Abstract
Endovascular repair of the ascending aorta and aortic arch is an emerging field of aortic surgery currently reserved for patients not candidates for traditional cardiac surgery. Techniques to avoid hypothermic circulatory arrest, cardiopulmonary bypass, and even chest incisions range from total endovascular procedures to a panoply of surgical debranching procedures in combination with thoracic endovascular aortic repair (TEVAR). Here, we describe the indications, approach, techniques, and outcomes of ascending and arch TEVAR. Ascending TEVAR can be performed using existing endografts designed for the descending and abdominal aorta or investigational devices which are specifically designed for the ascending aorta. Techniques for arch TEVAR is determined by the method of great vessel revascularization and includes cervical (extra-thoracic) debranching, thoracic debranching, chimney stent grafting, and branched stent grafting. Current challenges include device durability and minimizing the rates of neurological, vascular, and cardiac complications. Device innovation and demonstration of long-term efficacy may lead to expansion of the indications for endovascular repair of the ascending aorta and aortic arch to candidates of lower surgical risk.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Endovascular
- Ascending aorta
- Aortic arch
- Hybrid arch repair
- Chimney stent grafting
- Branched stent grafting
Introduction
Surgical pathologies of the ascending aorta and aortic arch are currently managed using an open approach in suitable candidates, with a mortality rate of 3% for the ascending aorta [1,2,3,4,5], 4–10% for the aortic arch [6, 7], and approximately 25% for acute type A dissections [8, 9]. Open surgery requires sternotomy, cardiopulmonary bypass, and cerebral protection. Patients with advanced frailty, multiple comorbidities, or unfavorable anatomic features are often considered prohibitive risk for open repair of the ascending aorta and aortic arch due to increased morbidity and mortality. Endovascular repair has emerged as a viable option for patients considered high risk for an open surgery. While the endovascular approach, devices, and technique have been well described for the descending and infrarenal aorta, endovascular repair of the ascending aorta and aortic arch is in its relative infancy, available only in an off-label fashion using devices approved for the descending and abdominal aorta. Here, we review the current knowledge on the indications, approach, techniques, and outcomes of endovascular repair of the ascending aorta and aortic arch.
Preoperative Diagnostic Imaging
Imaging the ascending aorta and aortic arch is essential in the evaluation and treatment of aortic pathology. Several imaging modalities including computed tomography angiography (CTA) and magnetic resonance angiography (MRA) have been successfully used to identify pathology of the ascending aorta and aortic arch. Multidetector CT can be used to determine operative candidacy, during preoperative planning, and in the postoperative surveillance of patients undergoing aortic surgery [10]. Images can be converted into three-dimensional reconstructions which enable angiographic evaluation of the ascending aorta, aortic arch, supra-aortic trunks, and access vessels. Motion artifacts can be reduced with electrocardiographic (ECG) gating, which can also be used to assess the coronary vasculature. Transesophageal echocardiography (TEE) can be used to assess cardiac hemodynamics and aortic valve pathology associated with thoracic aneurysm and left ventricular thrombus [11]. TEE is also useful for monitoring complications following graft deployment such as aortic regurgitation (AR) and coronary obstruction.
Intraoperative Monitoring
Intravascular ultrasound (IVUS) has emerged as one of the key instruments for performing endovascular repair in the ascending aorta and aortic arch, serving as the most accurate method of measuring intraluminal diameter [12]. IVUS provides real-time dynamic images that can be used to establish graft landing zones and graft selection, visualize thrombi or plaques, and inspect branch vessel anatomy.
Fusion imaging integrates preoperative CT images with intraoperative fluoroscopy and provides a nuanced method for developing a strategy for proximal aortic repair. This process has been used in complex aortic procedures including fenestrated branched endovascular repair and has been shown to increase the accuracy of endovascular graft placement and decrease the contrast load. Fusion imaging is also associated with lower operative and fluoroscopy times. Moreover, confirmation of postprocedural success using fusion imaging is comparable to multidetector CT (MDCT) [13].
Instrumentation of the ascending aorta and aortic arch increases the risk for developing neurologic complications due to the proximity of the supra-aortic vessels and atheroma burden of the aortic arch. Moreover, graft placement can involve several wire manipulations which may generate and propagate thrombi. Several modalities have been developed for intraoperative neurologic monitoring. Transcranial Doppler can be used intraoperatively to provide real-time detection of cerebral microemboli and changes in cerebral blood flow [14]. Variations in cerebral flow velocities can be monitored as endografts are deployed through the ascending aorta and aortic arch. Near-infrared spectroscopy can also be used to assess cerebral oxygenation during endovascular repair of the aorta [15, 16].
Indications and Contraindications
Traditional indications for thoracic endovascular aortic repair (TEVAR) have included asymptomatic thoracic aortic aneurysms larger than 5.5 cm, symptomatic thoracic aortic aneurysms (TAAs) or those with expansion greater than 5 mm over 6 months [17, 18], type B aortic dissection [19, 20], penetrating aortic ulcers, intramural hematomas [21], and traumatic aortic injury [22]. Several case reports and series have contributed to a growing body of literature seeking to expand TEVAR indications to include patients with type A dissections [23, 24] or those deemed prohibitive risk for surgery [25]. The primary contraindication to TEVAR is unfavorable anatomy. Patients with inadequate access vessels (heavily calcified vessels or iliac diameter <7 mm, unable to accommodate 22F or 24F sheaths), inadequate proximal or distal seal zones (<10 mm in length or at extremes of diameter [<16 mm or > 42 mm]), extensive aortic tortuosity, or an actively infected field may not qualify for endovascular repair. TEVAR is also generally avoided in patients with connective tissue disorders unless used as a salvage procedure before definitive open surgical management.
