Abstract
Estrogens (E), in oral contraceptives (COCs) and hormone replacement therapy (HRT) drugs used for the relief of climacteric symptoms of menopause, increase the synthesis of clotting factors, decrease the levels of coagulation inhibitors, and increase the risk of venous thromboembolic events (VTE). Ischemic stroke incidence in postmenopausal women during HRT use is also increased and is probably due to a thrombotic event. This suggests that a safer estrogen may reduce stroke and VTE incidence, with lower impact on hemostasis.
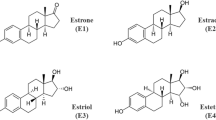
Estetrol (E4) is a relatively recently described new human-specific E produced exclusively by the fetal liver during pregnancy. This Native (human and natural) E has Selective actions in Tissues (NEST). Nest activities of E4 are the consequence of its unique dual role. It activates the nuclear estrogen receptor alpha (ERα) but antagonizes the membrane ERα in contrast to other E, which activate both types of receptors. Most beneficial effects of E on the vascular system have been ascribed to the activation of the membrane ERα of vascular endothelial cells, including enhancement of nitric oxide (NO) production, vasodilation, and prevention of atherosclerosis, of neointimal proliferation, and of hypertension. In a series of papers reviewed here, the INSERM team in Toulouse has demonstrated, by the combined use of pharmacological tools and of transgenic mice lacking either the nuclear ERα, the membrane ERα, or both, that the nuclear ERα plays a major role in controlling E activities in vessels. E4 is able to elicit the important vasculoprotective actions mediated by estradiol (E2). Phase 1 and 2 clinical studies of E4 in a contraceptive indication (in combination with drospirenone) or in postmenopausal women for the relief of climacteric complaints demonstrate that E4 has a minimal impact on hemostasis, coagulation factors, coagulation inhibitors, fibrinolysis, angiotensinogen, triglycerides, and cholesterol. Altogether, preclinical studies and phase 1 and 2 clinical data indicate that E4 could be a new E with a better safety/efficacy profile than other E for women’s healthcare.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Estrogens
- Estetrol
- Estrogen receptor alpha
- Vascular safety
- Coagulation
- Hemostasis
- HRT-hormone therapy of menopause
- Combined oral contraceptives (COCs)
12.1 Introduction
For several decades, the role of hormone replacement therapy (HRT) has been debated. Early observational data on HRT showed many benefits, including a reduction in coronary atherosclerosis, coronary heart disease (CHD), osteoporosis, CHD mortality, and all-cause mortality as well as the risk of Alzheimer’s disease [1,2,3]. As CHD has the highest case fatality rate, the reduction in CHD with HRT was considered to have the greatest impact on mortality, with all-cause mortality cases being decreased by 20–40% in the observational studies [4, 5]. Meta-analyses of these and other data suggested that use of HRT would result in one to two additional years of life, which is large in epidemiological terms. More recently, randomized trials in women with established coronary disease (secondary prevention trials), as well as the Women’s Health Initiative (WHI), which studied mostly women many years after the onset of menopause, showed no such benefit and an increased risk of CHD and breast cancer [6, 7]. In 2002, the NIH, who sponsored the WHI, emphatically stated that HRT caused more harm than good and that this pertained to women of all ages and occurred with all types of HRT.
In 2006, the first study from WHI emerged that carried out an age stratification for coronary disease outcomes. The use of conjugated equine estrogens (CEE) had a protective effect in women aged 50–59 years, but not in those women aged ≥60 years [8]. In 2007, another manuscript from the WHI showed that in women aged 50–59 years or those <10 years from menopause onset, total mortality decreased significantly by 30% [9]. This figure is in accordance with reports from the original observational studies and suggests that the protective effects of HRT are dependent on the “timing” of initiation, as was originally proposed by Clarkson. Recent randomized (ELITE, KEEP) and observational data (COMPREHEND) and several meta-analyses, including a Cochrane analysis, now consistently show reductions in CHD and mortality when HRT is initiated soon after menopause [10,11,12].
Finally the WHI writing group published in September 2017 that among 27,347 postmenopausal women, HRT with CEE plus medroxyprogesterone acetate (MPA) for a median of 5.6 years or with CEE alone for a median of 7.2 years was not associated with risk of all-cause, cardiovascular, or cancer mortality during a cumulative follow-up of 18 years. Younger women (aged 50–59 years) tended to have lower HRs than older women did for mortality due to CVD, cancer, and other causes during the intervention phases of the two trials [13].
In summary, the WHI had a considerable negative impact; the originally reported adverse cardiovascular effects have been proven incorrect, when taking into account the time of initiation of HRT. The relationship of HRT to breast cancer is also complex, controversial, and confusing. In WHI, CEE alone showed a decrease in breast cancer, and only prior users of HRT before randomization into the WHI trial who used combination CEE and MPA showed some increase in breast cancer risk over time.
Objective analyses of the data allow concluding that observational studies and randomized trials are concordant in showing some increase in stroke (RR 1.3–1.4), modest increase in endometrial cancer with estrogen alone, a decrease in colon cancer, and an increase in venous thrombosis with oral HRT [14]. Ischemic stroke in younger (aged <60 years) women is rare and has been proposed to be due to a thrombotic mechanism [15, 16]. This suggests that a non-oral route of estrogen administration or an estrogen, which is devoid of thrombotic tendencies, may reduce stroke incidence. Transdermal estradiol, in standard doses, does not to increase the risk of stroke [17].
Finally, in 2017, the WHI writing group published its latest long-term follow-up of the hormone trials. They acknowledged that WHI only assessed one dose, one formulation, and one route of administration in each trial. The group conceded that the results are not necessarily generalizable to other hormone preparations, in contrast to their initial statement [13, 18]. The predominance of data has been generated with CEE. However, clinical data suggest that estradiol has similar efficacy. Since orally administered estradiol increases the risk of deep venous thrombophlebitis and pulmonary embolism and is contraindicated in obese women with high levels of plasma triglycerides, transdermal E2, which avoids the first liver pass effect, has the benefit of not increasing the risks of thrombosis and is well suited for more high-risk women [19]. The type of estrogen is of critical importance, and the development of a new, safe, oral estrogen which would maintain the benefits of estrogens in use today but which provides a safer profile is a worthwhile goal.
Combined oral contraception (COC) use is associated with a three- to sevenfold relative increased risk of DVT and pulmonary embolism (PE) [20]. This increased risk is the consequence of the changes that occur in the coagulation cascade brought on by E [21]. E causes an increase in plasma levels of thrombogenic clotting factors (factors I, II, VII, VIII, X) as well as a decrease in plasma levels of clotting inhibitors (antithrombin, protein S, protein C, tissue factor pathway inhibitor (TFPI), etc.) that shift the balance to favor clot formation. Several studies have shown that the type of progestogen used in a COC influences the magnitude of the E-induced changes in these hemostatic proteins [22,23,24]. The biological mechanism of this progestogen effect seems to be through modification of the E impact on the liver rather than a direct effect, because progestogen-only contraception with levonorgestrel (LNG) or drospirenone (DRSP) does not increase VTE risk [25, 26].
The high levels of E seen by the liver following oral dosing are supraphysiologic. While E2 is rapidly converted to less potent estriol and estrone following the first pass, synthetic ethinylestradiol (EE) is more stable and reactivates during prolonged period of time the liver cell synthesis of clotting factors during recirculation. The lower estrogenic imprinting of E2 versus EE explains why the E2-based COC is less thrombogenic than the third- and fourth-generation COCs that contain EE [27]. The risk of thromboembolism from using COCs with norethisterone, levonorgestrel, desogestrel, or gestodene decreases with decreasing E dose [20]. The same progestins and DRSP in the absence of EE do not increase the VTE risk ([20, 25] [26]). These data indicate that it is the E and not the progestin that causes the DVT risk.
The second-generation pills containing EE + LNG as a progestin cause a threefold increased risk of DVT, while the third- and fourth-generation pills containing desogestrel, gestodene, or DRSP are associated with a six- to sevenfold increase and with considerably higher plasma levels of clotting factors [20]. The third- or fourth-generation progestin has a lower androgenic profile of activity, with less acne and seborrhea, weight gain, and oily hair in comparison to LNG. Their antiestrogenic activity decreased also significantly [28, 29]. Consequently, the liver impact of EE, the E predominantly used in COCs, is more important in users of third- and fourth-generation pills [30]. This causes a significantly higher increase in coagulation factors and a more profound decrease in coagulation inhibitors.
