Abstract
Traumatic brain injury (TBI) arises from disruptions in the structural connectivity of brain, which further manifests itself as alterations in the functional connectivity, eventually leading to cognitive and behavioral deficits. Although patient-specific measures quantifying the severity of disease is crucial due to the heterogeneous character of the disease, neuroimaging based measures that can assess the level of injury in TBI using structural and functional connectivity is very scarce. Taking a graph theoretical approach, we propose a measure to quantify how dissimilar a TBI patient is relative to healthy subjects using their structural and functional connectomes. Over a TBI dataset with 39 moderate-to-severe TBI patients that are examined 3, 6, and 12 months post injury, and 35 healthy controls, we demonstrate that the dissimilarity scores obtained by the proposed measure distinguish patients from controls using both modalities. We also show that the dissimilarity scores significantly correlate with post-traumatic amnesia, processing speed, and executive function among TBI patients. Our results indicate the applicability of the proposed measure in quantitatively assessing the extent of injury. The measure is applicable to structural and functional connectivity, paving the way for a joint analysis in the future.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Traumatic brain injury (TBI) is mainly considered as a white matter disorder arising from axonal injuries, which can result from causes such as fall or traffic accidents. Injury of axons cause disruptions in neural communication, eventually leading to psychological, cognitive, and emotional disturbances, making it a significant public health burden [1]. Although the relationship between trauma induced white matter injury and the neuropsychological outcomes in TBI has been established for over two decades [2], neuroimaging measures that effectively quantify the injury burden based on connectivity disruptions started emerging recently and are still limited [3, 4]. Heterogeneous character of TBI necessitates subject-specific investigations for providing patient-specific diagnostics and prognostics, which further highlights the need for imaging based measures.
Several studies in the literature have investigated the relationship between brain structure and behavioral outcomes in TBI on the basis of diffusion characteristics such as FA and MD [5]. Another major direction of research in elucidating this relationship has focused on changes in the topology of the structural brain network [6]. Recently, a summary measure of diffuse connectivity alterations to quantify overall injury burden was proposed in [4] which demonstrated high correlations with cognitive deficits. However, measures to quantify structural and functional disruptions in TBI together and investigating its relationship with cognitive outcomes of the disease has been sparsely studied.
Connectomes can naturally be represented as graphs, making a wide array of graph theoretical tools available for connectomic analysis. This, in turn, makes it possible to investigate structural and functional characteristics of the brain while preserving its organizational features [7]. Although fundamental graph theory measures such as centrality and small worldness are widely applied to connectomic analysis [8], application of advanced graph theory tools to brain data is still in its early stages. Graph similarity measures are prime examples of such tools that found very limited applications in brain analysis to date despite their great potentials.
Since the brain is commonly represented as connectomes, which are weighted graphs in essence, finding similarities between them can be considered as a graph matching problem. The main idea in here is to find a mapping between nodes and edges of two graphs while minimizing an overall similarity score. Although widely applied in pattern recognition and computer vision over several decades [9, 10], use of graph matching as a similarity measure in neuroscience very scarce [11]. One of the early uses of graph similarity measures in connectomics is the application of graph edit distance in network classification of the epileptic brain [12]. Graph embedding [13] and graph kernels [14] are utilized in decoding of brain states in fMRI, which in turn is used for performing classification. Further utilization of graph similarity measures for quantifying neurological diseases and disorders is still a fertile area of investigation.
In this preliminary study, we explore graph similarity measures as a quantifier of injury on TBI using structural and functional connectomes, and investigate its relationship with emerging cognitive deficits. Specifically, over a longitudinal TBI dataset of 39 patients and 35 controls, we consider connectomes as weighted graphs and define dissimilarity between patients and controls by taking a graph matching approach to calculate similarity between two subjects. We demonstrate that there is significant group difference between patients and controls in both structural and functional connectomes, when graph based dissimilarity of subjects is used as the metric of injury. We further show that the proposed dissimilarity score also correlates well with the cognitive scores of the patients.
2 Materials and Methods
2.1 Dataset
We use a traumatic brain injury dataset consisting of 35 controls and 39 TBI patients [4]. For each subject, DTI and rs-fMRI data was acquired, once for healthy controls and three times for patients with TBI at approximately 3, 6, and 12 months post-injury, respectively. In addition to neuroimaging, TBI patients were also subjected to behavioral assessment at these time points.