Ascending Aorta
Endovascular intervention in the ascending aorta has traditionally been limited by several inherent anatomic features including its angulation, short length, complex spatial geometry, hemodynamic throughput, large diameter fixation sites, and proximity to the aortic valve and coronary vessels. Thus, the endovascular approach to the ascending aorta has usually been reserved for patients at prohibitive risk for open intervention. Initial reports described the use of ascending TEVAR for type A dissections, pseudoaneurysms, and penetrating atherosclerotic ulcers [26, 27].
The anatomical considerations for ascending TEVAR are listed in Table 20.1. Access for endovascular repair of both the ascending aorta and aortic arch is most commonly achieved with a transfemoral approach using commercially available endografts designed for the descending thoracic aorta. Transapical, transseptal, transaxillary, and carotid approaches have also been described for patients in whom femoral access is not possible [11, 29, 30] or when using aortic extension endografts designed to reach the abdominal aorta (and too short to the thoracic aorta).
Several reports have described the ascending TEVAR with the use of thoracic stent grafts that have been modified for the ascending aorta, usually with proximal extensions of thoracic endografts [31,32,33]. We have used the extension cuff from an abdominal aortic stent graft to perform an aortic reconstruction for an ascending aortic pseudoaneurysm in a patient deemed prohibitive risk for open surgery. Kolvenbach described the use of stent grafting the ascending aorta in 11 patients [27]. Technical success was achieved in 91% of the cohort with one endoleak, one cerebrovascular accident, and one death due to left ventricular perforation by a wire. Li recently reported the long-term outcomes of a series of 15 patients who had undergone endovascular repair of ascending aortic dissections [34]. Although no deaths occurred in the median 72 months of follow-up, there were eight major complications and four reinterventions. One patient developed a new dissection in the aortic arch distal to the endograft at 3 months and was treated with a branched stent graft . Another patient experienced a retrograde type A aortic dissection 29 months following endografting and underwent replacement of the ascending aorta and proximal arch. There was also one endoleak which occurred at 71 months which was managed conservatively. At 12 months, significant decreases in false lumens and total aortic diameter were observed along with an increase in the true lumen. These changes in aortic remodeling remained stable over 3 years, thereby demonstrating the sustained effect of endovascular exclusion.
Devices for the Ascending Aorta
The Zenith Ascend TAA endovascular graft (Cook Medical) is a single-component tubular endograft which consists of polyester fabric sewn onto self-expanding nitinol stents (Fig. 20.1). Both the proximal and distal ends of the graft contain uncovered stents which can be used to improve graft deployment and subsequent apposition in the aorta. It is 65 mm long and comes in diameters ranging from 28 to 46 mm. Endograft deployment is performed using a 100-cm pre-curved introducer using sequential deployment which enables a staged release. Using either a transfemoral or transapical approach, the device can then be deployed under rapid ventricular pacing, adenosine-induced cardiac arrest, or vena cava occlusion technique.
Cook Medical Zenith Ascend TAA endovascular graft. (From Tsilimparis et al. [35]. Reprinted with permission from Elsevier)
Metcalf reported the first successful clinical implantation of a dedicated ascending aortic endograft in a patient with a type A dissection [36]. Tsilimparis later reported outcomes using a modified version of this graft in a series of 10 patients with ascending aortic pathology deemed unsuitable for open surgery [35]. There was one perioperative death which occurred in a patient who developed a persistent type Ia endoleak after undergoing ascending aortic grafting for an intraoperative aortic valve implantation dissection in the setting of transcatheter aortic valve replacement (TAVR). Late outcomes included three additional deaths and two graft replacements for endoleaks.
The Valiant PS-IDE was available in two configurations, one with a proximal closed-web design with distal stent and a second one with proximal FreeFlo stent (Fig. 20.2). The device comes in 5-, 7-, and 9-cm lengths with diameters ranging from 28 to 44 mm. Bilateral femoral arterial and venous access is established for IVUS, device delivery, and ventricular pacing, respectively. Khoynezhad reported the early results of a feasibility study using the Valiant Captiva (Medtronic, Inc.) in a series of six patients who received investigational device exemption [37]. There were no perioperative deaths, but one patient died 4 months after undergoing ascending aorta repair for de novo ulceration in the mid-aortic arch which required a total arch replacement and frozen elephant repair. One patient developed a lacunar infarct and type I endoleak and an additional patient experienced wire perforation of the left ventricle with resultant pericardial effusion which resolved with conservative management.
Medtronic Valiant PS-IDE. (From Khoynezhad et al. [37]. Reprinted with permission from Elsevier)
Aortic Arch
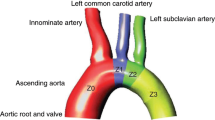
The aorta is divided into five landing zones from 0 to 4 (Fig. 20.3). Placement of endografts into the aortic arch (Zones 0 through 2) results in occlusion of the aortic arch branches and requires additional techniques for branch revascularization. Endovascular repair of the aortic arch can be achieved with hybrid arch repair, chimney stent grafting, fenestrated stent grafting, or branched stent grafting. In the hybrid approach, endovascular techniques are combined with anatomic and extra-anatomic surgical revascularization of the arch vessels to extend the proximal seal zone.