Due to the low absolute incidence of DVT (0.02–0.04%), prospective trials require 5–10 years to obtain reliable estimates for the DVT risk associated with the use of a particular COC. Surrogate hemostasis markers are therefore useful tools to estimate the thrombogenic risk of new COCs or E. Measurement of activated protein C (APC) resistance via thrombin generation is a validated test for determining the thrombogenicity of hormonal contraceptives [31, 32]. Sex hormone-binding globulin (SHBG) has also been suggested to be a marker for the risk of venous thrombosis [31, 33].
In summary, the E used in COCs are responsible for procoagulatory changes in hemostasis markers. The development of new safer E that would have a minimal impact on liver metabolism and on coagulation would provide great vascular advantage to women’s healthcare.
In this review, we highlight recent data on the impact of estetrol (E4) on the vascular system in comparison to that of E2 and propose that it may provide a more attractive risk-benefit ratio compared to other oral estrogens in use today. We will also report on recent clinical data of the effects of E4 for the relief of the climacteric symptoms and for contraception.
12.2 Estetrol (E4)
Estetrol (E4) is an estrogen of fetal origin, which was the last estrogen discovered. E4 differs from all other natural estrogens because it is present only in pregnant primates (at 100-fold higher levels in human pregnant women compared to other primates). It is exclusively synthesized by the human fetal liver (which has the capacity to hydroxylate estradiol at the 15 and 16 carbon positions) and circulates at high levels (up to 20–30 ng/mL) in fetal blood. It is present in maternal blood and urine from the ninth week of gestation and reaches the maternal circulation through the placenta. Maternal plasma levels increase during pregnancy to high concentrations toward the end of gestation (≥3–6 ng/mL) [34, 35]. E4 maternal plasma levels are about 12–20 times levels lower than in the fetal plasma at parturition.
12.3 Estetrol: The NEST Concept
E4 can be described as the first NEST [Native (natural and human) Estrogen with Specific action in Tissues]. The Nest property is the consequence of its unique mode of action, which is distinctly different from a selective estrogen receptor modulator (SERM). All estrogens bind to two subpopulations of estrogen receptor alpha: the nuclear ERα which induces gene transcription and the membrane ERα which initiates rapid signaling also called “membrane-initiated steroid signaling” (MISS) effects (for a review, see [36]). In a similar fashion to other estrogens, E4 activates the nuclear ERα and induces gene transcription. However, in contrast to the other estrogens, E4 antagonizes the activity of membrane ERα (Fig. 12.1) [37]. Evidence has accumulated over the last two decades that ERα is not only present in the cell nucleus but is associated with the plasma membrane and activates nonnuclear signaling from this site. These rapid/nongenomic/MISS actions have been characterized in a variety of cell lines and in particular in cancer cell lines and in endothelial cells. The development of selective pharmacological tools that specifically activate MISS and the generation of knock-out mice expressing either an ERα protein which is impeded for membrane action or for the nuclear action has allowed for the more precise delineation of the role of the nuclear and membrane forms of ERα [36, 38]. Thus the vascular profile of E4 activity can be compared with that of E2 17β. These actions of E4 (NEST activity) are distinctly different from SERMs which induce conformational changes in the ER and accordingly recruit coactivators or co-inhibitors in different tissues to allow for varied expression of agonistic or antagonistic activities.
ERα localization in the nucleus and at the cell surface. ERα is localized in the nucleus and at the cell surface. E2 and classical E bind and activate both types of ERα (green arrows). The nuclear ERα activation upon E binding induces gene transcription, while the cell surface-associated ERα initiates a series of rapid membrane-initiated steroid signaling (MISS) that contribute to regulate cyclic AMP synthesis, Ca++ entry, and kinase activation (receptor tyrosine kinase-RTK, MAPKs, ERK1/2, and/or PI3K/Akt, SRC) that are essential for the modulation of transcription and nongenomic functions in many target cells. As an example, the MISS effect may enhance cell proliferation and endothelial NO synthase (eNOS) activity (for a review, see [36]). E4 binds and activates the nuclear ERα (green arrow), but its binding to the membrane ERα results in a blockade of the MISS signaling (red arrow) [37]. E4 displays thus mixed agonist and antagonist estrogenic activities
12.4 Experimental Data
12.4.1 E4 Does Not Accelerate Endothelial Healing and Does Not Enhance Endothelial NO Production but Induces Endothelium-Dependent Vasodilation
E2 accelerates re-endothelialization after removal of the carotid artery endothelium by electric injury [39]. It also increases endothelial nitric oxide synthase (eNOS) phosphorylation and thereby endothelial NO production [40]. Stimulation of endothelial healing and eNOS activation is now recognized as two established actions of E2 dependent on membrane ERα activation [41, 42].
E4 (at doses of 0.3, 1, and 6 mg/kg/day) failed to accelerate endothelial cell proliferation/migration as well as to stimulate eNOS phosphorylation or endothelial NO production. E4 was not only devoid of ERa membrane-initiated steroid signaling (MISS) in the endothelium but was also able to antagonize the E2 effects, in line with its NEST activity profile that antagonizes the membrane ERα [37].
Does this mean that E4 is less effective than E2 on the vascular system?
Acceleration of endothelial healing and NO production are usually considered as features, which are protective for arteries. Would an absence of endothelial NO synthase stimulation by E4 result in a lack of E4-mediated vasodilation and in a poorer endothelium-mediated vascular protection in comparison to E2? The answer to this essential question is no. Several studies have confirmed that NO production, which is essential for proper vasodilation and endothelial function, is controlled by multiple factors besides estrogen. In addition, E4 causes vasodilation of animal arteries by a specific mechanism distinct from NO production.
Vascular tone by endothelium-derived nitric oxide is mediated by multiple controlling mechanisms, including physical factors, such as increases in shear stress and reduction in temperature, as well as a large number of neurohumoral mediators through the activation of specific endothelial cell membrane receptors and/or posttranslational modifications (Fig. 12.2). The main physiological driver of NO production is not estrogen but shear stress [43,44,45], and estrogen is considered to play a limited role in regulating endothelial-derived NO production and vasodilation.
Regulation of vascular tone by endothelium-derived nitric oxide (NO). Physical factors (increases in shear stress and temperature lowering) and neurohumoral mediators (through activation of specific endothelial cell membrane receptors) cause instantaneous increases in the release of endothelium-derived NO by endothelial nitric oxide synthase (eNOS). Color code: Signals leading to activation of eNOS dependent mainly on increases in Ca2+ concentration, mainly on posttranslational modifications, or on both are indicated by blue, yellow, and green, respectively. These factors include α2: alpha adrenergic mediators, E (epinephrine); NE (norepinephrine); Ach (acetylcholine); ADP (adenosine diphosphate); Ang1–7 (angiotensin1–7); adiponectin; AVP (arginine vasopressin); bradykinin; PGE2 (prostaglandin E2); estrogen; ET (endothelin-1); GLP1 (glucagon-like peptide 1); histamine; HDL (high-density lipoprotein); 5HT (serotonin, 5-hydroxytryptamine); insulin; PGI2 (prostacyclin); SP1 (sphingosine 1-phosphate); VEGF (vascular endothelial growth factor); VD (vitamin D); melanocortin; thrombin; ATP; cannabinoids; oxytocin; etc. (Reproduced from Vanhoutte PM, Zhao Y, Xu A, et al. Thirty years of saying NO: sources, fate, actions, and misfortunes of the endothelium-derived vasodilator mediator. Circ Res 2016; 119: 375–96, by permission of Wolters Kluwer)
In addition, pharmacological studies suggest that E4 induces vasodilation through a distinct mechanism. It has been shown that E4 induces vasodilation at very high concentrations (10 μM or more) in ewe uterine arteries [46] and relaxing responses in rat uterine, thoracic, aortic, carotid, mesenteric, pulmonary, renal, middle cerebral, and septal coronary arteries [47]. The vasodilation induced by E4 in rat arteries was ER dependent since it was abrogated by ICI 182 780, an ERα antagonist. Blockade of eNOS by N(ω)-nitro-l-arginine methyl ester (L-NAME) blunted E2-mediated but not E4-mediated relaxing responses, demonstrating that E2 but not E4 induces vasodilation by stimulating eNOS activity. Only, the soluble guanylate cyclase (sGC) inhibitor, ODQ, blocked E4 relaxation. These studies concluded that E4 caused relaxation of precontracted rat uterine arteries via both an endothelium-dependent mechanism involving ER and a guanylate cyclase mechanism. Furthermore, E4 inhibited smooth muscle cell Ca++ entry and contraction. However, a discrepancy between the micromolar range estrogenic effects on ex vivo vasorelaxation and the nanomolar range of circulating estrogen levels in vivo is unclear. It may be the consequence of the experimental conditions of the in vitro perfusion model used. The observations made on the effects of estrogens using ex vivo vasorelaxation models are pharmacological in nature and require further validation in vivo and if possible in human.
