Abstract
Endophytic fungi are abundant and have been reported from all tissues such as roots, stems, leaves, flowers, and fruits. In recent years, research into the beneficial use of endophytic fungi has increased worldwide. In this chapter, we critically review the production of a wide range of secondary metabolites, bioactive compounds from fungal endophytes that are a potential alternative source of secondary plant metabolites and natural producers of high-demand drugs. One of the major areas in endophytic research that holds both economic and environmental potential is bioremediation. During their life span, microbes adapt fast to environmental pollutants and remediate their surrounding microenvironment. In the last two decades, bioremediation has arisen as a suitable alternative for remediating large polluted sites. Endophytic fungi producing ligninolytic enzymes have possible biotechnological applications in lignocellulosic biorefineries. This chapter highlights the recent progress that has been made in screening endophytic fungi for the production and commercialization of certain biologically active compounds of fungal endophytic origin.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Anticancerous molecule
- Bioactive compounds
- Biofertilizers
- Bioremediation
- Endophytic fungi
- Lignocellulosic biorefineries
- Secondary metabolites
1.1 Introduction
Microbes such as fungi, bacteria, cyanobacteria, and actinomycetes belonging to a class of plant symbionts residing within plant tissue are referred to as “endophytes” (De Bary 1866). From the germination of seeds to the development of fruits, endophytic microorganisms are associated with different parts of the plant, such as the spermosphere (in seeds), rhizosphere (roots), caulosphere (in stems), phylloplane (in leaves), anthosphere (in flowers), and laimosphere and carposphere (in fruits) (Clay and Holah 1999). To adapt to abiotic and biotic stress factors , endophytic microbes produce bioactive substances (Guo et al. 2008). The associations of endophytic microbes with plants, and in many cases their tolerance to biotic stress factors, have correlated with fungal natural products or biologically active metabolites, such as enzymes, phytohormones, nutrients, and minerals, and also enhance the resistance of the host against herbivores, insects, disease, drought, phytopathogens, and variations in temperature and salinity (Breen 1994; Brem and Leuchtmann 2001; Schulz et al. 2002). Endophytic microbes enhance the resistance of plants to abiotic stress factors such as increasing drought tolerance, high temperature, low temperature, low pH, high salinity, and the presence of heavy metals in the soil (Jalgaonwala et al. 2017). On the other hand, plants provide a protective environment for the growth and multiplication of endophytic microbes, protection from aridness, and longevity via seed transmission to the next generation of host (Khan et al. 2015). One widespread phenomenon in nature is the symbiotic association between fungus and plant.
Initial information about fungal endophytes was found during the year 1904, from endophytes isolated from the seeds of darnel ryegrass (Bezerra et al. 2012; Freeman 1904). Endophytic fungi are a diverse and useful group of microorganisms reported to colonize plants in different parts of world, such as the Arctic (Fisher et al. 1995) and Antarctic (Rosa et al. 2009), and in geothermal lands (Redman et al. 2002), deserts (Bashyal et al. 2005), oceans (Wang et al. 2006b), rainforests (Strobel 2002), mangrove swamps (Lin et al. 2008b), and coastal forests (Suryanarayanan et al. 2005). Various secondary metabolites , for instance, alkaloids, cyclohexanes, flavonoids, hydrocarbons, quinines, and terpenes, have been reported to be synthesized by fungal endophytes and have various biological properties including antimicrobial, antioxidant, antidiabetic, anticancer, antihypercholesterolemic, and antiproliferative activities and cytotoxicity, and they are used in biofuel manufacturing (Fernandes et al. 2015; Naik and Krishnamurthy 2010; Ruma et al. 2013). Endophytic fungi produce various kinds of extracellular enzymes, i.e., hydrolases, lyases, oxidoreductases, and (Traving et al. 2015). In another study, endophytic microbes producing enzymes could help to initiate the symbiotic process (Hallmann et al. 1997). Fungal endophytes have been reported to produce hydrolytic enzymes such as cellulase, lipoidase, pectinase, proteinase, and phenol oxidase so as to overcome the defense response against the host (Krishnamurthy and Naik 2017; Naik et al. 2009; Oses et al. 2006). Various organic compounds, for instance, cellulose, glucose, hemicelluloses, keratin, lignin, lipids, oligosaccharides, pectin, and proteins, have been reported to be degraded by the endophytic fungi (Kudanga and Mwenje 2005; Tomita 2003). Endophytic microbes have been reported in almost all plant studies (Suman et al. 2016; Verma et al. 2013, 2014a, 2015a). This chapter describes the biodiversity of endophytic fungi from diverse plants, producing wide groups of extracellular hydrolytic enzymes, bioactive compounds, and secondary metabolites useful for plant growth and soil health for sustainable agriculture, for environment bioremediation , and for different processes in industry.
1.2 Biodiversity and Distribution of Fungal Endophytes
Recently, a greater progress has been made in fungal endophytic research. Fungal endophytes have been found to colonize land plants everywhere on earth. They have been isolated from boreal forests, tropical climates, diverse xeric environments, extreme arctic environments, ferns, gymnosperms , and angiosperms (Mohali et al. 2005; Selim et al. 2017; Šraj-Kržič et al. 2006; Suryanarayanan et al. 2000). Endophytic fungi play an important role in protecting their host from attack by phytopathogens and also facilitate the solubilization of the macronutrients phosphorus, potassium, and zinc; the fixation of atmospheric nitrogen; and the production of various hydrolytic enzymes, ammonia, siderophore, and hydrogen cyanide (HCN) (Maheshwari 2011; Rana et al. 2016a, b, 2017; Verma et al. 2015b, c, 2016a, b).
From a review of the diverse research on endophytic fungi diversity, it can be concluded that reported fungi belong to diverse phyla including Ascomycota, Basidiomycota, and Mucoromycota (Fig. 1.1a). Figure 1.1b presents the biodiversity and abundance of endophytic fungi reported from chick pea, common pea, maize, pigeon pea, rice, soybean, tomato, and wheat. Figure 1.1c presents the relative distribution and biodiversity of endophytic fungi reported from different host plants, showing the common and host-specific endophytic fungi. Figure 1.1d is a Venn diagram showing the endophytic fungal diversity of leguminous and nonleguminous crops. There are many reports of the microbiomes as niche-specific diversity caused by diverse environmental conditions, including low temperature (Yadav 2015; Yadav et al. 2015a, b, 2016, 2017c), high temperature (Kumar et al. 2014; Sahay et al. 2017), salinity (Yadav et al. 2015c, 2018a), drought (Verma et al. 2014a, 2016b), pH (Verma et al. 2013), and multiple extreme conditions (Saxena et al. 2016; Verma et al. 2017; Yadav et al. 2015c, 2018b). Suman et al. (2016) reported niche-specific endophytic microbes from 17 different host plants. Table 1.1 presents the biodiversity of endophytic fungi reported from these diverse host plants.