Preprocessing of DTI Data: For each subject, DTI data was acquired on a Siemens 3T TrioTim scanner with a 8 channel head coil (single shot spin echo sequence, TR/TE = 6500 / 84 ms, \(b = 1000\,\mathrm{s/mm}^{2}\), 30 gradient directions). 86 region of interests from the Desikan atlas [15] were extracted to represent the nodes of the structural network. A mask was defined using voxels with an FA of at least 0.1 for each subject. Deterministic tractography was performed to generate and select 1 million streamlines, seeded randomly within the mask. Angle curvature threshold of 60\(^{\circ }\), and a min and max length threshold of 5 mm and 400 mm were applied, resulting in an \(86\times 86\) adjacency matrix of weighted connectivity values, where each element represents the number of streamlines between regions.
Preprocessing of rs-fMRI Data: For each subject, resting state fMRI data was acquired on a Siemens 3T TrioTim scanner with a 8-channel head coil (single-shot, multi-slice, gradient-echo (GE) echoplanar (EPI) sequence, TR/TE = 3000/3 ms, interleaved acquisition, 3 mm isotropic voxel dimensions). The resting state fMRI data were pre-processed using preprocessing pipeline in [16]. The first 6 volumes of the BOLD 4-D time series data were discarded to allow signal stabilization. All functional time series were slice-time corrected, motion corrected to the median image using a rigid registration, and co-registered with the anatomical MPRAGE image. The DVARS method [17] was used to estimate the degree of image intensity change across volumes attributed to motion where volumes with excessive motion were flagged and not used for further analysis. Confound regression was performed to regress out the average BOLD signal from non-gray matter tissue compartments. Pairwise correlations were calculated across 86 nodes of the Desikan atlas [15] using Pearson’s correlation, and finally an \(86\times 86\) resting state functional connectivity matrix was built for each subject.
Cognitive Measures: In our analysis, we included four clinical measures for assessing outcomes of injury that are widely used in TBI research: first three measures are used for quantifying cognitive function, and the fourth is used for measuring injury severity.
-
(i)
Processing speed (PS): We used the Processing Speed Index from the Wechsler Adult Intelligence Scale-IV [18] to determine the speed of mental processing.
-
(ii)
Executive function (EF): Following five tests were used to measure different aspects of executive function: Controlled Oral Word Association Test [19], Trail Making Test-Part B [20], Color-Word Interference Test, and Digits Backward and Letter-Number Sequencing subtests from the Wechsler Memory Scale IV [21]. In order to reduce type I error and increase signal-to-noise ratio, we constructed a composite score by identifying the rank of a participant on each individual measure, and then averaging the ranks across five measures.
-
(iii)
Verbal learning (VL): The Rey Auditory-Verbal Learning Test [22] was used to evaluate verbal learning.
-
(iv)
Duration of post-traumatic amnesia (PTA): PTA is a behavioral index of the severity of the neurological injury, which is calculated as the number of days between the TBI and the time within 72 h that the participant was fully oriented.
2.2 Graph Matching as a Similarity Measure
Having obtained structural and functional connectomes of patients and controls, our goal is to investigate connectivity based (dis)similarities across the two groups. Since graph theory provides a rich repertoire of tools that can be utilized to characterize the properties of brain networks and solve various network related problems [23], we considered connectomes as graphs. A graph is an ordered pair \(G=(V,E)\) consisting of a set of nodes V, and a set of edges \(E \subset V \times \ V\) that define a relation between node pairs. Connectomes can be effectively represented with weighted graphs where nodes correspond to brain regions and the weighted edges correspond to connectivity between brain regions with edge weights quantifying the strength of pairwise connectivity. Since the structural connectomes that we obtained from tractography and functional connectomes that we obtained via Pearson’s correlation are symmetrical matrices indicating the nondirectional nature of relation between regions, we represented the connectomes with undirected weighted graphs.