As we will see below, E2-mediated acceleration of endothelial healing and NO production does not summarize the “physiological vascular benefit,” and E4, as E2, can mediate numerous vasculoprotective actions that are independent on membrane ERα and fully dependent on nuclear ERα. In summary, we believe that the E2-mediated acceleration of endothelial healing and NO production should not convey a unique “physiological benefit” of E2 17β, and the impact of E4 on arterial and capillary vessels also should be as beneficial.
12.4.2 E4 Has an Atheroprotective Effect as Mediated Through Nuclear ERa
The scientific community believes that the increase in endothelium-derived NO plays an important role in the vascular protective actions of estrogen, by preventing or attenuating arterial atheroma formation [48, 49]. A validated model to study atheroprotection by estrogens [50, 51] was used to compare the effect of E2 (80 μg/kg/day) and E4 (0.6 and 6 mg/kg/day). Lipid deposition was evaluated at the aortic sinus from ERa+/+LDLr−/− or ERa−/−LDLr−/− (low-density lipoprotein receptor) mice fed with a high-cholesterol diet. E4 in a dose-dependent fashion prevented lipid deposition in ovariectomized ERa+/+LDLr−/− mice (Fig. 12.3), decreasing atheroma deposition by up to 80% [37], a level of protection similar to that obtained using a high dose of E2 [53, 54]. Deletion of ERα in ERa−/−LDLr−/− mice abolished this E4 protection, indicating that ERa is necessary to mediate the atheroprotective effect of E4 [37]. Taken together these studies indicate that E4 is able to prevent atherosclerosis, by activating the nuclear ERα, without enhancement of NO. The capacity of E4 to prevent atherosclerosis and the persistence of the atheroprotection by estrogen in transgenic mice lacking the membrane ERα indicate that this membrane receptor subpopulation does not contribute to atheroprotection [55]. Accordingly, we propose that the atheroprotective effects of E4 should be similar to the effects of E2 17β and are not likely to be less in spite of not activating the membrane receptor subpopulation and not enhancing NO production.
E4 prevents aortic sinus lipid deposition in hypercholesterolemic mice. Four-week-old ovariectomized ERa+/+LDL-r−/− (estrogen receptor α-positive/low-density lipoprotein receptor-negative) or ERa−/−LDL-r−/− (estrogen receptor α-negative/low-density lipoprotein receptor-negative) mice were switched to atherogenic diet from the age of 6 to 18 weeks added with placebo (Ctrl) or E4 (0.6 or 6 mg/kg/day). (a, b) Representative micrographs of oil red-O (ORO) lipid-stained cryosections of the aortic sinus (a) and quantification of lipid deposition (b) are represented (from Abot et al. 2014 EMBO Mol Med. 2014, 10:1328–46 Fig. 5, p. 1338; reproduced with permission from Wiley online Library)
12.4.3 E4 Protects Against Neointimal Hyperplasia
Neointimal hyperplasia arises when vascular smooth muscle cells cross the internal elastic lamina to migrate and proliferate in the intima [56, 57]. In human pathology, this process frequently occurs after the treatment of symptomatic atherosclerosis, which involves mechanical endovascular ballooning (angioplasty) followed by stenting. Neointimal hyperplasia leads to a narrowing of the arterial lumen and restenosis [58]. E2 antagonizes this process [59].
We used an experimental model of femoral artery injury to trigger neointimal hyperplasia of vascular smooth muscle cells (VSMC) [60]. Using transgenic mice and selective deletion of ERα in VSMC or in endothelial cells, we demonstrated that, contrary to a common belief, E2 and E4 prevent neointimal hyperplasia by a direct inhibitory effect on SMC proliferation and migration but not by acting on endothelial cells. E4 prevented neointimal hyperplasia formation to the same extent as E2 (Fig. 12.4).
E4 protects against neointimal hyperplasia. Four-week-old wild-type female mice were ovariectomized and subcutaneously implanted with control or E4 (6 mg/Kg/day) eluting osmotic minipumps . Two weeks later, animals were submitted to mechanical injury of the femoral artery. Arteries were harvested 28 days after the injury for morphometric analysis. Left, representative image of cross section of femoral arteries of mice stained with Masson trichrome. Bars, 100 μm. Right, quantitative analysis of neointima/media ratio of mice. Values are presented as mean ± SEM (n = 7–12 mice per group) and statistically compared with Mann–Whitney U test. ***p < 0.001 (from Smirnova NF et al. (Fig. 12.5b) Circ Res. 2015; 117:770–8 reproduced with permission of Wolters Kluwer)
12.4.4 E4 Favors Flow-Mediated Arteriolar Remodeling (FMR)
Chronic increases in blood flow induce outward hypertrophic remodeling in resistance arteries and improvement of nitric oxide (NO)-dependent dilation [61,62,63]. Resistance arteries control local blood flow , and their capacity to remodel in response to chronic changes in the hemodynamic environment is necessary to maintain their full efficiency. Chronic increases in blood flow occur in physiological situations, such as growth, pregnancy, or exercise. In response to a chronic increase in blood flow, arterial diameters increase until the shear stress is normalized. In addition to angiogenesis, high-flow-mediated remodeling of resistance arteries plays a key role in revascularization of ischemic tissues after occlusion of a large artery [64]. Physiologically, chronic increases in blood flow in resistance arteries cause the diameter to expand with a compensatory increase in wall mass and an increased responsiveness of the endothelium to vasodilator stimuli. Besides pressure-associated medial hypertrophic remodeling that characterizes hypertension, outward remodeling at the level of arterioles occurs in response to increased blood flow and shear stress at the surface of endothelial cells.
Flow-mediated arteriolar remodeling (FMR) consists in vascular enlargement or outward remodeling induced by flow (shear stress) in small collateral arteries surrounding ischemic areas. This remodeling is essential in postischemic revascularization or collateral arteries growth since it contributes to the prevention of further tissue injury, in limb or myocardial ischemia [65]. FMR has been shown to be reduced by hypertension [66, 67], diabetes [68, 69], and aging [70, 71].
Two weeks after arterial ligation of some mesenteric arteries, the arterial diameter of the intact arteries was determined in vitro in response to stepwise increases in intraluminal pressure (Fig. 12.5a). As expected, passive arterial diameter was significantly higher in high-flow (HF) than in normal-flow (NF) arteries in WT mice. Ovariectomy caused a complete loss of FMR (Fig. 12.5b). A chronic treatment with E4 allowed flow arteriolar remodeling to occur in ovariectomized mice (Fig. 12.5c). Importantly, we assessed the short-term acute flow-mediated dilation in pressurized precontracted mesenteric arteries, i.e., in physiological ex vivo conditions (compared to vascular rings). E4 or vehicle caused quite similar vasodilating response to stepwise increase in intraluminal flow, demonstrating that E4 does not impair the vasodilatory response to the most important and physiological stimulus of NO production: endothelial shear stress [55].
