Abstract
Abdominal aortic aneurysms (AAA) are a major cause of morbidity and mortality. Rupture of an AAA is a catastrophic event that leads to death in 80–90% of patients overall, including 50–70% of patients that reach medical attention. AAA can be described by their location and/or morphologic features. The pathophysiology is driven by abnormalities in the connective tissue of the aortic wall, namely elastin. While abnormalities in elastin lead to aneurysm formation, disruption of collagen in the aortic wall is what ultimately leads to rupture.
Major risk factors for this entity include smoking, advanced age, male gender, and family history. There are a variety of modalities that are used to image abdominal aortic aneurysms. Most commonly, ultrasound is used and is recommended by several societies for screening patients at risk of this disease. While behavioral modifications are recommended in patients with AAA to improve overall cardiac health, the mainstay of management is aneurysm repair. Generally, repair of an AAA is considered once it is ≥5.0 cm. Current options for intervention include open surgical repair and endovascular aneurysm repair (EVAR). Large randomized trials to date thus far suggest that while EVAR has improved perioperative mortality, long-term mortality outcomes appear to be better in patients that undergo surgical repair. Surveillance imaging with CT is recommended in patients that undergo open surgical repair of an AAA to assess for paranastomotic aneurysm formation, while CT or ultrasound surveillance is performed in patient post-EVAR to assess for endoleak formation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Abdominal aortic aneurysm
- Mycotic aneurysm
- Saccular aneurysm
- Infrarenal abdominal aortic aneurysm
- Endovascular aneurysm repair
- Aneurysm surveillance
Introduction and Definitions
Aneurysm derives from the Greek word ανɛυρυσμα (aneurusma), meaning widening, and can be defined as a permanent and irreversible localized dilatation of a vessel. An abdominal aortic aneurysm (AAA) is a permanent, localized dilatation of the abdominal aorta that exceeds the normal diameter by 50%.
The abdominal aorta begins at the level of the diaphragm and extends to its bifurcation into the left and right common iliac arteries. Normal aortic diameter varies with age, gender, and body habitus, but the average diameter of the adult human infrarenal aorta is about 2.0 cm and typically less than 3.0 cm. Thus, for the majority of patients, an infrarenal aorta with a maximum diameter ≥ 3.0 cm is considered aneurysmal [1].
Anatomy of the Abdominal Aorta
The abdominal aorta is a retroperitoneal structure that begins superiorly at the diaphragm and extends down to the level of the forth lumbar vertebra, where it bifurcates into the right and left common iliac arteries (Fig. 14.1) [3]. The aorta lies slightly left of midline, with the inferior vena cava adjacent to it on the right. The branches of the aorta include (superior to inferior) the left and right inferior phrenic arteries, left and right middle suprarenal arteries, the celiac axis, superior mesenteric artery, left and right renal arteries, left and right gonadal arteries, inferior mesenteric artery, left and right common iliac artery, middle sacral artery, and the paired lumbar arteries. The common iliac artery bifurcates into the external iliac and internal iliac arteries at the pelvic inlet.
Anatomy of the abdominal aorta . (From Tainter [2]. Reprinted with permission from Elsevier)
Similar to other arteries, the aortic wall is divided into three layers (from external to lumen): the tunica externa (or tunica adventitia), tunica media, and tunica intima. The vascular supply to the tunica externa and tunica media is provided by an extensive network of small blood vessels known as the vasa vasorum [3].
Classification
Aneurysms can be categorized by morphological characteristics, location, or etiology. The following are terms that define aneurysms based on morphology (Fig. 14.2) [5].
-
True aneurysm : An aneurysm that involves all three layers of the arterial wall (intima, media, and adventitia).
-
False aneurysm (pseudoaneurysm) : A collection of blood or hematoma that has leaked out of the artery but is then confined by the surrounding tissue.
-
Fusiform aneurysm : The circumference of the artery is impacted by the aneurysm (most aneurysms are fusiform).
-
Saccular aneurysm : Only a part of the circumference of the artery is impacted by the aneurysm.
-
Inflammatory aneurysm : Characterized by extensive perianeurysmal and retroperitoneal fibrosis and dense adhesions to adjacent abdominal organs [1].
-
Infectious (mycotic) aneurysm : Aneurysm caused by an infectious agent, most commonly bacterial (most commonly Staphylococcus aureus, Salmonella, and Streptococcus pneumonia) [6].
Aneurysm morphologies . (From Netscher et al. [4]. Reprinted with permission from Elsevier)
Another commonly used classification modality is based on location within the aorta (Fig. 14.3) [8]:
-
Suprarenal aneurysm : Involves the origins of one or more visceral arteries but does not extend into the chest.
-
Pararenal aneurysm : The renal arteries arise from the aneurysmal aorta; however, the aorta at the level of the superior mesenteric artery is not aneurysmal.
-
Juxtarenal aneurysm : Originates just beyond the origins of the renal arteries. There is no segment of nonaneurysmal aorta distal to the renal arteries, but the aorta at the level of the renal arteries is not aneurysmal.
-
Infrarenal aneurysm : Originates distal to the renal arteries. There is a segment of nonaneurysmal aorta that extends distal to the origins of the renal arteries.
-
Thoracoabdominal aneurysm : Originates in the chest and may involve the visceral or renal vessels.
Abdominal aortic aneurysms described by their location in relation to the renal arteries. (From Goldstone [7]. Reprinted with permission from Elsevier)
Abdominal aortic aneurysms (AAA) most often affect the segment of aorta between the renal and inferior mesenteric arteries [9].
Pathophysiology
The development of abdominal aortic aneurysms is associated with alterations of the connective tissue in the aortic wall. Elastic fibers and fibrillar collagen are the main determinants of the mechanical properties of the aorta. Elastin and associated proteins form a network of elastic fibers responsible for the viscoelastic properties of the aorta. Elastin is stabilized by cross-links between the molecules and is degraded by specific proteases that display elastase activity. Elastic fibers associated with smooth muscle cells are most abundant in the media of the aortic wall. Collagen, in polymeric form, is also a significant component of the media and the surrounding fibrous adventitia.
One of the major histological features of aneurysmal tissue is fragmentation of elastic fibers and a decreased concentration of elastin. The loss of elastic fibers seems to be an early step in aneurysm formation. Although elastin fragmentation and medial attenuation are the most important characteristics of the wall of an aneurysm, the adventitial tissue, in which collagen is predominant, is responsible for the resistance of the aorta in the absence of medial elastin. Therefore, while loss of elastin leads to aneurysm formation, collagen degradation is thought to be the ultimate cause of aneurysm rupture [5].
Collagen production continues throughout life and is even increased in the aneurysmal wall. Besides enhanced collagen synthesis, however, collagenolytic activity is increased in AAA as well. This increased lytic activity is why several hereditary connective tissue disorders (e.g., Ehlers-Danlos and Marfan’s syndromes) are associated with aneurysm formation at an early age [8].
The alteration of elastin and collagen in the aortic wall is dependent on production of proteases by nearby vascular wall cells (medial smooth muscle cells and adventitial fibroblasts) and by the cells of the lymphomonocytic infiltrate. These inflammatory cells in the media and adventitia come from the aortic blood and from a medial neovascularization, which characterizes abdominal aortic aneurysms. Leukocyte recruitment into the aortic wall is promoted by elastin degradation fragments as well as proinflammatory cytokines, chemokines, and prostaglandin derivatives produced by both the resident mesenchymal cells and the inflammatory cells themselves. Elastic and collagen fibers are degraded by proteolytic enzymes mostly represented by matrix metalloproteinases (MMP) locally activated by either other MMP or by plasmin generated by plasminogen activators.
Besides rarefaction of its extracellular matrix, the elastic media also undergo a reduction in the density of smooth muscle cells, which is regarded as a key event in the development of abdominal aortic aneurysms. Smooth muscle cells participate in vascular wall remodeling through localized expression of various extracellular matrix proteins as well as proteases and their inhibitors. Additionally, smooth muscle cells have a protective role against inflammation and proteolysis.
The development of abdominal aortic aneurysms is also associated with a mural thrombus in a number of patients. By contrast with arterial occlusive diseases, blood flow is maintained in aortic aneurysms resulting in a persistent remodeling activity of the components of the thrombus. Although the thrombus can substantially reduce aneurysmal wall stress, its increasing thickness leads to local hypoxia at the inner layer of the media, which can induce increased medial neovascularization and inflammation. This appears to play a role in aneurysmal degeneration associated with an adherent thrombus [1]. Some data suggests that thrombus may actually increase risk of aneurysm rupture, presumably due to localized tissue hypoxia and diminished wall strength [10,11,12,13].
