Abstract
Pluripotent stem cells (PSCs), including embryonic stem cells and induced pluripotent stem cells, show heterogeneity with respect to their pluripotency, self-renewal ability, and other traits. PSC heterogeneity may exist among cell lines, among cells within a line, and among temporal states of individual cells. Both genetic and epigenetic factors can cause heterogeneity among cell lines. Heterogeneity among cells within a cell line may arise during long-term culturing even when a PSC cell line is derived from a single cell. Moreover, the expression levels of genes and proteins in PSCs fluctuate continuously at a frequency ranging from a few hours to a few days. Such heterogeneity decreases the reproducibility of research. Thus, methods related to the detection, reduction, and control of heterogeneity in experiments involving human PSCs need to be developed. Further, the presupposition that PSCs are highly heterogeneous should be taken into account by all researchers not only when they plan their own studies but also when they review the studies of other researchers in this field.
Author contributions: Sections “Genetic Variability Among PSC Types,” “Epigenetic Differences Among PSC Lines” and “Heterogeneity Among Each Cell in a Cell Line,”(YH); Sections “Introduction,” “Temporal Fluctuation of PSCs,” and “Perspective and Conclusion” (KO), Sections “Imaging Methods for Detecting PSC Heterogeneity” and “Quality control of heterogeneous hPSCs” (MKF).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Pluripotent stem cells (PSCs)
- Human pluripotent stem cells (hPSCs)
- Embryonic stem cells (ESCs)
- Human embryonic stem cells (hESCs)
- Induced pluripotent stem cells (iPSCs)
- Human pluripotent stem cells (hiPSCs)
Introduction
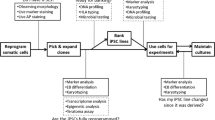
In this chapter, we present six points of view on pluripotent stem cells (PSCs), including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) (Fig. 6.1). Since a PSC may potentially develop into any type of cell (pluripotency) and proliferate limitlessly (self-renewal), PSCs are a promising source for gaining an understanding of early embryonic development and applying such understanding to regenerative medicine and drug discovery. However, in this regard, heterogeneity of PSCs is an important issue that remains unresolved and therefore in need of further research.
Many studies have indicated that PSCs may be heterogeneous. Although PSCs share a similar undifferentiated stem cell phenotype, each line has slightly different properties , such as the propensity for differentiation. Such line-to-line heterogeneity stems from not only the genetic background of the cells (Section “Genetic Variability Among PSC Types”) but also from epigenetic modifications (Section “Epigenetic Differences Among PSC Lines”). Moreover, heterogeneity also exists among cells within a PSC line (Section “Heterogeneity Among Each Cell in a Cell Line”). Cell-to-cell heterogeneity is also caused by dynamic (temporal) changes in gene expression levels in a cell (Section “Temporal Fluctuation of PSCs”). Thus, PSC heterogeneity exists among lines, among cells within a line, and among temporal states in individual cells.
A difficulty arises in that PSC heterogeneity may lead to poor reproducibility in cell quality or cell processing, especially in the case of human PSCs (hPSCs) including human ESCs (hESCs) and human iPSCs (hiPSCs). In order to utilize hPSCs as a tool in regenerative medicine or pharmaceutical research, stable and robust culturing is required. Many techniques have been proposed to overcome the issue of reproducibility. Microscopic imaging techniques are the most powerful methods that are available to detect hPSC heterogeneity (Section “Imaging Methods for Detecting PSC Heterogeneity”). Moreover, validation, standardization, and regulation of hPSC heterogeneity should be achieved prior to their implementation as tools in regenerative medicine and drug screening (Section “Quality Control of Heterogeneous hPSCs”).
Genetic Variability Among PSC Types
The causes of variability among PSC types may be categorized as (i) genetic and (ii) epigenetic factors [1]. Reasons for heterogeneity among PSC types are summarized (Fig. 6.2 ). Each reason is further explained below.
Donor Variation (Differences in Genetic Backgrounds)
Normally, hESCs and other embryo-derived PSCs may be obtained from any type of tissue of any type of individual, which allows for the generation of large quantities of genetically diverse cells. Since genetic diversity forms the basis of drug efficacy, toxicity, and adverse reactions, care must be taken in collecting PSCs from variable genetic backgrounds for use in drug development [2]. Genetic variations can affect stem cell behavior in vitro via variabilities in gene expression and in signaling pathways [1]. One study demonstrated that the donor genetic background accounted for more functional differences between hiPSCs , than either the donor cell type or the derivation method [3]. The authors compared hepatic differentiation efficiency among hiPSCs from peripheral blood and dermal fibroblasts from the same donor or different donors and reported that variations in hepatic differentiation were largely attributable to donor differences, rather than to the original cell type. These findings suggested that genetic variation among the normal population may contribute substantially to the variability observed in PSC behavior, compared to genetic mutations acquired during the reprogramming or to long-term culturing of PSCs.
Mutations and Other Genetic Changes During Derivation of PSCs
Two types of mutations and other genetic changes may occur during derivation of PSCs. One is clonal selection of existing somatic mutations, and the other is the occurrence of de novo mutations during PSC derivation.
Clonal Selection of Existing Somatic Mutations
Since each PSC line is derived from a single cell or a few founder cells, clonal selection of existing somatic mutations causes cell line variability, even among cells from the same donor. Several studies reported that much of the genetic variation found among PSCs was due to pre-existing variation in the cloned, original somatic cells [4,5,6,7,8,9]. PSC clones carried existing somatic mutations or largely random genetic changes. However, some pre-existing genetic variation can either facilitate or inhibit reprogramming of iPSCs, which are preferentially propagated by selective advantages or disadvantages [10]. Although, to our knowledge, no proof of preferential propagation has been obtained from a single donor carrying mosaic genetic variations, some mutations have been reported to affect iPSC derivation [11]. Mutations , which cause altered DNA repair (e.g., ataxia-telangiectasia, Fanconi anemia, and DNA ligase IV (LIG4) syndrome) [12,13,14], premature aging (e.g., Hutchinson-Gilford progeria syndrome and Néstor-Guillermo progeria syndrome) [15, 16], altered telomere homeostasis (e.g., dyskeratosis congenita) [17,18,19], mitochondrial respiratory dysfunction [20,21,22], chromosomal abnormalities [23, 24], and fibrodysplasia ossificans progressiva [25, 26], have all been shown to affect efficiency of reprogramming somatic cells into iPSCs.
By using this process, some researchers have successfully derived both normal and mutant PSCs from the same donor carrying mosaic mutations. NLRP3-mutant and nonmutant iPSC lines were generated from two chronic infantile neurologic cutaneous and articular (CINCA) syndrome patients with somatic mosaicism [27]. Nonmutant iPSCs may function as natural isogenic control cells in disease modeling and drug development. They found that mutant cells were predominantly responsible for pathogenesis because only mutant iPSC-derived macrophage cells showed the disease-relevant phenotype. These results indicated that mosaicism in a donor may be useful for analyzing somatic mosaicism using hiPSC technology.