Hybrid Repair
Hybrid repair combines supra-aortic artery debranching to create a proximal landing zone (Fig. 20.4). In its simplest form, the left subclavian artery (LSA) artery may be revascularized by either carotid–subclavian transposition or carotid–subclavian bypass for Zone 2 TEVAR. The transposition technique requires more extensive dissection in order to gain access proximal to the vertebral artery and has also been associated with a higher rate of complications [40]. The bypass technique, on the other hand, requires a bypass graft and an additional procedure to occlude the proximal portion of the subclavian artery. In its most complex form, the entire arch can be debranched and revascularized using a combination of anatomic and extra-anatomic configurations (Fig. 20.5).
Algorithm for hybrid aortic arch repair. *Note that these criteria are relative factors in the decision-making process but not absolute indications/contraindications. Ideally, the decision for conventional versus hybrid repair should be made by a surgical team with expertise in both techniques. Institutional results with each approach should further influence the decision-making process. (From Andersen et al. [39]. Reprinted with permission from Elsevier)
Hybrid aortic arch repair . (a) Scheme of the operative approach (I: aorto-brachiocephalic bypass; II: bypass side branch to the left common carotid artery; III: carotid–subclavian bypass). (b) Carotid–subclavian bypass (III). (c) Bypass to the brachiocephalic artery (I) and to the left common carotid artery (II) in the open aortic surgery. (d) Reconstructed, contrast-enhanced computed tomography scan with the main stent graft in the ascending aorta and aortic arch, covering the ostia of the brachiocephalic and the left common carotid artery (I: aorto-brachiocephalic bypass; II: bypass side branch to the left common carotid artery). (From Shah et al. [41]. Reprinted with permission from Ali Khoynezhad, Long Beach Medical Center)
Moulakakis et al. conducted a systematic review of hybrid arch replacement techniques including 26 studies with 956 patients who underwent debranching procedures and 20 studies with 1316 patients who underwent elephant trunk procedures [42]. Perioperative mortality was estimated at 11.9% in the debranching group and 9.5% in the elephant trunk group. Pooled rates of cerebrovascular complications were 7.6% and 6.2% in the arch debranching group and elephant trunk group, respectively.
Miao recently published an analysis comparing hybrid arch repair to open surgical approach [43]. Their work combined the results from seven studies with 727 patients, 269 of whom underwent hybrid arch repair and 458 who underwent open surgical repair. Although hybrid arch repair was associated with decreased ICU lengths of stay and overall hospital stay, there was a trend toward increased late mortality at 2 years compared to an open approach (OR 3.41; 95% CI 0.83–14.03; p = 0.09). Operative mortality (OR 0.75; 95% CI 0.41–1.30; p = 0.37), neurological complications (OR 1.24; 95% CI 0.73–2.13; p = 0.42), and renal failure (OR 0.80; 95% CI 0.40–1.61; p = 0.53) were comparable between the groups. Importantly, patients undergoing open repair had decreased need for reintervention compared to those undergoing hybrid arch repair (OR 3.43; 95% CI 1.72–6.84; p = 0.0005).
The cause of increased reinterventions in the hybrid arch group was likely the increased rate of type I endoleaks with continued growth of the aneurysm which could increase the risk of rupture. Type I endoleaks usually result from propagation of a pathological lesion, inadequate proximal or distal seal, or technical difficulties associated with the device. Lower rates of endoleak and reintervention were observed in patients undergoing hybrid arch repair in Zone 0 [43]. Type I endoleak may therefore be theoretically reduced with the use of an additional stent graft, which is extended to Zone 0. The increased reinterventions and late mortality associated with hybrid arch repair may also result from the increased risk associated with patients undergoing hybrid repair who often have multiple comorbidities which may preclude them from undergoing an open repair.
Chimney Stent Grafting
With chimney stent grating , multiple stent grafts are placed in the aortic arch branches in the same seal zone, entering the aorta parallel to the main aortic stent graft (Figs. 20.6 and 20.7). Although chimney stenting does not lengthen the seal zone, it does increase the available space for proximal fixation of the stent graft. It also enables blood to be simultaneously directed through the main aortic stent and chimney graft to provide both aortic and branch vessel perfusion. Greenberg et al. first described chimney stent grafting as a method of renal artery preservation in the management of abdominal aortic aneurysms with short proximal necks [44]. This technique was then adapted by Criado in a bailout operation following left common carotid artery coverage by a TEVAR graft [45]. The current indications for chimney stent grafting include poor candidacy for open surgery or hybrid procedures, insufficient landing zones for traditional TEVAR, and bailout revascularization following inadvertent over-stenting during endovascular operations.
Chimney technology. (a) Illustration of the chimney stent graft technology . The “chimney” stent graft (I) supplies the left common carotid artery and is located alongside the main stent graft. A carotid–subclavian bypass will ensure the perfusion of the left subclavian artery (II). (b) Scheme of the arrangement of the stent grafts in the aorta in the transversal section view. (c) Transverse computed tomography (CT) scan section with the chimney graft and main stent graft (arrow indicates the chimney stent graft). (d) Reconstructed CT angiogram with the chimney stent graft in the left common carotid artery (arrow). (From Shah et al. [41]. Reprinted with permission from Ali Khoynezhad, Long Beach Medical Center)
Chimney procedure. (a) Preoperative angiogram demonstrating aortic pseudoaneurysm on the lesser curve of the aortic arch at the origin of the left subclavian artery (LSA). (b) Fluoroscopic image demonstrating the chimney sheath protruding into the aortic arch adjacent to the deployed aortic stent graft. (c) Fully deployed aortic stent graft and LSA chimney stent graft. (d) Completion angiogram revealing successfully excluded aortic pseudoaneurysm with patent LSA stent graft and no endoleak. (From Shah et al. [41]. Reprinted with permission from Ali Khoynezhad, Long Beach Medical Center)
Unfortunately, the process inherently creates gutters between the parallel chimney graft and the main aortic stent graft, which may lead to type Ia endoleaks [46]. Oversizing by at least 20% enhances wall apposition, facilitates the formation of channels lateral to the graft, and decreases gutter development [47]. Adequate sealing and fixation can be brought about by using aortic neck lengths > 10 mm and ensuring appropriate stent-graft overlapping. The chimney stent graft provides a degree of interference along the endograft which enables the aortic length distal to the chimney graft to be available for preventing type Ia endoleak. The degree of overlap between the chimney graft and the thoracic endograft should be between 3 and 7 cm [48, 49].