Risk Factors
The common risk factors of AAA are smoking, male gender, white race, older age, chronic obstructive pulmonary disease (COPD), hypertension, dyslipidemia, coronary artery disease (CAD), peripheral artery disease (PAD), and positive family history [14]. Interestingly, although diabetes mellitus is a risk factor for PAD and CAD, it has been found to be a negative risk factor for AAA development and growth [15, 16].
Age
Elastin is not synthesized in the adult aorta. With a half-life of 70 years, the amount of elastin in the aortic wall decreases with age. The age-related alterations in the vessel wall affect the mechanical properties of the aorta. This explains why AAA is primarily a disease of the elderly [8].
Atherosclerosis
The historical association of AAA with atherosclerosis has now expanded into a multifactorial causation for the disease. It is unclear why atherosclerosis, normally causing narrowing of the arterial lumen, should in some cases result in dilation. There are epidemiological differences between patients with obstructive vascular and aneurysmal disease. Histological examination of the aneurysm wall reveals a chronic adventitial and medial inflammatory infiltrate of varying intensity. This distinguishes AAA from the purely atherosclerotic aorta, in which inflammatory cells are mainly associated with plaque. Patients with obstructive peripheral vascular disease also carry an increased risk for AAA. It is also important to remember that both AAA and peripheral arterial disease share many common risk factors (such as age, gender, smoking, hypertension, and hyperlipidemia, among others) [8].
Smoking
Smoking is the risk factor most strongly associated with AAA. Men who currently smoke more than 25 cigarettes per day have a 15-fold increased risk of AAA (hazard ratio [HR] 14.6, 95% CI 9.6–22) compared with men who have never smoked [17]. A smoker’s risk of developing AAA continues for at least 10 years following smoking cessation. In spite of this association, however, no causative link has been proven between smoking and AAA formation. The mechanism by which cigarette smoking contributes to aneurysm formation is independent of atherosclerosis. Theories behind the pathophysiology include disruption in collagen synthesis, altered expression of metalloproteinases, and the response to oxidative stress [18].
Patients with smoking history are more likely to develop COPD. As is the case in AAA, COPD is driven by excess matrix turnover and proteolysis. A large meta-analysis recently showed a 1.8-fold increase prevalence and incidence of AAA in patients with COPD compared to those without it [19]. Further, some studies suggest that COPD increases the risk of AAA rupture [20].
Gender
Men are at much higher risk of AAA than women. The reasons for this are unclear, but it is likely to be a function of hormonal factors, genetic susceptibility, and risk factor exposure [18].
Hypertension
Hypertension enhances the growth rate of aneurysms and is associated with an increased prevalence of AAA, which indicates that an increased load on the aortic wall may be involved in pathogenesis [5]. Hypertension also increases rupture risk in patients with established AAAs [14].
Hyperlipidemia
High-serum total cholesterol has a positive association with AAA prevalence, whereas high-density lipoprotein cholesterol has an inverse association. This correlation may be related to the increased risk of atherosclerosis, or may in part be a direct factor [21]. Similarly, obesity has also been shown to be an independent risk factor [18].
Family History/Genetic Factors
Positive family history has been shown to be a major risk factor for development of abdominal aortic aneurysm. A study by Larsson and colleagues showed the overall relative risk of AAA associated with family history compared to no family history was 1.9 (95% confidence interval [CI] 1.6–2.2) [22].
The development of AAAs is unlikely to be related to a single gene mutation, and multiple genetic factors are implicated. Susceptibility genes, rather than causal gene mutations, are likely to be important, particularly those regulating inflammatory mediators, tissue proteases, and smooth muscle cell biology [18].
Alcohol Intake
High levels of alcohol intake (>30 g/day) have been associated with increased risk of AAA (OR 1.65, 95% CI 1.03–2.64) [17]. The mechanism through which alcohol exposure increases the risk of AAA is unclear, but could be through upregulation of matrix metalloproteinases and focal elastin degradation.
Primary Disorders of the Aorta
A fraction of the cases of AAA are the direct consequence of disorders of the aorta itself or disruptions in the integrity of the aorta. Some of these causes include trauma, acute infection (bacterial or fungal), chronic infection (tuberculosis), inflammatory diseases (Behçet and Takayasu disease), and connective tissue disorders (Marfan’s syndrome, Ehlers-Danlos type IV) [1].
In spite of the nomenclature, mycotic aneurysms are most often caused by bacterial pathogens, with fungi rarely being associated. The most commonly implicated organisms include Staphylococcus aureus, Salmonella, and Streptococcus pneumonia. Syphilis (T. pallidum) once caused up to 50% of infected aneurysms [6].
The source of infection may be due to the direct inoculation of vessel wall or spread from an adjacent source of infection, which contributes to degradation or focal erosion of the arterial wall. Mycotic aneurysms may also arise from hematogenous spread [23].
Presentation
Nonruptured Abdominal Aortic Aneurysm
Nonruptured abdominal aortic aneurysms (AAA) are asymptomatic in most patients. Often the initial diagnosis is made as an incidental finding on abdominal ultrasound, abdominal computed tomography, or abdominal magnetic resonance imaging utilized for other purposes. When symptoms are present, they may include nonspecific abdominal pain, lower back pain, and mid-abdominal or flank pain with radiation to the back, groin, or scrotum [1]. The pain may be described as a gnawing sensation with episodes lasting hours to days [24]. Aneurysmal pain is typically not exacerbated by movement, though patients may be more comfortable in certain positions [25]. The presence of these symptoms is usually secondary to direct pressure or distention of intra-abdominal structures adjacent to the aorta [1]. Development of new or worsening pain that is severe, persistent, and/or localized to the back, lower abdomen, buttocks, or lower extremities may forebode impending rupture [25].
On physical exam, a pulsatile, typically nontender, mass can be present. Palpation of an AAA has been demonstrated to be safe and does not precipitate rupture [25]. The sensitivity of abdominal palpation is variable due to variabilities in AAA size and patient body habitus [26, 27]. Sensitivity of abdominal palpation in 15 studies of patients screened for AAA with both palpation and ultrasound was 29% for AAA 3.0–3.9 cm, 50% for 4.0–4.9 cm, and 76% for AAA ≥ 5 cm. The positive predictive value was 43% for AAA > 3.0 cm [26]. Abdominal obesity reduces sensitivity. One study demonstrated that palpation for AAA in patients with an abdominal girth of less than 100 cm (40-inch waistline) was 91% versus just 53% in patients with a girth of 100 cm or more (p < 0.001) [27].
Mycotic aneurysms are a distinct entity that classically present with a triad of fever, abdominal pain, and a palpable abdominal mass; however, the majority of patients with mycotic aneurysms do not have this triad of symptoms [28]. Laboratory evaluation reveals elevation of inflammatory markers such as erythrocyte sedimentation rate [29]. Blood cultures are positive in 50–90% of cases and can remain positive in spite of appropriate antimicrobial therapy [28].
Ruptured Abdominal Aortic Aneurysm
Ruptured AAAs classically present with the triad of abdominal or back pain, a pulsatile abdominal mass, and hypotension, though this triad is present in only about 33–50% of presentations [1, 25]. Alternatively, presenting symptoms may be secondary to hemorrhagic shock post rupture. These symptoms can include hypotension, vasoconstriction, mottled skin, diaphoresis, altered mental status, and oliguria. Terminal symptoms maybe are manifested by arrhythmias and/or cardiac arrest [25].
The clinical presentation varies depending on location of rupture. Rupture involving anterolateral wall into the peritoneal cavity causes abdominal distention and is usually rapidly fatal. Most patients with AAA rupture who survive long enough to reach medical attention have rupture of the posterolateral wall into the retroperitoneal space. On physical exam, ecchymosis in the flanks (Grey Turner sign) may be seen. A small tear can temporarily seal the rupture mitigating initial blood loss. Within hours, the rupture progresses necessitating acute intervention.
Rarely, an AAA can rupture into the inferior vena cava forming an aortocaval fistula. The triad of abdominal or lower back pain, an abdominal bruit, and a pulsatile abdominal mass is characteristic; however, this triad is found in the minority of patients. Other possible symptoms include lower extremity edema, congestive heart failure, hypotension, and hematuria [30]. Rarely, an aortocaval fistula may form leading to hematuria or shock [31, 32]. Rupture into the gastrointestinal tract or the formation of an aortoenteric fistula presents as massive gastrointestinal hemorrhage [25].
