needs to be swallowed, i.e., transported from the anterior esophagus to the stomach. Swallowing has not yet been studied well in any snakes (Cundall and Greene 2000). Small prey may be transported exclusively by esophageal peristalsis, although peristalsis in snakes remains poorly known. However, postcranial axial bending movements produced by skeletal muscles appear to be important in helping push larger food items from the anterior esophagus along a substantial length of the body to the stomach (Kley and Brainerd 2002; Moon 2000b; Fig. 14.11). Thus, swallowing looks somewhat like a kind of inside-out locomotion, with some of the same epaxial muscles involved in locomotion contributing to internal force exertion for swallowing (Moon 2000b). In the colubrid snakes, Pituophis melanoleucus and Lampropeltis getula, axial bending patterns used during swallowing appear to vary from undulatory to accordion-like concertina bends, and the associated epaxial muscle activity appears more variable than during steady locomotion (Moon 2000b). Kley and Brainerd (2002) used X-ray videography to study axial bending movements in detail during postcranial swallowing in P. melanoleucus, and found that three out of four distinct phases of postcranial prey transport involved undulatory or concertina-like movements of the anterior trunk. Kley and Brainerd (2002) characterized four phases of postcranial swallowing: an oral phase in which pterygoid walking movements of the jaws are used to advance the head forward over the prey, an orocervical phase in which unilateral jaw movements continue and become aided by concertina-like axial movements, a cervical phase that involves exclusively concertina-like movements of the neck, and a thoracic phase in which prey is transported to the stomach using axial undulations. Broadly similar postcranial transport mechanisms appear to be involved in diverse snakes that feed on relatively large prey (Kley and Brainerd 2002). Notable variation occurs in postcranial transport mechanisms with different prey types, particularly elongate prey, for which postcranial transport begins well before most of the prey has passed beyond the jaws (Jackson et al. 2004; Kley and Brainerd 2002).
Four-phase model of prey transport in alethinophidian snakes. The prey is represented by the black oval. During the oral phase, prey is transported into the mouth exclusively by unilateral, ratchet-like movements of the jaws. During the orocervical phase, these jaw movements are augmented by concertina-like movements of the anterior portion of the trunk. In this phase of transport, cervical extension is synchronized with jaw protraction so that extension of the neck functions to protract and rotate the braincase relative to the fixed jaw. During the brief cervical phase, the jaws are no longer in contact with the prey, and the snake advances over the prey in a concertina-like fashion. Finally, during the thoracic phase, the prey is propelled through the esophagus and into the stomach by a continuous, caudally directed wave of lateral undulation. Note that throughout all phases of transport, the prey remains in a relatively constant position as the snake advances over it (reproduced from Kley and Brainerd 2002)
3.4 Retention and Egestion
Few studies have addressed the mechanical consequences of waste retention in snakes. However, Lillywhite et al. (2002) found that gut passage times in snakes were highly variable and included some of the longest times of any vertebrates. For example, slender arboreal snakes may defecate as soon as 24–48 h after ingesting a meal, whereas stout and heavy terrestrial snakes may retain feces in the digestive tract for periods of months to a year or more. The retained mass can accumulate up to 20% of the body mass in some species, such as the viper Bitis gabonica (Lillywhite et al. 2002). The long retention of digested material in snakes may function adaptively as metabolically inert ballast, which may even enhance water and nutrient uptake. Lillywhite et al. (2002) argued that this “adaptive ballast” could increase the static friction and inertia of the posterior body and thus reduce rearward slippage of the body during vigorous strikes, or possibly anchor a large snake on open ground during biting or constriction. The adaptive-ballast hypothesis could be tested experimentally using weights attached to the posterior body of a snake while it strikes, bites, or constricts large or vigorous prey.
3.5 Feeding Energetics
Animal biomechanics and energetics are intimately related because force production requires energy. Resting metabolic rates have been measured in snakes from several families, but we know much less about the energetics of feeding movements, such as striking, constriction, ingestion, and swallowing. Here, we would like to highlight some important work on the energetics of feeding movements in snakes and briefly discuss their implications for the biomechanics of feeding.
3.5.1 Prey Capture
The energetics of biting and striking in snakes has not yet been studied. It would be challenging to use standard physiological techniques such as respirometry to quantify the metabolic energy of biting and striking because the movements are so rapid and brief. However, the kinetic energy and forces of striking could be quantified by modeling or inverse-dynamic approaches that derive kinetic energy or forces from the velocities or accelerations and masses of the head and anterior trunk during striking. Similarly, the contributions of elastic mechanisms and power amplification to strike performance and especially energetics are essentially unknown in snakes. Elastic recoil can partially decouple muscle contraction and movement by allowing muscles to contract over more optimal durations and lengths, briefly store the energy in elastic tissues such as tendons, and then release the energy abruptly to produce rapid striking movements. Such mechanisms could provide substantial temperature independence and perhaps energy savings in striking despite the rates of muscular shortening and force generation being moderately to strongly temperature dependent. Young (2010) reported that axial extensor muscles (specifically, the Mm. semispinalis and longissimus dorsi) were activated entirely before strike movements began in puff adders (Bitis arietans), indicating that elastic recoil contributes to strike performance in those robust snakes. Power amplification may be necessary for massive snakes to strike with high accelerations (Young 2010). Given the diversity of body forms and sizes among snakes and how few species have been studied thus far, we feel that the issue of elastic recoil and power amplification in snake strikes needs further study. Elastic mechanisms may be most apparent in large-bodied snakes and those that forage at low temperatures, and less beneficial in small, slender, and thermophilic snakes. Therefore, we may expect to find variation in the use and benefits of elastic mechanisms among species. Furthermore, quantifying the energetic cost of striking would help resolve the costs of different stages of feeding and determine the total cost of feeding, from prey capture through digestion.
3.5.2 Prey Handling
In one of the only publications to date on the energetic cost of constriction, Canjani et al. (2003) measured oxygen consumption during constriction, prey handling, and ingestion in boa constrictors feeding on rats that were 5–40% of the snake’s mass; this work was also cited by Cruz-Neto et al. (2001) as “unpublished data” at that time. During constriction, oxygen consumption in the boa constrictors increased nearly sevenfold higher than resting values for 8–16 min of constriction, although surprisingly the increase in metabolic rate was not significantly related to prey size. Canjani et al. (2003) also determined the energetic cost of prey handling and ingestion subsequent to constriction, and found oxygen consumption during 4–20 min of ingestion to be nearly fivefold higher than resting levels. They defined ingestion as including the period “when the snake first positioned its open jaws on the prey” to “when the prey had just disappeared into the mouth of the snake and the tongue was first protruded.” The large increases in oxygen consumption during constriction and ingestion are similar to the increases associated with other demanding activities such as locomotion (e.g., Walton et al. 1990; Secor et al. 1992).
In an early study of predation energetics in snakes, Feder and Arnold (1982) found that garter snakes (Thamnophis elegans) experienced 260% increases in whole-body lactate concentrations after 14 min of attacking and feeding on salamander prey (representing 14% of the snake’s mass on average) compared to resting levels before predation. These results reflect the entire sequence of prey capture, handling, ingestion, and swallowing, and suggest that the locomotor and feeding movements are mechanically and energetically quite demanding. However, the estimated total cost of predatory activity was less than 1% of the energy likely assimilated from the prey, making such vigorous predation energetically advantageous (Feder and Arnold 1982). The post-predation lactate concentration was only 58% of the concentration reached after 2 min of forced locomotor activity that fatigued the snakes, and thus the predation event may have fatigued the snakes to a limited degree. Similarly, the energetic demand of ingesting large prey in the rattlesnake Crotalus durissus can also approach the factorial aerobic scope for exercise and probably involves anaerobic metabolism, although the cost of ingestion was a very small percentage (0.02%) of the energy available in the prey (Cruz-Neto et al. 1999). The potential for fatigue after feeding suggests that muscular, and hence mechanical, performance may be reduced in second or later feeding bouts that follow soon after previous ones. This hypothesis remains to be tested, but suggests potentially interesting future research.