(a) Phylogenetic tree shows the relationship among different groups of endophytic fungi isolated from different host plants . (b) Abundance of endophytic fungi belonging to diverse phyla isolated from different host plants . (c) Diversity and distribution of endophytic fungi of different crops. (d) Venn diagram showing niche-specific microbes reported from leguminous and nonleguminous crops . Wheat (Triticum aestivum): (Colla et al. 2015; Comby et al. 2017; Fisher and Petrini 1992; Keyser et al. 2016; Köhl et al. 2015; Larran et al. 2002, 2007, 2018; Ofek-Lalzar et al. 2016; Sieber et al. 1988; Spagnoletti et al. 2017; Wakelin et al. 2004); rice (Oryza sativa): (Naik et al. 2009; Fig. 1.1 (continued) Potshangbam et al. 2017; Tian et al. 2004; Wang et al. 2016; Yuan et al. 2010); tomato (Solanum lycopersicum): (Bogner et al. 2016; Chadha et al. 2015; Larran et al. 2001; Tian et al. 2014); maize (Zea mays): (Amin 2013; Köhl et al. 2015; Nassar et al. 2005; Pan et al. 2008; Potshangbam et al. 2017; Renuka and Ramanujam 2016; Saunders and Kohn 2008; Xing et al. 2018); chickpea (Cicer arietinum): (Narayan et al. 2017; Singh and Gaur 2017); soybean (Glycine max): (de Souza Leite et al. 2013; Fernandes et al. 2015; Hamayun et al. 2017; Impullitti and Malvick 2013; Khan et al. 2011b, 2012b; Rothen et al. 2017; Tenguria and Firodiya 2013; Yang et al. 2014, 2018; Zhao et al. 2018); common bean (Phaseolus vulgaris): (dos Santos et al. 2016; Gonzaga et al. 2015; Marcenaro and Valkonen 2016; Parsa et al. 2016; Pierre et al. 2016); pigeon pea (Cajanus cajan): (Gao et al. 2011, 2012; Zhao et al. 2012, 2013, 2014)
Impullitti and Malvick (2013) reported fungal endophytes such as Alternaria sp., Cladosporium sp., Davidella sp., Diaporthe sp., Epicoccum sp., Fusarium sp., Phialophora sp., Phoma sp., Phomopsis sp., Plectosphaerella sp., Trichoderma sp., and Verticillium sp. in soybean plants; these were found by using culture-dependent and culture-independent methods. Tenguria and Firodiya (2013) isolated endophytic fungi, including Acremonium sp., Alternaria alternate, Aspergillus sp., Colletotrichum sp., Emericella nidulans, Fusarium sp., Penicillium sp., and Phoma sp. from leaves of fresh Glycine max collected from the central region of Madhya Pradesh, India. Fernandes et al. (2015) reported the diversity of fungal endophytes in the leaves and roots of G. max (dos Santos Souza and dos Santos 2017). In that study, Ampelomyces sp., Cladosporium cladosporioides, Colletotrichum gloeosporioides, Diaporthe helianthi, Guignardia mangiferae, and Phoma sp. were isolated from the leaves, and the dominance of Fusarium oxysporum, Fusarium solani, and Fusarium sp. was greater in the roots (Fernandes et al. 2015). Hamayun et al. (2017) reported Porostereum spadiceum AGH786 as a novel gibberellin (GA)-synthesizing fungal endophyte that promoted the growth of soybeans and was capable of producing six types of GAs (Onofre et al. 2013).
Larran et al. (2007) isolated Alternaria alternata, Cladosporium herbarum, Epicoccum nigrum, Cryptococcus sp., Rhodotorula rubra, Penicillium sp., and Fusarium graminearum with the highest colonization frequency from wheat (dos Santos Souza and dos Santos 2017). Amin (2013) isolated Acremonium sp., Aspergillus sp., Botryodiplodia sp., Fusarium sp., Penicillium sp., and Trichoderma sp. from the roots of Zea mays (Azevedo et al. 2000). Chadha et al. (2015) isolated endophytic fungi identified as Aspergillus niger, Aspergillus sp., A. versicolor, Chaetomium globosum, Fusarium fusarioides, F. moniliforme, F. oxysporum, F. semitectum, F. solani, Mucor hiemalis, Mucor sp., and Trichoderma pseudokoningii from the roots of tomato, and further screened for different plant growth-promoting attributes. All the isolates showed that they were capable of solubilizing phosphorus, 7 showed siderophore production, 4produced HCN, and 3 produced ammonia. The production of indole acetic acid (IAA) was found to be highest in Fusarium fusarioides. Renuka and Ramanujam (2016) determined Acremonium zeae, Coprinopsis cinerea, Fusarium fujikuroi, Gibberella moniliformis, Nemania sp., Penicillium sp., Cladosporium oxysporum, Rigidoporus vinctus, Colletotrichum boninense, Sarocladium zeae, Epicoccum sorghinum, Curvularia lunata, Scopulariopsis gracilis, and Colletotrichum gloeosporioides from the leaf, stem, and root fragments of different varieties of maize. Wang et al. (2016) isolated endophytic fungal and bacterial strains from sprouts, stems, and roots simultaneously in rice plants . Aspergillus, Cryptococcus, Eurotium, Fusarium, Penicillium, Septoria, and Wallemia were the most frequently detected genera in rice plants. The dominant fungal genera, including Aspergillus, Penicillium, and Trichosporon, coexisted in the stems and roots. Furthermore, Cryptococcus, Fusarium, Penicillium, Pestalotiopsis, and Verticillium were detected in the sprouts, stems, and roots simultaneously. Xing et al. (2018) isolated Alternaria alternata, Aspergillus flavus, A. niger, Bipolaris zeicola, Chaetomium murorum, Cladosporium sphaerospermum, Fusarium proliferatum, F. verticillioides, Penicillium aurantiogriseum, P. oxalicum, P. polonicum, Sarocladium zeae, and Trichoderma gamsii from maize seeds.
1.3 Biotechnological Applications of Endophytic Fungi
Over the past several decades, endophytic fungi separated from numerous plant sources have been recognized as valuable sources of natural products for agronomy, industry, and biomedical development, and also produce extracellular hydrolase enzymes, such as pectinases, cellulases, lipases, amylases, laccases, xylanase, and proteases, as one of the resistance mechanisms against pathogenic organisms and for gaining nutrition from the host. From medicinal plants, endophytic fungi synthesizing hydrolytic enzymes have been reported (Khan et al. 2017; Saxena et al. 2015a; Sunitha et al. 2013; Yadav et al. 2012). Extracellular enzymes target various macromolecules , e.g., lignin, proteins, carbohydrates, sugar-based polymers, to break them down into simpler ones. The production of extracellular enzymes has been measured qualitatively and quantitatively, from using agar plate-based to applying advanced spectrophotometric methods (Yadav et al. 2017a, b).
1.3.1 Bioresources of Hydrolytic Enzymes
Endophytic microorganisms are well known, as they spend the whole of their life cycle inhabiting the inside of tissues in host plants without causing them any obvious harm (Bezerra et al. 2012; Kaul et al. 2013; Tan and Zou 2001; Yadav et al. 2016). The endophytic microbes guard their host plants against attack by other microbes, insects, and herbivore animals, furthermore providing other benefits, for instance, the production of numerous plant growth regulators, enzymes, and other chemical compounds (Azevedo et al. 2000; Bezerra et al. 2012). In addition, these endophytic microbes have also been reported to produce diverse metabolites, including alkaloids, flavonoids, isocoumarin derivatives, peptides, phenolic acids, phenols, quinones, steroids, and terpenoids (Rana et al. 2016b; Yadav et al. 2015). In recent times, fungal endophytes have become responsive, as they are an appropriate reserve for the degradation of polycyclic aromatic hydrocarbons , which are well known as a toxic class of environmental contaminants (Bezerra et al. 2012; Dai et al. 2010). Additionally, endophytes are also known for the production of various extracellular enzymes, such as cellulases, esterases, lipases, pectinases, proteases, and xylanases, which play an important role in protecting themselves from the defense response of the host or in attaining nourishment from the soil (Bezerra et al. 2012; Suto et al. 2002). Therefore, endophytes are an enormous source of naturally active products that are of marked significance to the agricultural, industrial, and medical sectors (Hazalin et al. 2012). The major industries that utilize microbial enzymes include biomaterials, cellulose, cosmetics, detergents, energy, fine chemicals, food, leather, paper, pharmaceuticals, and textiles, (Bezerra et al. 2012; Suto et al. 2002; Yadav et al. 2015). Table 1.2 shows the diversity and abundance of diverse extracellular hydrolytic enzyme production by different groups of endophytic fungi reported from diverse host plants worldwide.
