Abstract
Treatment of patients with type 2 diabetes aims to avoid acute symptoms of hyperglycemia and to prevent macro- and microvascular complications. In recent years, the number of glucose-lowering drugs increased. All are proven to decrease HbA1c levels or postprandial glucose excursions, but evidence on patient-relevant outcomes, such as cardiovascular mortality, amputations, or retinopathy, is sparse. This chapter gives an overview of older classes of antidiabetic agents and their efficacy on patient-relevant outcomes. Pubmed and the Cochrane library were searched for systematic reviews and randomized controlled trials. Findings regarding metformin, sulfonylureas, thiazolidinediones, meglitinides, and alpha-glucosidase inhibitors are presented. An excerpt of a patient decision aid is given at the end of this chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Metformin
- Sulfonylureas
- Secretagogues
- Thiazolidinediones
- Glitazones
- Alpha-glucosidase inhibitors
- Hypoglycemia
- Shared Decision-Making
- Informed decisions
Introduction
Treatment of patients with type 2 diabetes aims to avoid acute symptoms of hyperglycemia and to prevent macro- and microvascular complications. In recent years, the number of glucose-lowering drugs increased. The American Diabetes Association (ADA) lists more than ten drug classes of available glucose-lowering agents in their standards of medical care in diabetes [1]. All are proven to decrease HbA1c levels or postprandial glucose excursions, but evidence on patient-relevant outcomes, such as cardiovascular mortality, amputations, or retinopathy, is sparse. Reduction of HbA1c values is often used as a surrogate outcome measure to assess the efficacy of antidiabetic medication. However, its appropriateness has been disproven [2, 3]. In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study [4] and the Veterans Affairs Diabetes Trial (VADT) [5], a rigid treatment regime with low HbA1c targets did not result in better patient-relevant outcomes. Patients in the intervention arm of the ACCORD study even had a higher risk of mortality. Consequently, the study was terminated early [4]. Some drugs were withdrawn from markets because of a negative benefit-risk ratio, e.g., phenformin, which increased lactic acidosis or rosiglitazone (in Europe) that reduced HbA1c values but may increase cardiovascular risk. In recent years, pharmaceutical companies decided to withdraw several new antidiabetic agents from the German market, such as vildagliptin and canagliflozin, because no additional benefit over usual care could be demonstrated, and therefore health insurances would not have covered additional costs.
In 2012, the ADA and the European Association for the Study of Diabetes (EASD) recommended patient-centered care including shared decision-making (SDM) [6] and reasserted this position in their recent statement [7]. SDM is a particular form of communication between patients and their healthcare professionals. It focuses on the mutual exchange of information in order to involve patients in the decision-making process [8]. Therefore, patients need understandable information on probabilities of benefits and harms of treatment options [9,10,11]. The question to be answered is: what option is the best to prevent diabetes-related complications and yet in line with individual patient values and preferences? Supportive tools in that process are patient decision aids which help patients to weigh up pros and cons of diabetes treatment [12, 13].
This chapter gives an overview of older classes of antidiabetic agents and their efficacy. It is based on a systematic inventory published in 2015 [3]. Sulfonylureas (SU) and biguanides are the oldest classes of oral glucose-lowering agents. Later, thiazolidinediones (TZDs), alpha-glucosidase inhibitors (AGIs), and meglitinides were approved. Table 32.1 shows the old drug classes and their compounds that are still available in the USA or Europe. Newer classes, such as sodium-glucose cotransporter-2 (SGLT-2) inhibitors and dipeptidyl peptidase-4 (DPP-4) inhibitors, will be described in the following chapters of this book.
According to recent guidelines [1, 7, 14, 15], this chapter focuses on the efficacy of metformin and SU monotherapies compared with other monotherapies as well as comparisons of metformin-based combinations. At the end of this chapter, we give an example of our decision aid for patients with type 2 diabetes and how diabetes educators share evidence-based information with their patients [16, 17].
Methods
We updated our search from April 2014 [3]. In a first step, we searched PubMed and the Cochrane library for systematic reviews and meta-analyses published from May 2014 to the end of July 2017. Systematic reviews were considered if they included randomized controlled trials on the efficacy of metformin, sulfonylureas, thiazolidinediones, meglitinides, or alpha-glucosidase inhibitors as monotherapy or combination of two or three drugs. There is a growing number of network analyses. They typically comprise indirect comparisons when there is no head-to-head comparison available. Network analyses are methodologically challenging and can lead to false results and interpretations if differences between studies were not adequately considered [18]. Treatment of type 2 diabetes is complex, and as a result, RCTs in meta-analyses are usually heterogeneous. We therefore excluded network meta-analyses. In addition, inclusion criteria, such as study duration, sample size, target group, and drug classes, vary between systematic reviews. Hence, following our previous methodological approach [3], we extracted RCTs from the reviews that fulfilled our inclusion criteria, (1) patient-relevant primary endpoint, i.e., macro- and microvascular complications, cardiovascular mortality, total mortality, and quality of life; (2) intention-to-treat analysis; (3) follow-up of at least 24 weeks and adequate sample size; and (4) hard clinical endpoints had to be reported. Finally, we searched for further studies and screened the websites of the National Institute for Health and Care Excellence (NICE), the Agency for Healthcare Research and Quality (AHRQ), and the German Institute for Quality and Efficiency in Health Care (IQWIG) for new reports and guidelines.