3.5.3 Digestion and Correlated Responses in Cardiovascular System
There is extensive literature on the energetics of digestion in snakes, particularly focusing on specific dynamic action. Excellent reviews of this work are available (e.g., McCue 2006b; Secor 2009), and we do not wish to repeat them here, except to note that intestinal upregulation may have biomechanical consequences for gut motility. Upregulation of the digestive tract after feeding in pythons has been studied extensively in recent years, and led to the remarkable finding that cardiac ventricular mass can increase 40% in pythons within 48 h of ingesting large meals (25% of the snake’s mass) after a period of fasting (Andersen et al. 2005; Secor and Diamond 1995), although such growth has not occurred in some studies (e.g., Jensen et al. 2011; Secor et al. 2012; Hansen et al. 2013). Stroke volume also increases to 50% higher levels in postprandial pythons than in faster individuals during maximal exercise, and systemic blood flow increases nearly fivefold after feeding (Secor et al. 2000), perhaps due in part to ventricular enlargement and greater cardiac filling (Enok et al. 2016). Cardiac enlargement, when it occurs, and possible changes in blood viscosity after nutrient absorption have important consequences for cardiac and circulatory mechanics (e.g., Secor and White 2010), and are likely to provide exciting avenues of future research.
3.6 Drinking
In addition to feeding, drinking is a key function of the oral system in snakes and many other animals. The mechanism of drinking in snakes has been challenging to resolve, and still needs further study. Early studies based on kinematic, radiographic, electromyographic, and pressure recordings indicated a two-stage buccal-pump mechanism of drinking in snakes (Bels and Kardong 1995; Berkhoudt et al. 1995; Kardong and Haverly 1993). Cundall (2000) questioned some elements of the buccal-pump mechanism, and later Cundall et al. (2012) determined that snakes use a sponge mechanism to drink, in addition to or perhaps instead of the buccal-pump mechanism. The sponge mechanism involves oropharyngeal and esophageal soft tissues, along with certain cranial muscles that compress the sponge mechanism to aid in swallowing. Using this mechanism, snakes can slowly take up water against the effect of gravity. Cundall et al. (2012) hypothesized that this sponge mechanism may have evolved from tissue properties associated with feeding (e.g., extensive mucosal folding in the oral cavity that allows expansion during ingestion) in macrostomate snakes. Some of these studies used different species to resolve the drinking mechanisms of snakes, and Cundall et al. (2012) noted that the particular drinking mechanism used depends on oral tissue morphology, properties, and movements, which may vary among species and body sizes. Hence, future research on drinking mechanisms in more species of snakes is likely to be productive.
4 Perspectives
Recent years have seen exciting advances in both techniques and results for the feeding biology of snakes. The increasing availability of detailed, noninvasive imaging methods allows researchers to better quantify the morphology of both external and internal structures without destructive sampling. These methods are becoming widely used on diverse live and fossil animals, including snakes, and nicely complement traditional research methods in morphology. Recent research has shown that snake strikes, bites, constriction, envenomation, prey transport, and digestion all involve mechanisms and performance that affect feeding success and have fitness consequences. The great diversity of snakes calls for many more studies of feeding biology, which are likely to lead to the discovery of new mechanisms, as recent research has shown. In addition, by further integrating robust phylogenies, detailed morphological data, functional mechanisms, and ecologically relevant performance measures in future research, we will surely gain important new insights into how feeding, locomotor, and other mechanisms may have driven evolution and diversification of snakes.
References
Albright RG, Nelson EM (1959) Cranial kinesis of the generalised colubrid snake Elaphe obsoleta quadrivittata. II. Functional morphology. J Morphol 105:241–291
Alfaro ME (2002) Forward attack modes of aquatic feeding garter snakes. Funct Ecol 16:204–215
Alfaro ME (2003) Sweeping and striking: a kinematic study of the trunk during prey capture in three thamnophiine snakes. J Exp Biol 206:2381–2392
Andersen JB, Rourke BC, Caiozzo VJ, Bennett AF, Hicks JW (2005) Postprandial cardiac hypertrophy in pythons. Nature 434:37–38
Anderson CV, Deban SM (2010) Ballistic tongue projection in chameleons maintains high performance at low temperature. Proc Natl Acad Sci USA 107:5495–5499
Anderson PSL, LaCosse J, Pankow M (2016) Point of impact: the effect of size and speed on puncture mechanics. Interface Focus 6:20150111
Andjelkovic M, Blagojevic V, Tomovic L, Ivanovic A (2016a) Ontogeny of pileus shape in Natrix natrix and N. tesselata. Herpetol J 26:3–9
Andjelkovic M, Tomovic L, Ivanovic A (2016b) Variation in skull size and shape of two snake species (Natrix natrix and Natrix tesselata). Zoomorphology 135:243–253
Arnold SJ (1993) Foraging theory and prey-size–predator-size relations in snakes. In: Seigel RA, Collins JT (eds) Snakes: ecology and behavior. McGraw Hill, New York, pp 87–116
Aubret F, Shine R (2009) Genetic assimilation and the postcolonization erosion of phenotypic plasticity in island tiger snakes. Curr Biol 19:1932–1936
Aubret F, Shine R, Bonnet X (2004) Adaptive developmental plasticity in snakes. Nature 431:261–262
Bakken GS, Krochmal AR (2007) The imaging properties and sensitivity of the facial pits of pitvipers as determined by optical and heat-transfer analysis. J Exp Biol 210:2801–2810
Barlow A, Pook CE, Harrison RA, Wüster W (2009) Coevolution of diet and prey-specific venom activity supports the role of selection in snake venom evolution. Proc R Soc Lond B 276:2443–2449
Bealor MT, Saviola AJ (2007) Behavioural complexity and prey-handling ability in snakes: gauging the benefits of constriction. Behaviour 144:907–929
Beaupre SJ, Montgomery CE (2007) The meaning and consequences of foraging mode in snakes. In: Reilly SM, McBrayer LD, Miles DB (eds) Lizard ecology: the evolutionary consequences of foraging mode. Cambridge University Press, Cambridge, pp 334–367
Bels V, Kardong KV (1995) Water drinking in snakes: evidence for an esophageal sphincter. J Exp Zool 272:235–239
Berkhoudt H, Kardong KV, Zweers G (1995) Mechanics of drinking in the brown tree snake, Boiga irregularis. Zoology 98:98–103