Flow-mediated remodeling is promoted by E4. Experimental model: Blood flow was increased in the mesenteric artery of 4- to 5-month-old female mice using a model in which two mesenteric arteries are ligated, leading to a chronic increase in blood flow in the central artery without a change in the systemic hemodynamic environment [52]. Arterial diameter was then measured in vitro after cannulation in response to stepwise increases in pressure in the mesenteric arteries submitted chronically to high flow (HF) or to normal flow (NF). Briefly, three consecutive first-order mesenteric arteries were used and surgery consisted in ligatures of second-order branches. The artery located between the two ligated arteries was designated as the high-flow (HF) artery. Arteries located at distance of the ligated arteries were used as controls (normal flow, NF). Mice were sacrificed after 14 days and mesenteric arteries were collected. For the measurement of the pressure–diameter relationship in mesenteric arteries, in vitro arterial segments were cannulated at both ends and mounted in a video-monitored perfusion system (Living System, LSI, Burlington, VT). (a) FMR was evaluated in mesenteric arteries isolated from ovariectomized mice treated chronically with vehicle or E4 during 2 weeks (n = 6). Arterial diameter was measured in response to stepwise increases in pressure in mesenteric arteries submitted chronically to high flow (HF) or to normal flow (NF). After ovariectomy, no FMR is observed in HF mesenteric arteries. (b) Treatment with E4 for 2 weeks restored the capacity of HF mesenteric arteries to develop FMR
Taken altogether these data indicate that E4 has a similar vasoprotective capacity compared to E2 and allows flow-mediated remodeling to occur, which is essential for proper arterial functioning in health and diseases.
12.4.5 E4 Protects Mice from Angiotensin II-Induced Hypertension
Hypertension affects one in four women worldwide, and its prevalence is particularly high among women over 60 years of age. The first decade after menopause is accompanied by an increase in blood pressure and has been associated with a higher risk of atherosclerosis, myocardial infarction, and stroke [72]. Up to the time of menopause, endogenous estrogen plays a protective role against high blood pressure. In line with these epidemiological data, clinical and preclinical studies show considerable evidence that estrogen modulates cardiovascular physiology and function and is cardioprotective [73].
We therefore tested the capacity of E4 to reduce the effects of angiotensin II (AngII) and to prevent hypertension in animals.
In the WT littermate control ovariectomized mice, 4 weeks of AngII infusion increased systolic blood pressure (SBP) [55]. The hypertensive effect of AngII was significantly more exacerbated in ovariectomized mice or in mice lacking ERα (ERα−/−), showing that the beneficial effect of endogenous estrogen is ERα dependent. This finding is in agreement with previous work showing that hypertension induced by angiotensin II is lower in females than in males [74, 75]. Furthermore, the hypertensive effect of AngII was significantly more exacerbated in mice lacking nuclear ERα but not in mice lacking membrane ERα. Importantly and quite consistently, both E2 and E4 protected WT female mice from AngII-induced hypertension (Fig. 12.6).
E4 protects against AngII-induced hypertension. Effect of E4 on AngII treatment was evaluated in wild-type female mice ovariectomized 2 weeks before sham-surgery or simultaneous implantation with osmotic minipumps delivering angiotensin II (1 month) (AngII, solubilized in NaCl 0.9%, 0.5 mg/kg per day and E4 6 mg/kg per day, solubilized in 50% PBS + 50% DMSO) (n = 7). Mean of systolic blood pressure (SBP) measurements for 5 days was represented as weekly evolution of blood pressure pre- (week 0) and during AngII treatment (reproduced with permission modified from Guivarc’h et al. 2018)
12.4.6 E4 Protects from Arterial and Venous Thrombosis in Mice
12.4.6.1 E4 Like E2 Has a Minimal Impact on Coagulation Factors
In the mouse, estrogens do not alter circulating coagulation factors toward a procoagulant profile [76] as they do in women [20, 77, 78]. We verified that chronic E4 treatment (6 mg/Kg/day) did not modify the platelet counts, prothrombin time, and the level of plasma fibrinogen. A small decrease of the activated partial thromboplastin time and a modest increase in factors II and IX were observed. The conclusion of these studies suggests that E4 only has a moderate effect on coagulation factors in the mouse.
12.4.6.2 Estetrol Increases the Bleeding Time in Mice
Chronic administration of E4 for 3 weeks led to an increased tail-bleeding time in mice. The tail-bleeding time of untreated ovariectomized mice was 5.2 min (SD ± 2.4, n = 12), which is in the normal range [79]. Among 18 mice treated with E4, the bleeding time was so prolonged that there was no overlapping time with the vehicle-treated mice. The tail-bleeding time was prolonged above 30 min in 6 out of 18 mice [80].
12.4.6.3 E4 Protects Against Arterial Thrombus Formation
All ovariectomized control mice (n = 12) died from occlusive thromboembolism within 10 min after an injection in the jugular vein of 0.4 mg/kg collagen and 60 μg/kg epinephrine. Six E4-treated mice (E4) died, while four mice were protected from thromboembolism (p = 0.028). Histological analysis of mouse lungs harvested 10 min after the injection showed marked protection from occlusive thrombi in vessels of surviving E4-treated mice compared to E4-treated and control mice that died. The occlusive pulmonary thrombi in surviving E4-treated mice were mostly observed in small vessels and rarely in large vessels [80]. Using transthoracic echocardiography, we found that left ventricular geometry, assessed by the sphericity index (SI, an indicator of right ventricular pressure overload), was altered from the moment of injection in ovariectomized mice and in E4-treated mice that died. In contrast, E4-treated mice protected from collagen-/epinephrine-induced thromboembolism showed normal left ventricular function.
12.4.6.4 E4 Protects from Venous Thrombus Formation
We induced venous thrombosis by partial or total ligation of the inferior vena cava (IVC) [81]. In these models, blood flow decreased or arrested leading to endothelial cell activation and extensive thrombosis. Thrombus mass was quantified 24 h (for total ligation) or 48 h (partial ligation) after stasis or stenosis induction. As compared to ovariectomized control mice, E4-treated mice had significantly smaller thrombi in both models of IVC stenosis. In the total ligation model, the mean thrombus weight was 6.1 mg (SD ± 1.9, n = 13) in control ovariectomized mice and 2.3 mg (SD ± 0.5, n = 7) following E4 treatment. In the partial ligation model, the mean thrombus weight was again significantly reduced by E4 treatment (4.2 mg SD ± 1.3, n = 8 and 0.7 mg SD ± 0.5, n = 7, respectively).
12.4.6.5 E4 Decreases Thrombus Growth on Collagen Under Ex Vivo Arterial Flow Conditions
Platelet adhesion and thrombus formation were evaluated ex vivo under arterial flow conditions (60 dynes/cm2, 1500 s−1) using a flow-based adhesion assay where heparinized whole blood was perfused over a collagen matrix. While blood from untreated mice exhibited robust formation of densely packed platelet thrombi on collagen, platelets from E4-treated mice formed 33% smaller thrombi. To assess the stability of the thrombi formed, a low shear rate (20 dynes/cm2, 500 s−1) was first applied during 2 min followed by an acute increase of blood flow to reach a pathological shear rate (160 dynes/cm2, 4000 s−1) for 2 min. In all groups, the thrombi formed did not detach from the collagen matrix and even grew further but at a slower rate with blood from E4-treated mice compared to control untreated mice. These results indicate that while platelet aggregation in suspension was not significantly modified, platelet thrombus growth under arterial shear rate on collagen matrix was reduced following E4 treatment. Finally, we generated hematopoietic chimera with bone marrow cells deficient for nuclear ERα. Deletion of hematopoietic nuclear ERα significantly reduced E4-induced protection against thromboembolism, while this deletion did not affect the increased tail-bleeding time. Taken altogether, these data, combined with the human studies (see below), concur to define E4 as a unique, native estrogen. It could be a promising candidate for oral contraception as well as for HRT, as it has minimal impact on coagulation and is even protective in the animal models used. To what extent this observed antithrombotic effect in animal studies relates to women remains to be determined in clinical studies. Such preliminary clinical data are emerging and will be reviewed below.
12.4.7 Other Effects
E4 Antagonizes E2-Stimulated Tumoral Angiogenesis.
Most solid tumors need a blood supply and can grow only if they induce the development of new blood vessels from pre-existing ones, a process known as tumor angiogenesis. Tumor vascularization is a complex event that involves tumor cells and host endothelial cells, stromal cells, immune cells, and circulating bone marrow stem cells. Multiple endocrine factors, cytokines, and chemokines regulate tumoral angiogenesis [82]. The physiopathology of tumoral angiogenesis and the role of estrogens in this process are beyond the scope of this review. It is however noteworthy to mention that in experimental models of tumoral angiogenesis, E4 antagonizes the effect of E2 on blood vessels formation and tumor growth (Fig. 12.7). This observation is in line with the demonstration that E4 antagonizes the stimulation of endothelial cells proliferation and migration elicited by E2 [37].