De Novo Mutations During Derivation of PSCs
Although the studies mentioned above indicated that a majority of PSC mutations pre-existed in founder somatic cells, some studies reported that the rate of de novo mutations was also increased during iPSC derivation [28, 29]. One study compared point mutation profiles between ESCs and iPSCs under closely identical conditions from the same mouse strain, and demonstrated that the rate of point mutations in iPSCs was much higher than that in ESCs [29]. A recent study reported that these mutations preferentially occurred in structurally condensed lamina-associated heterochromatic domains, but were underrepresented in protein-coding genes and in open chromatin regions, including transcription factor binding sites [30]. Other reports describing the effect of genetic changes in iPSC derivation use thorough genomic analysis [31, 32], and the genetic variants were generally benign in contrast to disease-causing single-nucleotide polymorphisms (SNPs) . However, a harmful de novo mutation may have a critical effect on the application of PSCs in regenerative medicine. A fine case in point is the first clinical trial using human iPSC-derived cells, where one patient could not undergo transplantation due to concerns regarding genetic changes that had occurred in the iPSCs [33].
Although the molecular mechanism by which reprogramming-induced mutations are introduced remains to be elucidated, mutation signature analysis indicated that oxidative stress associated with reprogramming may be a likely cause of point mutations [30, 31]. There is an urgent need for methods which may be used to decrease the rate of de novo mutations during the derivation and prolonged culturing of PSCs for use in clinical applications.
Epigenetic Differences Among PSC Lines
Types of PSCs
PSCs may be obtained from different sources. Two types of hPSCs are widely used: hESCs derived from the inner cell mass (ICM) of the blastocyst [34] and hiPSCs generated by introducing key transcription factors into somatic cells [35, 36]. There is much disagreement regarding the differences between hESCs and hiPSCs. However, both hESCs and successfully reprogrammed hiPSCs generally have similar gene expression patterns, differentiation potentials, and epigenetic signatures [37,38,39].
The Effect of Culture Conditions (Intracellular Signal State)
Although conventional hPSCs in bFGF-dependent culture conditions have been widely used, an alternative pluripotent state with different signal dependence has attracted much attention. The derivation of mouse epiblast stem cells (mEpiSCs) indicated that pluripotent cells may exhibit developmental stages [40, 41]. mESCs derived from the preimplantation inner cell mass represent the “naïve” stage, and mEpiSCs derived from the post-implantation epiblast represent the “primed” stage. mESC self-renewal has been achieved through exposure to the leukemia inhibitory factor (LIF) [42,43,44]. Addition of extracellular signal-regulated kinase (MEK) and glycogen synthase kinase 3 (GSK3) inhibitors (2i) in defined medium allowed the cells to attain a homogeneous ground state [45]. On the other hand, mEpiSCs are cultured in a medium containing bFGF and activin/nodal/TGF, which is similar to conventional human PSC culture medium. Although some early studies used LIF in feeder-free media for undifferentiated hESCs [46, 47], LIF and its downstream STAT3 signaling pathway were shown to be dispensable for maintaining primed human and primate PSC self-renewal in several independent studies [48,49,50]. Thus, currently, defined hPSC culture media usually do not contain LIF; however, it was reported that media for naïve hPSCs contained LIF and not bFGF [51,52,53,54]. STAT3 activation was reported to be crucial in reprogramming human PSCs to the naïve state [55]. A recent study also showed that LIF promoted X chromosome reactivation, which was one of the characteristics of naïve pluripotency in female hPSCs [56]. These studies indicated that naïve hPSCs required different culture conditions and signaling activation status compared to primed hPSCs. Based on their similarities in cytokine requirement and signal dependence, conventional hPSCs represent a primed state, similar to mEpiSCs [57]. Differences in the cytokine requirements and signal dependency under PSC conditions lead to variation in PSC behavior. It is commonly observed that differentiation efficiency obtained by an established method is dependent on the specific maintenance of the culture conditions of PSCs.
Recent studies highlighting different culture conditions demonstrated the different developmental potentials of PSCs. A study demonstrated the generation of mouse expanded potential stem cells, which can contribute both to the embryo proper and to the trophectoderm lineages in a chimeric mouse production assay [58].
Cell (Tissue)-Type Variation and “Epigenetic Memory”
Somatic cell nuclear transfer (NT) and transcription factor-based reprogramming can generate PSCs, which are designated as NT-ESCs and iPSCs. Through different mechanisms and kinetics, these two reprogramming methods mostly reset genomic methylation, an epigenetic modification of DNA that influences gene expression. The resulting PSCs may have different properties in their epigenetic status. Many studies have reported that iPSCs derived from adult tissues harbor residual epigenetic characteristics of their somatic tissue of origin, which causes their preferential differentiation propensity toward lineages related to the donor cell while restricting alternative cell fates [59, 60]. Such “epigenetic memory ” is harmful in terms of biased differentiation properties; however, a skewed differentiation potential may be advantageous in certain types of research and clinical applications. One study demonstrated that pancreatic beta-cell-derived iPSCs maintained an open chromatin structure at key beta-cell genes, together with a unique DNA methylation signature that distinguishes them from other PSCs. These iPSCs also demonstrated an increased ability to differentiate into insulin-producing cells both in vitro and in vivo, compared with other PSCs [61]. “Epigenetic memory ” of the donor tissue could be reset by differentiation and serial reprogramming via treatment with chromatin-modifying drugs [59] or telomerase overexpression [62].
Reprogramming Methods
It is believed that different reprogramming methods may give rise to variable PSCs in terms of their epigenetic status, pluripotency, and other functionalities. Using mouse PSCs, one study demonstrated that differentiation and methylation of NT-ESCs were more similar to conventional ESCs than were iPSCs. Therefore, the authors proposed that NT was more effective in establishing the ground state of pluripotency than iPSC reprogramming, which could leave an epigenetic memory of the tissue of origin that may influence the results of directed differentiation [59]. By contrast, one study demonstrated that differentiated cells derived from isogenic human iPSCs and NT-ESCs showed comparable lineage gene expression, cellular heterogeneity, physiological properties, and metabolic functions [63]. Genome-wide transcriptome and DNA methylome analysis indicated that iPSC-derived cardiomyocytes and endothelial cells and iPSC-ECs were similar to isogenic NT-ESC counterparts. Although iPSCs and NT-ESCs shared the same nuclear DNA and carried different sources of mitochondrial DNA, they claimed that molecular and functional characteristics of cells differentiated from PSCs are primarily attributed to genetic composition rather than the reprogramming method.
In order to avoid unnecessary misunderstandings stemming from cell line heterogeneity, standardized PSC lines are used as a partial solution for general research purposes. Also, using as many cell lines as possible is crucial to achieve plausible results. However, for rare diseases, it is sometimes hard to generate sufficient PSC lines. In these cases, using isogenic control cells to neutralize the genetic background effect may be effective in ensuring that the cellular phenotypes stem from the mutation. Our study also demonstrated that variations in hepatocyte differentiation efficiency could be predicted by studying the gene expression profile of undifferentiated hPSCs [64]. Development of such prediction methods may be crucial for avoiding misinterpretations caused by cell line heterogeneity.
Heterogeneity Among Each Cell in a Cell Line
Heterogeneity among cells in a cell line may be acquired during long-term culturing even when a PSC cell line is derived from a single cell (Fig. 6.3). In detecting cell-to-cell variation and heterogeneity, single-cell genome, epigenome, and transcriptome sequencing technologies serve as powerful tools to dissect comprehensive heterogeneity and to identify distinct characteristics, even within a PSC line in the same dish [65].