Chimney stents are available in balloon expandable or self-expanding stent forms. The balloon expandable stents create strong radial force and are associated with more accurate positioning. Self-expanding chimney stents are better able to conform complex geometry of aortic anatomy. Mangialardi et al. reported outcomes of 26 patients who underwent chimney stenting with TEVAR for various aortic pathologies including thoracic aortic aneurysm, complicated type B dissection, type I endoleak following prior TEVAR, and penetrating ulcer [50]. They reported a technical success rate of 100% with one perioperative death from a cerebral hemorrhage. At 18 months, chimney graft patency was 89.3%, and 23% of patients developed type I endoleaks. A recent analysis by Mangialardi et al. reviewed 182 patients who underwent 217 chimney graft implantations including 91 to the LCCA, 89 to the LSA, and 36 to the brachiocephalic artery [51]. They reported a technical success rate of 98%, a stroke rate of 5.3%, and endoleak rate of 18.4%.
Fenestrated Stent Grafting
Fenestrated stent grafts , or those which feature openings along the fabric to enable blood flow into branch vessels, have been used successfully in the management of distal aortic pathology. Newer devices have been developed in an attempt to apply fenestrated technology to the aortic arch. Kawaguchi described the results of the first generation of the Japan’s Najuta system (Kawasumi Laboratories, Tokyo, Japan), a preformed, stainless steel stent attached to PTFE [52]. From 1995 to 2008, approximately 1100 endovascular repairs were performed including 435 in the distal aortic arch, of which 288 involved the fenestrated endograft. The initial technical success rate (absence of type I or III endoleak) was 95.2% with a stroke rate of 5.5% in the cohort managed with the fenestrated endograft. The Najuta graft used in this trial required patients to have a proximal landing zone greater than 20 mm. The device was subsequently modified to allow placement in patients with proximal landing zones greater than 10 mm. Azuma et al. reported their experience in aortic arch reconstruction in 393 patients using 19 types of curved stent skeletons and eight types of graft fenestrations [53]. Technical success was achieved in 99.2% of patients, while hospital mortality rate was 1.5%, and 1.7% of patients experienced a cerebrovascular accident (CVA). The modified endograft therefore proved efficacious in cases with short landing zones.
Fenestrated graft deployment often requires substantial catheter manipulation to achieve accurate positioning, which can increase the risk of cerebrovascular complications and arterial embolization. By creating fenestrations directly in the graft across from the corresponding vessels, the in situ technique reduces the need for catheter manipulation and can be readily applied to off-the-shelf stent grafts. Retrograde fenestration is achieved from the common carotid approach using laser, radiofrequency, or a needle [54].
Branched Stent Grafting
In 1999, Inoue et al. described the use of branched stent grafts in 15 patients with aortic arch aneurysms [55]. Two failures were noted in which the stent graft did not pass through the 22F sheath due to increased tortuosity and small iliac artery diameter. Ultimately, complete aneurysm thrombosis was achieved in 11 (73%) patients. In the Chuter method, two stent grafts are inserted following CC and LCA-SA bypass creation to repair wide-necked aortic arch pseudoaneurysms (Fig. 20.8). The branched stent graft is positioned proximally in the ascending aorta and distally in the innominate artery and descending thoracic aorta. The technique, however, proved to be technically challenging and associated with high rates of morbidity and mortality. There was also a risk of modular disconnection. Modern devices for branched and fenestrated stent grafting are available as part of investigational device studies. Once approved, these devices have the potential to decrease the need for debranching techniques, chimney stent grafting, and ultimately open aortic arch repair.
Chuter branched stent technique. (From Chuter et al. [56]. Reprinted with permission from Elsevier) (a) Carotid-carotid bypass and subclavian-carotid reimplantation (b) Insertion of first sheath (c) Deployment of proximal stent (d) Deployment of short aortic limb
Single-Branched Endografts
Reconstruction of the distal aortic arch may require endograft occlusion of the left subclavian artery, following subclavian revascularization, to achieve an adequate proximal landing zone. Single-branched endografts were designed to maintain LSA patency, thereby obviating the need for revascularization during stent graft deployment in thoracic aortic aneurysms. The custom-made Inoue system features a Dacron stent graft, a detachable carrying wire, a balloon catheter, an introducer wire, and two detachable traction wires. After the graft is positioned using the carrying wire and traction wire, the aortic and branched sections are deployed using balloon dilation. Saito described the successful deployment of the Inoue system in 17 patients with thoracic aortic aneurysms with 3 patients developing endoleaks, 1 patient developing spinal ischemia, and no device-related mortality over the course of 28 months [57]. The Valiant Mona LSA system (Medtronic Inc., Santa Rosa, CA, USA) features a nitinol-containing main and branch stent grafts which are delivered separately (Fig. 20.9). Roselli reported the early feasibility results of the system in nine patients with four endoleaks, four minor CVAs, and no mortality was observed in nearly 6 months of follow-up [58]. The Gore Thoracic Branch Endoprosthesis (TBE) (W.L. Gore, Flagstaff, AZ, USA) is nitinol-based expanded polytetrafluoroethylene stent graft with an internal portal that accommodates a tapered, heparin-coated, stent graft oriented in a retrograde manner (Fig. 20.10). Patel reported early feasibility results of the system in 22 patients demonstrating no mortality, stroke, paraplegia, or type 1 endoleaks at 30 days and 1 patient with paraparesis [59]. The Gore TBE device is also currently being studied in Zone 2 TEVAR (NCT02777593) and Zone 0/1 TEVAR (NCT02777528) using a hybrid approach (Fig. 20.11).