Results
The search update for systematic reviews and meta-analyses resulted in 516 records. Most of them were network analyses. Although the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) changed licensing regulations toward cardiovascular outcome trials for glucose-lowering drugs in 2008, reviews mainly focused on surrogate endpoints, such as HbA1c level. We identified one systematic review on the efficacy of metformin compared to no intervention, placebo, or lifestyle intervention [19]. Another meta-analysis compared metformin and SU as monotherapy [20], and four evaluated the effects of TZDs [21,22,23,24]. With respect to alpha-glucosidase inhibitors and meglitinides, no additional review could be identified. A recently updated meta-analysis by the Agency for Healthcare Research and Quality (AHRQ) evaluated all available glucose-lowering drugs [25, 26]. The report includes RCTs and observational studies on (1) comparisons of monotherapies (metformin, thiazolidinediones, sulfonylureas, DPP-4 inhibitors, SGLT-2 inhibitors, and GLP-1 receptor agonists), (2) comparisons of metformin alone and metformin-based combinations, and (3) comparisons of metformin-based combinations where the second drug was one of the monotherapies or insulin treatment. The evidence was graded separately for both study types. The AHRQ search update was performed through December 2016. Our search for more recent RCTs from January 2016 to July 2017 yielded 222 records. No further eligible RCTs could be identified. Overall, we could not include any new relevant RCTs for this chapter.
Metformin
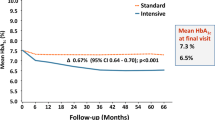
Metformin belongs to the class of biguanides. It is the only still licensed compound of its class after phenformin was withdrawn from the markets. In the University Group Diabetes Program (UGDP) [27, 28], the first large RCT that evaluated the efficacy of glucose-lowering drugs on macro- and microvascular outcomes, phenformin was associated with an increase of cardiac mortality. In contrast, metformin is internationally recommended as initial drug treatment for people with type 2 diabetes [1, 7, 15, 29, 30]. This is mainly based on the results of the United Kingdom Prospective Diabetes Study (UKPDS), published in 1998 [31]. About 4000 patients with newly diagnosed type 2 diabetes were enrolled in this RCT. The study objective was to assess the efficacy of intensive blood glucose-lowering therapy compared to conventional treatment (primarily with diet). Patients in the intensive treatment group were supposed to achieve a fasting plasma glucose level of less than 6 mmol/L. The fasting plasma glucose target of the conventional treatment arm was less than 15 mmol/L with no symptoms of hyperglycemia. Non-overweight patients were randomly assigned to intensive treatment with insulin, intensive treatment with sulfonylurea, or conventional therapy with diet. A subgroup of overweight patients had the additional possibility to be randomized to intensive treatment with metformin [31, 32]. A total of 342 patients were assigned to metformin and 411 patients to conventional control with diet [31].
The median HbA1c level of the intensive treatment group with metformin was 7.4% during the 10 years of follow-up. The conventional group had a median HbA1c level of 8.0%. Compared to conventional treatment, the metformin monotherapy arm showed significant reductions in any diabetes-related endpoint, a composite endpoint comprising the following outcome measures: sudden death, death from hyperglycemia or hypoglycemia, fatal or nonfatal myocardial infarction, angina, heart failure, stroke, renal failure, amputation of at least one digit, vitreous hemorrhage, retinopathy requiring photocoagulation, blindness in one eye, or cataract extraction. Moreover, diabetes-related death, all-cause mortality, and myocardial infarction significantly decreased in the intensive treatment group with metformin.
Based on these results, metformin became the first-line drug for patients with type 2 diabetes who do not achieve their HbA1c target with diet and other lifestyle interventions alone. However, the results of the UKPDS have not yet been reproduced [2, 33]. The UKPDS was a study with an open-label design which may lead to overestimated results. The protocol was changed during the study. The initially defined significance threshold of 1% was later changed to 5%. The significant difference in reduction of total mortality and myocardial infarction in the metformin group was above the threshold of 1% [33].
Antihypertensive treatment or statins may have a greater effect on mortality than metformin [34]. This may also explain the results of the UKPDS follow-up study [35] which reported significant reductions in total mortality and cardiovascular mortality for all intensive treatment groups 10 years after the main publication of the UKPDS results. Considering the high risks of bias of the UKPDS, the interpretation of the follow-up results as long-term effect of intensive early glucose control might be misleading [36]. In addition, only about one-third of the initially randomized patients were analyzed in this follow-up study.
A meta-analysis that included 13 studies comparing metformin as monotherapy or add-on therapy to diet, placebo, or no treatment found no significant effects on all-cause mortality, cardiovascular mortality, or microvascular complications [37]. Of the included RCTs that assessed patient-relevant outcomes as primary endpoint [31, 38,39,40], only UKPDS [31] showed a beneficial effect for treatment with metformin.
In the UKPDS, metformin monotherapy was also associated with a decrease in any diabetes-related endpoint and all-cause mortality compared to intensive treatment with sulfonylurea or insulin [31]. Data on metformin compared to SU alone were not reported in the UKPDS [20].
The study on the Prognosis and Effect of Antidiabetic Drugs on Type 2 Diabetes Mellitus With Coronary Artery Disease (SPREAD-DIMCAD) [41] compared metformin with the SU glipizide in 304 Chinese people with type 2 diabetes mellitus and coronary artery disease. The targeted HbA1c level was less than 7% for both groups. The primary endpoint was recurrent cardiovascular events, a composite outcome measure comprising nonfatal myocardial infarction, nonfatal stroke, arterial revascularization, cardiovascular death, and all-cause mortality. Study results showed a significant reduction in this endpoint in favor of the metformin group. However, there was a substantial risk of bias which limits the validity of the study results. The study was retrospectively registered, and there is no study protocol published. Data from 5 years of follow-up were analyzed, but the study drug was only administered for 3 years. It was not reported whether the study treatment was maintained after this time.