Once connectomes are represented as graphs, an efficient way of calculating (dis)similarity across subjects is through graph matching [9], where the goal is to find a mapping between the nodes of two graphs along with an overall score quantifying their similarity. In this study, we formulated the graph matching as an instance of the linear assignment problem [24]. Specifically, given two graphs \(\mathcal {P}=(V_{\mathcal {P}},E_{\mathcal {P}})\) and \(\mathcal {Q}=(V_{\mathcal {Q}},E_{\mathcal {Q}})\), the aim is to find the optimal one-to-one mapping \(f:V_\mathcal {P}\rightarrow V_\mathcal {Q}\) between their nodes while minimizing the following objective function:
where \(c(\cdot ,\cdot )\) is a cost function determining the cost of assigning each node in \(\mathcal {P}\) to a corresponding node in \(\mathcal {Q}\). In order to define assignment cost between nodes of graphs, we annotated each node with an 86-dimensional feature vector which represented their connectivity with the rest of the nodes in the graph. We then considered Euclidean distance between feature vectors of nodes as the cost function. We used the Hungarian algorithm [25] for solving the matching problem, obtaining the optimal one-to-one mapping f between the nodes of the two graphs.
Solution to the graph matching can provide two similarity measures. First one is a summary measure obtained by summing the assignment costs of nodes in (1) based on the calculated mapping f. Hence, matching connectomes of a healthy control and a severe TBI patient, one would expect to get a high dissimilarity score. The second measure, denoted matching accuracy, leverages apriori knowledge of true matching between nodes by quantifying the similarity as the ratio of nodes that matched to its counterpart in the other graph over total number of nodes. Contrary to the previous measure, one would expect to get a lower score for comparing a healthy control with a severe TBI patient. In this study, we investigated the first similarity measure and left the analysis with the second measure to an extensive future study.
2.3 Experimental Setup
In our analysis, we considered the dissimilarity of individual subjects relative to the control population as a measure of TBI severity. For each patient at all three time points, we calculated its dissimilarity with each healthy control subject using graph matching. We then considered the average of these distances as the dissimilarity of the patient with respect to healthy control population. In order to analyze the distribution of dissimilarities across subject groups, we similarly calculated the average dissimilarity of each healthy subject with respect to the rest of the healthy control population. We finally calculated the z-score of each of the dissimilarity scores with respect to the healthy control population to obtain a standardized dissimilarity measure. In our analysis, we used weighted graph representation of structural and functional connectomes of subjects separately for calculating dissimilarities. While investigating dissimilarity using functional data, we separately evaluated the complete functional connectome having both positive and negative correlations, and two additional connectomes that consist of only positive and only negative functional edges.
3 Results and Discussion
Here, we demonstrate the efficacy of the proposed connectomic dissimilarity measure over the TBI dataset in distinguishing subject groups and its correlation with cognitive scores of individual subjects.
3.1 Structural Connectivity
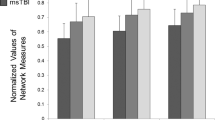
Boxplot of average structural dissimilarities of subjects with respect to the healthy control population is shown in Fig. 1. We observe that patient groups at 3, 6, and 12 months have higher dissimilarity scores relative to healthy controls, demonstrating significant group differences with effect sizes of 0.91, 0.68, and 0.88, respectively (Student’s t-test, \(p<0.01\), effect size calculated using Cohen’s d). Distribution of dissimilarities span a larger interval for the patient groups than controls, which can be attributed to heterogeneity of the TBI. We observe a decline in dissimilarity at 6 months which later on increases at 12 months, where the group difference between the 3–6 months and 6–12 months are significant with effect sizes 0.95 and 0.72, respectively (\(p<0.01\)). It is also interesting to note that the longitudinal trajectory of processing speed follows that of dissimilarity scores. This pattern resembles the curvilinear recovery trajectory of the PS reported in [26] over the same data set. Overall, these results indicate that the dissimilarity captured by graph matching between the graph representation of structural connectomes distinguishes TBI patients from controls, as well as patient groups at different time points from each other.
Z-score of graph dissimilarity of subjects in each group (healthy and patients in 3 time points) with respect to the healthy controls, where the distance is measured over the structural connectome of subjects. Red lines indicate means of distributions. Group differences are significant between patients at all time points and controls. (Color figure online)
We further calculated the correlation between dissimilarity scores of the patients with respect to healthy controls, and their cognitive scores, to investigate whether the dissimilarity score captures cognitive changes in TBI (Table 1). We observe significant correlations between the dissimilarity scores of patients and their cognitive scores and PTA duration. Specifically, a significant negative correlation was observed for processing speed at all time points, with correlation reaching its peak at 6 months. It is interesting to note the parallelism between the strength of correlation peaking at 6 months and the processing speed recovering at its highest level at 6 months post-injury. In addition, the negative correlation of executive function is significant in 6 and 12 months with an increase in the magnitude of correlation over time. We also observed verbal learning to significantly correlate with dissimilarity at 6 months.