Imaging
There are many modalities to screen, confirm, and monitor AAA. This section will discuss ultrasound, computed tomographic angiography and rotational angiography, magnetic resonance imaging, as well as newer techniques such as three-dimensional reconstruction and wall stress calculation.
Ultrasound
Ultrasound (US) is the most common imaging modality for AAA due to ease of use, relative accuracy, cost, and absence of radiation [33]. Routine evaluation measures the anteroposterior, transverse, and longitudinal dimensions of the suprarenal, juxtarenal, pararenal, and infrarenal aorta. Iliac arteries should be included. Bowel gas or abdominal fat may block the suprarenal aorta or iliac arteries and for these reasons may misjudge the extent of an AAA. If possible, patients should fast prior to examination to reduce bowel gas interference. Despite these factors, it is rare to be unable to image the aorta properly, with less than 2% of studies limited by technical factors [34]. If US is unable to provide reliable images, an alternative imaging modality should be pursued.
While dependent on operator experience and patient characteristics, there is less than 5 mm inter-rater variability of AAA size in more than 80% of cases [35], though some prior studies have shown US can underestimate the size of a AAA by up to 1 cm when compared directly with CT angiography [36, 37]. Generally, US is used as the initial diagnostic test for screening and surveillance of AAA. The US Preventative Services Taskforce (USPSTF) recommends a one-time screening US for men age 65–75 with a smoking history. See Table 14.1 for additional screening recommendations. US is also very useful when patients present to the emergency department with hemodynamic instability in the setting of either a known or unknown AAA. While not a requirement prior to diagnosis, bedside ultrasonography can be performed, while patient is still in emergency department or in route to the operating room without causing unnecessary delay. Emergency department physicians have become much more comfortable with the abdominal US due to continued use of the Focused Assessment with Sonography in Trauma (FAST) exam and can quickly identify abnormal findings, such as an enlarged aorta, abdominal ascites, or retroperitoneal hematoma [38].
Spiral Computed Tomographic and Angiography
Spiral computed tomographic and computed tomographic angiography (CT and CTA) studies are costlier than US and expose the patient to radiation and intravenous contrast; however, they provide more anatomic detail, which is needed for perioperative planning [35, 37]. Different methods, such as magnification, electronic calipers, and other standardized techniques, have brought variability to less than 2 mm in 90% of cases. Three-dimensional reconstruction is added to assess for symmetry as a tortuous aorta can show oblique cross sections and AAA diameters could be overestimated.
CT is recommended in hemodynamically stable patient suspected of having an AAA or aneurysm rupture [39]. Signs of rupture on CT include an indistinct aortic wall, retroperitoneal hematoma, extravasation of intravenous contrast, retroperitoneal stranding, or loss of the fat plane between the aorta and surrounding tissue (Fig. 14.4, Table 14.2). If rupture is not seen, it may reveal certain findings associated with unstable aneurysms suggesting impending rupture such as crescent sign, discontinuous circumaortic calcification, aortic bulges or blebs, and aortic draping. Combined with aneurysm size of 5 cm or greater, these signs have been shown to be predictive of impending rupture [41].
The crescent sign and drape sign seen on CT are suggestive of impending rupture. (From Dalrymple et al. [40]. Reprinted with permission from Elsevier)
CT imaging of the aortic wall can also show signs of inflammation or infection consistent with an inflammatory or mycotic aneurysm [42, 43]. The aorta can become primarily infected by bacteria and cause a rapidly expanding AAA, or a pre-existing AAA can be secondarily infected. Features that suggest infected aneurysm include soft tissue inflammation surrounding the aorta, perivascular fluid collection, an AAA with air around the vessel or intramurally, or multilobular, eccentric, or saccular AAA. An inflammatory AAA (IAAA) can show thickening of the adventitia, defined on CT as greater than a 1 cm ring surrounding the aorta. Fibrosis or adherence to adjacent structures, such as the duodenum, ureters, and generalized retroperitoneum, may be seen. Though inflammation is present, periaortic air or fluid is not seen as noted for infected aneurysms.
Recent guidelines from the American Heart Association recommend CTA as the initial imaging modality when there is suspicion for a mycotic aneurysm (Class IIa, level of evidence B). Findings suggestive of a mycotic aneurysm on CTA include a saccular appearance, an irregular lobular contour, minimal or absent calcifications, periaortic soft tissue stranding, and periaortic gas [28].
Currently, CTA is the most commonly used imaging modality for preoperative planning and endografting (Fig. 14.5). It can be used in both elective and acute settings and can exclude rupture. It also images the renal and iliac arteries more accurately than US, which is beneficial for preoperative planning as the presence of juxtarenal or suprarenal aneurysms can affect placement of vascular cross-clamps or help determine which type of graft is used (fenestrated or branched). Rotational angiography before and after EVAR is commonly used. Benefits include confirmation of rupture within the operating room, and the images can be used for operative planning and graft sizing. However, image quality may be less than spiral CT angiography, and branch vessels (renal and iliacs) may not display as well [44].
Regular follow-up imaging of postoperative EVAR is typically done with CT and usually performed for the life of the patient. Monitoring is performed to detect aneurysm expansion, graft deformation or migration, and endoleaks. CTA may be more sensitive than spiral CT in detecting endoleaks.
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) are accurate in determining size and morphology of AAA, but increased cost, time, and less standardized techniques make it a less favorable study compared to CT and US. Moreover, MRI/MRA does not visualize calcium plaque as well as CT. It does have the benefit of no radiation exposure, and it was previously thought that gadolinium contrast would be safe in patient with renal insufficiency until studies described a link with nephrogenic sclerosing fibrosis [45]. Nevertheless, gadolinium does prove useful if intravenous contrast is precluded due to allergies or other reasons.
Other Modalities
Commonly AAA may be incidentally seen on nonvascular imaging studies that were performed for other reasons. Plain film x-rays can delineate the abdominal aorta if enough calcified plaque is present or a large-enough soft tissue density is visualized, signaling presence of an aneurysm. While aneurysm presence or size may be inferred from other imaging, dedicated vascular imaging should be performed to confirm details.
While US, CT, and MR have become standardized imaging modalities for assessing AAA, functional and molecular imaging are becoming more prevalent and may aid in learning the pathophysiology behind AAA.
Functional imaging can reveal the physiological changes within an organ or tissue via radiolabeled tracers or probes. With AAA, this is most commonly performed with single-photon emission computer tomography (SPECT). SPECT imaging using radiolabeled red blood cells or platelets has been performed for years for noninvasive vascular flow studies, though their sensitivity in detecting AAA or leak has been surpassed by CT and MR. Radiolabeled leukocytes can also be used to detect inflammatory AAA.
Molecular imaging may provide insight into the earlier biomolecular and mechanical changes of the aorta prior to aneurysm formation [46]. Molecular probes can be used to mark different molecular processes at different stages of disease. This could help determine other factors that lead to growth and rupture aside from the anatomic characteristics currently known. SPECT and optical imaging can be performed for this purpose; however, PET nuclear imaging may have the most promise. PET, primarily used as the gold standard in cancer diagnosis and surveillance, can be used to show wall inflammation and instability using the same 18F-fluoro-deoxy-glucose (18F-FDG) radiotracer [47]. Increased metabolic activity can indicate infection or inflammatory processes within the aortic wall which may help pursue repair when other anatomic imaging suggests surveillance. Other studies show that asymptomatic AAA shows increased 18F-FDG uptake compared to nonaneurysmal controls irrespective of AAA size, alluding to additional factors that contribute to AAA pathophysiology outside of anatomical characteristics [48].
Surveillance
AAAs are frequently asymptomatic until they rupture, and the overall mortality of a ruptured AAA approaches 85–90% with improvement to 50–70% in patients who are able to reach the hospital [32]. By contrast, elective aneurysm repair, whether a surgical or endovascular approach, is associated with an overall 30-day mortality of less than 5%. Additionally, given the ease and availability of a low-cost, low-risk, and high accuracy test (ultrasonography), it follows that screening of appropriate patients prior to development of symptoms may help to prevent undue mortality, particularly given that AAAs have a significant asymptomatic phase. One meta-analysis of four randomized controlled trials (RCTs) of screening for AAAs in older men demonstrated a significant decrease in AAA-related mortality and emergency operations (with an expected increase in elective procedures) in both mid-term and long-term analysis [49].