A meta-analysis on the effects of SU monotherapy compared to metformin monotherapy did not find any differences between treatment groups regarding all-cause or cardiovascular mortality [20]. A potential benefit of SU over metformin was identified in nonfatal macrovascular outcomes, but definitions of that composite endpoint were heterogeneous. There were no data on microvascular outcomes for a meta-analysis. Results of that meta-analysis were mainly based on “A Diabetes Outcome Progression Trial” (ADOPT), a multicenter, randomized controlled, double-blind trial with 4 years of follow-up [42]. Patients with untreated diabetes were randomized to metformin, glibenclamide, or rosiglitazone. Primary endpoint was time to treatment failure, defined as fasting plasma glucose level of more than 180 mg per deciliter after 6 weeks at maximum tolerated dose of the study drug. As this is not a clinical hard endpoint, we have excluded this trial from our overview. However, there was no difference regarding all-cause mortality or fatal myocardial infarction between the glibenclamide and metformin groups [20, 42].
Compared to sulfonylurea alone, the combination of metformin and sulfonylurea significantly increased death from any cause and diabetes-related death in overweight and non-overweight patients in the UKPDS [31]. The meta-analysis by Boussageon et al. [37] confirmed a significant increase in all-cause and cardiovascular mortality for metformin plus SU compared to metformin monotherapy. The results were mainly based on the UKPDS. After excluding this study, no group difference was seen in both endpoints.
The HOME (Hyperinsulinemia: the Outcome of its Metabolic Effects) trial evaluated the efficacy of metformin in the Netherlands [40]. The RCT included 390 overweight and obese patients with type 2 diabetes. Metformin added to insulin therapy was compared to insulin monotherapy. After about 4 years, there was no difference between groups regarding cardiovascular and total mortality or microvascular outcomes (progression of retinopathy, nephropathy, and neuropathy) but a significant reduction in a combined macrovascular endpoint for patients with metformin plus insulin treatment. This composite endpoint included a total of 13 separate outcome measures, e.g., myocardial infarction, heart failure, stroke, diabetic foot, percutaneous transluminal coronary angioplasty, non-traumatic amputation, and sudden death. Patients’ characteristics were unequally distributed between the study groups at baseline. For example, in the metformin group, there were fewer smokers (19% vs. 30%) and more patients with antihypertensive medication (47% vs. 39%). In addition, the number of non-completers differed between the metformin plus insulin study arm (n = 65) and the insulin alone arm (n = 48), mainly because of adverse events.
A recent systematic review which analyzed RCTs published until February 2017 [19] found similar results regarding the efficacy of metformin. The authors identified no more recent RCTs than earlier meta-analyses. However, study selection was not completely transparent. The UKPDS [31] was included in the meta-analysis, but only the combination of metformin and SU compared to SU alone, not the comparison of metformin with diet or metformin monotherapy with SU. Moreover, the authors included the 10-year follow-up UKPDS in their analysis. Observational studies were excluded. In fact, the level of evidence of the UKPDS follow-up publication [35] is quite similar to observational studies due to the already mentioned risks of bias [33, 36].
Compared with other interventions, metformin does not increase the risk of mild or severe hypoglycemia. The main adverse events associated with metformin are gastrointestinal side effects, in particular diarrhea. There have been warnings of lactic acidosis due to metformin. The latest Cochrane review [43] and the AHRQ report [25] did not find an increased risk of lactic acidosis with metformin use. Up to 2016, metformin was not recommended for patients with moderate to severe kidney function. Following this advice by practicing physicians might be one reason for a low number of reported cases of lactic acidosis. In 2016, the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) changed recommendations to allow metformin use in patients with moderately reduced kidney function (GFR = 30–59 ml/min) [44, 45]. The FDA explicitly recommends the assessment of benefits and risks in patients with metformin whose GFR fall below 45 mL/minute/1.73 m2. Starting metformin in patients with eGFR between 30 and 45 mL/minute/1.73 m2 is not recommended [45].
Sulfonylurea
The first-generation SU tolbutamide and chlorpropamide were introduced in the 1950s. In the UGDP, tolbutamide increased mortality risk. Nonetheless, both substances were extensively used even after publication of the UGDP in many countries. Today, the first-generation SU have been replaced by the second- and third-generation SU. SU are recommended as initial drug therapy if metformin is contraindicated or not tolerated in patients [15, 46]. Effects of SU compared to metformin are already described in the metformin part of this chapter. We additionally searched for systematic reviews and RCTs on the efficacy of SU as monotherapy compared to diet, placebo, or lifestyle interventions.
As in our previous overview [3], the only RCT that met our inclusion criteria was the UKPDS [32]. In the UKPDS 33, effects of intensive blood glucose control with either SU or insulin were compared to conventional treatment. A total of 615 patients were assigned to glibenclamide, and 896 received conventional treatment which comprised dietary advice. Over 10 years, median HbA1c values were 7.2% for glibenclamide and 7.9% for conventional therapy. More patients in the conventional treatment arm had the primary endpoint any diabetes-related endpoint and microvascular complications, but there were no significant effects on macrovascular outcomes. The effect on the microvascular outcome was mainly attributed to fewer cases of retinal photocoagulation [32].
Patients of the SU group gained more weight (1.7 kg) than the conventional treatment group, and more had major hypoglycemic events (1.4% vs. 0.7%) over 10 years (Table 32.2).