We observe significant negative correlations between PTA and the similarity scores of patients at 3, 6, and 12 months, with a steady increase in the magnitude of the correlation over time. Noting that PTA is a measure of trauma severity which is calculated once for each patient based on the duration of post traumatic amnesia, the increase in correlation over time can be attributed to the structural connectomes at 12 months post-injury revealing the degree of injury severity better due to progressed neurodegeneration. PTA duration is assumed to reflect the overall amount of axonal injury, suggesting that graph matching based dissimilarity score captures the injury level of the patients.
3.2 Functional Connectivity
Boxplots of average functional dissimilarities of subjects with respect to the healthy control population is shown in Fig. 2. We evaluated the functional dissimilarity of subjects using full functional connectome, and positive and negative functional connectomes separately. We observe significant group difference between healthy controls and patients at three time points for full functional connectome with effect sizes 0.99, 0.98, and 1.09 (\(p<0.01\), effect size calculated using Cohen’s d), respectively (Fig. 2a). A similar pattern is observed in the positive functional connectome where the patients were significantly different than controls at 3 and 12 months with effect sizes 0.51 and 0.65 (\(p<0.05\)) (Fig. 2b). We did not observe any significant group difference between the dissimilarity scores of patients and controls over negative functional connectomes. We also calculated the correlation between the dissimilarities using full, positive, and negative functional connectomes and the cognitive scores, and did not observe any significant correlation between them.
4 Conclusions and Future Work
In this paper, we presented a graph matching based approach for calculating dissimilarity between TBI patients and controls in terms of structural and functional connectomes, and demonstrated its utility over a longitudinal TBI dataset. We showed that the proposed dissimilarity metric can distinguish patient groups at three time points from healthy controls as well as differentiating patient groups in different time points from each other using both modalities. We also demonstrated that, the structural dissimilarity of patients correlates well with neuropsychological scores and a clinical measure used to evaluate TBI severity.
We note that, application of the method we presented here can be considered as a proof of concept for the use of graph matching in the calculation of dissimilarities between connectomes to assess neuropathology and plasticity in TBI. We will extend this study to develop a trauma specific dissimilarity measure by focusing on subgraphs with edges that are significantly affected by the disease across the population as suggested in [4].
As the trauma causes disconnections in the structural connectivity of the brain, one might expect to observe larger differences across patients and controls in the connectivity strength between indirectly connected regions. We will further extend this study to investigate the dissimilarities across subject groups by considering communication between regions over structural pathways by using message passing schemes such as shortest path or communicability. Another interesting question to be addressed is whether the brain changes its communication scheme post trauma, which requires an in depth structure-function coupling analysis of the TBI patients.
In this study, we utilized the value of the objective function of graph matching as a summary measure of dissimilarity between connectomes. Further similarity measures, such as (mis)matching nodes, can be extracted as a by-product of the same approach, which can help in detailed analysis of the dissimilarities at multiple scales of network, which we will investigate in the extended version of this study.