Bilcke J, Herrel A, Aerts P (2006) Effect of prey- and predator size on the capture success of an aquatic snake. Belg J Zool 137:191–195
Boback SM, Hall AE, McCann KJ, Hayes AW, Forrester JS, Zwemer CF (2012) Snake modulates constriction in response to prey’s heartbeat. Biol Lett 8:473–476
Boback SM, McCann KJ, Wood KA, McNeal PM, Blankenship EL, Zwemer CF (2015) Snake constriction rapidly induces circulatory arrest in rats. J Exp Biol 218:2279–2288
Boltt RE, Ewer RF (1964) The functional anatomy of the head of the puff adder, Bitis arietans (Merr.). J Morphol 114:83–106
Bonnet X, Shine R, Naulleau G, Thiburce C (2001) Plastic vipers: influence of food intake on the size and shape of Gaboon vipers (Bitis gabonica). J Zool 255:341–351
Borczyk B (2015) Allometry of head size and shape dimorphism in the grass snake (Natrix natrix L.). Turk J Zool 39:340–343
Brecko J, Vervust B, Herrel A, Van Damme R (2011) Head morphology and diet in the dice snake (Natrix tessellata). Mertensiella 18:20–29
Britt EJ, Clark AJ, Bennett AF (2009) Dental morphologies in gartersnakes (Thamnophis) and their connection to dietary preferences. J Herpetol 43:252–259
Cadena V, Andrade DV, Bovo RP, Tattersall G (2013) Evaporative respiratory cooling augments pit organ thermal detection in rattlesnakes. J Comp Physiol A 199:1093–1104
Canjani C, Andrade DV, Cruz-Neto AP, Abe AS (2003) Aerobic metabolism during predation by a boid snake. Comp Biochem Physiol A 133:487–498
Catania KC (2009) Tentacled snakes turn C-starts to their advantage and predict future prey behavior. Proc Natl Acad Sci USA 106:11183–11187
Catania KC (2010) Born knowing: tentacle snakes innately predict future prey behavior. PLoS ONE 5:e10953–e10953
Catania KC, Leitch DB, Gauthier D (2010) Function of the appendages in tentacled snakes (Erpeton tentaculatus). J Exp Biol 213:359–367
Chippaux J-P, Huchzermeyer FW (2006) Snake venoms and envenomations. Krieger Publ Co, Malabar
Chu CW, Tsai TS, Tsai IH, Lin YS, Tu MC (2009) Prey envenomation does not improve digestive performance in Taiwanese pit vipers (Trimeresurus gracilis and T. stejnegeri stejnegeri). Comp Biochem Physiol A 152:579–585
Clark RW, Tangco S, Barbour MA (2012) Field video recordings reveal factors influencing predatory strike success of free-ranging rattlesnakes (Crotalus spp.). Anim Behav 84:183–190
Close M, Cundall D (2012) Mammals as prey: estimating ingestible size. J Morphol 273:1042–1049
Close M, Cundall D (2014) Snake lower jaw skin: extension and recovery of a hyperextensible keratinized integument. J Exp Zool 321A:78–97
Close M, Perni S, Franzini-Armstrong C, Cundall D (2014) Highly extensible skeletal muscle in snakes. J Exp Biol 217:2445–2448
Conrad JL (2008) Phylogeny and systematics of Squamata (Reptilia) based on morphology. Bull Am Mus Nat Hist 310:1–182
Crowe-Riddell JM, Snelling EP, Watson AP, Suh AK, Partridge JC, Sanders KL (2016) The evolution of scale sensilla in the transition from land to sea in elapid snakes. Open Biol 6:160054
Cruz-Neto AP, Andrade DV, Abe AS (1999) Energetic cost of predation: aerobic metabolism during prey ingestion by juvenile rattlesnakes, Crotalus durissus. J Herpetol 33:229–234
Cruz-Neto AP, Andrade DV, Abe AS, (2001) Energetic and physiological correlates of prey handling and ingestion in lizards and snakes. Comp Biochem Physiol A 128:515–533
Cundall D (1983) Activity of head muscles during feeding by snakes: a comparative study. Am Zool 23:383–396
Cundall D (1987) Functional morphology. In: Seigel RA, Collins JT, Novak SS (eds) Snakes: ecology and evolutionary biology. Macmillan, New York, pp 106–140
Cundall D (1995) Feeding behaviour in Cylindrophis and its bearing on the evolution of alethinophidian snakes. J Zool 237:353–376
Cundall D (2000) Drinking in snakes: kinematic cycling and water transport. J Exp Biol 203:2171–2185
Cundall D (2009) Viper fangs: functional limitations of extreme teeth? Physiol Biochem Zool 82:63–79
Cundall D, Beaupre SJ (2001) Field records of predatory strike kinematics in timber rattlesnakes, Crotalus horridus. Amphibia-Reptilia 22:492–498
Cundall D, Deufel A (1999) Striking patterns in booid snakes. Copeia 1999:868–883
Cundall D, Deufel A (2006) Influences of the venom delivery system on intraoral prey transport in snakes. Zool Anz 245:193–210
Cundall D, Gans C (1979) Feeding in water snakes: an electromyographic study. J Exp Zool 209:189–208
Cundall D, Greene HW (2000) Feeding in snakes. In: Schwenk K (ed) Feeding: form, function and evolution in tetrapod vertebrates. Academic Press, London, pp 293–333
Cundall D, Irish F (2008) The snake skull. In: Gans C, Gaunt AS, Adler K (eds) Biology of the reptilia, vol 20. Morphology H. The Skull of lepidosauria. Society for the Study of Amphibans and Reptiles, pp 349–692
Cundall D, Deufel A, Irish F (2007) Feeding in boas and pythons: motor recruitment patterns during striking. In: Henderson RW, Powell R (eds) Biology of the boas and pythons. Eagle Mountain Publishing, Utah, pp 169–197
Cundall D, Brainerd EL, Constantino J, Deufel A, Grapski D, Kley NJ (2012) Drinking in snakes: resolving a biomechanical puzzle. J Exp Zool 317:152–172
Cundall D, Tuttman C, Close M (2014) A model of the anterior esophagus in snakes, with functional and developmental implications. Anat Rec 297:586–598
Da Silva FO, Fabre A-C, Savriama Y, Ollonen J, Mahlow K, Herrel A, Müller J, Di-Poï N (2018) The ecological origins of snakes as revealed by skull evolution. Nat Commun 9:376. https://doi.org/10.1038/s41467-017-02788-3
Daghfous G, Smargiassi M, Libourel P-A, Wattiez R, Bels V (2012) The function of oscillatory tongue-flicks in snakes: insights from kinematics of tongue flicking in the banded water snake (Nerodia fasciata). Chem Senses 37:883–896
Daltry JC, Wüster W, Thorpe RS (1996) Diet and snake venom evolution. Nature 379:537–540
Deban SM, Lappin AK (2011) Thermal effects on the dynamics and motor control of ballistic prey capture in toads: maintaining high performance at low temperature. J Exp Biol 214:1333–1346
Deban SM, Richardson JC (2011) Cold-blooded snipers: thermal independence of ballistic tongue projection in the salamander Hydromantes platycephalus. J Exp Zool 315A:618–630
Deban SM, Scales JA (2016) Dynamics and thermal sensitivity of ballistic and non-ballistic feeding in salamanders. J Exp Biol 291:431–444
de Groot JH, van der Sluijs I, Snelderwaard PC, van Leeuwen J (2004) A three-dimensional analysis of tongue flicking in Python molurus. J Exp Biol 207:827–839
de Oliveira L, Prudente ALC, Zaher H (2014) Unusual labial glands in snakes of the genus Geophis Wagler, 1830 (Serpentes: Dipsadinae). J Morphol 275:87–99