Genetic Heterogeneity Among Each Cell in a PSC Line
Chromosomal and Subchromosomal Changes
The International Stem Cell Initiative (ISCI) analyzed 125 hESC lines and 11 hiPSC lines , from 38 laboratories worldwide, for genetic changes occurring during culture [66]. In this study, most lines remained karyotypically normal between early and late passages, but some lines tended to acquire karyotypic changes after prolonged culture, commonly affecting chromosomes 1, 12, 17, and 20. Subchromosomal structural variants also appeared sporadically. No common variants related to culture were observed on chromosomes 1, 12, and 17, but a minimal amplicon in chromosome 20q11.21 occurred in >20% of the lines. In the following study, PSC lines containing this amplicon were shown to have higher population doubling rates, attributable to enhanced cell survival through resistance to apoptosis [67]. Overexpression of BCL2L1 (BCL-XL isoform), with a locus situated within the minimal amplicon, provides control cells with growth characteristics similar to those of CNV-containing cells , whereas inhibition of BCL-XL suppresses the growth advantage of CNV cells, establishing BCL2L1 as a driver mutation.
Other studies also reported that the karyotypic heterogeneity generated by mosaic aneuploidy may contribute to the reported functional and phenotypic heterogeneity of hPSCs lines, as well as their therapeutic efficacy and safety following transplantation [68, 69].
Copy Number Variations (CNVs)
During derivation and maintenance culture of PSCs, subtle changes in genomic DNA may occur. One study demonstrated that 17 different hESC lines maintained in different laboratories identified 843 CNVs of 50 kb–3 Mb in size [70]. Twenty-four percent of the loss of heterozygosity (LOH) sites and 66% of the CNVs changed in culture between early and late passages of the same line. Thirty percent of the genes detected within CNV sites had altered expression compared to samples with normal copy number states, of which >44% were functionally linked to cancer. These results suggested the importance of monitoring the genomic DNA status during PSC culturing for research and clinical purposes.
Epigenetic Heterogeneity Among Each Cell in a Cell Line
Heterogeneity of DNA Methylation Among Each Cell
A hiPSC line comprises a heterogeneous population characterized by variable levels of aberrant DNA methylation [71]. These aberrations are induced during somatic cell reprogramming, and their levels are associated with the type of hiPSC source cell. Heterogeneity of DNA methylation status in the hiPSC population was reduced during prolonged culture to a level similar to that of hESCs. The ISCI reported that, in a large scale analysis using mainly hESCs , DNA methylation patterns changed haphazardly with no link to the time in culture [66].
The Effect of Cell Cycle Stages on PSC Behaviors
Undifferentiated PSCs display an unusual mode of cell cycle regulation , with shorter G1 and G2 phases. When individual PSCs are exposed to differentiation stimuli, they remodel the cell cycle so that the length of G1 and overall cell division times increase. Heterogeneous responses of individual stem cells to pro-differentiation signals result in asynchronous differentiation. The reason for different cells in the same culture responding in a nonuniform manner to identical exogenous signals remains unclear. Recent studies demonstrated that the cell cycle position may directly influence lineage specification and suggested that cells in G1 were uniquely “poised” to undergo cell specification [72, 73]. G1 cells served as a “Differentiation Induction Point,” which may explain the heterogeneity of stem cell cultures.
Temporal Fluctuation of PSCs
PSCs are not temporally stable and fluctuate between multiple states. Although the cell lines are stable, the expression levels of PSC-related genes and proteins fluctuate dynamically at a frequency ranging from a few hours to a few days (Fig. 6.1). Since the fluctuation does not specifically occur in vitro but may also occur in vivo, its dynamics are considered to be closely related to maintaining the “stemness” of the cells during embryonic development. In this section, we introduce PSC heterogeneity based on temporal expression dynamics.
Temporal Fluctuation of PSC-Related Gene Expression
Temporal fluctuation of Nanog is a well-known phenomenon. Nanog is a homeobox protein, which is thought to be one of the most important transcription factors for maintaining pluripotency in PSCs including ES and iPS cells of human and other animals [74,75,76]. Hence, it was believed that all PSCs may homogeneously express a high level of Nanog as well as other PSC-related transcription factors. However, it was shown that Nanog of mouse and human PSCs displayed mosaic expression, although both high- and low-Nanog cells expressed high levels of other PSC-related transcription factors [77, 78]. Later, it was shown that low-Nanog cells are not a stable subpopulation. Sorting and culturing of low-Nanog cells resulted in the production of high-Nanog cells eventually [79]. Gene expression tracking techniques using single molecule in situ fluorescence hybridization also showed that the gene expression of estrogen-related receptor beta (Esrrb), which is co-expressed with Nanog , also changes with time [80, 81]. Moreover, time-lapse imaging of the Nanog fluorescent protein fusion ESC line showed that Nanog expression may fluctuate over several generations [82].
Other PSC-related genes such as Rex1, Hes1, Stella, and Zscan4 are also known to fluctuate temporally [83,84,85]. Similar to Nanog, all these genes fluctuate temporally, except Zscan4. Zinc finger and SCAN domain containing 4 (ZSCAN4), which has functions related to telomere elongation and genomic stability, is specifically expressed in the two-cell stage mouse embryo and in ESCs [86]. The expression of Zscan4 is intermittent; it is expressed in only 5% of the population at a time but in all cells eventually [87,88,89]. All these reports indicated that expression of many PSC-related genes is not stable but fluctuates temporally during the maintenance of their population.
Temporal Expression May Be Essential for Maintaining Pluripotency
The question may arise as to whether temporal fluctuation is an in vitro-specific artifact. The inner cell mass of the blastocyst is the origin of mouse and human ESCs. Although mouse PSCs share the same properties with the inner cell mass, human and monkey PSCs share the same properties with the epiblast, which arises from the inner cell mass [41]. It is reported that Nanog and other PSC-related gene expression in the mouse inner cell mass and monkey epiblast may not be homogeneous but mosaic [77, 90, 91]. Since it is well-known that the fate of cells in the inner cell mass remains undetermined and that the cells have powers of regulation, mosaic expression may not be stable but changeable. Thus, although there is no direct evidence that PSC-related gene expression temporally fluctuates in vivo, fluctuation may not be an in vitro-specific artifact.
Issues of whether all main genes corresponding to PSCs temporally fluctuate, as well as whether all fluctuating genes correspond to pluripotency, may need to be clarified. Oct3/4 (Pou5f1), which is a master gene of pluripotency similar to Nanog , is known to have a rather stable expression level in mouse and human PSCs [82, 92]. Zscan4, not showing any correlation with Rex1, is thought to be correlated with telomere shortening, irrespective of the pluripotency [89]. Thus, temporal fluctuation of these genes may closely be correlated with PSC population maintenance. However, it is not always correlated to the maintenance of pluripotency.
Two hypotheses have been suggested to explain why temporal fluctuation is displayed by so many PSC-related genes. The first is that fluctuation is required for preparing subpopulations that are origins of multiple lineages. Reportedly, a gene being expressed at a high level at a specific time indicates its readiness for differentiation into a specific lineage [84, 93,94,95]. For example, mouse ESCs expressing low and high levels of Hes1 tended to differentiate into neural and mesodermal cells, respectively [84]. The second hypothesis is that higher “flexibility” is required to keep the cells in a state of larger “stemness” (pluripotency). Since there are so many genes that temporally fluctuate, the networks of these dynamics should be considered to understand the mechanisms involved. Many reports indicated that fluctuation is essential for maintaining pluripotency and that gene network dynamics should be considered to understand the complex dynamics [96,97,98]. These cells are able to develop into all types of cells, suggesting that PSCs are most “flexible” cells.