Medtronic Valiant Mona LSA. (From Roselli et al. [58]. Reprinted with permission from Elsevier)
Branched/fenestrated thoracic endovascular aortic repair. (a) Illustration of a branched stent graft (I) from the main stent graft supplying the brachiocephalic artery (red). To ensure sufficient blood supply for the covered brachiocephalic vessels, a carotid–carotid bypass (II) and a carotid–subclavian bypass (III) can be performed. (b) Branched stent graft (Gore TBE). The arrow indicates the stent graft that will be deployed into the brachiocephalic vessel. (c) Fluoroscopy of a branched brachiocephalic trunk (arrow). (From Shah et al. [41]. Reprinted with permission from Ali Khoynezhad, Long Beach Medical Center)
Double-Branched Endografts
The custom-made Cook arch branched system (Cook Medical Inc., Denmark) features a curved body endograft with two side branches (Fig. 20.12). Using a 22 or 24 Fr delivery system, the device is designed for a Zone 0 landing with a diameter ≤ 38 mm. The main graft is delivered through femoral access, while the left axillary and right common carotid arteries are cannulated to obtain access to the left common carotid and innominate arteries, respectively. Haulon et al. reported the outcomes of a multicenter study involving 38 patients who underwent endovascular exclusion of arch aneurysms using a branched endograft with two inner branches [60]. Perioperative mortality was 13.2%, and technical success was achieved in 84.2% of patients. Cerebrovascular complications were noted in 6 (15.8%) patients, while endoleaks were noted in 11 (28.8%) patients. The authors also reported a learning curve of ten operations, after which reductions in perioperative mortality (two vs. three; p = 0.066), intraoperative complications (three vs. four; p = 0.04), secondary procedures for endoleak (zero vs. three; p = 0.014), and operative time (248 vs. 320 min; p = 0.03) were achieved. Ascending aorta diameters ≥ 38 mm were associated with an increased risk of combined early mortality and cerebrovascular events (p = 0.026). The authors reasoned the increased risk was due to the less accurate endograft deployment in a large ascending aorta which itself could represent a less stable sealing zone.
The Double Branch Arch system (Bolton Medical, Sunrise, FL, USA) includes a fixed branch configuration with a large opening for two nitinol internal branches inside the main endograft (Fig. 20.13). Locking barbs in both internal tunnels help to prevent component migration and disconnection. Riambiau recently reported the early results of 26 patients undergoing treatment with the double-branched endograft for thoracic aortic aneurysm or dissection [61]. In this cohort, there were three endoleaks, one stroke (which resulted in death), and a perioperative mortality of 7.7%.
Triple-Branched Endograft
Triple-branched stenting was developed as part of a hybrid technique in which the transverse arch and proximal descending aorta are repaired in an open approach. Chen et al. described the successful repair of the ascending aorta and aortic arch and 3 arch vessels simultaneously with an open placement of a triple-branched stent graft combined with graft replacement of the ascending aorta as part of the primary repair in 30 patients with acute type A dissection [62]. This technique was successfully applied in a cohort of 121 patients with selective antegrade perfusion with excellent results [63]. Perioperative mortality was 3.3%, and although neurovascular complications were noted in 13 patients, no permanent dysfunction was identified.
Surveillance Imaging Following Endovascular Repair
Patients undergoing endovascular interventions of the ascending aorta and aortic arch should be followed closely in the first year after intervention. Scheduled exams should occur at 1 month of treatment followed by surveillance visits at 6 months, 12 months, and then yearly. Clinical evaluation should focus on blood pressure management and detection of complications, which may present subtly. CT is presently the first-line imaging modality in surveillance following TEVAR (Level IC; [64]). Although the principal disadvantage of CT is the amount of radiation delivered during scans, several device innovations such as prospective gating, low tube voltage, and dose reduction protocols have been developed to mitigate the risks [65, 66]. MRI has also been successfully used in the surveillance of nitinol stent grafts [67]. Endografts containing stainless steel components may generate artifacts on MRI which may limit its clinical applicability [17]. In patients with contraindications to CT or MRI, a combination of TEE and chest radiography can be used for postoperative surveillance .