de Oliveira L, Scartozzoni RR, de Almeida-Santos SM, Jared C, Antoniazzi MM, da Graça Salomão M (2016) Morphology of Duvernoy’s glands and maxillary teeth and a possible function of the Duvernoy’s gland secretion in Helicops modestus Günther, 1861 (Serpentes: Xenodontinae). S Amer J Herpetol 11:54–65
de Oliveira L, Guerra-Fuentesa RA, Zaher H (2017) Embryological evidence of a new type of seromucous labial gland in neotropical snail-eating snakes of the genus Sibynomorphus. Zool Anz 266:89–94
de Queiroz A (1984) Effects of prey type on the prey-handling behavior of the bullsnake, Pituophis melanoleucus. J Herpetol 18:333–336
Dell’Aglio DD, Toma TSP, Muelbert AE, Sacco AG, Tozetti AM (2012) Head triangulation as anti-predatory mechanism in snakes. Biota Neotrop 12:1912032012
Deufel A, Cundall D (1999) Do booids stab prey? Copeia 1999:1102–1107
Deufel A, Cundall D (2003) Feeding in Atractaspis (Serpentes: Atractaspididae): a study in conflicting functional constraints. Zoology 106:43–61
dos Santos MM, da Silva FM, Hingst-Zaher E, Machado FA, Zaher HE, Prudente ALC (2017) Cranial adaptations for feeding on snails in species of Sibynomorphus (Dipsadidae: Dipsadinae). Zoology 120:24–30
Dullemeijer P (1956) A comparative functional-anatomical study of the heads of some Viperidae. Morph Jb 99:881–985
Dwyer CM, Kaiser H (1997) Relationships between skull form and prey selection in the thamnophiine snake genera Nerodia and Regina. J Herpetol 31:463–475
Ebert J, Westhoff G (2006) Behavioural examination of the infrared sensitivity of rattlesnakes (Crotalus atrox). Comp Physiol A 192:941–947
Ebert J, Müller S, Westhoff G (2007) Behavioural examination of the infrared sensitivity of ball pythons. J Zool 272:340–347
Enok S, Leite GSPC, Leite CAC, Gesser H, Hedrick MS, Wang T (2016) Improved cardiac filling facilitates the postprandial elevation of stroke volume in Python regius. J Exp Biol 219:3009–3018
Esquerré D, Keogh JS (2016) Parallel selective pressures drive convergent diversification of phenotypes in pythons and boas. Ecol Lett 19:800–809
Ernst CH, Ernst EM (2003) Snakes of the United States and Canada. Smithsonian Institution, Washington, DC
Fabre A-C, Bickford D, Segall M, Herrel A (2016) The impact of diet, habitat use and behaviour on head shape evolution in homalopsid snakes. Biol J Linn Soc 118:634–647
Feder ME, Arnold SJ (1982) Anaerobic metabolism and behavior during predatory encounters between snakes (Thamnophis elegans) and salamanders (Plethodon jordani). Oecologia 53:93–97
Figueroa A, McKelvy AD, Grismer LL, Bell CD, Lailvaux SP (2016) A species-level phylogeny of extant snakes with description of a new colubrid subfamily and genus. PLoS ONE 11:e0161070
Filoramo NI, Schwenk K (2009) The mechanism of chemical delivery to the vomeronasal organs in squamate reptiles: a comparative morphological approach. J Exp Zool 311A:20–34
Forsman A (1996) An experimental test for food effects on head size allometry in juvenile snakes. Evolution 50:2536–2542
Forsman A, Lindell LE (1993) The advantage of a big head: swallowing performance in adders, Vipera berus. Funct Ecol 7:183–189
Forsman A, Shine R (1997) Rejection of non-adaptive hypotheses for intraspecific variation in trophic morphology in gape-limited predators. Biol J Linn Soc 62:209–223
Frazzetta TH (1966) Studies on the morphology and function of the skull in the Boidae (Serpentes). Part II. Morphology and function of the jaw apparatus in Python sebae and Python molurus. J Morph 118:217–295
Frazzetta TH (1999) Adaptations and significance of the cranial feeding apparatus of the sunbeam snake (Xenopeltis unicolor): Part I. Anatomy of the skull. J Morphol 239:27–43
Friedel P, Young BA, van Hemmen JL (2008) Auditory localization of ground-borne vibrations in snakes. Phys Rev Lett 100:048701-1–048701-4
Fry B (ed) (2015) Venomous reptiles and their toxins: evolution, pathophysiology and biodiscovery. Oxford University Press, Oxford
Fry BG, Undheim EAB, Ali SA, Jackson TNW, Debono J, Scheib H, Ruder T, Morgenstern D, Cadwallader L, Whitehead D, Nabuurs R, van der Weerd L, Vidal N, Roelants K, Hendrikx I, Gonzalez SP, Koludarov I, Jones A, King GF, Antunes A, Sunagar K (2013) Squeezers and leaf-cutters: differential diversification and degeneration of the venom system in Toxicoferan reptiles. Moll Cell Proteom 12:1881–1899
Gans C (1952) The functional morphology of the egg-eating adaptations in the snake genus Dasypeltis. Zoologica 37:209–244
Gans C (1961) The feeding mechanism of snakes and its possible evolution. Am Zool 1:217–227
Gans C (1974) Biomechanics: an approach to vertebrate biology. The University of Michigan Press, Ann Arbor
Gans C, Oshima M (1952) Adaptations for egg eating in the snake Elaphe climacophora (Boie). Amer Mus Novitates 1571:1–16
Gartner GEA, Greene HW (2008) Adaptation in the African egg-eating snake: a comparative approach to a classic study in evolutionary functional morphology. J Zool 275:368–374
Gauthier JA, Kearney M, Maisano JA, Rieppel O, Belhke ADB (2012) Assembling the squamate tree of life: perspectives from the phenotype and the fossil record. Bull Peabody Mus Nat Hist 53:3–308
Gibbons W, Dorcas M (2015) Snakes of the Southeast, 2nd edn. University of Georgia Press, Athens
Gracheva EO, Ingolia NT, Kelly YM, Cordero-Morales JF, Hollopeter G, Chesler AT, Sánchez EE, Perez JC, Weissman JS, Julius D (2010) Molecular basis of infrared detection by snakes. Nature 464:1006–1011
Greene HW (1983) Dietary correlates of the origin and radiation of snakes. Am Zool 201:315–329
Greene HW (1984) Feeding behavior and diet of the eastern coral snake, Micrurus fulvius. In: Seigel RA, Hunt LE, Knight JL, Malaret L, Zuschlag NL (eds) Vertebrate ecology and systematics—a tribute to Henry S. Fitch. Museum of natural history. The University of Kansas, Lawrence, pp 147–161
Greene HW (1994) Homology and behavioral repertoires. In: Hall BK (ed) Homology: the hierarchical basis of comparative biology. Academic Press, San Diego, pp 369–391
Greene HW (1997) Snakes: the evolution of mystery in nature. University of California Press, Berkeley
Greene HW, Burghardt GM (1978) Behavior and phylogeny: constriction in ancient and modern snakes. Science 200:74–77
Greenwald OE (1974) Thermal dependence of striking and prey capture by gopher snakes. Copeia 1974:141–148
Greenwald OE (1978) Kinematics and time relations of prey capture by gopher snakes. Copeia 1978:263–268
Grudzien TA, Huebner BJ, Cvetkovic A, Joswiak GR (1992) Multivariate analysis of head shape in Thamnophis s. sirtalis (Serpentes: Colubridae) among island and mainland populations from northeastern Lake Michigan. Am Midl Nat 127:339–347