Whether fluctuations in gene expression levels of the PSC gene are important in maintaining PSC pluripotency is debatable [82, 99,100,101]. To clarify mechanisms of temporal fluctuation in gene dynamics, gene expression feedback control techniques, which enable regulation of the gene expression level to fit its preset function, such as the patch clamp technique of electrophysiology, are required.
Similarity Between Sister Cells at the Onset of Differentiation
We studied PSC heterogeneity related to temporal expression dynamics [102]. The main purpose of our experiment was to determine whether the origin of heterogeneity was caused by the production of many subpopulations (Fig. 6.4a) or by an increase in each cell’s heterogeneity (Fig. 6.4b). The former and the latter are related to the first and the second hypothesis we described in the last subsection, respectively. We focused on the heterogeneity , or to be exact, similarity, between sister cells derived from a mother ESC at the onset of differentiation. Cells at the onset of differentiation are expected to resemble those in vivo, since the cells emerge from the artificial self-renewal condition in vitro, to start differentiation. The cells are required not only to proliferate but also to differentiate into many lineages at the onset of differentiation.
Green fluorescence protein expression driven by the Nanog promoter (Nanog-GFP) was used to monitor the state of cells. Nanog-GFP is active only in undifferentiated cells [103]. Population analysis using flow-cytometry showed that the peak width of the cell population broadened at the onset of differentiation, suggesting that the population of ESCs had reached heterogeneity. Single-cell analysis via time-lapse imaging was used to compare the difference in Nanog-GFP intensity of the sister cells to those of randomly chosen non-sister cell pairs, which were used as an unsynchronized control. The Nanog-GFP-level difference between sister cells was smaller than that between non-sister cells in the maintenance medium, but not at the onset of differentiation. Similar results were obtained in the Nanog-GFP mouse iPS cell line, suggesting that the sister cells were close to each other in the maintenance medium but were not close at the onset of differentiation.
The results suggested that the cells produced different cells. For example, the high-Nanog-GFP cells are produced from both high- and low-Nanog-GFP cells. Thus, there is a possibility that the PSCs do not produce many subpopulations with similar properties but increase temporal fluctuation of each cell to produce heterogeneity at the onset of differentiation (Fig. 6.4b). After increasing heterogeneity, some external factors such as cell-cell interaction may be necessary to produce differences between each lineage.
In this section, we introduced PSC heterogeneity based on temporal and intermittent expression dynamics of PSCs. The cell state is dynamic, and such dynamics may be important for maintaining pluripotency.
Imaging Methods for Detecting PSC Heterogeneity
Whereas previous studies have revealed genetic instability and heterogeneity in hPSCs, the true extent of genetic variation in hPSCs is only likely to become apparent when whole-genome sequence analysis is performed [104]. A number of candidate genes for aberrant phenotype were reported [66, 67, 105]; however, morphology is a practically important criterion that is used to continuously evaluate the undifferentiated state and heterogeneity of hPSCs. Healthy undifferentiated hPSCs form a typical colony that appears as tightly packed, round cells with large nuclei and notable nucleoli without spaces between cells [106, 107]. Altered propensity of aberrant hPSCs, such as rapid cell growth, may affect their colony appearance, compared with that of healthy parent hPSCs. Several studies demonstrated that morphology is correlated to hPSC quality [106, 108,109,110,111,112]. Therefore, morphological analysis may be valuable for evaluating the heterogeneity of hPSCs.
Recent developments in image analysis have facilitated the evaluation of cellular growth and status using non-labelled as well as labelled images [107, 111,112,113,114,115,116]. Time-lapse live-cell phase-contrast images acquired using a culture observation system revealed that hPSCs exhibit variation in colony and cell areas (Fig. 6.5) [116]. Using this system, we developed a non-labelled imaging method for calculating hPSC growth using formulae to calculate the cell number in hPSC colonies driven from hPSC colony areas and number of nuclei [116]. Although the relationships between the colony areas and nuclei numbers are linear, equation coefficients are dependent on the cell line used, colony size, colony morphology, and culture conditio ns. When culture conditions are improper, the change in cell condition can be detected by these morphological analysis using phase-contrast images (Fig. 6.6) [116]. This indicates that image analysis may be used to quantify heterogeneity.
Colony areas during culture. The hiPSC lines, Tic, or iPS-TIG114-4f1 cells were seeded using feeder-free culture conditions or KSR-based conventional culture conditions with feeder cells in 6-well-plates and cultured for 5 days. After the cell clumps had settled on the plate surfaces (48 h), phase-contrast images of these cells were acquired every 12 h and analyzed using the culture observation system and software. Cell clump areas of >0.25 μm2 were recognized as undifferentiated hiPSC colonies for analysis. (a) Colony areas for Tic feeder-free cell culture. (b) Colony areas for iPS-TIG114-4f1 feeder-free cell culture. (c) Colony areas for Tic on feeder. (d) Colony areas for iPS-TIG114-4f1 on feeder. Modified from Fig. 2 and Supplementary Fig. 1 in STEM CELLS TRANSLATIONAL MEDICINE 2015;4:1–11
Detection of change in cell growth by the imaging system. Tic cells were seeded on Matrigel in TeSR-E8 medium. After 3 days, cells were cultured for more than 2 days in TeSR-E8 medium (a, b, g–i), E5 medium that did not contain insulin, fibroblast growth factor-2, or transforming growth factor-b, or TeSR-E8 medium with an inhibitor of phosphatidylinositol 3 kinase of LY294002 at a concentration of 20 mM (e, f, m–o). (a–f) Phase contrast images were analyzed using the imaging analysis system. Type A colonies were recognized as blue and type B colonies as yellow. (g, j, m) The colony areas were extracted from the images and analyzed for type A or type B colonies <1 mm2 or >1 mm2. (h, k, n) Cell numbers calculated using the equation with the ratio of type A or type B colonies. (i, l, o) The cells dissociated by trypsin/EDTA were counted using a hemocytometer. Scale bars = 2 mm. Modified from Fig. 5 in STEM CELLS TRANSLATIONAL MEDICINE 2015;4:1–11
Machine learning including pattern recognition, automated recognition, and autonomous prediction is gaining recognition as a potential analysis tool. Tokunaga et al. [112] reported that supervised machine learning pattern recognition can distinguish completely reprogrammed iPSCs from improperly reprogrammed cells. Generally, iPSC colonies are manually selected based on their morphology via expert observation. Utilizing representative iPSC colony images of incompletely reprogrammed iPSCs selected by experts, it is possible to train computers to classify colonies according to morphological patterns. This finding confirms that cell morphology is a valuable marker, which noninvasive computation image analysis can evaluate as hPSC quality. We reported that the non-labelled live-cell image analysis can classify morphological heterogeneity of hPSC colonies based on the statistical analysis of images with unbiased morphological parameters [107]. Colony morphology of an aberrant hPSC subclone (#12 trisomy) and parent cell line was compared. Whereas most of the population of the aberrant hPSC subclone appeared to have partially differentiated colony morphology, most of the population of a parent cell line seemed to show typical embryonic stem cell-like morphology. Classification utilizing statistical analysis of colony images revealed morphological heterogeneity in both cell lines that was similar to heterogeneity in gene expression profiles. The analysis indicated that the aberrant subclone had unhealthy colonies at a ratio of 27.3% that were characterized by the loss of clear colony edges, a comparatively flatter cytoplasm, and a low nucleus-to-cytoplasm ratio. The parent line had unhealthy colonies at a ratio of 13.3%, suggesting that non-label image analysis of morphology may predict unhealthy state by monitoring variations in hPSC heterogeneity.