1.3.1.1 Cellulases
Cellulases are basically the enzymes that catalyze cellulolysis, which involves the degradation of the cellulose and certain related polysaccharides. Certain bacteria, fungi, and protozoans are known to synthesize the enzyme (Singh 2006). Different types of cellulases are known that differ from each other structurally and mechanistically, and these include endocellulases, exocellulases, also known as cellobiohydrolases, cellobiases or beta-glucosidases, oxidative cellulases, cellulose phosphorylases. Cellulases from microbes find diverse applications such as use with a supplement of hemicellulases, pectinases, ligninases, and associated enzymes (Adav and Sze 2014). In addition to lignocellulosic bioenergy, cellulase are important in the agricultural, animal feed, brewing, food, laundry, paper and pulp, textile, and wine industries (Adav and Sze 2014; Bhat and Bhat 1997; Mandels 1985; Ryu and Mandels 1980). The most commonly studied cellulolytic fungi include the species of Aspergillus, Humicola, Penicillium, and Trichoderma (Sukumaran et al. 2005).
Peng and Chen (2007), obtained 141 isolates of fungal endophytes from the stems of seven oleaginous plant species. These isolates belonged to genera including Cephalosporium, Microsphaeropsis, Nigrospora, Phomopsis, and Sclerocystis. The oil content of these isolates ranged from 21.3% to 35.0% of dry cell weight. Further, the strains also produced cellulase in addition to microbial oil when cultured on solid-state medium consisting of steam-exploded wheat straw, wheat bran, and water. The yield of cellulase ranged from 0.31 to 0.69 filter paper unit per gram of initial dry substrate. Bezerra et al. (2012) isolated 44 isolates of fungal endophytes from Opuntia ficus-indica and assessed their ability to synthesize hydrolytic enzymes such as cellulases, pectinases, proteases, and xylanases. The cellulase producers were identified as Acremonium terricola, Aspergillus japonicas, Cladosporium cladosporioides, Fusarium lateritium, Nigrospora sphaerica, Penicillium aurantiogriseum, P. glandicola, Pestalotiopsis guepini, and Xylaria sp.
Cabezas et al. (2012) isolated 100 fungal endophytes from Espeletia sp. and estimated their cellulolytic potential. The research showed that only four isolates could synthesize cellulases, of which Penicillium glabrum displayed the highest cellulolytic activity, with the highest CMCase, exoglucanase, and β-glucosidase enzyme activities of 44.5 U/ml, 48.3 U/ml, and 0.45 U/ml respectively. Syed et al. (2013) identified the endophytic fungus Penicillium sp. CPF2 (NFCCI 2862). Different substrates were assessed for optimal synthesis of cellulase by CPF2. The best activities for FPase (1.2 IU/ml), endocellulase (19 IU/ml), xylanase (40 IU/ml), and β-glucosidase (2.8 IU/ml) with a protein content of 0.86 mg/ml were detected when cellulose (1.5 % w/v) was used in association with peptone (0.2 % w/v) in the growth medium. Optimal temperature and pH for the extracellular cellulase production were 28 °C and 5.5 °C respectively. Onofre et al. (2013) evaluated the production of cellulases by endophytic fungi, Fusarium oxysporum isolated from Baccharis dracunculifolia. The results showed that after 55 days of fermentation, the maximum peak of enzyme production with a yield of 55.21 ± 10.54 IU/g of fermented substrate was at pH 5.96.
Patil et al. (2015) screened Aspergillus sp., Bisporus sp., Chaetomium sp., Cladosporium sp., Colletotrichum sp., Curvularia sp., Fusarium sp., and Rhizoctonia sp., isolated from seven medicinal plants and screened both qualitatively and quantitatively for the synthesis of hydrolytic extracellular enzymes, such as amylases, cellulases, lipases, and proteases. The study revealed that Aspergillus sp., Bisporus sp., Cladosporium sp., and Colletotrichum sp. showed cellulase production qualitatively, whereas quantitatively, Rhizoctonia sp. produced maximum cellulase of about 0.3 U/ml. However, other isolates, including Bisporus sp., Chaetomium sp., and Fusarium sp., exhibited moderate to low activity. Toghueo et al. (2017) reported the fungal endophytes from Cameroonian medicinal plants and screened for their extracellular cellulase activity. The two assays, enzyme and plate-clearing, were used for the screening of effective cellulolytic fungal endophytes. Penicillium sp., and P. chermesimum were the most effective producers.
1.3.1.2 Xylanase
Xylanases are glycosidases comprising endo-1,4-b-xylanaseand β-xylosidase and catalyzing the endohydrolysis of 1,4-b- D-xylosidic linkages in xylan (Collins et al. 2005; Thomas et al. 2017). These enzymes basically cause the hydrolysis of the xylan present in the hemicelluloses of plants and convert them into monomeric sugars; this function is not performed alone, but rather with the assistance of certain other hydrolytic enzymes, for instance, acetyl xylan esterase, α-L-arabinofuranosidase, α-glucuronidase, and phenolic acid, including ferulic and p-coumaric acid esterase (Collins et al. 2005; Thomas et al. 2017). The chief substrate of xylanases is xylan, which is the key structural polysaccharide of plant cells and the second most abundant polysaccharide in nature, accounting for approximately one third of all renewable organic carbon on earth (Collins et al. 2005; Prade 1996). Xylanases possess numerous applications in the food, de-inking, biofuels, baking, animal feed, and paper and pulp industries (Kumar et al. 2017a; Polizeli et al. 2005; Singh et al. 2016; Suman et al. 2015; Thomas et al. 2017). In the baking industry, xylanases improve the strength of the gluten and ultimately the superiority of the bread as they are capable of absorbing water and collaborating with gluten (Butt et al. 2008; Gray and Bemiller 2003; Harris and Ramalingam 2010; Nuyens et al. 2001). Xylanases are also used with other enzymes to improve the yield of juices from fruit and vegetables; the firmness of fruit pulp; and the regaining of aromas, edible dyes, essential oils, hydrolysis substances, mineral salts, etc. (Polizeli et al. 2005). These enzymes have been repoprted from different microorganisms such as algae, arthropods, bacteria, fungi, gastropods, and protozoa (Collins et al. 2005).