The USPSTF released recommendations in 2005 and again in 2014 to screen men and women aged 65–75 both with and without a history of smoking [50, 51]. Men in this age group with a smoking history would benefit from one-time screening for AAA (grade B recommendation), and this is largely based on the aforementioned RCTs. Selective screening for nonsmoking men may demonstrate a small net benefit (grade C recommendation), and it is the low prevalence (approximately 2%) in this population that decreases the absolute benefit [52]. There is insufficient data to assess the benefits of AAA screening in women in this age group with a smoking history as only one RCT demonstrated no difference in AAA-related mortality, though the trial was underpowered to detect these differences [53]. Women who have never smoked have a lower prevalence than men (less than 1%) and do not benefit from screening [52]. Additional screening recommendations from national guidelines are outlined in Table 14.1.
Once an AAA is detected, it is primarily the diameter of the aneurysm which determines subsequent evaluation. While the annual risk of rupture of AAAs < 5.5 cm is ≤1.0%, those from 5.5 to 5.9 cm have a risk of 9.4%, 6.0–6.9 cm have a risk of 10.2%, and ≥7.0 cm have a risk of 32.5% (Table 14.3) [54,55,56]. Generally, referral for elective repair is indicated in patients with AAA diameter ≥ 5.5 cm with a high level of evidence, as this was the cut-off used in multiple screening trials [51, 57]. For patients with diameters < 4 cm, surveillance is generally recommended.
There is still debate regarding the intermediate patients with AAA diameters between 4 and 5.4 cm, and the decision to treat may depend on other risk factors and clinical variables. The UKSAT and ADAM trials demonstrated equivalent long-term survival in both surgical and surveillance groups, though there may be a trend toward improved survival in younger patients with larger aneurysms [54, 58]. The CAESAR and PIVOTAL trials compared surveillance with endovascular repair, and neither trial showed a clear benefit of EVAR over surveillance in AAAs with diameters < 5.5 cm [59, 60]. Current guidelines recommend ultrasound surveillance at varying intervals depending on the size of the AAA (Table 14.4) [57]. Due to inter-observer variability in ultrasound measurements, there has been some interest in using CT to monitor AAA growth [61], though ultrasound remains favored due to its relative cost and lack of radiation exposure.
Following repair of the AAA, imaging surveillance is still necessary given complications of the repair itself. The concerning complication of surgical repair is late paranastomotic aneurysm formation, the risk of which increases over time and approximates 1%, 5%, and 20% in patients 5, 10, and 15 years following surgical repair, respectively [57]. The Society for Vascular Surgery therefore recommends screening with CT imaging at five-year intervals following open surgical intervention.
For endovascular aneurysm repair (EVAR) , the primary concern for postprocedural surveillance is monitoring for endoleak, the most frequent complication following EVAR. Endoleak is persistent blood flow in the aneurysm sac outside the endograft. There are five types of endoleak that have been described (Table 14.5) [57]. Type I endoleak occurs as a result of incomplete sealing at the end of the stent graft and is associated with continuous risk of rupture; therefore, these should be repaired at the time of EVAR. Type II endoleaks are the most common and describe retrograde filling of the sac by collateral vessels (Fig. 14.6). These may resolve spontaneously or persist, and repair may be indicated depending on the patient, aneurysm size, vessels involved, and other factors, though generally risk of rupture is uncommon. Type III endoleaks are the result of poorly seated components, degradation, disconnection, or erosion of the material and should be treated. Type IV endoleaks refer to benign leak due to porosity of the graft material itself and do not need treatment. Finally, type V endoleak, also known as endotension, leads to persistently elevated pressures in the aneurysm sac. While no endoleak is noted in endotension, it can result in aneurysmal sac enlargement and rupture [29].
Type II endoleak . Contrast is seen filling the aortic sac, most likely due to filling from a lumbar vessel. (From Titus [62]. Reprinted with permission from Springer)
The EUROSTAR registry demonstrated many of these initial concerns when published in 2000 [63]. The cumulative rate of rupture was approximately 1% per year, and rate of late conversion to open surgical repair was about 2% per year; endoleak was noted to be a statistically significant risk factor for both endpoints of late failure.
CT angiography (CTA) remains the gold standard for postprocedural surveillance following EVAR with current recommendations suggesting 1-month, 6-month, and 12-month surveillance postrepair with annual lifelong screening thereafter [57]. Given the risks associated with this extensive radiation exposure, recent studies have investigated alternate forms of imaging for postrepair surveillance. Duplex ultrasound (DUS) was compared to CTA in a study of 132 patients and found a sensitivity and specificity of 86% and 67%, respectively [64]. The limitation was a significant number of false positives, with a positive predictive value of only 45%. A recent study of contrast-enhanced ultrasonography (CEUS) comparing DUS, CEUS, and CTA found significantly improved sensitivity and specificity for CEUS of 93% and 95%, respectively [65]. DUS was inferior to CTA in this study (p = 0.002), but CEUS and CTA were equivalent, and all endoleaks that required intervention detected on CTA were also detected on CEUS. Endoleaks missed by CEUS were type II without sac expansion that did not require intervention. Prior studies of CEUS did not demonstrate as strong results that were limited by low sensitivity and high false positive rates, possibly highlighting the importance of ultrasonographer technique and experience (in addition to patient habitus limitations) when performing these studies [66, 67]. Magnetic resonance angiography (MRA) has also been shown to be comparable to CTA and may be an alternative to patients with nitinol stents or iodinated contrast allergies, and though lacking in radiation, it is obviously limited by cost [68].
One recent study by Garg et al. interestingly questions the current dogma on postrepair surveillance [69]. Approximately 10,000 patients from a Medicare database who had underwent EVAR were retrospectively evaluated for long-term outcomes including mortality, late rupture, and reintervention with a mean follow-up of 6 years. Two cohorts divided into complete or incomplete surveillance based on follow-up imaging (“complete” defined as at least 1 imaging event within 15 months of repair and every 15 months thereafter), and these were propensity matched based on demographic variables. Incomplete surveillance was seen in about 50% of patients after propensity score matching. Analysis of outcomes demonstrated no statistical significant differences in aneurysm-related mortality between the two groups. Moreover, the incomplete surveillance group was noted to have lower rates of complication, reintervention, and all-cause mortality. The authors suggest that patients with other comorbidities may undergo more surveillance, are more likely to receive additional imaging not necessarily for surveillance, and are overall subject to increased mortality.
Treatment Options
Ruptured abdominal aortic aneurysms are associated with a mortality of 80–90% overall and approximately 50–70% among those that reach the hospital [70, 71]. The aim of therapy is therefore to prevent aneurysm rupture. While this goal is reached through several modalities, including behavioral modifications, pharmacologic therapies, screening, and surveillance, the mainstay of treatment is elective surgical or endovascular repair of the aneurysm.
Behavioral Modifications
Cigarette smoking is strongly associated with the presence of abdominal aortic aneurysms. In a cohort study examining more than 3 million patients, duration and amount of cigarette smoking were both directly correlated with the presence of an AAA. Among patients that quit smoking, the risk of aneurysm formation decreased over time; those that quit less than 5 years prior had an odds ratio of AAA formation of 0.87 (95% CI 0.84–0.912) compared with current smokers; those that quit greater than 10 years prior had an odds ratio of 0.42 (95% CI 0.41–0.43) [72].
Patients with AAA should be encouraged to participate in moderate physical activity as a means of decreasing their overall risk of cardiovascular morbidity and death. Some data has shown that blood flow to AAA increases with exercise [73]. Animal models demonstrated that increased blood flow to AAAs was associated with limited aneurysm expansion [74]. When viewed in concert, it seems plausible that exercise may limit aneurysm expansion; however to date, this has not been demonstrated clinically [75].
Although exercise increases blood pressure and wall tension, which theoretically could lead to expansion and rupture, there is currently no data to suggest this is the case. One study examined 262 patients with an abdominal aortic aneurysm (mean size 5.5 cm ± 1.1 cm) undergoing stress test. Only one patient suffered aneurysm rupture in the 72 hours following stress testing (aneurysm diameter was 6.1 cm in that patient), and the authors therefore concluded treadmill exercise could safely be performed [76].