Conclusions and Future Directions
The ascending aorta and aortic arch remain the last frontier of endovascular aortic intervention. Innovations in endovascular technology for the ascending aorta and aortic arch continue to rapidly evolve with direct applications for treating aortic pathology. In the USA, surgeons have modified the preexisting technology for thoracic/abdominal EVAR to create solutions for the ascending aorta and aortic arch. Newer devices are needed which can conform to the unique anatomical constraints of the ascending aorta and aortic arch with low profile delivery systems. Approval of devices specifically designed for the ascending aorta and aortic arch will expand the armamentarium for managing patients at advanced surgical risk. Long-term outcomes and device durability remain prominent concerns of the new devices. As the technical considerations and complications are minimized, endovascular repair of the ascending aorta and aortic arch may prove a viable option for lower risk surgical patients. Until then, open surgery remains the standard of care for diseases of the ascending aorta and aortic arch.
References
Moon MC, Morales JP, Greenberg RK. The aortic arch and ascending aorta: are they within the endovascular realm? Semin Vasc Surg. 2007;20(2):97–107.
Gott VL, Gillinov AM, Pyeritz RE, Cameron DE, Reitz BA, Greene PS, et al. Aortic root replacement. Risk factor analysis of a seventeen-year experience with 270 patients. J Thorac Cardiovasc Surg. 1995;109(3):536–44; discussion 44–5.
Lakew F, Pasek P, Zacher M, Diegeler A, Urbanski PP. Femoral versus aortic cannulation for surgery of chronic ascending aortic aneurysm. Ann Thorac Surg. 2005;80(1):84–8.
Svensson LG, Crawford ES, Coselli JS, Safi HJ, Hess KR. Impact of cardiovascular operation on survival in the Marfan patient. Circulation. 1989;80(3 Pt 1):I233–42.
Cohn LH, Rizzo RJ, Adams DH, Aranki SF, Couper GS, Beckel N, et al. Reduced mortality and morbidity for ascending aortic aneurysm resection regardless of cause. Ann Thorac Surg. 1996;62(2):463–8.
Kazui T, Yamashita K, Washiyama N, Terada H, Bashar AH, Suzuki T, et al. Usefulness of antegrade selective cerebral perfusion during aortic arch operations. Ann Thorac Surg. 2002;74(5):S1806–9; discussion S25–32.
Estrera AL, Miller CC 3rd, Lee TY, Shah P, Safi HJ. Ascending and transverse aortic arch repair: the impact of retrograde cerebral perfusion. Circulation. 2008;118(14 Suppl):S160–6.
Geirsson A, Szeto WY, Pochettino A, McGarvey ML, Keane MG, Woo YJ, et al. Significance of malperfusion syndromes prior to contemporary surgical repair for acute type a dissection: outcomes and need for additional revascularizations. Eur J Cardiothorac Surg. 2007;32(2):255–62.
Appoo JJ, Pozeg Z. Strategies in the surgical treatment of type a aortic arch dissection. Ann Cardiothorac Surg. 2013;2(2):205–11.
Godoy MC, Cayne NS, Ko JP. Endovascular repair of the thoracic aorta: preoperative and postoperative evaluation with multidetector computed tomography. J Thorac Imaging. 2011;26(1):63–73.
Heye S, Daenens K, Maleux G, Nevelsteen A. Stent-graft repair of a mycotic ascending aortic pseudoaneurysm. J Vasc Interv Radiol. 2006;17(11 Pt 1):1821–5.
Pearce BJ, Jordan WD Jr. Using IVUS during EVAR and TEVAR: improving patient outcomes. Semin Vasc Surg. 2009;22(3):172–80.
Dijkstra ML, Eagleton MJ, Greenberg RK, Mastracci T, Hernandez A. Intraoperative C-arm cone-beam computed tomography in fenestrated/branched aortic endografting. J Vasc Surg. 2011;53(3):583–90.
Bismuth J, Garami Z, Anaya-Ayala JE, Naoum JJ, El Sayed HF, Peden EK, et al. Transcranial Doppler findings during thoracic endovascular aortic repair. J Vasc Surg. 2011;54(2):364–9.
Murkin JM, Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth. 2009;103(Suppl 1):i3–13.
Etz CD, von Aspern K, Gudehus S, Luehr M, Girrbach FF, Ender J, et al. Near-infrared spectroscopy monitoring of the collateral network prior to, during, and after thoracoabdominal aortic repair: a pilot study. Eur J Vasc Endovasc Surg. 2013;46(6):651–6.
Grabenwoger M, Alfonso F, Bachet J, Bonser R, Czerny M, Eggebrecht H, et al. Thoracic endovascular aortic repair (TEVAR) for the treatment of aortic diseases: a position statement from the European Association for Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2012;33(13):1558–63.
Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE Jr, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121(13):e266–369.
Nienaber CA, Kische S, Ince H, Fattori R. Thoracic endovascular aneurysm repair for complicated type B aortic dissection. J Vasc Surg. 2011;54(5):1529–33.
Szeto WY, McGarvey M, Pochettino A, Moser GW, Hoboken A, Cornelius K, et al. Results of a new surgical paradigm: endovascular repair for acute complicated type B aortic dissection. Ann Thorac Surg. 2008;86(1):87–93; discussion -4.
Eggebrecht H, Plicht B, Kahlert P, Erbel R. Intramural hematoma and penetrating ulcers: indications to endovascular treatment. Eur J Vasc Endovasc Surg. 2009;38(6):659–65.
Dake MD, White RA, Diethrich EB, Greenberg RK, Criado FJ, Bavaria JE, et al. Report on endograft management of traumatic thoracic aortic transections at 30 days and 1 year from a multidisciplinary subcommittee of the Society for Vascular Surgery Outcomes Committee. J Vasc Surg. 2011;53(4):1091–6.