Reduction by E4 of E2 stimulated B16 melanoma tumor growth. Four weeks old, ovariectomized C57 black/6 mice were inoculated with a pellet releasing E2 (80 μg/kg/day) as described in Pequeux et al. [83]. Two weeks later, mice were inoculated with 4 × 105 ERα-negative B16K2 melanoma cells. Some mice were injected with an E4 pellet (1.3 mg/kg/day) 4 days after tumor cell injection. Tumor growth was monitored for 15 days (*p < 0.05; **p < 0.01). While E2 stimulates the ERα-negative B16 melanoma tumor growth, E4 antagonizes this effect
E2 17β potentiates tumor growth even when cancer cells lack ERα and are therefore E insensitive [83]. E2 activates normal host stromal (endothelial) cells ERα to increase intratumoral vessel density. E2 improves vessels structure and functionality that deliver more oxygen to the tumor [83]. E4, which does not stimulate endothelial cells proliferation and migration (see above), is able to partially antagonize the enhancement of tumoral angiogenesis and tumor growth elicited by E2 in ER-negative tumors (Fig. 12.7). These data confirm that E4 is an antagonist of the membrane ERα subpopulation and antagonizes the effect of E2 on tumoral angiogenesis.
12.5 Clinical Data
12.5.1 Five or Ten Milligram E4 Has a Minimal Impact on Hemostasis in Young Reproductive Age Women
Hepatic estrogenicity and hemostasis markers are in the list of the European Medicines Agency (EMA) advised to evaluate potential risk for thrombotic side effects of hormonal contraceptives [84]. Five or ten milligram E4 was therefore evaluated, in a phase 2 clinical trial, as an oral contraceptive in combination with 3 mg drospirenone (DRSP) [77] and compared to a classical combined oral contraceptive containing 20 μg EE combined with 3 mg DRSP (Yaz®). Healthy women 18–35 years of age with a body mass index (BMI) of 18–30 kg/m2 were eligible for inclusion. Spontaneous ovulation between day 9 (±1) and day 24 (±1) was verified in the pretreatment cycle by a progesterone concentration ≥ 16 nM (5 ng/mL) and a luteal phase duration of ≥6 days. The study included three treatment groups: 20 μg EE combined with 3 mg DRSP (EE/DRSP), 5 mg E4 combined with 3 mg DRSP (5 mg E4/DRSP), and 10 mg E4 combined with 3 mg DRSP (10 mg E4/DRSP). All subjects were stratified according to the day of ovulation in the pretreatment cycle and then assigned to a treatment group. Oral treatment was started on the first day of menstruation following the pretreatment cycle and continued daily for 24 days followed by a 4-day break.
In the EE/DRSP group, both SHBG and angiotensinogen increased significantly in the third cycle (to 381% and 256% of baseline, respectively) (Fig. 12.8a, b) . In contrast, SHBG and angiotensinogen were 100% (i.e., no change) and 125% of baseline in the 5 mg E4/DRSP group and 143% and 131% of baseline in the 10 mg E4/DRSP group. Thus, compared to 20 μg EE, both 5 mg E4 and 10 mg E4 had considerably lower effect on SHBG and minor impact on angiotensinogen.
Estrogenicity markers on liver. Changes in median plasma levels of SHBG (a) and angiotensinogen (b), relative to the pretreatment value (=100%), in women receiving for 3 cycles either 5 or 10 mg E4 combined with 3 mg DRSP. A classical COC containing 20 μg EE + the same dose of DRSP was used as a comparator. Paired statistics: Wilcoxon signed rank test see Table 12.1. In the EE/DRSP group, both SHBG and angiotensinogen increased significantly in the third cycle (to 381% and 256% of baseline, respectively) (a, b). In contrast, SHBG and angiotensinogen were 100% (i.e., no change) and 125% of baseline in the 5 mg E4/DRSP group and 143% and 131% of baseline in the 10 mg E4/DRSP group. Thus, compared to 20 μg EE, both 5 mg E4 and 10 mg E4 had nearly no effect on SHBG and minor on angiotensinogen (modified from Kluft et al. 2017 reproduced with permission from Elsevier)
At variance to EE + DRSP which induced dramatic changes in the hemostasis markers (Fig. 12.9), E4, at the daily dose of 5 or 10 mg, had little or no effect on coagulation factors (Fig. 12.9 and Table 12.1 [77]). The influence of EE and E4 was also evaluated on the three most relevant coagulation inhibitors (free TFPI, protein S, and antithrombin) and on the global coagulation inhibition test (ETP-based APCr). Five and 10 mg E4 had no effect on antithrombin, protein S activity, or APCr (Fig. 12.10) and had only a considerably smaller impact on free TFPI than EE which considerably decreased the plasma levels of these coagulation inhibitors, thereby promoting coagulation.
Changes in hemostasis versus baseline in women receiving either a classical combined oral contraceptive (COC) containing 20 μg EE + 3 mg DRSP or 5 or 10 mg E4 + 3 mg DRSP. The markers of estrogenicity in liver (SHBG, angiotensinogen) of coagulation (prothrombin, prothrombin fragment 1.2, ETP-based APCr, APTT APCr, D-dimers, protein C, fibrinogen), of coagulation inhibitors (free TFPI, protein S, antithrombin), and of fibrinolysis (tissue plasminogen activator (TPA)) show considerably lower changes versus baseline (spontaneous ovulatory cycle) in the groups treated with 5 or 10 mg E4 + DRSP than in the group treated with EE 20 μg + DRSP. Ideally a COC would not modify any surrogate marker of hemostasis
Changes in ETP-based APC resistance (a), D-Dimer (b), and prothrombin fragment 1.2 (c), in the presence of 5 or 10 mg E4 + 3 mg DRSP versus 20 μg EE + 3 mg DRSP. The global marker of coagulation inhibition test (ETP-based APC resistance) and of coagulation activation (D-Dimers and prothrombin fragment 1.2) demonstrates considerable differences of EE versus E4 in association with the same dose of DRSP. EE causes major procoagulatory changes, while E4 does not. (Modified from Kluft et al. 2017 with permission from Elsevier)
Five weeks after stopping treatment, previous exposure to, 20 μg EE + 3 mg DRSP still caused an elevation of SHBG, angiotensinogen, and ETP-APC resistance that remained 10–15% of the maximal values observed. Protein S activity and free TFPI were still decreased [77].
Finally, the global activation of coagulation was evaluated. EE + DRSP increased D-dimer and prothrombin fragments F1.2, corresponding to a global activation of coagulation. COCs containing 5 or 10 mg E4 did not increase either marker, but rather decreased them.
The considerably lower impact of E4/DRSP compared to EE/DRSP on a number of hemostatic parameters confirms the importance of the E selected for COC use and indicates how neutral DRSP is in terms of coagulation.
Recently the influence of three COCs on hemostasis parameters was compared in three groups of about 30 women exposed for six cycles to either 20 μg EE + 3 mg DRSP or 30 μg EE + 150 μg LNG or 15 mg E4 + 3 mg DRSP. Changes of hemostasis parameters during EE/LNG and EE/DRSP were similar to those reported in literature. The effects of E4/DRSP and EE/LNG on hemostasis parameters were less important than those of EE/DRSP. Several coagulation parameters were lower in the E4/DRSP group than in the EE + LNG group (Mithra data on file).
Altogether these studies indicate that the lower impact of E4/DRSP compared to EE/DRSP on a number of hemostatic parameters confirms the importance of the estrogen selected for COC use. Larger studies on DRSP alone and on E4/DRSP combination are not yet available and will be required in order to document the putative reduced incidence of VTE among women using COCs containing E4 and DRSP.
12.5.2 Fifteen Milligram E4 Has No Impact on Hemostasis in Postmenopausal Women
E4 relief is a prospective, multicenter, randomized, placebo-controlled, double-blinded, dose-finding study in both hysterectomized and non-hysterectomized postmenopausal women. The primary objective was to select the optimal effective oral dose of E4 for the treatment of VMS in postmenopausal women. Secondary efficacy objectives included the evaluation of safety, the effects of different doses of E4 on vulvar and vaginal atrophy (VVA scoring system; maturation index), bone biomarkers, and health-related quality of life. No significant changes in any markers of coagulation or angiotensinogen were documented in the 250 women receiving for 12 weeks 2.5, 5, 10, or 15 mg E4 (Mithra data on file).