Pharmacologic Interventions
In searching for a pharmacologic intervention that can slow the rate of AAA expansion, many classes of medications have been investigated, including beta blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, statins, anti-platelets, and antibiotics. While some have shown promise in animal studies, to date no class of medications has definitively been found to slow the rate of expansion of abdominal aortic aneurysms [77,78,79,80,81,82]. One study found the use of ACE inhibitors to be associated with increased rate of growth of AAA [78]. Another study examined the effect of propranolol on the growth rate of AAA, and found it to have no impact (growth of 0.26 cm/year with placebo versus 0.22 cm/year with propranolol, p = 0.11). Further, the patients taking propranolol had worse quality of life scores and had no improvement in mortality [77].
As AAA is a cardiovascular disease risk equivalent, it is recommended that these patients be placed on aspirin. Additionally, while statins have not been found to slow the rate of AAA expansion, statin therapy has been associated with improved survival following surgical or endovascular repair of AAA [83].
Surgical and Endovascular Aneurysm Repair
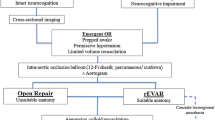
When indicated, abdominal aortic aneurysm repair is the gold standard of treatment. This can be accomplished either via open surgical repair or EVAR. In open surgical repair, a midline abdominal incision or a retroperitoneal incision is made. Once isolated, the abdominal aortic aneurysm is replaced with a prosthetic graft or tube [83]. In EVAR, an endograft is inserted via the femoral or iliac arteries, thereby excluding any blood flow in the aneurysm sac (Figs. 14.7 and 14.8) [84, 85].
(a) Aortogram showing a large infrarenal abdominal aortic aneurysm. (b) Completion aortogram demonstrating aneurysm sac exclusion. (From Annambhotla [84]. Reprinted with permission from Springer)
There have been several major trials that have compared EVAR and open surgical repair. In the Dutch Randomized Endovascular Aneurysm Management (DREAM) trial, 351 patients were randomized to either open surgical repair or EVAR. While there was no significant difference in the primary endpoint (a composite of operative mortality and moderate or severe complications), there was a nonsignificant reduction in mortality at 30 days with EVAR [86]. At 2 years of follow-up, however, this reduction in mortality was no longer evident (cumulative survival rate of 89.6% versus 89.7% in open versus EVAR, respectively). Further, while aneurysm-related death was significantly lower in the EVAR group, this was entirely accounted for by differences in perioperative mortality [87]. In subsequent follow-up at 6 years, there was still no difference in survival between the two groups; however, more patients initially randomized to the EVAR group had required secondary interventions (freedom from intervention was 81.9% for open repair versus 70.4% for endovascular repair). Additionally, a larger proportion of secondary interventions performed in the EVAR group were due to graft-related indications, while the majority of secondary interventions in the open repair group were hernia repairs [88].
The UK Endovascular Aneurysm Repair trial 1 (EVAR trial 1) found similar results [89, 90]. In this trial, 1082 patients were randomly assigned to either EVAR or open repair. Thirty-day mortality was significantly lower in the EVAR group when compared to the open repair group (1.7% versus 4.7% respectively, odds ratio of 0.35, p = 0.009) [90]. At 4 years of follow-up, all-cause mortality was not significantly different between the two groups. There was, however, a persistent reduction in aneurysm-related mortality in the EVAR group that was attributable to the observed reduction in perioperative mortality [89]. Recently, the EVAR trial 1 investigators reported that at a mean of 12.7 years of follow-up, there was no difference in overall mortality or aneurysm-related mortality. Of note, there was an increase in late mortality from aneurysm-related deaths in the EVAR group [91].
The Open Versus Endovascular Repair (OVER) study trial also assessed whether endovascular repair may have benefit over open repair. At an interim assessment at 2 years, there was no significant difference in mortality between the groups [92]. Once again, perioperative mortality was lower in the endovascular group than the open surgical repair group. Notably, mortality rates overall were much lower in this more recent trial, with 30-day mortality following EVAR of 0.5% (compared with 2.1% in EVAR-1 and 1.2% in DREAM trial) and 3.0% following open surgical repair (compared with 6.2% in EVAR-1 and 4.6% in DREAM trial). At the conclusion of the 9-year follow-up period, there was no difference in survival between the two groups. Interestingly, younger patients seemed to derive more benefit from EVAR compared with older patients [93].
Timing of Intervention
Given the catastrophic consequences of rupture of an abdominal aortic aneurysm, the mainstay of therapy is either surgical or endovascular repair prior to rupture. However, both of these surgeries carry significant perioperative risks, including death. Therefore, it is appropriate to intervene on an AAA only when it carries a significant risk of rupture. A great deal of research has been conducted to delineate the optimum time for intervention.
In the UK Small Aneurysm Trial, 1090 patients with an AAA of 4.0–5.5 cm were randomized to either ultrasound surveillance or early elective surgery. Those that were randomized to ultrasound surveillance underwent surgery if the AAA grew to greater than 5.5 cm, grew more than 1 cm in a year, became tender, or repair of an iliac or thoracic aneurysm was needed. At the end of 6 years of follow-up, about one third of patients in each group had died. Further, the rate of death in the first 6 months following randomization was 2.5 times higher for the early surgery group due to perioperative mortality [94]. A similar trial performed in the United States randomized 1136 patients to early surgery or ultrasound surveillance. Once again, no difference in outcomes with early surgery or routine ultrasound surveillance was observed at a mean of 4.9 years of follow-up [54].
Data also suggests that there is no benefit to EVAR for aneurysms less than 5.5 cm in diameter. The CAESAR trial randomized 360 patients with AAA sized 4.1–5.4 cm to early EVAR or ultrasound surveillance. At 54 months of follow-up, there was no difference in all-cause mortality [59]. The PIVOTAL trial, which randomized 728 patients with AAA sized 4.0–5.0 cm to early EVAR or ultrasound surveillance, also found no difference in overall mortality after a mean follow-up period of 20 months [60].
Current Guidelines
The European Society for Vascular Surgery recommends ultrasound surveillance for small abdominal aortic aneurysms (4.0–5.5 cm) and referral to a vascular surgeon when the AAA grows to greater than 5.5 cm in men (greater than 5.0 cm in women), the rate of growth is greater than 1 cm in a year, or the patient develops symptoms [95]. These recommendations are the same for open surgical repair and EVAR. Likewise, the American College of Cardiology and American Heart Association (AHA) guidelines recommend repair of infrarenal or juxtarenal AAAs measuring 5.5 cm or larger and imaging surveillance every 6–12 months for those AAAs measuring 4.0–5.4 cm [96].
In spite of the current guidelines , significant variability in the timing of surgical or endovascular intervention remains. A recent study compared practice patterns and outcomes for AAAs in the United States and England. The study found that aneurysm repair was less common in England than the United States; however, aneurysm-related death was more common in England (odds ratio of 3.6, p < 0.001). Further, the mean aneurysm diameter at the time of repair was larger in England than in the United States (6.37 cm vs. 5.83 cm, respectively, p < 0.001) [97].
Special Considerations
Juxtarenal, Suprarenal, and Thoracoabdominal Aneurysms
While the use of EVAR is well established for the treatment of infrarenal abdominal aortic aneurysms , until recently it was not used for treatment of juxtarenal or suprarenal AAAs. Technical advances have allowed an expansion of EVAR into these territories which were previously exclusive to surgical repair. A retrospective analysis of endovascular aneurysm repair of juxtarenal, suprarenal, and thoracoabdominal aneurysms with a fenestrated aortic endograft found it to be safe and effective for patients deemed too high risk for surgical repair [98]. Alternatively, chimney grafts have been found to be a suitable alternative intervention in those patients who are not eligible for fenestrated endografts [99].
Mycotic Aneurysms
Treatment of mycotic aneurysms is multidimensional and includes antibiotic therapy directed at common organisms, as well as surgical or endovascular intervention. The most common pathogens include Salmonella and Staphylococcus [100]. As such, treatment with beta-lactam antibiotics is indicated. Due to the increasing prevalence of methicillin-resistant Staphylococcus aureus (MRSA), vancomycin is frequently part of the antibiotic regimen [101]. According to recent AHA guidelines, antimicrobial therapy should be continued for 6 weeks to 6 months (Class IIb, level of evidence B), and in some cases, lifelong suppressive therapy may be considered [28].