Wipusaree et al. (2011) isolated 54 endophytic fungi from the Thai medicinal plant, Croton oblongifolius Roxb, and screened the isolates for xylanase production. In primary screening, xylanase activity was found in 30 isolates by growing them on solid xylan agar plates. After secondary screening for xylanase activity in xylan liquid culture, the isolate with the highest xylanase production, identified as Alternaria alternata, was selected for further evaluation. The study revealed this xylanase to be monomeric, possessing molecular weight of 54.8 kDa. It showed a broadly similar substrate affinity to other xylanases, with a Km of 2.37 mg/ml, and was thermostable up to 40 °C. The enzyme was also shown to be inhibited to some extent by all tested divalent metal cations, but especially by Hg2+ and Cu2+. Sorgatto et al. (2012) characterized xylanase synthesized by the endophytic fungus Aspergillus terreus, isolated from Memora peregrine. The research revealed an optimal temperature of 55 °C and a pH value of 4.5. The enzyme was thermotolerant at 45 °C and 50 °C, with a half-life of 55 and 36 min respectively. Tasia and Melliawati (2017) found Acremonium sp. and a member of the class Coelomycetes to be xylanase producers. The study by Marques et al. (2018) also reported Acremonium sp., Botryosphaeria sp., Chaetomium sp., Cladosporium cladosporioides, Colletotrichum crassipes, Coniella petrakii, Coniothyrium minitans , Myrothecium gramineum, Paecilomyces sp., Phomopsis stipata, Saccharicola sp., Trichoderma viridae, and Ustilaginoidea sp. to be xylanase producers.
1.3.1.3 Lipase
Lipases belong to serine hydrolases and do not require any cofactors. They are involved in diverse conversion reactions, such as transesterification, inter esterification, esterification, aminolysis, alcoholysis, and acidolysis (Gopinath et al. 2013; Panjiar et al. 2017; Savitha et al. 2007; Yadav et al. 2017a). Triacylglycerol acyl hydrolases are lipases that are involved in the hydrolysis of fats and oils (Gopinath et al. 2013; Singh and Mukhopadhyay 2012). Lipases are of great importance to the food industry. Phospholipases are being used in treating egg yolk, which is useful for the processing of baby foods, custard, dressings, and mayonnaise; for dough preparation; and for sauces, such as Hollandaise, Béarnaise, and Café de Paris (Aravindan et al. 2007; Reimerdes et al. 2004). Lipase-modified butter fat has extensive applications in different food processes (Aravindan et al. 2007; Uhlig 1998). Chocolates with cocoa butter substitutes, bread, structured lipids such as human milk fat replacers, low calorie health oils, and nutraceuticals are some of lipase-mediated food products available (Aravindan et al. 2007). The addition of lipases to noodles results in appreciably softer textural characteristics (Undurraga et al. 2001). Furthermore, lipases are also used to increase the flavor content of bakery products (Ray 2012).
Lipases are produced by bacteria, yeasts, protozoans, molds, and even viruses are known to encode genes for lipases (Abrunhosa et al. 2013; Anbu et al. 2011; Ginalska et al. 2004). The production of lipases has been demonstrated in ascomycetes and coelomycetes (Gopinath et al. 2013). Lipolytic activity has been shown in Rhizopus sp., Penicillium sp., Mucor sp., Lipomyces starkeyi, Humicola lanuginose, Cunninghamella verticillata, Candida rugosa, Acremonium strictum, and Aspergillus sp. (Tsujisaka et al. 1973; Jacobsen et al. 1990; Petrović et al. 1990; Sztajer and Maliszewska 1989). Microbial lipases are of commercial importance because of the broader availability, greater stability, and low production costs compared with plant and animal lipases.
Torres et al. (2003) rendered a mycelium-bound lipase from Rhizopus oryzae that catalyzed the esterification of fatty acids in iso-octane. The enzyme was active over the entire pH range studied, from pH 3 to pH 8, but maximal activity was obtained at pH 4 and pH 7. The study by Costa-Silva et al. (2011) deals with improvement in the production and stabilization of lipases from the endophytic fungi Cercospora kikuchii isolated from Tithonia diversifolia. Amirita et al. (2012) reported Colletotrichum falcatum, Curvularia brachyspora, Curvularia vermiformis, Drechslera hawaiiensis, and Phyllosticta sp. to be producers of lipase enzymes from different medicinal plants. Panuthai et al. (2012) screened 65 endophytic fungal isolates for the production of lipases, of which only 10 were found to produce extracellular lipases, with Fusarium oxysporum, isolated from the leaves of Croton oblongifolius Roxb. (Plao yai), yielding the highest level. The enriched lipase showed optimal activity at 30 °C and pH 8, and was reasonably stable up to 40 °C and at a pH of 8.0–12. Venkatesagowda et al. (2012) isolated species of Trichoderma, Stachybotrys, Sclerotinia, Rhizopus, Phyllosticta, Phomopsis, Phoma, Pestalotiopsis, Penicillium, Mucor, Lasiodiplodia, Fusarium, Drechslera, Curvularia, Colletotrichum, Cladosporium, Chalaropsis, Aspergillus, and Alternaria, showing strong lipolytic activity. Sunitha et al. (2013) isolated lipase-producing Acremonium implicatum, Alternaria sp., Aspergillus niger, Chaetomium sp., Colletotrichum falcatum, C. gleosporoides, C. truncatum, Cylindrocephalum sp., Drechslera sp., Fusarium oxysporum, Isaria sp., Mycelia streilia sp., Penicillium sp., Pestalotiopsis sp., Phoma sp., Phomopsis longicolla, and Xylaria sp. from Alpinia calcarata, Bixa orellana, Calophyllum inophyllum, and Catharanthus roseus. Fareed et al. (2017) revealed Aspergillus calidoustus, A. fumigates, Microsporum gypseum , Penicillium marneffei, P. viridicatum, and Trichophyton tonsurans to be lipase producers.
1.3.1.4 β-glucosidase
Periconia sp. produce a thermotolerant β-glucosidase . This enzyme shows high activity toward cellobiose and carboxymethylcellulose. β-glucosidase hydrolyzes rice straw into simple sugars. Hydrolytic enzymes have the potential to convert lignocellulosic biomass to biofuels and chemicals (Harnpicharnchai et al. 2009). The major decomposers of lignocelluloses are fungi, which play an essential role in the cycling of carbon and other nutrients. Exo- and endoglucanases, exo- and endoxylanases, β-xylosidases, and β-glycosidase are the main hydrolytic enzymes involved in the degradation of lignocelluloses (Van Dyk and Pletschke 2012).
1.3.1.5 Tannases
Tannases comprise two classes of enzymes, tannin acyl hydrolases and ellagitannin acyl hydrolases, also called ellagitannases. Tannin acyl hydrolases are used in the beverage, food, leather, and pharmaceutical industries (González et al. 2017). Vegetable and animal tissues are easily available sources of tannases; however, on an industrial scale, microbial sources are preferred. Tannases have been obtained from fungi, including Aspergillus sp., Paecilomyces variotii, and Penicillium sp. (Battestin and Macedo 2007; González et al. 2017). There are some reports of tannase production by endophytic fungi. Cavalcanti et al. (2017) isolated 16 endophytic fungal strains and screened them for the production of tannases. All the isolates produced tannases, with Aspergillus fumigatus and A. niger being the highest producers. The study revealed that the optimal temperature and pH of enzymes from the two strains were 30 °C and 4.0 respectively.
1.3.1.6 Pectinases
Pectinase is an enzyme that actually breaks down pectin, which is a polysaccharide found in plant cell walls. This enzyme has shown a robust rise on the market and has also held a leading position amongst commercially produced industrial enzymes (Garg et al. 2016). In the industrial sector, this enzyme plays an important role in decreasing viscosity and improving yield (Garg et al. 2016; Makky and Yusoff 2015). In the processing of citrus juice, the enzyme helps to eliminate the cloudiness of the juice and stabilize it (Braddock 1981; Garg et al. 2016).