Haas G (1973) Muscles of the jaws and associated structures in the Rhynchocephalia and Squamata. In: Gans C, Parsons TS (eds) Biology of the reptilia, vol 4. Morphology D. Academic Press, London, pp 285–490
Hampton PM (2011) Comparison of cranial form and function in association with diet in natricine snakes. J Morphol 272:1435–1443
Hampton PM (2014) Allometry of skull morphology, gape size and ingestion performance in the banded watersnake (Nerodia fasciata) feeding on two types of prey. J Exp Biol 217:472–478
Hampton P, Kalmus T (2014) The allometry of cranial morphology and gape size in red-bellied mudsnakes (Farancia abacura). Herpetologica 70:290–297
Hampton PM, Moon B (2013) Gape size, its morphological basis, and the validity of gape indices in western diamond-backed rattlesnakes (Crotalus atrox). J Morphol 274:194–202
Hansen K, Pedersen PBM, Pedersen M, Wang T (2013) Magnetic resonance imaging volumetry for noninvasive measures of phenotypic flexibility during digestion in Burmese pythons. Physiol Biochem Zool 86:149–158
Hardy DL (1994) A re-evaluation of suffocation as the cause of death during constriction by snakes. Herpetol Rev 25:45–47
Hart NS, Coimbra JP, Collin SP, Westhoff G (2012) Photoreceptor types, visual pigments, and topographic specializations in the retinas of Hydrophiid sea snakes. J Comp Neurol 520:1246–1261
Hayes WK, Herbert SS, Rehling GC, Gennaro JF (2002) Factors that influence venom expenditure in viperids and other snake species during predatory and defensive contexts. In: Schuett GW, Hoggren M, Douglas ME, Greene HW (eds) Biology of the vipers. Eagle Mountain Publishing, Utah, pp 267–278
Henao-Duque AM, Ceballos CP (2013) Sex-related head size and shape dimorphism in Mapaná snakes (Bothrops asper) kept in captivity. Revista Colombiana de Ciencias Pecuarias 26:201–210
Henderson RW, Pauers MJ, Colston TJ (2013) On the congruence of morphology, trophic ecology, and phylogeny in Neotropical treeboas (Squamata: Boidae: Corallus). Biol J Linn Soc 109:466–475
Herrel A, Vincent SE, Alfaro ME, Van Wassenbergh S, Vanhooydonck B, Irschick DJ (2008) Morphological convergence as a consequence of extreme functional demands: examples from the feeding system of natricine snakes. J Evol Biol 21:1438–1448
Herrel A, Huyghe K, Okovic P, Lisicic D, Tadic Z (2011) Fast and furious: effects of body size on strike performance in an arboreal viper Trimeresurus (Cryptelytrops) albolabris. J Exp Zool 315:22–29
Hibbitts TJ, Fitzgerald LA (2005) Morphological and ecological convergence in two natricine snakes. Biol J Linn Soc 85:363–371
Higham TE (2007) The integration of locomotion and prey capture in vertebrates: morphology, behavior, and performance. Integr Comp Biol 47:82–95
Higham TE, Clark RW, Collins CE, Whitford MD, Freymiller GA (2017) Rattlesnakes are extremely fast and variable when striking at kangaroo rats in nature: three-dimensional high-speed kinematics at night. Sci Rep 7:40412
Hisaw FL, Gloyd HK (1926) The bull snake as a natural enemy of injurious rodents. J Mammal 7:200–205
Hoso M, Asami T, Hori M (2007) Right-handed snakes: convergent evolution of asymmetry for functional specialization. Biol Lett 3:169–172
Hsiang AY, Field DJ, Webster TH, Behlke ADB, Davis MB, Racicot RA, Gauthier JA (2015) The origin of snakes: revealing the ecology, behavior, and evolutionary history of early snakes using genomics, phenomics, and the fossil record. BMC Evol Biol 15:87
Hutchinson DA, Mori A, Savitzky AH, Burghardt GM, Wu X, Meinwald J, Schroeder FC (2007) Dietary sequestration of defensive steroids in nuchal glands of the Asian snake Rhabdophis tigrinus. Proc Natl Acad Sci USA 104:2265–2270
Hutchinson DA, Savitzky AH, Burghardt GM, Nguyen C, Meinwald J, Schroeder FC, Mori A (2013) Chemical defense of an Asian snake reflects local availability of toxic prey and hatchling diet. J Zool 289:270–278
Jackson K (2003) The evolution of venom-delivery systems in snakes. Zool J Linn Soc 137:337–354
Jackson K, Fritts TH (2004) Dentitional specialization for durophagy in the Common Wolf snake, Lycodon aulicus capucinus. Amphibia-Reptilia 25:247–254
Jackson K, Kley NJ, Brainerd EL (2004) How snakes eat snakes: the biomechanical challenges of ophiophagy for the California kingsnake, Lampropeltis getula californiae (Serpentes: Colubridae). Zoology 107:191–200
Janoo A, Gasc J-P (1992) High speed motion analysis of the predatory strike and fluographic study of oesophageal deglutition in Vipera ammodytes: more than meets the eye. Amphibia-Reptilia 13:315–325
Jayne BC, Riley MA (2007) Scaling of the axial morphology and gap-bridging ability of the brown tree snake, Boiga irregularis. J Exp Biol 210:1148–1160
Jayne BC, Voris HK, Ng PKL (2002) Snake circumvents constraints on prey size. Nature 418:143
Jensen B, Larsen CK, Nielsen JM, Simonsen LS, Wang T (2011) Change of cardiac function, but not form, in postprandial pythons. Comp Biochem Physiol A 160:35–42
Johnston P (2014) Homology of the jaw muscles in lizards and snakes: a solution from a comparative gnathostome approach. Anat Rec 297:574–585
Kardong KV (1982) Comparative study of changes in prey capture behavior of the cottonmouth (Agkistrodon piscivorus) and Egyptian cobra (Naja haje). Copeia 1982:337–343
Kardong KV, Bels VL (1998) Rattlesnake strike behavior: kinematics. J Exp Biol 201:837–850
Kardong KV, Haverly JE (1993) Drinking by the common boa, Boa constrictor. Copeia 1993:808–818
King RB (2002) Predicted and observed maximum prey size–snake size allometry. Funct Ecol 16:766–772
Klaczko J, Sherratt E, Setz EZF (2016) Are diet preferences associated to skulls shape diversification in xenodontine snakes? PLoS ONE 11:e148375
Klauber LM (1972) Rattlesnakes: their habits, life histories, and influence on mankind. University of California Press, Berkeley
Kley NJ (2001) Prey transport mechanisms in blindsnakes and the evolution of unilateral feeding systems in snakes. Amer Zool 41:1321–1337
Kley NJ (2006) Morphology of the lower jaw and suspensorium in the Texas Blindsnake, Leptotyphlops dulcis (Scolecophidia: Leptotyphlopidae). J Morphol 267:494–515
Kley NJ, Brainerd EL (1999) Feeding by mandibular raking in a snake. Nature 402:369–370
Kley NJ, Brainerd EL (2002) Post-cranial prey transport mechanisms in the black pinesnake Pituophis melanoleucus lodingi: an X-ray videographic study. Zoology 105:153–164
Kochva E (1978) Oral glands of the reptilia. In: Gans C, Gans KA (eds) Biology of the reptilia, vol 8. Physiology B. Academic Press, London, pp 43–162