Flow cytometry is the central methodology for canonical markers of stem cells [117], but recent developments in imaging analysis encourage the use of immunocytochemistry. This is because two-dimensional imaging cytometry enables evaluation of the heterogeneity of undifferentiated state hPSCs to analyze localization and morphological information regarding immunopositive cells in the culture [118]. Whole images of cells in a culture vessel acquired by the image analyzer can demonstrate the population ratio and staining intensity of the cells with positional information. Two-dimensional imaging cytometry reveals spatially heterogenic expression of the hPSC markers in undifferentiated hPSCs (Fig. 6.7) [118].
Localization of stem cell markers in hiPSC 201B7 colonies analyzed for expression profiles. Representative cells stained with Oct3/4 and SSEA1 (a, b) and with OCT3/4 and SSEA3 (c, d) were tracked back to the images from plots. (a, c) Merged image of immunostaining . A representative field of the images taken by an imaging cytometer is shown. Regions containing each target cell are marked with a yellow rectangle. Scale bar, 100 μm. (b, d) Analyzed fluorescent intensity profile of each cell in the field. Red plots represent target cells. Fluorescent images stained with SSEA1 or SSEA3 with OCT-3/4 antibody and Hoechst33342. The nucleic region of each target cell is marked as cyan Modified from Fig. 4 in In Vitro Cell. Dev. Biol.—Animal (2017) 53:83–91 DOI 10.1007/s11626-016-0084-3
Quality Control of Heterogeneous hPSCs
As a result of the heterogeneities described above, the quality of hPSCs used in research may cause concern. Because hPSCs are generally adapted for in vitro culture , they tend to have increased growth and less apoptotic cell death [70, 119,120,121,122,123]. When adapted aberrant hPSCs appear in the cell population, they rapidly replace the parent cell population, resulting in altered propensity for survival and retention of an undifferentiated phenotype [121]. Therefore, it is recommended that the quality of hPSCs be routinely checked during culturing for a long period. The International Stem Cell Banking Initiative (ISCBI) established in 2007 with funding from the International Stem Cell Forum (http://www.stemcell-forum.net/) provided guidance for banking and supplying of human embryonic stem cell lines for research purposes in 2009 [117] and points to be considered prior to clinical applications in 2015 [124]. These papers which describe best practices for the banking of hPSCs may also be valid for basic research using hPSCs in laboratories. An international study conducted on characteristic phenotypes of hPSCs by the ISCI summarized typical surface marker profiles and stem cell-related marker genes [76]. Based on these studies, the following five measurements are recommended to evaluate the quality of hPSCs in laboratories.
Cell Morphology
Cell morphology is an important criterion, as described above. When culturing cells obtained from a supplier such as a cell bank, it is necessary to acquire phase contrast images of cultured cells at the earliest possible time and compare them with the images provided by the supplier. Ideally, it is recommended to acquire cell images at every passage. At minimum, it is necessary to acquire both images of undifferentiated and differentiated cell areas every five passages at weak and strong enlargements to confirm whether the cells are able to maintain their original morphology.
Growth Rate
Growth rate is an important characteristic because an increase in growth rate may indicate transformation [124]. Changing culture conditions affects the growth rate. Just after the cells are thawed, they grow slowly. Three or four passages following thawing may be a suitable time to calculate the growth rate. It is necessary to calculate growth rate , at least every five passages after that. Although it is ideally recommended to count cell numbers of the culture vessel at every passage, dissociation into a single cell in the presence of an ROCH inhibitor is not always used under all culture conditions. Either counting cell numbers separately or monitoring live cell images is recommended, as described above.
Characterization of Stem Cell Marker Genes and Antigen Expression
Characterization of stem cell marker genes and antigen expression provides useful fundamental information on cell state and the variability and consistency of cultures [124]. Although an assay using the whole-genome expression arrays may be valuable in a cell bank where analyzed data from various cell lines are accumulated, analysis of extensive information derived from the array would be difficult in a small laboratory and also less cost-effective. A PCR array for human stem cell markers or selective typical genes, such as typical genes, Nanog, Oct 4, DNMT 3B, TDGF, GABRB3, GDF3 [117], and also BCL2L1 as an anti-apoptotic gene [66], may be useful in a laboratory. Quantitative analysis by flow cytometry is generally used for a typical surface marker set: SSEA-1 (negative or very low), SSEA-3, SSEA-4, TRA-1-60, and TRA-1-81. As recent developments in digital imaging technology have enabled the acquisition of an entire surface image of a culture vessel, immunocytochemical analysis with imaging has also become a useful tool to provide quantitative characterization, reflecting even a minute aberrance without losing spatial and morphological information of the cells [118, 125, 126]. Alkaline phosphatase-positive colony-forming assays are useful for quantitation of stem cell lines [127]. However, it should be taken into consideration that hPSC cell cultures vary in gene and antigen expression from one passage to another [76].
Genotype
Several genotyping techniques may be available in the laboratory as well as in the cell bank [124]. Karyotyping by Giemsa banding is the most common technique used to identify changes in chromosomal numbers as well as translocations and rearrangements. Spectral karyotyping or fluorescent in situ hybridization can also be performed in the laboratory. While a specific expensive instrument is not necessary for these analyses, proficient techniques for karyological study are required. Ideally, it is recommended to analyze the genotype of hPSCs every five passages. At a minimum, the genotype of hPSCs should be determined after every ten passages.
Comparative genome hybridization (CGH) microarray or multiple single-nucleotide polymorphism (SNP) analysis may be suitable for the laboratory, and the number of cells analyzed may be critical for sensitivity to abnormal clones [76]. Deep sequencing or whole-genome sequencing can be performed at large institutes or universities with well-equipped facilities [128,129,130]. However, the sensitivity and accuracy of these methods should be considered, and the results should also be interpreted with caution.
Differentiation Potential
Assessing the ability of stem cells to form teratomas in SCID mice has long been considered the “gold standard” for confirming pluripotency of cells [131]. However, this may not be realistic in the research laboratory, as the teratoma assay is time-consuming and costly. An alternative method is the formation and characterization of embryoid bodies [132,133,134]. Recently, various differentiation protocols for specific lineages using defined culture conditions have been developed. Determination of specific differentiation induction methods to measure differentiation potential is easy to perform in the laboratory.
Considering progressive phenotypes of aberrant clones, these measurements are recommended at least every ten passages. However, performing all of these measurements is time-consuming and costly. It is recommended that cryopreserved stocks of cells intended for use be prepared at the earliest possible time and analyzed once in the laboratory to assure that reliable stocks can be obtained [135, 136] and that cells newly cultured from the cryopreserved stock are used within 3 months to minimize the effects of heterogeneity from prolonged culture.