Chen LW, Wu XJ, Dai XF, Liao DS, Hu YN, Zhang H, et al. Repair of acute type a aortic dissection with ascending aorta replacement combined with open fenestrated stent graft placement. Ann Thorac Surg. 2016;101(2):644–9.
Kolbel T, Detter C, Carpenter SW, Rohlffs F, von Kodolitsch Y, Wipper S, et al. Acute type a aortic dissection treated using a tubular stent-graft in the ascending aorta and a multibranched stent-graft in the aortic arch. J Endovasc Ther. 2017;24(1):75–80.
Roselli EE, Idrees J, Greenberg RK, Johnston DR, Lytle BW. Endovascular stent grafting for ascending aorta repair in high-risk patients. J Thorac Cardiovasc Surg. 2015;149(1):144–51.
Chan YC, Cheng SW. Endovascular management of Stanford type a (ascending) aortic dissection. Asian Cardiovasc Thorac Ann. 2009;17(6):566–7.
Kolvenbach RR, Karmeli R, Pinter LS, Zhu Y, Lin F, Wassiljew S, et al. Endovascular management of ascending aortic pathology. J Vasc Surg. 2011;53(5):1431–7.
Muehle A, Shah A, Khoynezhad A. Thoracic endovascular aortic repair in the ascending aorta. Innovations (Philadelphia, Pa). 2015;10(5):363–7.
Lin PH, Kougias P, Huynh TT, Huh J, Coselli JS. Endovascular repair of ascending aortic pseudoaneurysm: technical considerations of a common carotid artery approach using the zenith aortic cuff endograft. J Endovasc Ther. 2007;14(6):794–8.
Szeto WY, Moser WG, Desai ND, Milewski RK, Cheung AT, Pochettino A, et al. Transapical deployment of endovascular thoracic aortic stent graft for an ascending aortic pseudoaneurysm. Ann Thorac Surg. 2010;89(2):616–8.
Roselli EE, Brozzi N, Albacker T, Lytle BW. Transapical endovascular ascending repair for inoperable acute type a dissection. JACC Cardiovasc Interv. 2013;6(4):425–6.
Preventza O, Henry MJ, Cheong BY, Coselli JS. Endovascular repair of the ascending aorta: when and how to implement the current technology. Ann Thorac Surg. 2014;97(5):1555–60.
Ronchey S, Serrao E, Alberti V, Fazzini S, Trimarchi S, Tolenaar JL, et al. Endovascular stenting of the ascending aorta for type a aortic dissections in patients at high risk for open surgery. Eur J Vasc Endovasc Surg. 2013;45(5):475–80.
Li Z, Lu Q, Feng R, Zhou J, Zhao Z, Bao J, et al. Outcomes of endovascular repair of ascending aortic dissection in patients unsuitable for direct surgical repair. J Am Coll Cardiol. 2016;68(18):1944–54.
Tsilimparis N, Debus ES, Oderich GS, Haulon S, Terp KA, Roeder B, et al. International experience with endovascular therapy of the ascending aorta with a dedicated endograft. J Vasc Surg. 2016;63(6):1476–82.
Metcalfe MJ, Karthikesalingam A, Black SA, Loftus IM, Morgan R, Thompson MM. The first endovascular repair of an acute type a dissection using an endograft designed for the ascending aorta. J Vasc Surg. 2012;55(1):220–2.
Khoynezhad A, Donayre CE, Walot I, Koopmann MC, Kopchok GE, White RA. Feasibility of endovascular repair of ascending aortic pathologies as part of an FDA-approved physician-sponsored investigational device exemption. J Vasc Surg. 2016;63(6):1483–95.
Azizzadeh AVJ, Estrera AL, Charlton-Ouw KM, Safi HJ. Thoracic endovascular aortic repair (TEVAR): a focus on complications. Ciru Cardiovasc. 2010;17(1):11–23.
Andersen ND, Williams JB, Hanna JM, Shah AA, McCann RL, Hughes GC. Results with an algorithmic approach to hybrid repair of the aortic arch. J Vasc Surg. 2013;57(3):655–67; discussion 66–7.
Domenig CM, Linni K, Mader N, Kretschmer G, Magometschnigg H, Holzenbein TJ. Subclavian to carotid artery transposition: medial versus lateral approach. Eur J Vasc Endovasc Surg. 2008;35(5):551–7.
Shah A, Bombien R, Khoynezhad A. Thoracic endovascular aortic repair: are we approaching total endovascular solutions for thoracic aortic disease? Multimed Man Cardiothorac Surg. 2014;2014. https://doi.org/10.1093/mmcts/mmu009.
Moulakakis KG, Mylonas SN, Markatis F, Kotsis T, Kakisis J, Liapis CD. A systematic review and meta-analysis of hybrid aortic arch replacement. Ann Cardiothorac Surg. 2013;2(3):247–60.
Miao L, Song L, Sun SK, Wang ZG. Meta-analysis of open surgical repair versus hybrid arch repair for aortic arch aneurysm. Interact Cardiovasc Thorac Surg. 2017;24(1):34–40.
Greenberg RK, Clair D, Srivastava S, Bhandari G, Turc A, Hampton J, et al. Should patients with challenging anatomy be offered endovascular aneurysm repair? J Vasc Surg. 2003;38(5):990–6.
Criado FJ. Chimney grafts and bare stents: aortic branch preservation revisited. J Endovasc Ther. 2007;14(6):823–4.
Moulakakis KG, Mylonas SN, Avgerinos E, Papapetrou A, Kakisis JD, Brountzos EN, et al. The chimney graft technique for preserving visceral vessels during endovascular treatment of aortic pathologies. J Vasc Surg. 2012;55(5):1497–503.