Krause MA, Burghardt GM (2007) Sexual dimorphism of body and relative head size in neonatal common garter snakes. J Zool 272:156–164
Krochmal AR, Bakken GS (2003) Thermoregulation is the pits: use of thermal radiation for retreat site selection by rattlesnakes. J Exp Biol 206:2539–2545
Krochmal AR, Bakken GS, LaDuc TJ (2004) Heat in evolution’s kitchen: evolutionary perspectives on the functions and origin of the facial pit of pitvipers (Viperidae: Crotalinae). J Exp Biol 207:4231–4238
LaBonte JP, Welch KC Jr, Suarez RK (2011) Digestive performance in neonatal Southern Pacific Rattlesnakes (Crotalus oreganus helleri). Can J Zool 89:705–713
LaDuc TJ (2002) Does a quick offense equal a quick defense? Kinematic comparisons of predatory and defensive strikes in the western diamond-backed rattlesnake (Crotalus atrox). In: Schuett GW, Hoggren M, Douglas ME, Greene HW (eds) Biology of the vipers. Eagle Mountain Publishing, Utah, pp 267–278
Lee MSY, Bell GL Jr, Caldwell MW (1999) The origin of snake feeding. Nature 400:655–659
Lillywhite HB (2014) How snakes work: structure, function, and behavior of the world’s snakes. Oxford University Press, New York
Lillywhite HB, de Delva P, Noonan BP (2002) Patterns of gut passage time and the chronic retention of fecal mass in viperid snakes. In: Schuett GW, Hoggren M, Douglas ME, Greene HW (eds) Biology of the vipers. Eagle Mountain Publishing, Utah, pp 497–506
Liu Y, Chen Q, Papenfuss TJ, Lu F, Tang YZ (2016) Eye and pit size are inversely correlated in Crotalinae: implications for selection pressure relaxation. J Morphol 277:107–117
Longrich NR, Bhullar B-AS, Gauthier JA (2012) A transitional snake from the Late Cretaceous period of North America. Nature 488:205–208
Lopez MS, Manzano AS, Prieto YA (2013) Ontogenetic variation in head morphology and diet in two snakes (Viperidae) from northeastern Argentina. J Herpetol 47:406–412
Lourdais O, Brischoux F, DeNardo D, Shine R (2004) Protein catabolism in pregnant snakes (Epicrates cenchria maurus, Boidae) compromises musculature and performance after reproduction. J Comp Physiol B 174:383–391
Lourdais O, Brischoux F, Shine R, Bonnet X (2005) Adaptive maternal cannibalism in snakes (Epicrates cenchria maurus, Boidae). Biol J Linn Soc 84:767–774
Mackessy SP (ed) (2009) Handbook of venoms and toxins of reptiles. CRC Press, Boca Raton
Mackessy SP (2010) Evolutionary trends in venom composition in the western rattlesnakes (Crotalus viridis sensu lato): toxicity vs. tenderizers. Toxicon 55:1463–1474
Mackessy SP, Saviola AJ (2016) Understanding biological roles of venoms among the Caenophidia: the importance of rear-fanged snakes. Integr Comp Biol 56:1004–1021
Mangiacotti M, Limongi L, Sannolo M, Sacchi R, Zuffi MAL, Scali S (2014) Head shape variation in eastern and western Montpellier snakes. Acta Herpetol 92:167–177
Margres MJ, Wray KP, Seavy M, McGivern JJ, Sanader D, Rokyta DR (2015) Phenotypic integration in the feeding system of the eastern diamondback rattlesnake (Crotalus adamanteus). Mol Ecol 24:3405–3420
Martill DM, Tischlinger H, Longrich NR (2015) A four-legged snake from the Early Cretaceous of Gondwana. Science 349:416–419
McCue MD (2006a) Cost of producing venom in three North American pitviper species. Copeia 2006:818–825
McCue MD (2006b) Specific dynamic action: a century of investigation. Comp Biochem Physiol A 144:381–394
McCue MD (2007) Prey envenomation does not improve digestive performance in western diamondback rattlesnakes (Crotalus atrox). J Exp Zool 307A:568–577
McDowell SB (1986) The architecture of the corner of the mouth in colubroid snakes. J Herpetol 20:353–407
McDowell SB (2008) The skull of serpentes. In: Gans C, Gaunt AS, Adler K (eds) Biology of the reptilia, vol 21. Morphology I. The Skull and appendicular locomotor apparatus of lepidosauria. Society for the Study of Amphibians and Reptiles, pp 467–620
McLaughlin RL (1989) Search modes of birds and lizards: evidence for alternative movement patterns. Am Nat 133:654–670
McLees F (1928) Killing by constriction. Bull Antivenin Inst Amer 1:105
Mehta RS (2003) Prey-handling behavior of hatchling Elaphe helena (colubridae). Herpetologica 59:469–474
Mehta RS (2009) Early experience shapes the development of behavioral repertoires of hatchling snakes. J Ethol 27:143–151
Mehta RS, Burghardt GM (2008) Contextual flexibility: reassessing the effects of prey size and status on prey restraint behaviour of macrostomate snakes. Ethol 114:133–145
Meik JM, Setser K, Mociño-Deloya E, Lawing AM (2012) Sexual differences in head form and diet in a population of Mexican lance-headed rattlesnakes, Crotalus polystictus. Biol J Linn Soc 106:633–640
Miller DE, Mushinsky HR (1990) Foraging ecology and prey size in the mangrove water snake, Nerodia fasciata compressicauda. Copeia 1990:1099–1106
Mizuno T, Kojima Y (2015) A blindsnake that decapitates its termite prey. J Zool 297:220–224
Moon BR (2000a) The mechanics and muscular control of constriction in gopher snakes (Pituophis melanoleucus) and a king snake (Lampropeltis getula). J Zool 252:83–98
Moon BR (2000b) The mechanics of swallowing and the muscular control of diverse behaviors in gopher snakes. J Exp Biol 203:2589–2601
Moon BR, Mehta RS (2007) Constriction strength in snakes. In: Henderson RW, Powell R (eds) Biology of the boas and pythons. Eagle Mountain Publishing, Utah, pp 241–246
Mori A (1998) Prey-handling behavior of three species of homalopsine snakes: features associated with piscivory and Duvernoy’s Glands. J Herpetol 32:40–50
Mori A, Vincent SE (2008) An integrative approach to specialization: relationships among feeding morphology, mechanics, behaviour, performance and diet in two syntopic snakes. J Zool 275:47–56
Murta-Fonseca RA, Fernandes DS (2016) The skull of Hydrodynastes gigas (Duméril, Bibron & Duméril, 1854) (Serpentes: Dipsadidae) as a model of snake ontogenetic allometry inferred by geometric morphometrics. Zoomorphol 135:233–241
Natusch DJD, Lyons JA (2012) Relationships between ontogenetic changes in prey selection, head shape, sexual maturity, and colour in an Australasian python (Morelia viridis). Biol J Linn Soc 107:269–276
Natusch DJD, Lyons JA (2014) Geographic and sexual variation in body size, morphology, and diet among five populations of Green Pythons (Morelia viridis). J Herpetol 48:317–323
Nicholson J, Mirtschin P, Madaras F, Venning M, Kokkinn M (2006) Digestive properties of the venom of the Australian coastal taipan, Oxyuranus scutellatus (Peters, 1867). Toxicon 48:422–428
Olori JC, Bell CJ (2012) Comparative skull morphology of uropeltid snakes (Alethinophidia: Uropeltidae) with special reference to disarticulated elements and variation. PLoS ONE 7:e23450