Perspective and Conclusion
In this chapter, we focused on the heterogeneity of PSCs from six points of view (Fig. 6.1). PSCs are heterogeneous. Such heterogeneity unfortunately decreases reproducibility of research. Thus, research related to verification, reduction, and regulation of heterogeneity is important, especially for hPSCs. However, as heterogeneity is closely related to pluripotency, a fundamental property of PSCs, there is a possibility that complete elimination of heterogeneity may also cause the elimination of pluripotency. Under the present circumstances, the presupposition that PSCs are highly heterogeneous should be taken into account by all researchers not only when they plan their own studies but also when they review the studies of other researchers in this field.
References
Cahan P, Daley GQ (2013) Origins and implications of pluripotent stem cell variability and heterogeneity. Nat Rev Mol Cell Biol 14:357–368
Fakunle ES, Loring JF (2012) Ethnically diverse pluripotent stem cells for drug development. Trends Mol Med 18:709–716
Kajiwara M et al (2012) Donor-dependent variations in hepatic differentiation from human-induced pluripotent stem cells. Proc Natl Acad Sci U S A 109:12,538–12,543
Howden SE et al (2011) Genetic correction and analysis of induced pluripotent stem cells from a patient with gyrate atrophy. Proc Natl Acad Sci U S A 108:6537–6542
Gore A et al (2011) Somatic coding mutations in human induced pluripotent stem cells. Nature 471:63–67
Quinlan AR et al (2011) Genome sequencing of mouse induced pluripotent stem cells reveals retroelement stability and infrequent DNA rearrangement during reprogramming. Cell Stem Cell 9:366–373
Abyzov A et al (2012) Somatic copy number mosaicism in human skin revealed by induced pluripotent stem cells. Nature 492:438–442
Cheng L et al (2012) Low incidence of DNA sequence variation in human induced pluripotent stem cells generated by nonintegrating plasmid expression. Cell Stem Cell 10:337–344
Young MA et al (2012) Background mutations in parental cells account for most of the genetic heterogeneity of induced pluripotent stem cells. Cell Stem Cell 10:570–582
Liang G, Zhang Y (2013) Genetic and epigenetic variations in iPSCs: potential causes and implications for application. Cell Stem Cell 13:149–159
Hayashi Y (2017) Human mutations affecting reprogramming into induced pluripotent stem cells. AIMS Cell Tissue Eng 1:31–46
Kinoshita T et al (2011) Ataxia-telangiectasia mutated (ATM) deficiency decreases reprogramming efficiency and leads to genomic instability in iPS cells. Biochem Biophys Res Commun 407:321–326
Nayler S et al (2012) Induced pluripotent stem cells from ataxia-telangiectasia recapitulate the cellular phenotype. Stem Cells Transl Med 1:523–535
Fukawatase Y et al (2014) Ataxia telangiectasia derived iPS cells show preserved x-ray sensitivity and decreased chromosomal instability. Sci Rep 4:5421
Zhang J et al (2011) A human iPSC model of Hutchinson Gilford Progeria reveals vascular smooth muscle and mesenchymal stem cell defects. Cell Stem Cell 8:31–45
Liu GH et al (2011) Recapitulation of premature ageing with iPSCs from Hutchinson-Gilford progeria syndrome. Nature 472:221–225
Agarwal S et al (2010) Telomere elongation in induced pluripotent stem cells from dyskeratosis congenita patients. Nature 464:292–296
Winkler T et al (2013) Defective telomere elongation and hematopoiesis from telomerase-mutant aplastic anemia iPSCs. J Clin Invest 123:1952–1963
Batista LF et al (2011) Telomere shortening and loss of self-renewal in dyskeratosis congenita induced pluripotent stem cells. Nature 474:399–402
Yokota M, Hatakeyama H, Okabe S, Ono Y, Goto Y (2015) Mitochondrial respiratory dysfunction caused by a heteroplasmic mitochondrial DNA mutation blocks cellular reprogramming. Hum Mol Genet 24:4698–4709
Zhou Y et al (2017) Mitochondrial spare respiratory capacity is negatively correlated with nuclear reprogramming efficiency. Stem Cells Dev 26:166–176
Hung SS et al (2016) Study of mitochondrial respiratory defects on reprogramming to human induced pluripotent stem cells. Aging (Albany NY) 8:945–957
Bershteyn M et al (2014) Cell-autonomous correction of ring chromosomes in human induced pluripotent stem cells. Nature 507:99–103
Yu Y et al (2015) Chromosome microduplication in somatic cells decreases the genetic stability of human reprogrammed somatic cells and results in pluripotent stem cells. Sci Rep 5:10,114
Hamasaki M et al (2012) Pathogenic mutation of Alk2 inhibits ips cell reprogramming and maintenance: mechanisms of reprogramming and strategy for drug identification. Stem Cells 30:2437–2449
Hayashi Y et al (2016) BMP-SMAD-ID promotes reprogramming to pluripotency by inhibiting p16/INK4A-dependent senescence. Proc Natl Acad Sci U S A 113:13,057–13,062
Tanaka T et al (2012) Induced pluripotent stem cells from CINCA syndrome patients as a model for dissecting somatic mosaicism and drug discovery. Blood 120:1299–1308
Ji J et al (2012) Elevated coding mutation rate during the reprogramming of human somatic cells into induced pluripotent stem cells. Stem Cells 30:435–440
Sugiura M et al (2014) Induced pluripotent stem cell generation-associated point mutations arise during the initial stages of the conversion of these cells. Stem Cell Rep 2:52–63
Yoshihara M et al (2017) Hotspots of de novo point mutations in induced pluripotent stem cells. Cell Rep 21:308–315
Rouhani FJ et al (2016) Mutational history of a human cell lineage from somatic to induced pluripotent stem cells. PLoS Genet 12:e1005932
Bhutani K et al (2016) Whole-genome mutational burden analysis of three pluripotency induction methods. Nat Commun 7:10,536
Mandai M et al (2017) Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med 376:1038–1046
Thomson JA et al (1998) Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147
Takahashi K et al (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872
Yu J et al (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318:1917–1920
Mallon BS et al (2014) Comparison of the molecular profiles of human embryonic and induced pluripotent stem cells of isogenic origin. Stem Cell Res 12:376–386
Koyanagi-Aoi M et al (2013) Differentiation-defective phenotypes revealed by large-scale analyses of human pluripotent stem cells. Proc Natl Acad Sci U S A 110:20569–20574
Riera M et al (2016) Comparative study of human embryonic stem cells (hESC) and human induced pluripotent stem cells (hiPSC) as a treatment for retinal dystrophies. Mol Ther Methods Clin Dev 3:16010
Brons IG et al (2007) Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 448:191–195
Tesar PJ et al (2007) New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448:196–199
Smith AG et al (1988) Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature 336:688–690
Williams RL et al (1988) Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature 336:684–687
Furue M et al (2005) Leukemia inhibitory factor as an anti-apoptotic mitogen for pluripotent mouse embryonic stem cells in a serum-free medium without feeder cells. In Vitro Cell Dev Biol Anim 41:19–28