In wine processing, pectinases are used to promote filtration, increase the juice yield, and strengthen the flavor and color (Chaudhri and Suneetha 2012; Garg et al. 2016). Additionally, in biorefineries, pectinases used to hydrolyze pectin are present in agro-industrial waste (Biz et al. 2014; Garg et al. 2016). The agro-waste is converted into simple sugars and bioethanol, or could also be used as fermentable sugars (Alshammari et al. 2011; Garg et al. 2016). The fermentation of tea can be speeded up by breaking down the pectin present in the cell walls of tea leaves (Garg et al. 2016). Further, pectinases are used in textile processing, the extraction of vegetable oil, the processing of animal feed, the biobleaching of kraft pulp, and the recycling of wastepaper (Garg et al. 2016). The most important sources of pectinases include bacteria, fungi, and plants, and recently microbial pectinases have been gaining a lot of attention.
Sunitha et al. (2013) reported Acremonium implicatum, Aspergillus fumigatus, Colletotrichum gleosporoides, Coniothyrium sp., Cylindrocephalum sp., Drechslera sp., Fusarium chlamydosporum, F. oxysporum, Fusicoccum sp., Nigrospora sphaerica, Paecilomyces variotii, Pestalotiopsis disseminata, Phoma sp., Pyllosticta sp., Talaromyces emersonii, and Xylaria sp. to be pectinase producers isolated from Alpinia calcarata, Bixa orellana, Calophyllum inophyllum, and Catharanthus roseus. Fouda et al. (2015) isolated pectinase producers, including Alternaria alternata, Penicillium chrysogenum, and the third fungal strain, described as sterile hyphae from the medicinal plant of Asclepias sinaica. Heidarizadeh et al. (2018) produced pectinases from Piriformospora indica. After 6 days, the maximum dry cell weight was 10.21 g/L, the growth yield was about 0.65 g/g, the specific growth rate 0.56 day−1, and pectinase activity was found to be 10.47 U/mL on pectin-containing medium (P+). In another case of pectin-free medium (P−), all parameters were kept lower than for P+ medium. It was found in the study that the synthesis of pectinase on P+ was 2.7 times greater than on the P− medium (Maheshwari 2011). About 5 and 50 °C are the ultimate pH and temperature required for polygalacturonase activity respectively (Kirti and Reddy 2013; Singh 2006). Indeed, this is the leading note of synthesis of pectinase by Piriformospora indica; the optimal pH of enzyme was additionally submitted and noted as a would-be contender for imminent use in the fruit juice industries (Bezerra et al. 2012; Mercado-Blanco et al. 2016). Uzma et al. (2016) reported Aspergillus sp., Cladosporium sp., Colletotrichum sp., Fusarium sp., Mucor sp., Mycelia sterilia, Penicillium sp., Phoma sp., Phomopsis sp., and Rhizopus sp. and found that these fungal species exhibited pectinase production attributes (Kaul et al. 2013; Kirti and Reddy 2013).
1.3.1.7 Phytases
Phytases , or myoinositol hexakisphosphate phosphohydrolase, are phytate-degrading enzymes. Phytases catalyze the hydrolysis of phytic acid to inositol phosphates, myoinositol, and inorganic phosphate (Gontia-Mishra and Tiwari 2013; Kaur et al. 2017; Kumar et al. 2016, 2017b; Mitchell et al. 1997). Phytases have been gaining a lot of interest and have become a center of focus for scientists and entrepreneurs in the fields of nutrition, environmental protection, and biotechnology (Yadav 2018; Yadav et al. 2017b, d). In plants, these enzymes are usually expressed during seed germination, bring about the degradation of the phytate, provide the growing seedling with orthophosphate, and lower inositol polyphosphates, free myoinositol, and previously bound cations, including Ca2+, K+, Mg2+, and Zn2+, and hence provide nutrition for plant growth (Gontia-Mishra and Tiwari 2013; Reddy et al. 1989). In animals, phytases play a role in the maintenance of the cell's metabolic reservoirs of inositol hexaphosphate and other inositol polyphosphates. Phytases have many applications. The activity of some yeasts and fungi is generally regarded as safe for consumption by humans and animals, for example, Saccharomyces cerevisiae (Gontia-Mishra and Tiwari 2013; Nayini 1984) could be used as a probiotic in a range of food formulations to improve the utilization of phosphate. Phytases can also be utilized in bakery products, especially in the bread-making process (Gontia-Mishra and Tiwari 2013; Haros et al. 2001). The addition of phytase is known to reduce the phytate content in dough and shorten the fermentation time. Further, it improves the bread shape, volume, and softness of the crumb. More phytases are also added in the fractionation of cereal bran, the absorption of iron, and in animal nutrition. In fact, numerous microbial phytases are already on the market and expansively used as animal feed supplements, for instance, phytase from Aspergillus ficuum as Natuphos, A. niger as Allzyme, A. awamori as Finase and Avizyme, A. oryzae as AMAFERM, SP, SF, TP, and Phyzyme, and Peniophora lycii as Ronozyme, Roxazyme, and Bio-Feed phytase (Gontia-Mishra and Tiwari 2013). Additionally, phytases are utilized in feed for fish, poultry, and pigs, as biofertilizers , in paper manufacturing, and in the wet milling of maize (Gontia-Mishra and Tiwari 2013). Although phytases have been described in plants, animals, and in a range of bacteria, filamentous fungi, and yeasts, here we concentrate primarily on those from endophytic fungi (Venugopalan and Srivastava 2015).
Marlida et al. (2010) obtained 34 isolates of fungal endophytes and screened them for phytase synthesis. Renuka and Ramanujam (2016) reported that phytase production could be achieved only in Fusarium verticillioides and Rhizoctonia sp., which were also best induced by phytic acid and rice bran compared with other inducers in the submerged fermentation medium used. The phytases produced by Fusarium verticillioides and Rhizoctonia sp. showed optimal pH of 5.0 and 4.0 respectively. Phytase from F. verticillioides showed an optimal temperature of 50 °C and stability up to 60 °C, optimal pH at 5.0 and pH stability at 2.5–6.0. Mehdipour-Moghaddam et al. (2010) isolated Azospirillum strains from rice and wheat and screened the strains for cellulase, pectinase, and phytase activity. The study revealed that the Azospirillum strain isolated from rice showed considerably greater phytase activity than that isolated from wheat. In fact, to our knowledge, this is the first study to report phytase activity and its zymogram for Azospirillum with different activity profiles exhibited by various isolates.
1.3.1.8 Ligninolytic Enzymes
White rot fungi are the most efficient ligninolytic organisms described to date. Owing to the extracellular nonspecific and nonstereoselective enzyme system in white rot fungi, the ability to degrade lignin is more efficient (Barr and Aust 1994). Recently, some microorganisms isolated from the hardwood forests of Zimbabwe (Tekere et al. 2001), Tunisia (Dhouib et al. 2005), Spain (Barrasa et al. 2009), Northern China (Sun et al. 2011a), and Norway (Kim et al. 2015) have been reported in the production of ligninolytic enzymes. For the study of lignin-degrading enzymes in endophytes, different substrates such as ABTS (2,2’-azinobis-3-ethylbenzothiazoline-6-sulfonic acid), naphthol, and Poly R-478 have been isolated from living plants (Fillat et al. 2016; Oses et al. 2006; Sun et al. 2011a). From tree species Drimys winteri and Prumnopitys andina, endophytic fungi producing lignocellulolytic enzymes have been isolated. In D. winteri, Bjerkandera sp. and Mycelia sterilia (Dw-2) were identified, whereas in P. andina, an unidentified basidiomycete (Pa-1) and also M. sterilia (Pa-2) were recognized (Oses et al. 2006). Rodriguez et al. (2009) reported in the forest region that a basidiomycete and a deuteromycete use a combination of enzymes, 1,4-b-D-glucan cellobiohydrolases , endo-1,4-b-D-glucanases, and 1,4-b-D-glucosidase, which break glycoside linkages between B-D-xylopyranosyl and glucopyranosyl residues, thus promoting the biodegradation of wood.