Thiazolidinediones
Thiazolidinediones were introduced in the 1990s. The first agent of this class, troglitazone, was withdrawn from the market because of increased liver damage and toxicity. The remaining compounds, rosiglitazone and pioglitazone, were under selling restrictions or withdrawn in some countries due to safety issues. Meta-analyses showed an increased risk of myocardial infarction in patients who received rosiglitazone [47, 48]. One of the included studies was the RECORD trial with a mean follow-up of 5.5 years [49]. A total of 4447 patients who were treated with metformin or SU monotherapy were randomized to additional rosiglitazone or additional metformin/SU. Patients of the rosiglitazone group had a twofold greater risk of fatal and nonfatal heart failure compared to patients with metformin plus SU treatment. There was no difference between groups regarding the combined primary endpoint, cardiovascular death, or cardiovascular hospitalization. Patients with rosiglitazone therapy reported significantly more bone fractures. Further adverse effects of rosiglitazone comprised weight gain and edema [49]. ADOPT confirmed probable cardiovascular risks and other adverse effects associated with rosiglitazone [42]. The FDA restricted access to rosiglitazone which was part of the Risk Evaluation and Mitigation Strategy (REMS). RECORD had some risk of bias. It was an open-label trial with low statistical power. An unplanned interim analysis was conducted which could have repealed blinding. Patients’ compliance to rosiglitazone was low. In December 2015, based on an independent review of the study, the FDA stated that REMS is no longer needed and that the benefits of rosiglitazone outweigh the risks. In their Standards of Medical Care in Diabetes, the American Diabetes Association recommends TZD as add-on therapy or monotherapy if metformin is contraindicated [46].
With our updated search, we identified a meta-analysis on the effect of pioglitazone on cardiovascular outcomes which also included participants with prediabetes and insulin resistance [23]. Primary endpoint was MACE (major adverse cardiovascular events) comprising cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke. In patients with diabetes pioglitazone was associated with lower risk of MACE. Incidence of myocardial infarction or stroke did not differ between pioglitazone and control groups. Pioglitazone was also associated with an increased risk of heart failure, bone fracture, edema, weight gain, and hypoglycemia [23]. The largest included RCT was the PROactive trial [50]. Patients with type 2 diabetes and previous stroke were randomized to pioglitazone or placebo. Mean study duration was 34.5 months. There was a reduction in the combined endpoint, death from any cause, nonfatal MI, and stroke, for patients randomized to pioglitazone. However, pioglitazone significantly increased risk of heart failure, edema, and weight gain. In addition, a nonsignificant higher rate of bladder cancer was observed [50]. In the meta-analysis, no significant differences were found in bladder or any cancer risk [23]. Another meta-analysis reported a significantly increased risk [22], but was mainly based on the PROactive trial. A systematic review on the effects of TZD on bone fractures confirmed an increased risk of fractures in women who use rosiglitazone or pioglitazone [21]. The National Institute for Clinical Excellence (NICE) recommends pioglitazone when metformin is contraindicated, but explicitly points out the risks of adverse events [15] (Table 32.3).
Alpha Glucosidase Inhibitors and Meglitinides
ADA and EASD [7] do not explicitly recommend the use of AGIs due to their modest effects. AGIs may be tried in specific situations [7]. Two Cochrane reviews including patients with type 2 diabetes and patients with impaired glucose tolerance did not find significant effects of AGIs on mortality or morbidity [51, 52]. We did not include the STOP-NIDDM trial in this overview because of its high risk of bias which was extensively discussed in the literature. The Acarbose Cardiovascular Evaluation (ACE) Trial [53] evaluated the efficacy of acarbose on cardiovascular death, nonfatal MI, and nonfatal stroke in patients with impaired glucose tolerance and coronary heart disease. As mentioned in our former publication [3], the trial may deliver further information. The RCT has been completed in April 2017. However, results were not published at the time of the preparation of this chapter.
Same as SU, meglitinides belong to the drug class of insulin secretagogues. Compounds of this class are nateglinide and repaglinide. In contrast to SU, they are rapid-acting secretagogues. ADA and EASD stated that meglitinides may be used as an alternative to SU in patients with irregular meal schedules [7]. In case repaglinide is considered as alternative to metformin, the NICE guidance on type 2 diabetes in adults suggests physicians to inform patients that there is no licensed non-metformin-based combination with repaglinide [15]. There is no evidence on effects regarding clinically relevant and long-term outcomes [54].
Conclusion
In conclusion, older classes of oral antidiabetic agents still play central roles in diabetes care, but evidence on macro- and microvascular risk is lacking or insufficient.
The applicability of study results is limited due to the short duration of studies [25]. Most studies assess the efficacy of medications on intermediate outcomes rather than long-term hard clinical endpoints. Intermediate outcomes or surrogates must be interpreted with caution. Medication that decreases HbA1c values does not necessarily reduce morbidity or mortality. In some cases of withdrawn drugs, blood glucose levels decreased, while risks of hard clinical endpoints did not change or even increased. Whenever RCTs included patient-relevant endpoints, they were mostly assessed as secondary endpoints or adverse effects. Available studies were often too small to identify any differences between groups. Composite outcome measures, such as any diabetes-related endpoint or macrovascular complications, which usually comprise endpoints of varying importance and validity are challenging to interpret and may lead to overinterpretation of single outcomes.
The authors of the AHRQ report [26] concluded that the efficacy of all diabetes medications regarding all-cause mortality, cardiovascular and cerebrovascular morbidity as well as retinopathy, nephropathy, and neuropathy is still uncertain. The report showed moderate strength of evidence that sulfonylurea monotherapy compared with metformin alone was associated with an increased risk of cardiovascular mortality. This result was mainly based on two RCTs: ADOPT with patients with newly diagnosed diabetes and SPREAD-DIMCAD which included patients with coronary heart disease. In contrast, the meta-analysis by Hemmingsen et al. [20] did not find any differences between SU and metformin monotherapy of total or cardiovascular mortality but a potential benefit of SU regarding nonfatal macrovascular outcomes. However, definition of the composite endpoint differed between studies [20].