Taken altogether these phase 2 clinical data indicate that E4 is a unique molecule, which does not appear to increase the risk of thrombosis in that it does not affect coagulation factors when compared to effects elicited by orally administered classical and synthetic estrogens.
12.6 Conclusion
Experimental and clinical observational and prospective randomized trials indicate that estrogens convey multiple benefits. Their use is also associated with unwanted side effects. For example, women using COCs or oral HRT have an increased incidence of DVT and PE. There is also an increased incidence of stroke among postmenopausal women treated with oral HRT. The identification of safer estrogenic compounds for HRT and for combined oral contraceptives (COCs) that would selectively preserve the beneficial effects of estrogens on symptoms of menopause, the bone, and urogenital system while reducing their unwanted side effects is largely needed. In particular, the precise delineation of the activity/safety profile of E4 requires both preclinical and clinical studies. This estrogen is human (primate) specific and synthesized in large amounts (3–5 mg/day) by the human fetus. Selected by natural evolution, this fetal estrogen acts selectively in tissues, with mixed agonist and antagonist activities. This NEST (Native Estrogen that has Selective action in Tissues) property is the consequence of the selective activation by E4 of the nuclear ERα and of the blockade of the membrane ERα as demonstrated by combined genetic and pharmacological approaches [37, 85].
This review has summarized the preclinical (and limited clinical) information about the impact of E4 on the vascular system. The impact of E4 on the vascular system may be summarized in one integrated concept (Fig. 12.11). The membrane ERα is neither necessary nor sufficient for eliciting beneficial effect of estrogens on arteries (i.e., protection against neointimal hyperplasia, atheroma, hypertension, and induction of FMR). E4 by selectively activating the nuclear ERα is able to convey, at least in animal models, all the beneficial vascular effects of E2. In contrast to E2, E4 does not enhance endothelial NO production but does not alter the flow-induced vasodilation, the most potent physiological stimulus of NO production. This neutrality could confer an advantage in a tumoral context, as E4 does not enhance tumoral angiogenesis in contrast to E2 and may even antagonize this unwanted effect elicited by E2. Finally, E4 may induce an arterial vasodilation by a guanylate cyclase dependent mechanism. In clinical studies, E4 in contrast to EE and E2 has a minimal impact on the synthesis of hemostasis parameters and of liver proteins induced by estrogens, including SHBG and angiotensinogen.
The key role of nuclear ERα activation in controlling the E4 vascular effects. The beneficial actions of ERα activation by E4 on atheroma, hypertension, neointimal hyperplasia and arterial remodeling all rely on nuclear ERα. ERα membrane-initiated signaling is not stimulated by E4 which does not enhance tumoral angiogenesis (reproduced with permission modified from Guivarc’h et al. 2018)
E4, a fetal estrogen, which is devoid of thrombogenic activity but carries the vascular beneficial effects of E2 17β, may prove to be an efficacious and safer estrogen for clinical use. Phase 3 clinical studies in progress should contribute to further confirm these encouraging data.
Abbreviations
- Ang II:
-
Angiotensin II
- CEE:
-
Combined equine estrogen
- CHD:
-
Coronary heart disease
- COCs:
-
Combined oral contraceptives
- CVD:
-
Cardiovascular disease
- DRSP:
-
Drospirenone
- DVT:
-
Deep venous thrombophlebitis
- E:
-
Estrogen
- E2:
-
Estradiol
- E4:
-
Estetrol
- eNOS:
-
Endothelial nitric oxide synthase
- ERα:
-
Estrogen receptor alpha
- ERα−/−:
-
Estrogen receptor alpha KO
- FMR:
-
Flow-mediated arteriolar remodeling
- HF:
-
High flow
- HT:
-
Hormone therapy
- LNG:
-
Levonorgestrel
- MPA:
-
Medroxyprogesterone acetate
- NEST:
-
Native estrogen with selective actions in tissues
- NF:
-
Normal flow
- NIH:
-
National Institutes of Health
- NO:
-
Nitric oxide
- PE:
-
Pulmonary embolism
- RR:
-
Relative risk
- VTE:
-
Venous thromboembolism
- WHI:
-
Women’s Health Initiative
References
Grodstein F, Stampfer MJ, Colditz GA. Postmenopausal hormone therapy and mortality. N Engl J Med. 1997;336:1769–75.
Stampfer MJ, Colditz GA. Estrogen replacement therapy and coronary heart disease: a quantitative assessment of the epidemiologic evidence. Prev Med. 1991;20:47–63.
Yaffe K, Sawaya G, Lieberburg I, Grady D. Estrogen therapy in postmenopausal women: effects on cognitive function and dementia. JAMA. 1998;279:688–95.
Grady D, et al. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med. 1992;117:1016–37.
Henderson BE, Paganini-Hill A, Ross RK. Decreased mortality in users of estrogen replacement therapy. Arch Intern Med. 1991;151:75–8.
Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–13.
Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J, Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–33.
Hsia J, et al. Conjugated equine estrogens and coronary heart disease: the Women’s Health Initiative. Arch Intern Med. 2006;166:357–65.
Rossouw JE, et al. Postmenopausal hormone therapy and cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–77.
Carrasquilla GD, et al. The association between menopausal hormone therapy and coronary heart disease depends on timing of initiation in relation to menopause onset: results based on pooled individual participant data from the Combined Cohorts of Menopausal Women — Studies of Register Based Health Outcomes in Relation to Hormonal Drugs (COMPREHEND) study [abstract S17]. Menopause. 2015;22:1373.
Hodis HN, Mack WJ, Henderson VW. Effects of early versus late postmenopausal treatment with estradiol. N Engl J Med. 2016;374:1221–31.
Lobo Pickar JH, Stevenson JC, Mack WJ, Hodis HN. Back to the future: Hormone replacement therapy as part of a prevention strategy for women at the onset of menopause. Atherosclerosis. 2016;254:282–90.
Manson JE, Aragaki AK, Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Chlebowski RT, Howard BV, Thomson CA, Margolis KL, Lewis CE, Stefanick ML, Jackson RD, Johnson KC, Martin LW, Shumaker SA, Espeland MA, Wactawski-Wende J, Investigators WHI. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the women’s health initiative randomized trials. JAMA. 2017;318:927–38.
Lobo RA. Hormone-replacement therapy: current thinking. Nat Rev Endocrinol. 2017;13:220–31.
Grodstein F, Manson JE, Stampfer MJ, Rexrode K. Postmenopausal hormone therapy and stroke: role of time since menopause and age at initiation of hormone therapy. Arch Intern Med. 2008;168:861–6.
Lobo RA, Clarkson TB. Different mechanisms for benefit and risk of coronary heart disease and stroke in early postmenopausal women: a hypothetical explanation. Menopause. 2011;18:237–40.
Sare GM, Gray LJ, Bath PM. Association between hormone replacement therapy and subsequent arterial and venous vascular events: a meta-analysis. Eur Heart J. 2008;29:2031–41.
NHLBI. NHLBI stops trial of estrogen plus progestin due to increased breast cancer risk and lack of overall benefit. South Med J. 2002;95:795–7.
Mohammed K, Abu Dabrh AM, Benkhadra K, Al Nofal A, Carranza Leon BG, Prokop LJ, Montori VM, Faubion SS, Murad MH. Oral vs transdermal estrogen therapy and vascular events: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100:4012–20.
Lidegaard Ø, Nielsen LH, Skovlund CW, Skjeldestad FE, Løkkegaard E. Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001–9. BMJ. 2011;25:343.
Han L, Jensen JT. Does the progestogen used in combined hormonal contraception affect venous thrombosis risk? Obstet Gynecol Clin N Am. 2015;42:683–98.
Kemmeren JM, Algra A, Meijers JC, Tans G, Bouma BN, Curvers J, Rosing J, Grobbee DE. Effect of second- and third-generation oral contraceptives on the protein C system in the absence or presence of the factor VLeiden mutation: a randomized trial. Blood. 2004;103:927–33.
Oral Contraceptive and Hemostasis Study Group. The effects of seven monophasic oral contraceptive regimens on hemostatic variables: conclusions from a large randomized multicenter study. Contraception. 2003;67:173–85.
Vandenbroucke JP, Koster T, Briët E, Reitsma PH, Bertina RM, Rosendaal FR. Increased risk of venous thrombosis in oral-contraceptive users who are carriers of factor V Leiden mutation. Lancet. 1994;344(8935):1453–7.