The endophytic community of Acer truncatum, the main woody tree species of northern Chinese forests, was investigated, with 17 isolates belonging to the taxa Alternaria alternata, A. arborescens, Ascochytopsis vignae, Coniothyrium olivaceum, Diaporthe sp. 2, Drechslera biseptata, Glomerella miyabeana, Gnomoniella sp. 1, Leptosphaeria sp. 1, Melanconis sp. 1, Melanconis sp. 2, Microsphaeropsis arundinis, Paraconiothyrium brasiliense, Phoma sp. 4, P. glomerata, Sirococcus clavigignenti-juglandacearum, and Coelomycetes sp. reported to oxidize the substrate naphthol (Sun et al. 2011a). The medicinal plants Adhatoda vasica, Costus igneus, Coleus aromaticus, and Lawsonia inermis were collected from Sathyamangalam, Tamil Nadu (India) for the isolation of endophytic fungi and screened for the synthesis of laccase enzyme (Kaul et al. 2013; Vasundhara et al. 2016; Venugopalan and Srivastava 2015). The fungal isolates were grown on GYP agar medium amended with 1-naphthol. Out of 12 different species, only two endophytes, Xylaria sp. and Curvularia brachyspora, were positive in naphthol (Amirita et al. 2012). From the medicinal plants Alpinia calcarata Roscoe, Calophyllum inophyllum L, Bixa orellana L, and Catharanthus roseus, 50 strains of fungal endophyte were isolated. Very few endophytic strains, Phomopsis longicolla (Bo13), Discosia sp. (Ci5), Fusicoccum sp. (Ac26), and Chaetomium sp., were able to produce laccase, i.e., showed oxidation of naphthol (Sunitha et al. 2013).
A total of 127 strains of fungal endophytes were isolated from Eucalyptus globulus trees of Spain: Cantabria, Asturias 128 (AS), Seville, (SE), Extremadura (EX), and Toledo (TO). Out of 127 strains of endophytic fungi, 21 showed positive ABTS oxidation in an agar plate medium containing ABTS . Hormonema sp., Pringsheimia smilacis, Ulocladium sp., Neofusicoccum luteum, and N. australe in liquid medium confirmed laccase production. Copper sulfate and ethanol were examined as inducers for increasing the production of laccase. Pringsheimia smilacis belonging to the family Dothioraceae were reported for the first time for the production of laccase (Bezerra et al. 2012; Fillat et al. 2016). Trametes sp. I-62 was optimized for the production of laccase and was applied to solve problems associated with pulp bleaching. Maximal laccase activity was obtained on the addition of wheat straw and copper sulfate in combination as inducers (Martin-Sampedro et al. 2013). Ligninolytic fungi were collected in Huejutla and characterized as having laccase activity as part of their fundamental enzymatic pool to mineralize lignin. Out of the 100 fungal isolates, 60 had laccase activity, indicating that the isolated fungi have great biotechnological potential (Ramírez et al. 2012).
Two isolates of Fusarium proliferatum from different global locations and ecological sites were reported to display similar abilities to degrade natural lignin from wheat (14C-MWL) and synthetic polymers (Anderson et al. 2005). Shi et al. (2004) demonstrated that the fungal endophyte Phomopsis sp. almost decays straw by degrading lignin. In another study, laccase and peroxide synthesized by fungal endophytes contributed reliably to the decomposition of litter lignin (Dai et al. 2010; Krishnamurthy and Naik 2017). Various researchers have observed the laccase activity of fungal endophytes in liquid medium: Phomopsis liquidambari (Diaporthaceae ), Xylaria sp. (Xylariaceae ), Fusarium sp., F. proliferatum (Nectriaceae ), Chaetomium sp., C. globosum (Chaetomiaceae), Podospora anserina (Lasiosphaeriaceae ), Colletotrichum gloeosporioides (Glomerellaceae ), Trichoderma harzianum (Hypocreaceae), Botryosphaeria sp., Neofusicoccum australe, N. luteum, Botryosphaeria rhodina (Lasiodiplodia theobromae ), Botryosphaeria obtuse, B. dothidea, B. ribis (Botryosphaeriaceae ), Monotospora sp. (Hysteriaceae ), and Hormonema sp. (Dothioraceae ) (Anderson et al. 2005; Durand et al. 2013; El-Zayat 2008; Fillat et al. 2016; Muthezhilan et al. 2014; Sara et al. 2016; Srivastava et al. 2013; Urairuj et al. 2003; Wang J et al. 2006a; Xie and Dai 2015).
1.3.2 Bioresources for Secondary Metabolites
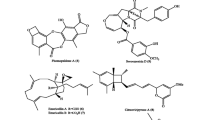
It is evident that numerous important compounds in the pharmaceutical and agronomy industries are synthesized by endophytes (Arora and Ramawat 2017). Numerous vital medicines have been acquired from plants, for instance, camptothecin, quinine, Taxol, vincristine, and vinblastine (Ramawat et al. 2009), whereas more than 8500 bioactive metabolites with fungi as a source are well-known (Arora and Ramawat 2017; Demain and Sanchez 2009; Goyal et al. 2016). With the fungal endophyte Taxomyces andreanae, research into fungal endophytes was initiated, synthesizing certain bioactive molecules such as Taxol (Nicoletti and Fiorentino 2015). There are numerous tasks that have been encountered to synthesize these commercialized bioactive molecules (Arora and Ramawat 2017; Kusari and Spiteller 2011). In Oryza sativa, Fusarium oxysporum was reported to cause foolish seedling disease, as the fungus was also reported to produce gibberellin (Arora and Ramawat 2017). Further, for the synthesis of secondary metabolites, the bio-transformation process has been efficaciously realized using endophytes (Pimentel et al. 2011; Wang and Dai 2011). The process of chemical variation of any substance is referred to as bio-transformation in a biological system (Arora and Ramawat 2017; Wang and Dai 2011). The changes or transformation in the basic molecule result in a further effective active compound, e.g., semisynthetic compounds established from Taxol and podophyllotoxin have a supplementary effect to the basic molecule (Arora and Ramawat 2017; Ramawat et al. 2009). Figure 1.2 presents the chemical structures of secondary metabolites produced by different groups of endophytic fungi. Table 1.3 shows the diversity and abundance of diverse bioactive compound or secondary metabolite production by different groups of endophytic fungi reported from diverse host plants worldwide.