In the AHRQ report [26], evidence on intermediate outcomes, such as HbA1c values, was graded as high. Effects on HbA1c values were comparable between most oral antidiabetic agents. Monotherapy comparisons of metformin with sulfonylurea and metformin with TZDs showed similar effects with respect to the reduction in HbA1c values. Moreover, metformin monotherapy reduced body weight more than TZDs or SU, though the clinical relevance of these differences may be debatable. Metformin monotherapy showed greater weight reduction when compared with the combination of metformin and SU or metformin plus TZDs, respectively [26]. In addition, metformin was favored over SU monotherapy, the combination of metformin and TZDs, and over the combination of metformin and SU regarding hypoglycemia. Risk of hypoglycemia was higher for SU than for TZDs [26].
Despite that there is only one RCT with a small sample size which demonstrated an effect on hard clinical endpoints, metformin is internationally recommended as first-line drug for patients with type 2 diabetes. It is used as comparator for the evaluation of new medications although high-quality evidence on patient-relevant outcomes is missing. Thus, the role of metformin as “gold standard” is questionable.
Shared Decision-Making
Even though there is no single perfect treatment of hyperglycemia in patients with type 2 diabetes, decisions about treatment policies and diabetes drug therapy are made for thousands of patients every day.
Shared decision-making is a personalized and patient-centered approach [55] that helps patients and clinicians to select the treatment that best fits individual patient needs, values, and preferences. It is a special way of conversation between patients and healthcare professionals comprising various elements, such as clarifying the patient’s situation, noticing that there is more than one treatment option, information about benefits and harms of the treatment options, and weighing up the pros and cons considering patient values and expectations. Patient decision aids are tools to promote SDM. They are proved to improve patients’ knowledge about treatment options and about probabilities of benefits and adverse effects of each option. Moreover, they help patients to find the option which is most important to them [13]. Decision aids can be used to prepare patients for the consultation with their clinician or within consultations [12]. We have developed an evidence-based patient decision aid on the prevention of myocardial infarction and a corresponding group counseling session in which diabetes educators help patients to understand the information and to define and prioritize own treatment goals regarding statin uptake, smoking cessation, and HbA1c and blood pressure goals [16, 17]. The intervention (informed shared decision-making programme; ISDM) was evaluated in a proof of concept RCT [16]. Patients of the ISDM group achieved higher levels of risk comprehension and realistic expectations about benefits and harms of treatment options. For the following cluster RCT with family practices, we added a structured SDM training for physicians and a patient-held documentation sheet to the intervention in order to optimize the consultation in terms of SDM [56, 57]. Study results showed that the whole ISDM program could be successfully implemented in every day practice. Patients and clinicians of the ISDM group pursued common treatment goals significantly more frequently than the control group [57].
Figure 32.1 displays a 100-stick figure pictogram and bar graphs to visualize probable effects of more or less intensified glucose control on the combined diabetes-related endpoint (UKPDS 34) as used in our patient decision aid and group teaching session [16, 17, 57].
Effects on any diabetes-related event can be explained as follows:
-
The term “any diabetes-related event” is a collective term for different complications of diabetes. It included death from hyperglycemia (high blood sugar) or hypoglycemia, heart attack, angina, heart failure, stroke, kidney failure, amputation, vitreous hemorrhage in the eye (bleeding from abnormal blood vessels in the eye which can lead to blindness), damage to the retina, blindness of one or both eyes, or eye surgery for cataract.
-
In the following you can see the results from the UKPDS [31]. This is a study which was performed in Great Britain and lasted 10 years.
-
Imagine two groups, each with 100 patients with type 2 diabetes followed for 10 years.
-
One group was treated intensively with medication to control blood sugar levels. Patients of that group achieved an average HbA1c of 7%.
-
The comparator (control group) was treated conventionally with less intensive medication and achieved an average HbA1c of 8%.
-
In the group with conventional treatment, “any diabetes-related event” occurred in 46 of the 100 patients during the 10-year period.
-
In the group with intensive control, “any diabetes-related event” occurred in 41 of the 100 patients during the 10-year period.
-
-
That means, intensive blood sugar control over 10 years prevented “any diabetes-related event” in 5 of 100 patients. The remaining 95 of 100 people had no benefit from the intensive treatment over a period of 10 years because they also experienced a diabetes-related event (41 patients) or because they would not have experienced any event even with conventional treatment (54 patients).
Intensively treated patients also experienced harm due to hypoglycemia. An additional 7 out of 100 people suffered severe hypoglycemia with intensive treatment compared to the comparator group over 10 years [31].
Communication of uncertainties is challenging. No one can say if one particular patient would benefit from intensive treatment. Presenting the data helps patients to weigh up the pros and cons making a decision which meets personal preferences and values. Moreover, the effects of antihypertensive treatment and statin intake should be taken into consideration. For example, intensive blood pressure lowering over 8 years (achieved RR 145/82 mmHg) prevented “any diabetes-related event” in 16 out of 100 patients [58].
According to the recent ADA and EASD recommendations [6, 7], clinicians should talk with their patients about the pros and cons of medications to achieve individual treatment goals. In our ISDM program, diabetes educators explain benefits and harms of evidence-based options to prevent cardiovascular complications. They guide patients to estimate their individual heart attack risk and then calculate their risks with and without statin intake and to estimate comparable effects of hypertensions or blood glucose control [16, 56].