Mantha S, Karp R, Raghavan V, Terrin N, Bauer KA, Zwicker JI. Assessing the risk of venous thromboembolic events in women taking progestin-only contraception: a meta-analysis. BMJ. 2012;345:e4944.
Regidor PA, Colli E, Schindler AE. Drospirenone as estrogen-free pill and hemostasis: coagulatory study results comparing a novel 4 mg formulation in a 24 + 4 cycle with desogestrel 75 μg per day. Gynecol Endocrinol. 2016;32:749–51.
Dinger J, Do Minh T, Heinemann K. Impact of estrogen type on cardiovascular safety of combined oral contraceptives. Contraception. 2016;94:328–39.
Sitruk-Ware R. New progestagens for contraceptive use. Hum Reprod Update. 2006;12:169–78.
Wiegratz I, Lee JH, Kutschera E, Winkler UH, Kuhl H. Effect of four oral contraceptives on hemostatic parameters. Contraception. 2004;70:97–106.
van Vliet HA, Frolich M, Christella M, Thomassen LG, Doggen CJ, Rosendaal FR, Rosing J, Helmerhorst FM. Association between sex hormone-binding globulin levels and activated protein C resistance in explaining the risk of thrombosis in users of oral contraceptives containing different progestogens. Hum Reprod. 2005;20:563–8.
Raps M, Helmerhorst F, Fleischer K, Thomassen S, Rosendaal F, Rosing J, Ballieux B, VAN Vliet H. Sex hormone-binding globulin as a marker for the thrombotic risk of hormonal contraceptives. J Thromb Haemost. 2012;10:992–7.
Rosing J, Middeldorp S, Curvers J, Christella M, Thomassen LG, Nicolaes GA, Meijers JC, Bouma BN, Büller HR, Prins MH, Tans G. Low-dose oral contraceptives and acquired resistance to activated protein C: a randomised cross-over study. Lancet. 1999;354:2036–40.
Odlind V, Milsom I, Persson I, Victor A. Can changes in sex hormone binding globulin predict the risk of venous thromboembolism with combined oral contraceptive pills? Acta Obstet Gynecol Scand. 2002;81:482–90.
Hickey M, Hart R, Keelan JA. The relationship between umbilical cord estrogens and perinatal characteristics. Cancer Epidemiol Biomark Prev. 2014;23:946–5.
Kundu N, Wachs M, Iverson GB, Petersen LP. Comparison of serum unconjugated estriol and estetrol in normal and complicated pregnancies. Obstet Gynecol. 1981;58(3):276–81.
Arnal JF, Lenfant F, Metivier R, Flouriot G, Henrion D, Adlanmerini M, Fontaine C, Gourdy P, Chambon P, Katzenellenbogen B, Katzenellenbogen J. Membrane and nuclear estrogen receptor alpha actions: from tissue specificity to medical implications. Physiol Rev. 2017;3:1045–87.
Abot A, Fontaine C, Buscato M, Solinhac R, Flouriot G, Fabre A, Drougard A, Rajan S, Laine M, Milon A, Muller I, Henrion D, Adlanmerini M, Valéra MC, Gompel A, Gerard C, Péqueux C, Mestdagt M, Raymond-Letron I, Knauf C, Ferriere F, Valet P, Gourdy P, Katzenellenbogen BS, Katzenellenbogen JA, Lenfant F, Greene GL, Foidart JM, Arnal JF. The uterine and vascular actions of estetrol delineate a distinctive profile of estrogen receptor α modulation, uncoupling nuclear and membrane activation. EMBO Mol Med. 2014;10:1328–46.
Gourdy P, Guillaume M, Fontaine C, Adlanmerini M, Montagner A, Laurell H, Lenfant F, Arnal JF. Estrogen receptor subcellular localization and cardiometabolism. Mol Metab. 2018;15:56–69.
Brouchet L, Krust A, Dupont S, Chambon P, Bayard F, Arnal JF. Estradiol accelerates reendothelialization in mouse carotid artery through estrogen receptor-alpha but not estrogen receptor-beta. Circulation. 2001;103:423–8.
Wu Q, Chambliss K, Umetani M, Mineo C, Shaul PW. Non-nuclear estrogen receptor signaling in the endothelium. J Biol Chem. 2011;286:14737–43.
Adlanmerini M, Solinhac R, Abot A, Fabre A, Raymond-Letron I, Guihot AL, Boudou F, Sautier L, Vessieres E, Kim SH, et al. Mutation of the palmitoylation site of estrogen receptor alpha in vivo reveals tissue-specific roles for membrane versus nuclear actions. Proc Natl Acad Sci U S A. 2014;111:E283–90.
Chambliss KL, Wu Q, Oltmann S, Konaniah ES, Umetani M, Korach KS, Thomas GD, Mineo C, Yuhanna IS, Kim SH, et al. Non-nuclear estrogen receptor alpha signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. J Clin Invest. 2010;120:2319–30.
Vanhoutte PM. Nitric oxide: from good to bad. Ann Vasc Dis. 2018;11:41–51.
Vanhoutte PM, Zhao Y, Xu A, Leung SW. Thirty years of saying no: sources, fate, actions, and misfortunes of the endothelium-derived vasodilator mediator. Circ Res. 2016;119:375–96.
Yamazaki Y, Kondo Y, Kamiyama Y. Estimation of shear-stress-induced endothelial nitric oxide production from flow-mediated dilation. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:4521–4.
Levine MG, Miodovnik M, Clark KE. Uterine vascular effects of estetrol in nonpregnant ewes. Am J Obstet Gynecol. 1984;148(6):735–8.
Hilgers RH, Oparil S, Wouters W, Coelingh Bennink HJ. Vasorelaxing effects of estetrol in rat arteries. Endocrinology. 2012;215:97–106.
Arnal JF, Fontaine C, Billon-Gales A, Favre J, Laurell H, Lenfant F, Gourdy P. Estrogen receptors and endothelium. Arterioscler Thromb Vasc Biol. 2010;30:1506–12.
Chambliss KL, Shaul PW. Estrogen modulation of endothelial nitric oxide synthase. Endocr Rev. 2002;23:665–86.
Mallat Z, Tedgui A. Cytokines as regulators of atherosclerosis in murine models. Curr Drug Targets. 2007;8:1264–72.
Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8:802–15.
Tarhouni K, Guihot AL, Freidja ML, Toutain B, Henrion B, Baufreton C, Pinaud F, Procaccio V, Grimaud L, Ayer A, Loufrani L, Lenfant F, Arnal JF, Henrion D. Key role of estrogens and endothelial estrogen receptor alpha in blood flow-mediated remodeling of resistance arteries. Arterioscler Thromb Vasc Biol. 2013;33:605–11.
Billon-Gales A, Fontaine C, Douin-Echinard V, Delpy L, Berges H, Calippe B, Lenfant F, Laurell H, Guery JC, Gourdy P, Arnal JF. Endothelial estrogen receptor-alpha plays a crucial role in the atheroprotective action of 17beta-estradiol in low-density lipoprotein receptor-deficient mice. Circulation. 2009;120:2567–76.
Billon-Gales A, Krust A, Fontaine C, Abot A, Flouriot G, Toutain C, Berges H, Gadeau AP, Lenfant F, Gourdy P, et al. Activation function 2 (AF2) of estrogen receptor-{alpha} is required for the atheroprotective action of estradiol but not to accelerate endothelial healing. Proc Natl Acad Sci U S A. 2011;108:13311–6.
Guivarc’h E, Buscato M, Guihot A.L, Favre J, Vessières E, Grimaud L, Wakim J, Melhem NJ, Zahreddine R, Adlanmerini M., Loufrani M, Knauf C, Katzenellenbogen JA, Katzenellenbogen BS, Foidart JM, Gourdy P, Lenfant F, Arnal JF, Henrion D, Fontaine C Predominant role of nuclear versus membrane estrogen receptor (ER)α in arterial protection: implications for ERα modulation in cardiovascular prevention/safety. J Am Heart Assoc. 2018. https://doi.org/10.1161/JAHA.118.008950.
Hui DY. Intimal hyperplasia in murine models. Curr Drug Targets. 2008;9:251–60.