Ying QL et al (2008) The ground state of embryonic stem cell self-renewal. Nature 453:519–523
Li Y, Powell S, Brunette E, Lebkowski J, Mandalam R (2005) Expansion of human embryonic stem cells in defined serum-free medium devoid of animal-derived products. Biotechnol Bioeng 91:688–698
Amit M, Shariki C, Margulets V, Itskovitz-Eldor J (2004) Feeder layer- and serum-free culture of human embryonic stem cells. Biol Reprod 70:837–845
Sumi T, Fujimoto Y, Nakatsuji N, Suemori H (2004) STAT3 is dispensable for maintenance of self-renewal in nonhuman primate embryonic stem cells. Stem Cells 22:861–872
Daheron L et al (2004) LIF/STAT3 signaling fails to maintain self-renewal of human embryonic stem cells. Stem Cells 22:770–778
Humphrey RK et al (2004) Maintenance of pluripotency in human embryonic stem cells is STAT3 independent. Stem Cells 22:522–530
Hanna J et al (2010) Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc Natl Acad Sci U S A 107:9222–9227
Buecker C et al (2010) A murine ESC-like state facilitates transgenesis and homologous recombination in human pluripotent stem cells. Cell Stem Cell 6:535–546
Hu Z et al (2015) Generation of naivetropic induced pluripotent stem cells from parkinson’s disease patients for high-efficiency genetic manipulation and disease modeling. Stem Cells Dev 24:2591–2604
Zimmerlin L et al (2016) Tankyrase inhibition promotes a stable human naive pluripotent state with improved functionality. Development 143:4368–4380
Chen H et al (2015) Reinforcement of STAT3 activity reprogrammes human embryonic stem cells to naive-like pluripotency. Nat Commun 6:7095
Tomoda K et al (2012) Derivation conditions impact X-inactivation status in female human induced pluripotent stem cells. Cell Stem Cell 11:91–99
Pera MF, Tam PP (2010) Extrinsic regulation of pluripotent stem cells. Nature 465:713–720
Yang J et al (2017) Establishment of mouse expanded potential stem cells. Nature 550:393–397
Kim K et al (2010) Epigenetic memory in induced pluripotent stem cells. Nature 467:285–290
Nazor KL et al (2012) Recurrent variations in DNA methylation in human pluripotent stem cells and their differentiated derivatives. Cell Stem Cell 10:620–634
Bar-Nur O, Russ HA, Efrat S, Benvenisty N (2011) Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells. Cell Stem Cell 9:17–23
Pomp O et al (2011) Unexpected X chromosome skewing during culture and reprogramming of human somatic cells can be alleviated by exogenous telomerase. Cell Stem Cell 9:156–165
Zhao MT et al (2017) Molecular and functional resemblance of differentiated cells derived from isogenic human iPSCs and SCNT-derived ESCs. Proc Natl Acad Sci U S A 114:E11111–E11120
Yanagihara K et al (2016) Prediction of differentiation tendency toward hepatocytes from gene expression in undifferentiated human pluripotent stem cells. Stem Cells Dev 25:1884–1897
Wen L, Tang F (2016) Single-cell sequencing in stem cell biology. Genome Biol 17:71
The International Stem Cell Initiative (2011) Screening ethnically diverse human embryonic stem cells identifies a chromosome 20 minimal amplicon conferring growth advantage. Nat Biotechnol 29:1132–1144
Avery S et al (2013) BCL-XL mediates the strong selective advantage of a 20q11.21 amplification commonly found in human embryonic stem cell cultures. Stem Cell Rep 1:379–386
Peterson SE et al (2011) Normal human pluripotent stem cell lines exhibit pervasive mosaic aneuploidy. PLoS One 6:e23018
Dekel-Naftali M et al (2012) Screening of human pluripotent stem cells using CGH and FISH reveals low-grade mosaic aneuploidy and a recurrent amplification of chromosome 1q. Eur J Hum Genet 20:1248–1255
Narva E et al (2010) High-resolution DNA analysis of human embryonic stem cell lines reveals culture-induced copy number changes and loss of heterozygosity. Nat Biotechnol 28:371–377
Tesarova L, Simara P, Stejskal S, Koutna I (2016) The aberrant DNA methylation profile of human induced pluripotent stem cells is connected to the reprogramming process and is normalized during in vitro culture. PLoS One 11:e0157974
Singh AM (2015) Cell cycle-driven heterogeneity: on the road to demystifying the transitions between “poised” and “restricted” pluripotent cell states. Stem Cells Int 2015:219514
Dalton S (2015) Linking the cell cycle to cell fate decisions. Trends Cell Biol 25:592–600
Mitsui K et al (2003) The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 113:631–642
Chambers I et al (2003) Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 113:643–655
The International Stem Cell Initiative (2007) Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat Biotechnol 25:803–816
Hatano S-Y et al (2005) Pluripotential competence of cells associated with Nanog activity. Mech Dev 122:67–79
Wu J, Tzanakakis ES (2012) Contribution of stochastic partitioning at human embryonic stem cell division to NANOG heterogeneity. PLoS One 7:e50715
Chambers I et al (2007) Nanog safeguards pluripotency and mediates germline development. Nature 450:1230–1234
van den Berg DL et al (2008) Estrogen-related receptor beta interacts with Oct4 to positively regulate Nanog gene expression. Mol Cell Biol 28:5986–5995
Frieda KL et al (2017) Synthetic recording and in situ readout of lineage information in single cells. Nature 541:107–111
Filipczyk A et al (2015) Network plasticity of pluripotency transcription factors in embryonic stem cells. Nat Cell Biol 17:1235–1246
Toyooka Y, Shimosato D, Murakami K, Takahashi K, Niwa H (2008) Identification and characterization of subpopulations in undifferentiated ES cell culture. Development 135:909–918
Kobayashi T et al (2009) The cyclic gene Hes1 contributes to diverse differentiation responses of embryonic stem cells. Genes Dev 23:1870–1875
Hayashi K, de Sousa Lopes SMC, Tang F, Surani MA (2008) Dynamic equilibrium and heterogeneity of mouse pluripotent stem cells with distinct functional and epigenetic states. Cell Stem Cell 3:391–401
Falco G et al (2007) Zscan4: a novel gene expressed exclusively in late 2-cell embryos and embryonic stem cells. Dev Biol 307:539–550
Zalzman M et al (2010) Zscan4 regulates telomere elongation and genomic stability in ES cells. Nature 464:858–863
Amano T et al (2013) Zscan4 restores the developmental potency of embryonic stem cells. Nat Commun 4:1966
Nakai-Futatsugi Y, Niwa H (2016) Zscan4 is activated after telomere shortening in mouse embryonic stem cells. Stem Cell Rep 6:483–495
Yamanaka Y, Lanner F, Rossant J (2010) FGF signal-dependent segregation of primitive endoderm and epiblast in the mouse blastocyst. Development 137:715–724
Nakamura T et al (2016) A developmental coordinate of pluripotency among mice, monkeys and humans. Nature 537:57
Bhadriraju K et al (2016) Large-scale time-lapse microscopy of Oct4 expression in human embryonic stem cell colonies. Stem Cell Res 17:122–129
Morgani SM et al (2013) Totipotent embryonic stem cells arise in ground-state culture conditions. Cell Rep 3:1945–1957
Pauklin S, Vallier L (2013) The cell-cycle state of stem cells determines cell fate propensity. Cell 155:135–147