Surgery is the cornerstone of treatment for mycotic abdominal aortic aneurysms. Options for intervention include resection of the aneurysm with extra-anatomic revascularization or in situ reconstruction. The AHA recommends resection and in situ revascularization in most cases (Class IIa; level of evidence B), with extra-anatomic revascularization reserved for patients with gross pus in the operative field, retroperitoneal or psoas abscess, vertebral osteomyelitis, ongoing signs of fever in spite of preoperative antibiotics, and certain patients with aortoenteric fistula (Class IIb, level of evidence C) [28]. While EVAR has been used for treatment in patient with prohibitive surgical risk, mortality is worse than with open repair and is therefore reserved for those with prohibitive surgical risk [102].
Inflammatory Aneurysms
Inflammatory aneurysms can be treated with either EVAR or open surgical repair, though EVAR is currently favored as surgical repair of inflammatory aneurysms, it is technically difficult and associated with worse outcomes [103]. One recent meta-analysis that included 999 patients than underwent open surgical repair and 121 patients that underwent EVAR for management of an inflammatory AAA found a reduction in mortality at 1 year with EVAR (2% versus 14%, p = 0.002) [104]. If open surgical repair is pursued, a retroperitoneal approach is preferred as the most inflamed section of the aneurysm is typically the anterior most aspect [57].
Abbreviations
- 18F-FDG:
-
18F-fluoro-deoxy-glucose
- AAA:
-
Abdominal aortic aneurysm
- ACE:
-
Angiotensin-converting enzyme
- ADAM:
-
Aneurysm Detection and Management
- CAD:
-
Coronary artery disease
- CAESAR:
-
Comparison of surveillance versus aortic endografting for small aneurysm repair
- CEUS:
-
Contrast-enhanced ultrasonography
- CI:
-
Confidence interval
- COPD:
-
Chronic obstructive pulmonary disease
- CTA:
-
Computed tomographic angiography
- DREAM:
-
Dutch Randomized Endovascular Aneurysm Management
- DUS:
-
Duplex ultrasound
- EVAR:
-
Endovascular aneurysm repair
- FAST:
-
Focused Assessment with Sonography in Trauma
- HR:
-
Hazard ratio
- IAAA:
-
Inflammatory abdominal aortic aneurysm
- MMP:
-
Matrix metalloproteinases
- MRA:
-
Magnetic resonance angiography
- MRI:
-
Magnetic resonance imaging
- OR:
-
Odds ratio
- OVER:
-
Open Versus Endovascular Repair
- PAD:
-
Peripheral artery disease
- PET:
-
Positron emission tomography
- PIVOTAL:
-
Positive Impact of Endovascular Options for treating Aneurysms Early
- SPECT:
-
Single-photon emission computer tomography
- UKSAT:
-
UK Small Aneurysm Trial
- US:
-
Ultrasound
- USPSTF:
-
US Preventative Services Task Force
References
Sakalihasan N, Limet R, Defawe OD. Abdominal aortic aneurysm. Lancet. 2005;365(9470):1577–89.
Tainter CR. Abdominal aorta. In: Soni N, Arntfield R, Pierre K, editors. Point-of-care ultrasound. 1st ed. Philadelphia: Saunders, an imprint of Elsevier Inc; 2015. p. 167–73.e3.
Legg JS, Legg LM. Abdominal aortic aneurysms. Radiol Technol. 2016;88(2):145–63.
Netscher D, Fiore N, Rodgers B. Vascular injuries and disorders of the upper extremity. In: Guyuron B, Eriksson E, Persing J, Chung K, Disa J, Gosain A, et al., editors. Plastic surgery: indications and practice. 1st ed. Philadelphia: Elsevier Inc; 2009. p. 1299–322.
Dobrin PB, Mrkvicka R. Failure of elastin or collagen as possible critical connective tissue alterations underlying aneurysmal dilatation. Cardiovasc Surg. 1994;2(4):484–8.
Moneta GL, Taylor LM Jr, Yeager RA, Edwards JM, Nicoloff AD, McConnell DB, et al. Surgical treatment of infected aortic aneurysm. Am J Surg. 1998;175(5):396–9.
Goldstone J. Aneurysms of the aorta and iliac arteries. In: Moore W, editor. Vascular and endovascular surgery: a comprehensive review. 8th ed. Philadelphia: Saunders, an imprint of Elsevier Inc.; 2013. p. 651–75.e4.
van der Vliet JA, Boll AP. Abdominal aortic aneurysm. Lancet. 1997;349(9055):863–6.
Evans GH, Stansby G, Hamilton G. Suggested standards for reporting on arterial aneurysms. J Vasc Surg. 1992;15(2):456.
Satta J, Laara E, Juvonen T. Intraluminal thrombus predicts rupture of an abdominal aortic aneurysm. J Vasc Surg. 1996;23(4):737–9.
Wiernicki I, Szumilowicz P, Kazimierczak A, Falkowski A, Rutkowski D, Gutowski P. The blood flow channel index as novel predictor of abdominal aortic aneurysm impending rupture based on the intraluminal thrombus angio-CT study. Eur J Radiol. 2015;84(4):662–7.
Vorp DA, Lee PC, Wang DH, Makaroun MS, Nemoto EM, Ogawa S, et al. Association of intraluminal thrombus in abdominal aortic aneurysm with local hypoxia and wall weakening. J Vasc Surg. 2001;34(2):291–9.
Koole D, Zandvoort HJ, Schoneveld A, Vink A, Vos JA, van den Hoogen LL, et al. Intraluminal abdominal aortic aneurysm thrombus is associated with disruption of wall integrity. J Vasc Surg. 2013;57(1):77–83.
Takayama T, Yamanouchi D. Aneurysmal disease: the abdominal aorta. Surg Clin North Am. 2013;93(4):877–91, viii.
Takagi H, Takuya Umemoto for the ALICE (All-Literature Investigation of Cardiovascular Evidence) Group. Association of diabetes mellitus with presence, expansion, and rupture of abdominal aortic aneurysm: “Curiouser and curiouser!” cried ALICE. Semin Vasc Surg. 2016;29(1–2):18–26.
De Rango P, Farchioni L, Fiorucci B, Lenti M. Diabetes and abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2014;47(3):243–61.
Wong DR, Willett WC, Rimm EB. Smoking, hypertension, alcohol consumption, and risk of abdominal aortic aneurysm in men. Am J Epidemiol. 2007;165(7):838–45.
Nordon IM, Hinchliffe RJ, Loftus IM, Thompson MM. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat Rev Cardiol. 2011;8(2):92–102.
Takagi H, Umemoto T, ALICE (All-Literature Investigation of Cardiovascular Evidence) Group. A meta-analysis of the association of chronic obstructive pulmonary disease with abdominal aortic aneurysm presence. Ann Vasc Surg. 2016;34:84–94.
Takagi H, Umemoto T. Association of chronic obstructive pulmonary, coronary artery, or peripheral artery disease with abdominal aortic aneurysm rupture. Int Angiol. 2016;36(4):322–31.
Tornwall ME, Virtamo J, Haukka JK, Albanes D, Huttunen JK. Life-style factors and risk for abdominal aortic aneurysm in a cohort of Finnish male smokers. Epidemiology. 2001;12(1):94–100.
Larsson E, Granath F, Swedenborg J, Hultgren R. A population-based case-control study of the familial risk of abdominal aortic aneurysm. J Vasc Surg. 2009;49(1):47–50; discussion 51.
Norman PE, Powell JT. Site specificity of aneurysmal disease. Circulation. 2010;121(4):560–8.
Isselbacher EM. Thoracic and abdominal aortic aneurysms. Circulation. 2005;111(6):816–28.
Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113(11):e463–654.
Lederle FA, Simel DL. The rational clinical examination. Does this patient have abdominal aortic aneurysm? JAMA. 1999;281(1):77–82.
Fink HA, Lederle FA, Roth CS, Bowles CA, Nelson DB, Haas MA. The accuracy of physical examination to detect abdominal aortic aneurysm. Arch Intern Med. 2000;160(6):833–6.
Wilson WR, Bower TC, Creager MA, Amin-Hanjani S, O’Gara PT, Lockhart PB, et al. Vascular graft infections, mycotic aneurysms, and endovascular infections: a scientific statement from the American Heart Association. Circulation. 2016;134(20):e412–60.
Mix J, Pitta S, Schwartz J, Tuchek J, Dieter R, Freeman M. Abdominal aorta. In: Dieter R, Dieter RJ, Dieter RI, editors. Peripheral arterial disease. New York: McGraw-Hill Education; 2008. p. 569–92.
Davis PM, Gloviczki P, Cherry KJ Jr, Toomey BJ, Stanson AW, Bower TC, et al. Aorto-caval and ilio-iliac arteriovenous fistulae. Am J Surg. 1998;176(2):115–8.