Smirnova NF, Gayral S, Pedros C, Loirand G, Vaillant N, Malet N, Kassem S, Calise D, Goudounèche D, Wymann MP, Hirsch E, Gadeau AP, Martinez LO, Saoudi A, Laffargue M. Targeting PI3Kγ activity decreases vascular trauma-induced intimal hyperplasia through modulation of the Th1 response. J Exp Med. 2014;211:1779–92.
Costa MA, Simon DI. Molecular basis of restenosis and drug eluting stents. Circulation. 2005;111:2257–73.
Chandrasekar B, Sirois MG, Geoffroy P, Lauzier D, Nattel S, Tanguay JF. Local delivery of 17beta-estradiol improves reendothelialization and decreases inflammation after coronary stenting in a porcine model. Thromb Haemost. 2005;94:1042–7.
Smirnova NF, Fontaine C, Buscato M, Lupieri A, Vinel A, Valera MC, Guillaume M, Malet N, Foidart JM, Raymond-Letron I, Lenfant F, Gourdy P, Katzenellenbogen BS, Katzenellenbogen JA, Laffargue M, Arnal JF. The activation function-1 of estrogen receptor alpha prevents arterial neointima development through a direct effect on smooth muscle cells. Circ Res. 2015;117:770–8.
Bouvet C, Belin de Chantemèle E, Guihot AL, Vessieres E, Bocquet A, Dumont O, Jardel A, Loufrani L, Moreau P, Henrion D. Flow-induced remodeling in resistance arteries from obese Zucker rats is associated with endothelial dysfunction. Hypertension. 2007;50:248–54.
Dumont O, Loufrani L, Henrion D. Key role of the NO-pathway and matrix metalloprotease-9 in high blood flow-induced remodeling of rat resistance arteries. Arterioscler Thromb Vasc Biol. 2007;27:317–24.
Pourageaud F, De Mey JG. Vasomotor responses in chronically hyperperfused and hypoperfused rat mesenteric arteries. Am J Phys. 1998;274:H1301–7.
Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–6.
Silvestre JS, Smadja DM, Levy BI. Postischemic revascularization: from cellular and molecular mechanisms to clinical applications. Physiol Rev. 2013;93:1743–802.
Dumont O, Kauffenstein G, Guihot AL, Guerineau NC, Abraham P, Loufrani L, Henrion D. Time-related alteration in flow- (shear stress-) mediated remodeling in resistance arteries from spontaneously hypertensive rats. Int J Hypertens. 2014;2014:859793.
Tuttle JL, Sanders BM, Burkhart HM, Fath SW, Kerr KA, Watson WC, Herring BP, Dalsing MC, Unthank JL. Impaired collateral artery development in spontaneously hypertensive rats. Microcirculation. 2002;9:343–51.
Belin de Chantemele EJ, Vessieres E, Guihot AL, Toutain B, Maquignau M, Loufrani L, Henrion D. Type 2 diabetes severely impairs structural and functional adaptation of rat resistance arteries to chronic changes in blood flow. Cardiovasc Res. 2009;81:788–96.
Freidja ML, Tarhouni K, Toutain B, Fassot C, Loufrani L, Henrion D. The age-breaker alt-711 restores high blood flow-dependent remodeling in mesenteric resistance arteries in a rat model of type 2 diabetes. Diabetes. 2012;61:1562–72.
Dumont O, Pinaud F, Guihot AL, Baufreton C, Loufrani L, Henrion D. Alteration in flow (shear stress)-induced remodelling in rat resistance arteries with aging: Improvement by a treatment with hydralazine. Cardiovasc Res. 2008;77:600–8.
Tarhouni K, Guihot AL, Vessieres E, Toutain B, Procaccio V, Grimaud L, Loufrani L, Lenfant F, Arnal JF, Henrion D. Determinants of flow-mediated outward remodeling in female rodents: respective roles of age, estrogens, and timing. Arterioscler Thromb Vasc Biol. 2014;34:1281–9.
Barton M, Meyer MR. Postmenopausal hypertension: mechanisms and therapy. Hypertension. 2009;54:11–8.
Regitz-Zagrosek V, Kararigas G. Mechanistic pathways of sex differences in cardiovascular disease. Physiol Rev. 2017;97:1–37.
Barsha G, Denton KM, Mirabito Colafella KM. Sex- and age-related differences in arterial pressure and albuminuria in mice. Biol Sex Differ. 2016;7:57.
Sampson AK, Moritz KM, Jones ES, Flower RL, Widdop RE, Denton KM. Enhanced angiotensin II type 2 receptor mechanisms mediate decreases in arterial pressure attributable to chronic low-dose angiotensin II in female rats. Hypertension. 2008;52:666–71.
Valera MC, Gratacap MP, Gourdy P, Lenfant F, Cabou C, Toutain CE, Marcellin M, Saint Laurent N, Sie P, Sixou M, Arnal JF, Payrastre B. Chronic estradiol treatment reduces platelet responses and protects mice from thromboembolism through the hematopoietic estrogen receptor alpha. Blood. 2012;120:1703–12.
Kluft C, Zimmerman Y, Mawet M, Klipping C, Duijkers IJ, Neuteboom J, Foidart JM, Bennink HC. Reduced hemostatic effects with drospirenone-based oral contraceptives containing estetrol vs. ethinyl estradiol. Contraception. 2017;95:140–7.
Shapiro S. Oral contraceptives, hormone therapy and cardiovascular risk. Climacteric. 2008;11:355–63.
Jirouskova M, Shet AS, Johnson GJ. A guide to murine platelet structure, function, assays, and genetic alterations. J Thromb Haemost. 2007;5:661–9.
Valéra MC, Noirrit-Esclassan E, Dupuis M, Fontaine C, Lenfant F, Briaux A, Cabou C, Garcia C, Lairez O, Foidart JM, Payrastre B, Arnal JF. Effect of estetrol, a selective nuclear estrogen receptor modulator, in mouse models of arterial and venous thrombosis. Mol Cell Endocrinol. 2018;477:132–9. S0303-7207(18) 30196-5
Geddings J, Aleman MM, Wolberg A, von Bruhl ML, Massberg S, Mackman N. Strengths and weaknesses of a new mouse model of thrombosis induced by inferior vena cava stenosis: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12:571–3.
Donnem T, Reynolds AR, Kuczynski EA, Gatter K, Vermeulen PB, Kerbel RS, Harris AL, Pezzella F. Non-angiogenic tumours and their influence on cancer biology. Nat Rev Cancer. 2018;18:323–36.
Péqueux C, Raymond-Letron I, Blacher S, Boudou F, Adlanmerini M, Fouque MJ, Rochaix P, Noël A, Foidart JM, Krust A, Chambon P, Brouchet L, Arnal JF, Lenfant F. Stromal estrogen receptor-α promotes tumor growth by normalizing an increased angiogenesis. Cancer Res. 2012;72:3010–9.
EMA. European Medicines Agency, Committee for Medicinal Products for Human Use. Guideline on clinical investigation of steroid contraceptives in women. London: EMA; 2005. EMEA/CPMP/EWP/519/98 rev 1
Benoit T, Valera MC, Fontaine C, Buscato M, Lenfant F, Raymond-Letron I, Tremollieres F, Soulie M, Foidart JM, Game X, Arnal JF. Estetrol, a fetal selective estrogen receptor modulator, acts on the vagina of mice through nuclear estrogen receptor α activation. Am J Pathol. 2017;187:2499–507.
Acknowledgments
The authors warmfully thank Professor Rogerio A. Lobo, Columbia University College of Physicians and Surgeons, and Professor Mitchell Creinin, Division of Family Planning, Department of Obstetrics and Gynecology, University of California, Davis, Sacramento, California, for helpful discussions and comments in the preparation and reviewing process of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 International Society of Gynecological Endocrinology
About this chapter
Cite this chapter
Foidart, J.M. et al. (2019). Unique Vascular Benefits of Estetrol, a Native Fetal Estrogen with Specific Actions in Tissues (NEST). In: Brinton, R., Genazzani, A., Simoncini, T., Stevenson, J. (eds) Sex Steroids' Effects on Brain, Heart and Vessels. ISGE Series. Springer, Cham. https://doi.org/10.1007/978-3-030-11355-1_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-11355-1_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-11354-4
Online ISBN: 978-3-030-11355-1
eBook Packages: MedicineMedicine (R0)