Hough SR et al (2014) Single-cell gene expression profiles define self-renewing, pluripotent, and lineage primed states of human pluripotent stem cells. Stem Cell Rep 2:881–895
Eldar A, Elowitz MB (2010) Functional roles for noise in genetic circuits. Nature 467:167–173
Furusawa C, Kaneko K (2012) A dynamical-systems view of stem cell biology. Science 338:215–217
Semrau S et al (2017) Dynamics of lineage commitment revealed by single-cell transcriptomics of differentiating embryonic stem cells. Nat Commun 8:1096
Macfarlan TS et al (2012) Embryonic stem cell potency fluctuates with endogenous retrovirus activity. Nature 487:57–63
Abranches E et al (2014) Stochastic NANOG fluctuations allow mouse embryonic stem cells to explore pluripotency. Development 141:2770–2779
Smith RCG et al (2017) Nanog fluctuations in embryonic stem cells highlight the problem of measurement in cell biology. Biophys J 112:2641–2652
Nakamura S et al (2018) Asymmetry between sister cells of pluripotent stem cells at the onset of differentiation. Stem Cells Dev 27:347–354
Okita K, Ichisaka T, Yamanaka S (2007) Generation of germline-competent induced pluripotent stem cells. Nature 448:313–317
Na J, Baker D, Zhang J, Andrews PW, Barbaric I (2014) Aneuploidy in pluripotent stem cells and implications for cancerous transformation. Protein Cell 5:569–579
Laurent LC et al (2011) Dynamic changes in the copy number of pluripotency and cell proliferation genes in human ESCs and iPSCs during reprogramming and time in culture. Cell Stem Cell 8:106–118
Amit M, Itskovitz-Eldor J (2011) Atlas of human pluripotent stem cells derivation and culturing. Humana Press, New York, pp 15–39
Kato R et al (2016) Parametric analysis of colony morphology of non-labelled live human pluripotent stem cells for cell quality control. Sci Rep 6:34009
Chan EM et al (2009) Live cell imaging distinguishes bona fide human iPS cells from partially reprogrammed cells. Nat Biotechnol 27:1033–1037
Pfannkuche K, Fatima A, Gupta MK, Dieterich R, Hescheler J (2010) Initial colony morphology-based selection for iPS cells derived from adult fibroblasts is substantially improved by temporary UTF1-based selection. PLoS One 5:e9580
Wakao S et al (2012) Morphologic and gene expression criteria for identifying human induced pluripotent stem cells. PLoS One 7:e48677
Gu M et al (2012) Microfluidic single-cell analysis shows that porcine induced pluripotent stem cell-derived endothelial cells improve myocardial function by paracrine activation. Circ Res 111:882–893
Tokunaga K et al (2014) Computational image analysis of colony and nuclear morphology to evaluate human induced pluripotent stem cells. Sci Rep 4:6996
Matsuoka F et al (2013) Morphology-based prediction of osteogenic differentiation potential of human mesenchymal stem cells. PLoS One 8:e55082
Matsuoka F et al (2014) Characterization of time-course morphological features for efficient prediction of osteogenic potential in human mesenchymal stem cells. Biotechnol Bioeng 111:1430–1439
Maddah M, Loewke K (2014) Automated, non-invasive characterization of stem cell-derived cardiomyocytes from phase-contrast microscopy. Med Image Comput Comput Assist Interv 17:57–64
Suga M, Kii H, Niikura K, Kiyota Y, Furue MK (2015) Development of a monitoring method for nonlabeled human pluripotent stem cell growth by time-lapse image analysis. Stem Cells Transl Med 4:720–730
The International Stem Cell Banking Initiative (2009) Consensus guidance for banking and supply of human embryonic stem cell lines for research purposes. Stem Cell Rev 5:301–314
Suga M, Tachikawa S, Tateyama D, Ohnuma K, Furue MK (2017) Imaging-cytometry revealed spatial heterogeneities of marker expression in undifferentiated human pluripotent stem cells. In Vitro Cell Dev Biol Anim 53:83–91
Draper JS et al (2004) Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat Biotechnol 22:53–54
Harrison NJ, Baker D, Andrews PW (2007) Culture adaptation of embryonic stem cells echoes germ cell malignancy. Int J Androl 30:275–281. discussion 281
Enver T et al (2005) Cellular differentiation hierarchies in normal and culture-adapted human embryonic stem cells. Hum Mol Genet 14:3129–3140
Hyka-Nouspikel N et al (2012) Deficient DNA damage response and cell cycle checkpoints lead to accumulation of point mutations in human embryonic stem cells. Stem Cells 30:1901–1910
Barbaric I et al (2014) Time-lapse analysis of human embryonic stem cells reveals multiple bottlenecks restricting colony formation and their relief upon culture adaptation. Stem Cell Rep 3:142–155
The International Stem Cell Banking Initiative (2015) Points to consider in the development of seed stocks of pluripotent stem cells for clinical applications: International Stem Cell Banking Initiative (ISCBI). Regen Med 10:1–44
Eliceiri KW et al (2012) Biological imaging software tools. Nat Methods 9:697–710
Chieco P, Jonker A, De Boer BA, Ruijter JM, Van Noorden CJ (2013) Image cytometry: protocols for 2D and 3D quantification in microscopic images. Prog Histochem Cytochem 47:211–333
O’Connor MD et al (2008) Alkaline phosphatase-positive colony formation is a sensitive, specific, and quantitative indicator of undifferentiated human embryonic stem cells. Stem Cells 26:1109–1116
Moralli D et al (2011) An improved technique for chromosomal analysis of human ES and iPS cells. Stem Cell Rev 7:471–477
Anguiano A et al (2012) Spectral Karyotyping for identification of constitutional chromosomal abnormalities at a national reference laboratory. Mol Cytogenet 5:3
Das K, Tan P (2013) Molecular cytogenetics: recent developments and applications in cancer. Clin Genet 84:315–325
The International Stem Cell Initiative (2018) Assessment of established techniques to determine developmental and malignant potential of human pluripotent stem cells. Nat Commun 9:1925
Tsankov AM et al (2015) A qPCR ScoreCard quantifies the differentiation potential of human pluripotent stem cells. Nat Biotechnol 33:1182
Bock C et al (2011) Reference Maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines. Cell 144:439–452
Ng ES, Davis RP, Azzola L, Stanley EG, Elefanty AG (2005) Forced aggregation of defined numbers of human embryonic stem cells into embryoid bodies fosters robust, reproducible hematopoietic differentiation. Blood 106:1601–1603
Coecke S et al (2005) Guidance on good cell culture practice. a report of the second ECVAM task force on good cell culture practice. Altern Lab Anim 33:261–287
OECD. Draft Guidance Document on Good In Vitro Method Practices (GIVIMP) for the Development and Implementation of In Vitro Methods for Regulatory Use in Human Safety Assessment, 2018
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Hayashi, Y., Ohnuma, K., Furue, M.K. (2019). Pluripotent Stem Cell Heterogeneity. In: Birbrair, A. (eds) Stem Cells Heterogeneity - Novel Concepts. Advances in Experimental Medicine and Biology, vol 1123. Springer, Cham. https://doi.org/10.1007/978-3-030-11096-3_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-11096-3_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-11095-6
Online ISBN: 978-3-030-11096-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)