Palci A, Lee MSY, Hutchinson MN (2016) Patterns of postnatal ontogeny of the skull and lower jaw of snakes as revealed by micro-CT scan data and three-dimensional geometric morphometrics. J Anat 229:723–754
Parker HW, Grandison AGC (1977) Snakes: a natural history. Cornell University Press, New York
Parker RM, Young BA, Kardong KV (2008) The forked tongue and edge detection in snakes (Crotalus oreganus): an experimental test. J Comp Psychol 122:35–40
Penning DA (2017a) The scaling of bite force and constriction performance in kingsnakes (Lampropeltis getula): proximate determinants and correlated performance. Integr Zool 12:121–131
Penning DA (2017b) The gluttonous king: the effects of prey size and repeated feeding on predatory performance in kingsnakes. J Zool 302:119–125
Penning DA, Cairns S (2016) Prey-handling behaviors of naïve Pantherophis guttatus. J Herpetol 50:196–202
Penning DA, Dartez SF (2016) Size, but not experience, affects the ontogeny of constriction performance in ball pythons (Python regius). J Exp Zool 325A:194–199
Penning DA, Moon BR (2017) The king of snakes: performance and morphology of intraguild predators (Lampropeltis) and their prey (Pantherophis). J Exp Biol 220:1154–1161
Penning DA, Dartez SF, Moon BR (2015) The big squeeze: scaling of constriction pressure in two of the world’s largest snakes, Python reticulatus and P. molurus bivittatus. J Exp Biol 218:2264–3367
Penning DA, Sawvel B, Moon BR (2016) Debunking the viper’s strike: harmless snakes kill a common assumption. Biol Lett 12:2016011
Pereira SC, Rodrigues JFM, Borges-Nojosa DM (2016) Single large or several small? The influence of prey size on feeding performance of Philodryas nattereri (Squamata: Serpentes). Acta Scientiarum 38:25–28
Phillips BL, Shine R (2004) Adapting to an invasive species: toxic cane toads induce morphological change in Australian snakes. Proc Natl Acad Sci USA 101:17150–17155
Pough FH (1980) The advantages of ectothermy for tetrapods. Am Nat 115:92–112
Pough FH, Groves JD (1983) Specializations of the body form and food habits of snakes. Am Zool 23:443–454
Povel D, Jvd Kooij (1997) Scale sensillae of the file snake (Serpentes: Acrochordidae) and some other aquatic and burrowing snakes. Neth J Zool 47:443–456
Pyron RA, Burbrink FT, Wiens JJ (2013) A phylogeny and revised classification of Squamata including 4161 species of lizards and snakes. BMC Evol Biol 13:93
Queral-Regil A, King RB (1998) Evidence for phenotypic plasticity in snake body size and relative head dimensions in response to amount and size of prey. Copeia 1998:423–429
Radcliffe CW, Chiszar DA (1980) A descriptive analysis of predatory behavior in the yellow lipped sea krait (Laticauda colubrina). J Herpetol 14:422–424
Randall JA, Matocq MD (1997) Why do kangaroo rats (Dipodomys spectabilis) footdrum at snakes? Behav Ecol 8:404–413
Rieppel O (1988) A review of the origin of snakes. In: Hecht MK, Wallace B, Prance GT (eds) Evolutionary biology, vol 22. Plenum, New York
Rieppel O, Kley NJ, Maisano JA (2009) Morphology of the skull of the white-nosed blindsnake, Liotyphlops albirostris (Scolecophidia: Anomalepididae). J Morphol 270:536–557
Rivas JA (2004) Eunectes murinus (green anaconda). Subduing behavior. Herp Rev 35:66–67
Ruane S (2015) Using geometric morphometrics for integrative taxonomy: an examination of head shapes of milksnakes (genus Lampropeltis). Zool J Linn Soc 174:394–413
Rudolph DC, Burgdorf SJ, Conner RN, Collins CS, Saenz D, Schaefer RR, Trees T, Duran CM, Ealy M, Himes JG (2002) Prey handling and diet of Louisiana pine snakes (Pituophis ruthveni) and black pine snakes (P. melanoleucus lodingi), with comparisons to other colubrid snakes. Herpetol Nat Hist 9:57–62
Sanders KL, Rasmussen AR, Mumpuni Elmberg J, De Silva A, Guinea ML, Lee MSY (2013) Recent rapid speciation and ecomorph divergence in Indo-Australian sea snakes. Mol Ecol 22:2742–2759
Sasa M (1999a) Diet and snake venom evolution: can local selection alone explain intraspecifc venom variation? Toxicon 37:249–252
Sasa M (1999b) Reply. Toxicon 37:259–260
Saviola AJ, Chiszar D, Busch C, Mackessy SP (2013) Molecular basis for prey relocation in viperid snakes. BMC Biol 11:20
Scanferla A (2016) Postnatal ontogeny and the evolution of macrostomy in snakes. R Soc Open Sci 3:160612
Scanlon JD (2012) Skull of the large non-macrostomatan snake Yurlunggur from the Australian Oligo-Miocene. Nature 439:839–842
Schuett GW, Hardy DLSr, Earley RL, Greene HW (2005) Does prey size induce head skeleton phenotyic plasticity during early ontogeny in the snake Boa constrictor. J Zool 267:363–369
Schwenk K (1994) Why snakes have forked tongues. Science 263:1573–1577
Secor SM (2009) Specific dynamic action: a review of the postprandial metabolic response. J Comp Physiol 179:1–56
Secor SM, Diamond J (1995) Adaptive responses to feeding in Burmese pythons: pay before pumping. J Exp Biol 198:1313–1325
Secor SM, White SE (2010) Prioritizing blood flow: cardiovascular performance in response to the competing demands of locomotion and digestion for the Burmese python, Python molurus. J Exp Biol 213:78–88
Secor SM, Jayne BC, Bennett AF (1992) Locomotor performance and energetic cost of sidewinding by the snake Crotalus cerastes. J Exp Biol 163:1–14
Secor SM, Hicks JW, Bennett AF (2000) Ventilatory and cardiovascular responses of a python (Python molurus) to exercise and digestion. J Exp Biol 203:2447–2454
Secor SM, Taylor JR, Grosell M (2012) Selected regulation of gastrointestinal acid–base secretion and tissue metabolism for the diamondback water snake and Burmese python. J Exp Biol 215:185–196
Segall M, Cornette R, Fabre A-C, Godoy-Diana R, Herrel A (2016) Does aquatic foraging impact head shape evolution in snakes? Proc R Soc B 283:20161645
Shine R, Schwaner T (1985) Prey constriction by venomous snakes: a review, and new data on Australian species. Copeia 1985:1067–1071
Shine R, Thomas J (2005) Do lizards and snakes really differ in their ability to take large prey? A study of relative prey mass and feeding tactics in lizards. Oecologia 144:492–498
Shine R, Sun L-X, Fitzgerald M, Kearney M (2002) Antipredator responses of free-ranging pit vipers (Gloydius shedaoensis, Viperidae). Copeia 2002:843–850
Simoes BF, Sampaio FL, Jared C, Antoniazzi MM, Loew ER, Bowmaker JK, Rodriguez A, Hart NS, Hunt DM, Partridge JC, Gower DJ (2015) Visual system evolution and the nature of the ancestral snake. J Evol Biol 28:1309–1320
Smith MT (2014) Induction of phenotypic plasticity in rattlesnake trophic morphology by diet manipulation. J Morphol 275:1339–1348