Since efficacy of single diabetes medication seems uncertain [26], information about antidiabetic agents can only focus on intermediate outcomes, such as weight change, HbA1c values, hypoglycemia, and other side effects. Montori’s research group developed and evaluated diabetes medication choice decision aid cards on intermediate effects to be used during the clinical encounter [59]. Patients had improved knowledge and were more involved in the decision-making process [59]. Another decision aid addressed statin choice to prevent myocardial infarction in patients with type 2 diabetes [60, 61]. There are also interactive and web-based decision aids which are supposed to foster shared decision-making and goal setting [62] and patient decision aids on special treatments, such as starting insulin [63].
Communication of quality of data is challenging. Patient decision aids are supposed to provide the best available evidence. However, sometimes there is no good evidence but patients have the right to know. Information on level of evidence is provided in guidelines and should be included in the patient information material.
Diabetes care is complex and has to be individualized. Level of evidence of antidiabetic agents on patient-relevant outcomes is low. Treatment of hypertension is more effective than treatment of blood glucose. Thus, involving patients in decision-making and making informed choices should be standard in the medical encounter.
Multiple Choice Questions
-
1.
Which is the aim of the treatment of type 2 diabetes?
-
(a)
Fasting blood glucose control
-
(b)
Avoid acute symptoms of hyperglycemia and to prevent macro and microvascular complications
-
(c)
Postprandial blood glucose control
-
(d)
Increase the use of medications
-
(e)
Weight reduction and control
-
(a)
-
2.
Rigid treatment regimens with low HbA1c targets:
-
(a)
Have resulted in better patient-relevant outcomes
-
(b)
Have produced equal patient-relevant outcomes
-
(c)
Are associated with higher risks of mortality
-
(d)
Improve health related-quality of life
-
(e)
Reduce hospital admissions and costs
-
(a)
-
3.
What was the argument to withdraw several new antidiabetic agents from the German market?
-
(a)
No additional benefit over usual care could be demonstrated and health insurances would not have covered additional costs
-
(b)
Higher costs compared with traditional medications
-
(c)
Higher risk of hypoglycemia
-
(d)
Unacceptable risk of nondiabetic ketoacidosis
-
(e)
All of the above
-
(a)
-
4.
According to the recent ADA and EASD recommendations, clinicians should not discuss with patients the pros and cons of medications to achieve individual treatment goals
-
(a)
False
-
(b)
True
-
(a)
-
5.
What is the mechanism of action of metformin?
-
(a)
Reduction of insulin resistance in target cells through transcription of several genes involved in glucose and lipid metabolism
-
(b)
Inhibition of alpha-glucosidase, delaying intestinal degradation of complex carbohydrates and prolonging postprandial glucose absorption
-
(c)
Multiple sites of action, including increase of insulin sensitivity by increasing peripheral glucose uptake, decrease of intestinal glucose absorption, and decrease of hepatic glucose production
-
(d)
Stimulation of insulin release in pancreatic beta cells. Decrease in hepatic clearance of insulin. Additional extra-pancreatic mechanisms
-
(e)
Increase of insulin sensitivity by skeletal muscle
-
(a)
-
6.
What is the mechanism of action of glyburide?
-
(a)
Reduction of insulin resistance in target cells through transcription of several genes involved in glucose and lipid metabolism
-
(b)
Inhibition of alpha-glucosidase, delaying intestinal degradation of complex carbohydrates and prolonging postprandial glucose absorption
-
(c)
Increase of insulin sensitivity by increasing peripheral glucose uptake, decrease of intestinal glucose absorption, and decrease of hepatic glucose production
-
(d)
Stimulation of insulin release in pancreatic beta cells. Decrease in hepatic clearance of insulin. Additional extra-pancreatic mechanisms
-
(e)
Increase of insulin sensitivity by skeletal muscle
-
(a)
-
7.
What is the mechanism of action of thiazolidinediones?
-
(a)
Reduction of insulin resistance in target cells through transcription of several genes involved in glucose and lipid metabolism
-
(b)
Inhibition of alpha-glucosidase, delaying intestinal degradation of complex carbohydrates and prolonging postprandial glucose absorption
-
(c)
Increase of insulin sensitivity by increasing peripheral glucose uptake, decrease of intestinal glucose absorption, and decrease of hepatic glucose production
-
(d)
Stimulation of insulin release in pancreatic beta cells. Decrease in hepatic clearance of insulin. Additional extra-pancreatic mechanisms
-
(e)
Increase of insulin sensitivity by skeletal muscle
-
(a)
-
8.
What is the mechanism of action of alpha-glucosidase inhibitors?
-
(a)
Reduction of insulin resistance in target cells through transcription of several genes involved in glucose and lipid metabolism
-
(b)
Inhibition of alpha-glucosidase, delaying intestinal degradation of complex carbohydrates and prolonging postprandial glucose absorption
-
(c)
Increase of insulin sensitivity by increasing peripheral glucose uptake, decrease of intestinal glucose absorption, and decrease of hepatic glucose production
-
(d)
Stimulation of insulin release in pancreatic beta cells. Decrease in hepatic clearance of insulin. Additional extra-pancreatic mechanisms
-
(e)
Increase of insulin sensitivity by skeletal muscle
-
(a)
-
9.
What is a patient relevant outcome?
-
(a)
HbA1c
-
(b)
Weight
-
(c)
Fasting plasma glucose
-
(d)
Hypoglycemia
-
(e)
Cholesterol levels
-
(a)
-
10.
Moderate strength of evidence suggest that the combination of sulfonylurea and metformin compared with metformin alone was associated with:
-
(a)
Higher risk of death from any cause and diabetes-related death
-
(b)
An increase in metabolic control
-
(c)
Lower weight gain
-
(d)
Reducing oxidative stress and pro-inflammatory molecules
-
(e)
Lower risk of severe hypoglycemia
-
(a)
Correct Answers
-
1.
(b) Avoid acute symptoms of hyperglycemia and to prevent macro and microvascular complications
-
2.