Salo JA, Verkkala KA, Ala-Kulju KV, Heikkinen LO, Luosto RV. Hematuria is an indication of rupture of an abdominal aortic aneurysm into the vena cava. J Vasc Surg. 1990;12(1):41–4.
Kent KC. Clinical practice. Abdominal aortic aneurysms. N Engl J Med. 2014;371(22):2101–8.
Hong H, Yang Y, Liu B, Cai W. Imaging of abdominal aortic aneurysm: the present and the future. Curr Vasc Pharmacol. 2010;8(6):808–19.
Beales L, Wolstenhulme S, Evans JA, West R, Scott DJ. Reproducibility of ultrasound measurement of the abdominal aorta. Br J Surg. 2011;98(11):1517–25.
Jaakkola P, Hippelainen M, Farin P, Rytkonen H, Kainulainen S, Partanen K. Interobserver variability in measuring the dimensions of the abdominal aorta: comparison of ultrasound and computed tomography. Eur J Vasc Endovasc Surg. 1996;12(2):230–7.
Chiu KW, Ling L, Tripathi V, Ahmed M, Shrivastava V. Ultrasound measurement for abdominal aortic aneurysm screening: a direct comparison of the three leading methods. Eur J Vasc Endovasc Surg. 2014;47(4):367–73.
Sprouse LR 2nd, Meier GH 3rd, Lesar CJ, Demasi RJ, Sood J, Parent FN, et al. Comparison of abdominal aortic aneurysm diameter measurements obtained with ultrasound and computed tomography: is there a difference? J Vasc Surg. 2003;38(3):466–71; discussion 471–2.
Reed MJ, Cheung LT. Emergency department led emergency ultrasound may improve the time to diagnosis in patients presenting with a ruptured abdominal aortic aneurysm. Eur J Emerg Med. 2014;21(4):272–5.
Chien DK, Chang WH, Yeh YH. Radiographic findings of a ruptured abdominal aortic aneurysm. Circulation. 2010;122(18):1880–1.
Dalrymple N, Oliphant M, Leyendecker J. Imaging evaluation of acute abdominal pain. In: Dalrymple N, editor. Problem solving in abdominal imaging. 1st ed. Philadelphia: Mosby Inc; 2009. p. 147–83.
Thammaroj J, Vungtal S, Srinakarin J. Predictive CT features in ruptured abdominal aortic aneurysm. J Med Assoc Thail. 2006;89(4):434–40.
Lin MP, Chang SC, Wu RH, Chou CK, Tzeng WS. A comparison of computed tomography, magnetic resonance imaging, and digital subtraction angiography findings in the diagnosis of infected aortic aneurysm. J Comput Assist Tomogr. 2008;32(4):616–20.
Litmanovich DE, Yildirim A, Bankier AA. Insights into imaging of aortitis. Insights Imaging. 2012;3(6):545–60.
Nordon IM, Hinchliffe RJ, Malkawi AH, Taylor J, Holt PJ, Morgan R, et al. Validation of DynaCT in the morphological assessment of abdominal aortic aneurysm for endovascular repair. J Endovasc Ther. 2010;17(2):183–9.
Shabana WM, Cohan RH, Ellis JH, Hussain HK, Francis IR, Su LD, et al. Nephrogenic systemic fibrosis: a report of 29 cases. AJR Am J Roentgenol. 2008;190(3):736–41.
Choke E, Cockerill G, Wilson WR, Sayed S, Dawson J, Loftus I, et al. A review of biological factors implicated in abdominal aortic aneurysm rupture. Eur J Vasc Endovasc Surg. 2005;30(3):227–44.
Kotze C, Menezes L, Endozo R, Groves A, Ell P, Yusuf S. Increased metabolic activity in abdominal aortic aneurysm detected by 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT). Eur J Vasc Endovasc Surg. 2009;38(1):93–9.
Reeps C, Essler M, Pelisek J, Seidl S, Eckstein HH, Krause BJ. Increased 18F-fluorodeoxyglucose uptake in abdominal aortic aneurysms in positron emission/computed tomography is associated with inflammation, aortic wall instability, and acute symptoms. J Vasc Surg. 2008;48(2):417–23; discussion 424.
Lindholt JS, Norman P. Screening for abdominal aortic aneurysm reduces overall mortality in men. A meta-analysis of the mid- and long-term effects of screening for abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2008;36(2):167–71.
Final Recommendation Statement: Abdominal Aortic Aneurysm: Screening. U.S. Preventive Services Task Force. 2014; Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/abdominal-aortic-aneurysm-screening. Accessed 3 Dec 2016.
LeFevre ML, U.S. Preventive Services Task Force. Screening for abdominal aortic aneurysm: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161(4):281–90.
Lederle FA, Johnson GR, Wilson SE, Chute EP, Littooy FN, Bandyk D, et al. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) Veterans Affairs Cooperative Study Group. Ann Intern Med. 1997;126(6):441–9.
Scott RA, Wilson NM, Ashton HA, Kay DN. Influence of screening on the incidence of ruptured abdominal aortic aneurysm: 5-year results of a randomized controlled study. Br J Surg. 1995;82(8):1066–70.
Lederle FA, Wilson SE, Johnson GR, Reinke DB, Littooy FN, Acher CW, et al. Immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346(19):1437–44.
Lederle FA, Johnson GR, Wilson SE, Ballard DJ, Jordan WD Jr, Blebea J, et al. Rupture rate of large abdominal aortic aneurysms in patients refusing or unfit for elective repair. JAMA. 2002;287(22):2968–72.
Powell JT, Brown LC, Forbes JF, Fowkes FG, Greenhalgh RM, Ruckley CV, et al. Final 12-year follow-up of surgery versus surveillance in the UK Small Aneurysm Trial. Br J Surg. 2007;94(6):702–8.
Chaikof EL, Brewster DC, Dalman RL, Makaroun MS, Illig KA, Sicard GA, et al. SVS practice guidelines for the care of patients with an abdominal aortic aneurysm: executive summary. J Vasc Surg. 2009;50(4):880–96.
United Kingdom Small Aneurysm Trial Participants. Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346(19):1445–52.
Cao P, De Rango P, Verzini F, Parlani G, Romano L, Cieri E, et al. Comparison of surveillance versus aortic endografting for small aneurysm repair (CAESAR): results from a randomised trial. Eur J Vasc Endovasc Surg. 2011;41(1):13–25.
Ouriel K, Clair DG, Kent KC, Zarins CK. Positive Impact of Endovascular Options for treating Aneurysms Early (PIVOTAL) Investigators. Endovascular repair compared with surveillance for patients with small abdominal aortic aneurysms. J Vasc Surg. 2010;51(5):1081–7.
Hendy K, Gunnarson R, Golledge J. Growth rates of small abdominal aortic aneurysms assessed by computerised tomography--a systematic literature review. Atherosclerosis. 2014;235(1):182–8.
Titus J, Butler B. Endoleak management. In: Dieter R, Dieter RJ, Dieter RI, editors. Endovascular interventions: a case based approach. New York: Springer Science + Business Media; 2014. p. 373–81.
Harris PL, Vallabhaneni SR, Desgranges P, Becquemin JP, van Marrewijk C, Laheij RJ. Incidence and risk factors of late rupture, conversion, and death after endovascular repair of infrarenal aortic aneurysms: the EUROSTAR experience. European Collaborators on Stent/graft techniques for aortic aneurysm repair. J Vasc Surg. 2000;32(4):739–49.
Manning BJ, O’Neill SM, Haider SN, Colgan MP, Madhavan P, Moore DJ. Duplex ultrasound in aneurysm surveillance following endovascular aneurysm repair: a comparison with computed tomography aortography. J Vasc Surg. 2009;49(1):60–5.
Bredahl KK, Taudorf M, Lonn L, Vogt KC, Sillesen H, Eiberg JP. Contrast enhanced ultrasound can replace computed tomography angiography for surveillance after endovascular aortic aneurysm repair. Eur J Vasc Endovasc Surg. 2016;52(6):729–34.
Ashoke R, Brown LC, Rodway A, Choke E, Thompson MM, Greenhalgh RM, et al. Color duplex ultrasonography is insensitive for the detection of endoleak after aortic endografting: a systematic review. J Endovasc Ther. 2005;12(3):297–305.