Smith TL, Povel GDE, Kardong KE (2002) Predatory strike of the tentacled snake (Erpeton tentaculatum). J Zool 256:233–242
Stevenson DJ, Norton TM, Bolt MR, Smith DJ, Enge KM, Hyslop NL, Dyer KJ (2010) Prey records for the eastern indigo snake (Drymarchon couperi). Southeastern Nat 9:1–18
Thomas RG, Pough FH (1979) The effects of rattlesnake venom on the digestion of prey. Toxicon 17:221–228
Underwood G (1997) An overview of venomous snake evolution. In: Thorpe RS, Wüster W, Malhotra A (eds) Venomous snakes: ecology, evolution, and snakebite. Symp Zool Soc Lond, No. 70. Clarendon Press, Oxford, pp 1–13
Urdaneta AH, Bolaños F, Gutiérrez JM (2004) Feeding behavior and venom toxicity of coral snake Micrurus nigrocinctus (Serpentes: Elapidae) on its natural prey in captivity. Comp Biochem Physiol C 138:485–492
Valkonen JK, Nokelainen O, Mappes J (2011) Antipredatory function of head shape for vipers and their mimics. PLoS ONE 6:e22272
Van Riper W (1954) Measuring the speed of a rattlesnake’s strike. Animal Kingdom 57:50–53
Van Wassenbergh S, Brecko J, Aerts P, Stouten I, Vanheusden G, Camps A, Van Damme R, Herrel A (2010) Hydrodynamic constraints on prey-capture performance in forward-striking snakes. J R Soc Interface 7:773–785
Vincent SE, Herrel A (2007) Functional and ecological correlates of ecologically-based dimorphisms in squamate reptiles. Integr Comp Biol 47:172–188
Vincent SE, Mori A (2008) Determinants of feeding performance in free-ranging pit-vipers (Viperidae: Ovophis okinavensis): key roles for head size and body temperature. Biol J Linn Soc 93:53–62
Vincent SE, Herrel A, Irschick DJ (2004) Ontogeny of intersexual head shape and prey selection in the pitviper Agkistrodon piscivorous. Biol J Linn Soc 81:151–159
Vincent SE, Herrel A, Irschick DJ (2005) Comparisons of aquatic versus terrestrial predatory strikes in the pitviper, Agkistrodon piscivorus. J Exp Zool 303A:476–488
Vincent SE, Dang PD, Herrel A, Kley NJ (2006a) Morphological integration and adaptation in the snake feeding system: a comparative phylogenetic study. J Evol Biol 19:1545–1554
Vincent SE, Vincent P-D, Irschick DJ, Rossell JM (2006b) Do juvenile gape-limited predators compensate for their small size when feeding. J Zool 268:279–284
Vincent SE, Moon BR, Herrel A, Kley NJ (2007) Are ontogenetic shifts in diet linked to shifts in feeding mechanics? Scaling of the feeding apparatus in the banded watersnake Nerodia fasciata. J Exp Biol 210:2057–2069
Vincent SE, Brandley MC, Herrel A, Alfaro ME (2009) Convergence in trophic morphology and feeding performance among piscivorous natricine snakes. J Evol Biol 22:1203–1211
Voris HK, Voris HH (1983) Feeding strategies in marine snakes: an analysis of evolutionary, morphological, behavioral and ecological relationships. Am Zool 23:411–425
Walton M, Jayne BC, Bennett AF (1990) The energetic cost of limbless locomotion. Science 249:524–527
Welsh HH Jr, Lind AJ (2000) Evidence of lingual-luring by an aquatic snake. J Herpetol 34:67–74
Werler JE, Dixon JR (2000) Texas snakes: identification, distribution, and natural history. University of Texas Press, Austin
Westhoff G, Fry BG, Bleckmann H (2005) Sea snakes are sensitive to low-amplitude water motions. Zoology 108:195–200
Whitaker PB, Ellis K, Shine R (2000) The defensive strike of the eastern brownsnake, Pseudonaja textilis (Elapidae). Funct Ecol 14:25–31
Willard DE (1977) Constricting methods of snakes. Copeia 1977:379–382
Wilson JD, Hopkins WA (2011) Prey morphology constrains the feeding ecology of an aquatic generalist predator. Ecology 92:744–754
Wüster W, Daltry JC, Thorpe RS (1999) Can diet explain intraspecific venom variation? Reply to Sasa. Toxicon 37:253–258
Yi H, Norell MA (2015) The burrowing origin of modern snakes. Sci Adv 1:e1500743
Young BA (1991) The influences of the aquatic medium on the prey capture system of snakes. J Nat Hist 25:519–531
Young BA (2003) Snake bioacoustics: toward a richer understanding of the behavioral ecology of snakes. Quart Rev Biol 78:303–325
Young BA (2007) Response of the yellow anaconda (Eunectes notaeus) to aquatic acoustic stimuli. In: Henderson RW, Powell R (eds) Biology of the boas and pythons. Eagle Mountain Publishing, Utah, pp 199–205
Young BA (2010) How a heavy-bodied snake strikes quickly: high-power axial musculature in the Puff Adder (Bitis arietans). J Exp Zool 313A:114–121
Young BA, Aguiar A (2002) Response of western diamondback rattlesnakes Crotalus atrox to airborne sounds. J Exp Biol 205:3087–3092
Young BA, Kardong KV (2007) Mechanisms controlling venom expulsion in the western diamondback rattlesnake, Crotalus atrox. J Exp Zool 307A:18–27
Young BA, Morain M (2002) The use of ground-borne vibrations for prey localization in the Saharan sand vipers (Cerastes). J Exp Biol 205:661–665
Young BA, Zahn K (2001) Venom flow in rattlesnakes: mechanics and metering. J Exp Biol 204:4345–4351
Young BA, Zahn K, Blair M, Lalor J (2000) Functional subdivision of the venom gland musculature and the regulation of venom expulsion in rattlesnakes. J Morphol 246:249–259
Young BA, Blair M, Zahn K, Marvin J (2001a) Mechanics of venom expulsion in Crotalus, with special reference to the role of the fang sheeth. Anat Rec 264:415–426
Young BA, Phelan M, Jaggers J, Nejman N (2001b) Kinematic modulation of the strike of the western diamondback rattlesnake (Crotalus atrox). Hamadryad 26:316–349
Zaher H, de Oliveira L, Grazziotin FG, Campagner M, Jared C, Antoniazzi MM, Prudente ALC (2014) Consuming viscous prey: a novel protein-secreting delivery system in neotropical snail-eating snakes. BMC Evol Biol 14:58
Zug GR (1993) Herpetology: an introductory biology of amphibians and reptiles. Academic Press, San Diego
Acknowledgements
We wish to thank Vincent Bels for inviting us to write this book chapter, and Stephen Deban, Paul Hampton, Timothy Higham, and Katherine Wadsworth for helpful input. We thank Raoul Van Damme and one anonymous reviewer for helpful and constructive comments on the manuscript. M. S. thanks the Région Ile de France, the doctoral school Frontières du Vivant (FdV), Programme Bettencourt, and the Fyssen Foundation for funding.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Moon, B.R., Penning, D.A., Segall, M., Herrel, A. (2019). Feeding in Snakes: Form, Function, and Evolution of the Feeding System. In: Bels, V., Whishaw, I. (eds) Feeding in Vertebrates. Fascinating Life Sciences. Springer, Cham. https://doi.org/10.1007/978-3-030-13739-7_14
Download citation
DOI: https://doi.org/10.1007/978-3-030-13739-7_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-13738-0
Online ISBN: 978-3-030-13739-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)