(c) Are associated with higher risks of mortality
-
3.
(a) No additional benefit over usual care could be demonstrated and health insurances would not have covered additional costs
-
4.
(a) False
-
5.
(c) Multiple sites of action, including increase of insulin sensitivity by increasing peripheral glucose uptake, decrease of intestinal glucose absorption, and decrease of hepatic glucose production
-
6.
(d) Stimulation of insulin release in pancreatic beta cells. Decrease in hepatic clearance of insulin. Additional extra-pancreatic mechanisms
-
7.
(a) Reduction of insulin resistance in target cells through transcription of several genes involved in glucose and lipid metabolism
-
8.
(b) Inhibition of alpha-glucosidase, delaying intestinal degradation of complex carbohydrates and prolonging post-prandial glucose absorption
-
9.
(d) Hypoglycemia
-
10.
(a) Higher risk of death from any cause and diabetes-related death
References
Chamberlain JJ, Herman WH, Leal S, et al. Pharmacologic therapy for type 2 diabetes: synopsis of the 2017 American Diabetes Association standards of medical care in diabetes. Ann Intern Med. 2017;166:572–8.
Boussageon R, Gueyffier F, Cornu C. Effects of pharmacological treatments on micro- and macrovascular complications of type 2 diabetes: what is the level of evidence? Diabetes Metab. 2014;40:169–75.
Buhse S, Mühlhauser I, Lenz M. The ‘old’ anti-diabetic agents: a systematic inventory. In: Stettler C, Christ E, Diem P (Hrsg.), editors. Novelties in diabetes. Endocr Dev. Basel: Karger. 2015;31:28–42.
Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. NEJM. 2008;358:2545–59.
Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. NEJM. 2009;360:129–39.
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2012;55(6):1577–96.
Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–9.
Charles C, Gafni A, Whelan T. Shared decision-making in the medical encounter: what does it mean? (or it takes at least two to tango). Soc Sci Med. 1997;44:681–92.
Marteau TM, Dormandy E, Michie S. A measure of informed choice. Health Expect. 2001;4:99–108.
Bunge M, Mühlhauser I, Steckelberg A. What constitutes evidence-based patient information? overview of discussed criteria. Patient Educ Couns. 2010;78(3):316–28.
Mühlhauser I, Berger M. Evidence-based patient information in diabetes. Diabet Med. 2000;17:823–9.
Montori VM, Kunneman M, Brito JP. Shared decision making and improving health care: the answer is not in. JAMA. 2017;318:617–8.
Stacey D, Legare F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:Cd001431.
Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm – 2017 executive summary. Endocr Pract. 2017;23:207–38.
National Institute for Health and Care Excellence (NICE). Type 2 diabetes in adults: management. NICE guideline. 2015. Available from nice.org.uk/guidance/ng28.
Buhse S, Mühlhauser I, Heller T, et al. Informed shared decision-making programme on the prevention of myocardial infarction in type 2 diabetes: a randomised controlled trial. BMJ Open. 2015;5:e009116.
Lenz M, Kasper J, Mühlhauser I. Development of a patient decision aid for prevention of myocardial infarction in type 2 diabetes – rationale, design and pilot testing. Psychosoc Med. 2009;6:Doc05.
Higgins JP, Welton NJ. Network meta-analysis: a norm for comparative effectiveness? Lancet. 2015;386:628–30.
Griffin SJ, Leaver JK, Irving GJ. Impact of metformin on cardiovascular disease: a meta-analysis of randomised trials among people with type 2 diabetes. Diabetologia. 2017;60:1620. https://doi.org/10.1007/s00125-017-4337-9.
Hemmingsen B, Schroll JB, Wetterslev J, et al. Sulfonylurea versus metformin monotherapy in patients with type 2 diabetes: a Cochrane systematic review and meta-analysis of randomized clinical trials and trial sequential analysis. CMAJ Open. 2014;2:E162–75.
Zhu ZN, Jiang YF, Ding T. Risk of fracture with thiazolidinediones: an updated meta-analysis of randomized clinical trials. Bone. 2014;68:115–23.
Turner RM, Kwok CS, Chen-Turner C, Maduakor CA, Singh S, Loke YK. Thiazolidinediones and associated risk of bladder cancer: a systematic review and meta-analysis. Br J Clin Pharmacol. 2014;78:258–73.
Liao HW, Saver JL, Wu YL, Chen TH, Lee M, Ovbiagele B. Pioglitazone and cardiovascular outcomes in patients with insulin resistance, pre-diabetes and type 2 diabetes: a systematic review and meta-analysis. BMJ Open. 2017;7:e013927.
Lee M, Saver JL, Liao HW, Lin CH, Ovbiagele B. Pioglitazone for secondary stroke prevention: a systematic review and meta-analysis. Stroke. 2017;48:388–93.
Maruthur NM, Tseng E, Hutfless S, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2016;164:740–51.
Bolen S, Tseng E, Hutfless S, et al. AHRQ comparative effectiveness reviews diabetes medications for adults with type 2 diabetes: an update. Rockville: Agency for Healthcare Research and Quality; 2016.
Meinert CL, Knatterud GL, Prout TE, Klimt CR. A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. II. Mortality results. Diabetes. 1970;19(Suppl):789–830.
The University Group Diabetes Program. A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. V. Evaluation of pheniformin therapy. Diabetes. 1975;24(Suppl 1):65–184.
Qaseem A, Barry MJ, Humphrey LL, Forciea MA. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017;166:279–90.
German Medical Association, National Association of Statutory Health Insurance Physicians, Association of the Scientific Medical Societies: National Disease Management Guidelines Programme: Typ-2-Diabetes mellitus – Therapy; 2013. Available from: http://www.versorgungsleitlinien.de/themen/diabetes2/dm2_therapie/pdf.