Raman KG, Missig-Carroll N, Richardson T, Muluk SC, Makaroun MS. Color-flow duplex ultrasound scan versus computed tomographic scan in the surveillance of endovascular aneurysm repair. J Vasc Surg. 2003;38(4):645–51.
Habets J, Zandvoort HJ, Reitsma JB, Bartels LW, Moll FL, Leiner T, et al. Magnetic resonance imaging is more sensitive than computed tomography angiography for the detection of endoleaks after endovascular abdominal aortic aneurysm repair: a systematic review. Eur J Vasc Endovasc Surg. 2013;45(4):340–50.
Garg T, Baker LC, Mell MW. Postoperative surveillance and long-term outcomes after endovascular aneurysm repair among Medicare beneficiaries. JAMA Surg. 2015;150(10):957–63.
Hoornweg LL, Storm-Versloot MN, Ubbink DT, Koelemay MJ, Legemate DA, Balm R. Meta analysis on mortality of ruptured abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2008;35(5):558–70.
Kent K. Clinical practice. Abdominal aortic aneurysms. NEJM. 2014;371:2101–8.
Kent KC, Zwolak RM, Egorova NN, Riles TS, Manganaro A, Moskowitz AJ, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg. 2010;52(3):539–48.
Tenforde AS, Cheng CP, Suh GY, Herfkens RJ, Dalman RL, Taylor CA. Quantifying in vivo hemodynamic response to exercise in patients with intermittent claudication and abdominal aortic aneurysms using cine phase-contrast MRI. J Magn Reson Imaging. 2010;31(2):425–9.
Nakahashi TK, Hoshina K, Tsao PS, Sho E, Sho M, Karwowski JK, et al. Flow loading induces macrophage antioxidative gene expression in experimental aneurysms. Arterioscler Thromb Vasc Biol. 2002;22(12):2017–22.
Myers J, McElrath M, Jaffe A, Smith K, Fonda H, Vu A, et al. A randomized trial of exercise training in abdominal aortic aneurysm disease. Med Sci Sports Exerc. 2014;46(1):2–9.
Best PJ, Tajik AJ, Gibbons RJ, Pellikka PA. The safety of treadmill exercise stress testing in patients with abdominal aortic aneurysms. Ann Intern Med. 1998;129(8):628–31.
Propanolol Aneurysm Trial Investigators. Propranolol for small abdominal aortic aneurysms: results of a randomized trial. J Vasc Surg. 2002;35(1):72–9.
Sweeting MJ, Thompson SG, Brown LC, Greenhalgh RM, Powell JT. Use of angiotensin converting enzyme inhibitors is associated with increased growth rate of abdominal aortic aneurysms. J Vasc Surg. 2010;52(1):1–4.
Takagi H, Matsui M, Umemoto T. A meta-analysis of clinical studies of statins for prevention of abdominal aortic aneurysm expansion. J Vasc Surg. 2010;52(6):1675–81.
Bailey MA, Dunne JA, Griffin KJ, Coughlin PA, Scott DJ. Systematic review and meta-analysis of the effects of statin therapy on abdominal aortic aneurysms (Br J Surg 2011; 98: 362-353). Br J Surg. 2011;98(5):744–5; author reply 745.
Meijer CA, Stijnen T, Wasser MN, Hamming JF, van Bockel JH, Lindeman JH, et al. Doxycycline for stabilization of abdominal aortic aneurysms: a randomized trial. Ann Intern Med. 2013;159(12):815–23.
Thompson A, Cooper JA, Fabricius M, Humphries SE, Ashton HA, Hafez H. An analysis of drug modulation of abdominal aortic aneurysm growth through 25 years of surveillance. J Vasc Surg. 2010;52(1):55–61.e2.
Sieunarine K, Lawrence-Brown MM, Goodman MA. Comparison of transperitoneal and retroperitoneal approaches for infrarenal aortic surgery: early and late results. Cardiovasc Surg. 1997;5(1):71–6.
Annambhotla S, Kibbe M. Infrarenal abdominal aortic aneurysm: EVAR. In: Dieter RS, Dieter Jr RA, Dieter III RA, editors. Endovascular interventions: a case-based approach. New York: Springer Science+Business Media; 2014. p. 355–65.
Aziz F. Endovascular abdominal aortic aneurysm repair for ruptured abdominal aortic aneurysm. In: Dean S, Satiani B, Abraham W, editors. Color atlas of vascular diseases. New York: McGraw-Hill; 2015.
Prinssen M, Verhoeven EL, Buth J, Cuypers PW, van Sambeek MR, Balm R, et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2004;351(16):1607–18.
Blankensteijn JD, de Jong SE, Prinssen M, van der Ham AC, Buth J, van Sterkenburg SM, et al. Two-year outcomes after conventional or endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2005;352(23):2398–405.
De Bruin JL, Baas AF, Buth J, Prinssen M, Verhoeven EL, Cuypers PW, et al. Long-term outcome of open or endovascular repair of abdominal aortic aneurysm. N Engl J Med. 2010;362(20):1881–9.
EVAR trial participants. Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet. 2005;365(9478):2179–86.
Greenhalgh RM, Brown LC, Kwong GP, Powell JT, Thompson SG, EVAR trial participants. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomised controlled trial. Lancet. 2004;364(9437):843–8.
Patel R, Sweeting MJ, Powell JT, Greenhalgh RM, EVAR trial investigators. Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet. 2016;388(10058):2366–74.
Lederle FA, Freischlag JA, Kyriakides TC, Padberg FT Jr, Matsumura JS, Kohler TR, et al. Outcomes following endovascular vs open repair of abdominal aortic aneurysm: a randomized trial. JAMA. 2009;302(14):1535–42.
Lederle FA, Freischlag JA, Kyriakides TC, Matsumura JS, Padberg FT Jr, Kohler TR, et al. Long-term comparison of endovascular and open repair of abdominal aortic aneurysm. N Engl J Med. 2012;367(21):1988–97.
The UK Small Aneurysm Trial Participants. Mortality results for randomised controlled trial of early elective surgery or ultrasonographic surveillance for small abdominal aortic aneurysms. Lancet. 1998;352(9141):1649–55.
Moll FL, Powell JT, Fraedrich G, Verzini F, Haulon S, Waltham M, et al. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg. 2011;41(Suppl 1):S1–S58.
Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss L, et al. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA Guideline Recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61(14):1555–70.
Karthikesalingram A, Vidal-Diez A, Holt P, Loftus I, Schermerhorn M, Soden P, et al. Thresholds for abdominal aortic aneurysm repair in England and the United States. N Engl J Med. 2016;375:2051–9.
Haulon S, Amiot S, Magnan PE, Becquemin JP, Lermusiaux P, Koussa M, et al. An analysis of the French multicentre experience of fenestrated aortic endografts: medium-term outcomes. Ann Surg. 2010;251(2):357–62.
Coscas R, Kobeiter H, Desgranges P, Becquemin JP. Technical aspects, current indications, and results of chimney grafts for juxtarenal aortic aneurysms. J Vasc Surg. 2011;53(6):1520–7.
Leon L, Ihnat D, Mills J. Native arterial infections. In: Hallett J, Mills J, Earnshaw J, Reekers J, editors. Comprehensive vascular and endovascular surgery. 2nd ed. Philadelphia: Elsevier; 2009.
Leon LR Jr, Mills JLS. Diagnosis and management of aortic mycotic aneurysms. Vasc Endovasc Surg. 2010;44(1):5–13.
Kan CD, Lee HL, Yang YJ. Outcome after endovascular stent graft treatment for mycotic aortic aneurysm: a systematic review. J Vasc Surg. 2007;46(5):906–12.
Hart T, Milner R. Surgical versus endovascular aortic aneurysm repair: evidence to guide the optimal approach for the individual patient. Curr Atheroscler Rep. 2016;18(12):76.
Paravastu SC, Ghosh J, Murray D, Farquharson FG, Serracino-Inglott F, Walker MG. A systematic review of open versus endovascular repair of inflammatory abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2009;38(3):291–7.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Newman, J.D., Motiwala, A., Turin, A., Chen, A., Maharaj, V.R., Dieter, R.S. (2019). Abdominal Aortic Aneurysms. In: Dieter, R., Dieter Jr., R., Dieter III, R. (eds) Diseases of the Aorta . Springer, Cham. https://doi.org/10.1007/978-3-030-11322-3_14
Download citation
DOI: https://doi.org/10.1007/978-3-030-11322-3_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-11321-6
Online ISBN: 978-3-030-11322-3
eBook Packages: MedicineMedicine (R0)