Gehringhoff B, Torsello G, Pitoulias GA, Austermann M, Donas KP. Use of chimney grafts in aortic arch pathologies involving the supra-aortic branches. J Endovasc Ther. 2011;18(5):650–5.
Lachat M, Frauenfelder T, Mayer D, Pfiffner R, Veith FJ, Rancic Z, et al. Complete endovascular renal and visceral artery revascularization and exclusion of a ruptured type IV thoracoabdominal aortic aneurysm. J Endovasc Ther. 2010;17(2):216–20.
Kolvenbach RR, Yoshida R, Pinter L, Zhu Y, Lin F. Urgent endovascular treatment of thoraco-abdominal aneurysms using a sandwich technique and chimney grafts – a technical description. Eur J Vasc Endovasc Surg. 2011;41(1):54–60.
Mangialardi N, Serrao E, Kasemi H, Alberti V, Fazzini S, Ronchey S. Chimney technique for aortic arch pathologies: an 11-year single-center experience. J Endovasc Ther. 2014;21(2):312–23.
Mangialardi N, Ronchey S, Malaj A, Fazzini S, Alberti V, Ardita V, et al. Value and limitations of chimney grafts to treat arch lesions. J Cardiovasc Surg. 2015;56(4):503–11.
Kawaguchi S, Yokoi Y, Shimazaki T, Koide K, Matsumoto M, Shigematsu H. Thoracic endovascular aneurysm repair in Japan: experience with fenestrated stent grafts in the treatment of distal arch aneurysms. J Vasc Surg. 2008;48(6 Suppl):24S–9S; discussion 9S.
Azuma T, Yokoi Y, Yamazaki K. The next generation of fenestrated endografts: results of a clinical trial to support an expanded indication for aortic arch aneurysm treatment. Eur J Cardiothorac Surg. 2013;44(2):e156–63; discussion e63.
Malina M, Sonesson B. In situ fenestration: a novel option for endovascular aortic arch repair. J Cardiovasc Surg. 2015;56(3):355–62.
Inoue K, Hosokawa H, Iwase T, Sato M, Yoshida Y, Ueno K, et al. Aortic arch reconstruction by transluminally placed endovascular branched stent graft. Circulation. 1999;100(19 Suppl):Ii316–21.
Chuter TA, Schneider DB, Reilly LM, Lobo EP, Messina LM. Modular branched stent graft for endovascular repair of aortic arch aneurysm and dissection. J Vasc Surg. 2003;38(4):859–63.
Saito N, Kimura T, Odashiro K, Toma M, Nobuyoshi M, Ueno K, et al. Feasibility of the Inoue single-branched stent-graft implantation for thoracic aortic aneurysm or dissection involving the left subclavian artery: short- to medium-term results in 17 patients. J Vasc Surg. 2005;41(2):206–12; discussion 12.
Roselli EE, Arko FR 3rd, Thompson MM. Results of the Valiant Mona LSA early feasibility study for descending thoracic aneurysms. J Vasc Surg. 2015;62(6):1465–71.e3.
Patel HJ, Dake MD, Bavaria JE, Singh MJ, Filinger M, Fischbein MP, et al. Branched endovascular therapy of the distal aortic arch: preliminary results of the feasibility multicenter trial of the Gore thoracic branch Endoprosthesis. Ann Thorac Surg. 2016;102(4):1190–8.
Haulon S, Greenberg RK, Spear R, Eagleton M, Abraham C, Lioupis C, et al. Global experience with an inner branched arch endograft. J Thorac Cardiovasc Surg. 2014;148(4):1709–16.
Riambau V. Application of the Bolton Relay device for thoracic endografting in or near the Aortic Arch. Aorta (Stamford, Conn). 2015;3(1):16–24.
Chen LW, Dai XF, Lu L, Zhang GC, Cao H. Extensive primary repair of the thoracic aorta in acute type a aortic dissection by means of ascending aorta replacement combined with open placement of triple-branched stent graft: early results. Circulation. 2010;122(14):1373–8.
Chen LW, Lu L, Dai XF, Wu XJ, Zhang GC, Yang GF, et al. Total arch repair with open triple-branched stent graft placement for acute type a aortic dissection: experience with 122 patients. J Thorac Cardiovasc Surg. 2014;148(2):521–8.
Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The task force for the diagnosis and treatment of aortic diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35(41):2873–926.
Holloway BJ, Rosewarne D, Jones RG. Imaging of thoracic aortic disease. Br J Radiol. 2011;84(3):S338–54.
McCollough CH, Primak AN, Braun N, Kofler J, Yu L, Christner J. Strategies for reducing radiation dose in CT. Radiol Clin N Am. 2009;47(1):27–40.
Eggebrecht H, Zenge M, Ladd ME, Erbel R, Quick HH. In vitro evaluation of current thoracic aortic stent-grafts for real-time MR-guided placement. J Endovasc Ther. 2006;13(1):62–71.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Iddriss, A., Nakano, J., Malaisrie, S.C. (2019). Endovascular Repair of the Ascending Aorta and Aortic Arch. In: Dieter, R., Dieter Jr., R., Dieter III, R. (eds) Diseases of the Aorta . Springer, Cham. https://doi.org/10.1007/978-3-030-11322-3_20
Download citation
DOI: https://doi.org/10.1007/978-3-030-11322-3_20
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-11321-6
Online ISBN: 978-3-030-11322-3
eBook Packages: MedicineMedicine (R0)