References
Johnson, V.E., Stewart, W., Smith, D.H.: Axonal pathology in traumatic brain injury. Exp. Neurol. 246, 35–43 (2013)
Gale, S.D., Johnson, S.C., Bigler, E.D., Blatter, D.D.: Nonspecific white matter degeneration following traumatic brain injury. J. Int. Neuropsychological Soc. 1(1), 17–28 (1995)
Hayes, J.P., Bigler, E.D., Verfaellie, M.: Traumatic brain injury as a disorder of brain connectivity. J. Int. Neuropsychological Soc. 22(2), 120–137 (2016)
Solmaz, B., et al.: Assessing connectivity related injury burden in diffuse traumatic brain injury. Hum. Brain Mapp. 38(6), 2913–2922 (2017)
Bonnelle, V., et al.: Default mode network connectivity predicts sustained attention deficits after traumatic brain injury. J. Neurosci. 31(38), 13442–13451 (2011)
Caeyenberghs, K., et al.: Altered structural networks and executive deficits in traumatic brain injury patients. Brain Struct. Function 219(1), 193–209 (2014)
Bullmore, E.T., Sporns, O., Solla, S.A.: Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 10(3), 186–198 (2009)
Sporns, O.: From simple graphs to the connectome: networks in neuroimaging. Neuroimage 62(2), 881–886 (2012)
Livi, L., Rizzi, A.: The graph matching problem. Pattern Anal. Appl. 16(3), 253–283 (2013)
Osmanlıoğlu, Y., Ontañón, S., Hershberg, U., Shokoufandeh, A.: Efficient approximation of labeling problems with applications to immune repertoire analysis. In: 2016 23rd International Conference on Pattern Recognition (ICPR), pp. 2410–2415. IEEE (2016)
Ktena, S.I., et al.: Metric learning with spectral graph convolutions on brain connectivity networks. NeuroImage 169, 431–442 (2018)
Raj, A., Mueller, S.G., Young, K., Laxer, K.D., Weiner, M.: Network-level analysis of cortical thickness of the epileptic brain. Neuroimage 52(4), 1302–1313 (2010)
Richiardi, J., Eryilmaz, H., Schwartz, S., Vuilleumier, P., Van De Ville, D.: Decoding brain states from fmri connectivity graphs. Neuroimage 56(2), 616–626 (2011)
Mokhtari, F., Hossein-Zadeh, G.-A.: Decoding brain states using backward edge elimination and graph kernels in fMRI connectivity networks. J. Neurosci. Methods 212(2), 259–268 (2013)
Desikan, R.S., et al.: An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31(3), 968–980 (2006)
Satterthwaite, T.D., et al.: An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage 64, 240–256 (2013)
Power, J.D., Barnes, K.A., Snyder, A.Z., Schlaggar, B.L., Petersen, S.E.: Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59(3), 2142–2154 (2012)
Wechsler, D., Coalson, D.L., Raiford, S.E.: WAIS-IV: Wechsler Adult Intelligence Scale. Pearson, San Antonio (2008)
Benton, A.L., deS. Hamsher, K., Sivan, A.B.: Multilingual Aphasia Examination: Token Test. AJA Associates, Iowa City (1994)
Reitan, R.M., Wolfson, D.: The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation, vol. 4. Reitan Neuropsychology (1985)
Wechsler, D.: Wechsler Memory Scale Fourth Edition (WMSIV). Pearson, San Antonio (2009)
Rey, A.: Memorisation d’une serie de 15 mots en 5 repetitions. L’examen clinique en psychologie (1958)
Fornito, A., Zalesky, A., Breakspear, M.: Graph analysis of the human connectome: promise, progress, and pitfalls. Neuroimage 80, 426–444 (2013)
Koopmans, T.C., Beckmann, M.: Assignment problems and the location of economic activities. Econometrica: J. Econometric Soc. 25, 53–76 (1957)
Kuhn, H.W.: The hungarian method for the assignment problem. Naval Res. Logistics Quart. 2(1–2), 83–97 (1955)
Rabinowitz, A.R., Hart, T., Whyte, J., Kim, J.: Neuropsychological recovery trajectories in moderate to severe traumatic brain injury: influence of patient characteristics and diffuse axonal injury. J. Int. Neuropsychological Soc. 24(3), 237–246 (2018)
Acknowledgements
This work was funded by NIH grants R01HD089390-01A1, 1 R01 NS096606, 5R01NS092398, and 5R01NS065980.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this paper
Cite this paper
Osmanlıoğlu, Y., Alappatt, J.A., Parker, D., Kim, J., Verma, R. (2019). A Graph Based Similarity Measure for Assessing Altered Connectivity in Traumatic Brain Injury. In: Crimi, A., Bakas, S., Kuijf, H., Keyvan, F., Reyes, M., van Walsum, T. (eds) Brainlesion: Glioma, Multiple Sclerosis, Stroke and Traumatic Brain Injuries. BrainLes 2018. Lecture Notes in Computer Science(), vol 11383. Springer, Cham. https://doi.org/10.1007/978-3-030-11723-8_19
Download citation
DOI: https://doi.org/10.1007/978-3-030-11723-8_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-11722-1
Online ISBN: 978-3-030-11723-8
eBook Packages: Computer ScienceComputer Science (R0)