U. K. Prospective Diabetes Study Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854–65.
U. K. Prospective Diabetes Study Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–53.
Boussageon R, Gueyffier F, Cornu C. Metformin as first-line treatment for type 2 diabetes: are we sure? BMJ. 2016;352:h6748.
Berger M, Mühlhauser I. Diabetes care and patient-oriented outcomes. JAMA. 1999;281:1676–8.
Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. NEJM. 2008;359:1577–89.
Mühlhauser I. Follow-up of intensive glucose control in type 2 diabetes (letter). NEJM. 2009;360:417. author reply 418.
Boussageon R, Supper I, Bejan-Angoulvant T, et al. Reappraisal of metformin efficacy in the treatment of type 2 diabetes: a meta-analysis of randomised controlled trials. PLoS Med. 2012;9:e1001204.
Rachmani R, Slavachevski I, Levi Z, Zadok B, Kedar Y, Ravid M. Metformin in patients with type 2 diabetes mellitus: reconsideration of traditional contraindications. Eur J Intern Med. 2002;13:428.
Cryer DR, Nicholas SP, Henry DH, Mills DJ, Stadel BV. Comparative outcomes study of metformin intervention versus conventional approach the COSMIC Approach Study. Diabetes Care. 2005;28:539–43.
Kooy A, de Jager J, Lehert P, et al. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med. 2009;169:616–25.
Hong J, Zhang Y, Lai S, et al. Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease. Diabetes Care. 2013;36:1304–11.
Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. NEJM. 2006;355:2427–43.
Salpeter SR, Greyber E, Pasternak GA, Salpeter EE. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev. 2009;4:CD002967.
European Medicines Agency. Use of metformin to treat diabetes now expanded to patients with moderately reduced kidney function (2016) EMA/603690/2016. Available from http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2016/10/WC500214248.pdf, Accessed 15 August 2017.
U.S. Food and Drug Administration. Metformin-containing drugs: drug safety communication – revised warnings for certain patients with reduced kidney function. 2016. Available from https://www.fda.gov/safety/medwatch/safetyinformation/safetyalertsforhumanmedicalproducts/ucm494829.htm.
American Diabetes Association. 8. Pharmacologic approaches to glycemic treatment. Diabetes Care. 2017;40:S64–s74.
Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–71.
Nissen SE, Wolski K. Rosiglitazone revisited: an updated meta-analysis of risk for myocardial infarction and cardiovascular mortality. Arch Intern Med. 2010;170:1191–201.
Home PD, Pocock SJ, Beck-Nielsen H, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373:2125–35.
Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–89.
Van de Laar FA, Lucassen PL, Akkermans RP, Van de Lisdonk EH, De Grauw WJ. Alpha-glucosidase inhibitors for people with impaired glucose tolerance or impaired fasting blood glucose. Cochrane Database Syst Rev. 2006;4:CD005061.
Van de Laar FA, Lucassen PLBJ, Akkermans RP, Van de Lisdonk EH, Rutten GEHM, Van Weel C. Alpha-glucosidase inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005;2:CD003639.
Holman RR, Bethel MA, Chan JC, et al. Rationale for and design of the Acarbose Cardiovascular Evaluation (ACE) trial. Am Heart J. 2014;168:23–29 e22.
Black C, Donnelly P, McIntyre L, Royle P, Shepherd JJ, Thomas S. Meglitinide analogues for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2009;2:CD004654.
Kunneman M, Montori VM, Castaneda-Guarderas A, Hess EP. What is shared decision making? (and what it is not). Acad Emerg Med. 2016;23:1320–4.
Buhse S, Mühlhauser I, Kuniss N, et al. An informed shared decision making programme on the prevention of myocardial infarction for patients with type 2 diabetes in primary care: protocol of a cluster randomised, controlled trial. BMC Fam Pract. 2015;16:43.
Buhse S, Kuniss N, Liethmann K, et al. Informed shared decision-making programme for patients with type 2 diabetes in primary care: cluster randomised controlled trial. BMJ Open. 2018;8(12):e024004.
U. K. Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–13.
Mullan RJ, Montori VM, Shah ND, et al. The diabetes mellitus medication choice decision aid: a randomized trial. Arch Intern Med. 2009;169:1560–8.
Weymiller AJ, Montori VM, Jones LA, et al. Helping patients with type 2 diabetes mellitus make treatment decisions: statin choice randomized trial. Arch Intern Med. 2007;167:1076–82.
Mann DM, Ponieman D, Montori VM, Arciniega J, McGinn T. The statin choice decision aid in primary care: a randomized trial. Patient Educ Couns. 2010;80:138–40.
Yu CH, Stacey D, Sale J, et al. Designing and evaluating an interprofessional shared decision-making and goal-setting decision aid for patients with diabetes in clinical care -systematic decision aid development and study protocol. Implement Sci. 2014;9:16.
Mathers N, Ng CJ, Campbell MJ, Colwell B, Brown I, Bradley A. Clinical effectiveness of a patient decision aid to improve decision quality and glycaemic control in people with diabetes making treatment choices: a cluster randomised controlled trial (PANDAs) in general practice. BMJ Open. 2012;2:e001469.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Buhse, S., Mühlhauser, I. (2019). The “Old” Oral Antidiabetics. In: Rodriguez-Saldana, J. (eds) The Diabetes Textbook. Springer, Cham. https://doi.org/10.1007/978-3-030-11815-0_32
Download citation
DOI: https://doi.org/10.1007/978-3-030-11815-0_32
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-11814-3
Online ISBN: 978-3-030-11815-0
eBook Packages: MedicineMedicine (R0)