1.3.2.1 Azadirachtin
Azadirachtin is a recognized insecticide found in three species of the neem tree, Azadirachta indica A. Juss., A. excelsa Jacobs, and A. siamensis Valeton (Verma et al. 2014b). Azadirachtin is a highly oxygenated tetranortriterpenoid (Verma et al. 2014b). It contains 16 stereogenic centers, 7 of which are fully replaced (Ley et al. 1993). It takes about 16 years for its first structural interpretation and improvements (Butterworth et al. 1972) and 25 years for its chemical synthesis (Veitch et al. 2007a) to take place. Azadirachtin has been synthesized chemically from a common intermediate “epoxide-2”; this molecule alone has the potential as an intermediate to synthesize compounds from all three groups of limonoids: azadirachtin, azadirachtol, and meliacarpin from the neem tree (Kusari et al. 2012; Veitch et al. 2007b, c). Inside the cellular metabolism, azadirachtin is designed via the “iso-prenoid pathway” (IPP ) (Kraus et al. 1985). Azadirachtin acts as an antifeedant, insect growth regulator, and sterilant in insects (Jennifer Mordue et al. 1998). Azadirachtin functions at a cellular level by disrupting protein synthesis, more precisely at the molecular level by altering the transcription and translation of protein expressed during rapid protein synthesis (Nisbet 2000). Azadirachtin has several structurally related isomers. Azadirachtin A and its several congeners have significant biological activity, specifically insecticidal and nematicidal (Klenk et al. 1986). To enable the synthesis of potential bioactive compounds, some novel biotechnological approaches have been used, such as callus culture (Krishnamurthy and Naik 2017; Prakash et al. 2002; Rafiq and Dahot 2010), cell culture (Jarvis et al. 1997), and hairy root culture of neem plants (Pimentel et al. 2011; Satdive et al. 2007).
1.3.2.2 Camptothecin
Camptothecin (CPT) is a quinoline alkaloid mainly isolated from Camptotheca acuminata, a deciduous tree native to China and Tibet (Kumara et al. 2014). The bark of the tree was extensively used in traditional Chinese medicine (Wall et al. 1966). Later, camptothecin was discovered in several other species belonging to the families Icacinaceae, Rubiaceae, Apocynaceae, and Loganiaceae, with the maximum concentration described in Nothapodytes nimmoniana (0.3% by dry weight from its bark) (Govindachari and Viswanathan 1972; Kumara et al. 2014). The biosynthetic pathway of CPT in plants is simply moderately categorized (Yamazaki et al. 2003, 2004). Further, Sun et al. (2011b) cloned and categorized three putative genes involved in CPT biosynthesis; namely, geraniol-10-hydroxylase, secologanin synthase, and strictosidine synthase from C. acuminata. In recent times, an effort was made to unravel the CPT biosynthetic gene from a CPT-producing endophytic fungus, Fusarium solani, isolated from C. acuminata (Kusari et al. 2011; Kaul et al. 2013; Kumara et al. 2014). However, the endophyte was revealed to synthesize CPT. Kusari et al. (2011) suggested that the endophyte might be using the host STR to synthesize CPT. However, as Sudhakar et al. (2013) debated, this proposition is unbelievable, because the endophyte was able to produce CPT in axenic cultures for numerous generations in the absence of the host tissue, where evidently the fungus cannot access the host STR. Anticancer drugs isolated from endophytic fungi include camptothecin, which is a potent anti-neoplastic agent isolated from C. acuminata Decaisne (Nyssaceae) from China (Premjanu and Jayanthy 2012; Wall et al. 1966).
1.3.2.3 Taxol
Paclitaxel, a greatly functionalized diterpenoid, occurs in Taxus plants (Suffness 1995). Derivatives of paclitaxel signify a leading group of anticancer agents that were earlier reported to be synthesized by endophytes (Kumara et al. 2014). In plants, the synthesis of Taxol occurs by the involvement of three genes, namely, ts (involved in the formation of the taxane skeleton), dbat (involved in baccatin-III formation), and bapt (involved in phenylpropanoyl side chain formation at C-13) (Xiong et al. 2013). Zhang P et al. (2009b) reported the gene 10-deacetylbaccatin-III-10-O-acetyl transferase to be accountable for the biosynthesis of Taxol in the endophyte Cladosporium cladosporioides MD213 isolated from Taxus media (yew species). In recent times, Xiong et al. (2013) revealed that in three Taxol-synthesizing endophytes isolated from Anglojap Yew, or T. media, the fungus resulted in positive successes for the three key genes, ts, dbat, and bapt. The fungus Taxomyces andreanae, an endophyte isolated from T. brevifolia, was found to produce Taxol (Stierle et al. 1993), subsequently drawing the attention of microbiologists to endophytes. Each plant is a repository of one or more fungal endophytes, and one endophytic species may possess several to a few hundred strains (Huang et al. 2007; Strobel and Daisy 2003). In recent years, the biosynthetic potential of endophytic fungi has gained more significance. It is thus imperative to study the complex relationship of endophytes with existing endophytes, host plants, insect pests, and other definitive herbivores, which standardizes the ability of endophytes to synthesize compounds similar to their hosts (Kusari et al. 2013b). Aegle marmelos, an extensively used medicinal plant, shelters Taxol-producing fungi (Gangadevi and Muthumary 2008). Taxol is an important and expensive anticancer drug generally used in clinics. The endophytic fungus Bartalinia robillardoides (strain AMB-9) produces 187.6 l g/l of Taxol. This confirms that the fungus can be genetically upgraded to increase the synthesis of Taxol. Taxanes such as Taxol are plentifully synthesized by members of the coniferous family Taxaceae (Wang and Dai 2011). It was found that a number of fungal endophytes isolated from yew trees (Taxus spp., Taxaceae) produce Taxol under in vitro conditions (Zhou et al. 2010).
1.3.2.4 Gibberellic Acid and Indole Acetic Acid
The biosynthetic pathway of gibberellic acid (GA) is compared with other secondary metabolites (Kumara et al. 2014). In plants, the conversion of GGDP to active GA requires the presence of three terpene synthases, two 450s, and a soluble 20 DDS. Compared with the fungus, the synthesis is made by only one bifunctional terpene cyclase (copalyl synthase/kaurene synthase) and by P450s. These results suggest that the biosynthetic pathways in plants and fungi might have evolved independently (Bömke and Tudzynski 2009; Kumara et al. 2014). GA production has also been reported from the endophytic fungus F. proliferatum, isolated from orchid roots. Research has specified that this fungus obtained the genes for GA biosynthesis from higher plants by horizontal gene transfer. Endophytic microorganisms have been found to produce phytohormones such as GA, abscisic acid, auxins, cytokinins, and ethylene (Kaul et al. 2013).
Hamayun et al. (2009b) isolated Cladosporium sphaerospermum from the roots of G. max (L.), which indicated the presence of bioactive GA3, GA4, and GA7. The endophytes isolated from medicinal plants have been found to encourage plant growth and development. Waqas et al. (2012) studied the endophytic fungi Phoma glomerata and Penicillium sp. in the promotion of shoot growth, related vegetative growth, and other characteristics of GA-deficient dwarf mutant Waito-C and Dongjin-byeo rice. Therefore, if cultured endophytes were to produce the same rare and important bioactive compounds as their host plants, this would diminish the harvesting of slow-growing rare plants, and also help to restore the world’s biodiversity (Waqas et al. 2012). Jerry (1994) revealed that during seed germination, the symbiotically associated endophytic fungi degrade cuticle cellulose and make carbon available to seedlings, which improves seed germination, vigor, and establishment. Endophytes have the ability to produce plant growth regulators and thereby promote seed germination in crop plants (Bhagobaty and Joshi 2009). Plant growth promotion is the major contribution of fungal symbiosis (Hassan et al. 2013). However, fungal endophytes enhance plant growth by the production of ammonia and plant hormones, particularly IAA (Bal et al. 2013). IAA acts as a plant growth promoter that enhances both cell elongation and cell division, and is essential for plant tissue differentiation (Taghavi et al. 2009). The ability of soil microorganisms to become involved in the production of IAA in culture plates and in soil has been recorded (Spaepen and Vanderleyden 2011). The endophytic microorganisms isolated from various plants have shown a high IAA production level compared with those isolated from root-free soil (Spaepen et al. 2007). Owing to the impact of IAA on the plant tissues, the ability of fungal endophytes to produce IAA has provoked a great response (Hamayun et al. 2010). Only a few fungi linked with plants have been stated to synthesize gibberellin (Kawaide 2006; Vandenbussche et al. 2007), for instance, Cladosporium sphaerospermum and Penicillium citrinum (Hamayun et al. 2009b; Khan et al. 2008). Hamayun et al. (2010) examined gibberellin production and the growth-promoting potential of a fungal strain belonging to Cladosporium sp. isolated from the roots of the cucumber. Khan et al. (2008) isolated P. citrinum, which showed growth promotion activity in dune plants owing to the presence of bioactive gibberellins in the filtrate of the fungi (Khan et al. 2008). Hasan (2002) revealed the growth promotion activity of endophytic Phoma herbarum and Chrysosporium pseudomerdarium in the soybean and proved that some endophytes are host-specific. Ahmad et al. (2010) studied the plant growth-promoting activity and stress resistance capability of endophytic Penicillium sp. and Aspergillus sp., which were shown to produce physiologically active gibberellins. Many fungal endophytes, such as Neurospora crassa (Rademacher 1994), Sesamum indicum (Choi et al. 2005), Penicillium citrinum (Khan et al. 2008), Scolecobasidium tshawytschae (Hamayun et al. 2009b), Arthrinium phaeospermum (Khan et al. 2009a), Chrysosporium pseudomerdarium (Hamayun et al. 2009a), Cladosporium sphaerospermum (Hamayun et al. 2009c), Cladosporium sp. (Hamayun et al. 2009c), Gliomastix murorum (Khan et al. 2009b), Fusarium fujikuroi, Sphaceloma manihoticola (Shweta et al. 2010), Phaeosphaeria sp. (Kawaide 2006), Phaeosphaeria sp., Penicillium sp. (Hamayun et al. 2010), Aspergillus fumigatus (Khan et al. 2011a), Exophiala sp. (Khan et al. 2011b), and P. funiculosum (Khan et al. 2011b), have been reported to be gibberellin producers. Hasan (2002) demonstrated gibberellin production with molds such as Aspergillus flavus, A. niger, Penicillium corylophilum, P. cyclopium, P. funiculosum, and Rhizopus stolonifera.
1.3.2.5 Siderophores
Endophytes help plants to take up solubilized phosphate (Wakelin et al. 2004), enhancing hyphal growth and mycorrhizal colonization (Will and Sylvia 1990), and by producing siderophores (iron-chelating molecules that increase the availability of phosphate to plants) (Costa and Loper 1994). Endophytic bacteria were found to be responsible for the allelopathic effects on maize observed with these plants, causing reduced plant emergence and plant height (Sturz et al. 1997). Dutta et al. (2008) reported improvement of plant growth and disease suppression in pea plants co-inoculated with fluorescent pseudomonads and Rhizobium. Hung et al. (2007) studied the effect of endophytes on soybean growth and development, and proved them to have a positive influence on root weight. Plant growth-promoting endophytic bacteria influence seed germination, root and hypocotyl growth and increase seedling vigor. Root endophytes in the cortical parenchymatous tissue of vetiver were used for the enhancement of essential oil metabolism (Del Giudice et al. 2008). Harish et al. (2009) studied the effect of the bio-formulations of consortial combinations of the rhizobacteria Pseudomonas fluorescens (Pf1) and endophytic Bacillus sp. (EPB22), which enhanced the yield of bananas. One of the bacterial endophytes, B. subtilis HC8, isolated from hogweed, Heracleum sosnowskyi, found the potential to stimulate plant growth and the biological control of foot and root rot diseases in tomato (Malfanova et al. 2011).
In field experiments, inoculation with endophytic bacteria resulted in sugarcane plants that were more superior in terms of plant height and shoot counts. Conventional manipulation of soil microorganisms has been practiced for decades. For example, sewage and manure applications for the enhancement of soil fertility dramatically affect autochthonous communities of soil biota (Biswas et al. 2018). The practice of monoculture is in itself instrumental in altering soil microbial populations at the field level. Thus, it may be possible to influence plant endophytic populations by seed bacterization, by soil inoculation, and by identifying the genetic (bacterial) component responsible for their beneficial effects. Endophytic microbes have merit over rhizospheric bacteria, as they deliver fixed nitrogen straight to the host plant tissue and are able to fix nitrogen more competently than free-living bacteria because of the lower oxygen pressure in the interior of plants than in soil. Jha et al. (2013) explored the potential of endophytic association with plants in agricultural sustainability in particular and yield enhancement in general. The potential of biofertilizers was formulated using endophytic bacteria for the enhanced production of bananas in a sustained way (Ngamau et al. 2014).
1.3.3 Pharmaceutically Important Bioactive Compounds
Throughout the year, natural products from microorganisms , plants, or animals play a key role in the search for novel drugs. These naturally derived products are nontoxic and inexpensive, and have been exploited for human use. The biggest store of bioactive compounds is fungal endophytes. Alexander Fleming, in 1928, discovered the first bioactive compound from Penicillium notatum , i.e., penicillin. During the 1990s one of the most useful anticancer drugs was paclitaxel. An endophyte of T. brevifolia, Taxomyces andreanae was reported to produce the drug paclitaxel. Later research suggested lateral gene transfer from host to fungus (Stierle et al. 1993). The fungal endophyte Fusarium was reported to produce subglutinol A and diterpene pyrones, providing immunosuppressive activity. The endophyte was isolated from the stem of Tripterygium wilfordii (Strobel and Pliam 1997). Isobenzofuranone as isopestacin, obtained from the fungal endophyte Pestalotiopsis microspora, possesses antifungal and antioxidant activity (Strobel et al. 2002). Antimicrobial activity of fungal endophytes was screened against the pathogenic organisms Staphylococcus aureus, Candida albicans, and Cryptococcus neoformans. Fungal endophytes were isolated from the leaves and branches of five different species of Garcinia plants. The fungal endophytes Phomopsis sp. and Botryosphaeria sp. showed antibacterial activity against Staphylococcus aureus. Botryosphaeria sp. also showed antifungal activity against M. gypseum. The results specify that the endophytic fungus of Garcinia plants are a potential source of antimicrobial compounds (Phongpaichit et al. 2006).
Endophytic fungi can be isolated from the bark of Juglans mandshurica. On the basis of the internal transcribed spacer sequence and morphological identification, the fungal endophyte belongs to Deuteromycotina, Hyphomycetes, Moniliales, and Trichoderma longibrachiatum. The fermentation of fungus FSN006 provides a possible mechanism for producing anticancer drugs with lower toxicity and greater efficiency (Li et al. 2009). The crude extract of the endophytic fungus Pichia guilliermondii was separated using bioassay-guided fractionation. Helvolic acid exhibited strong, broad-spectrum, antimicrobial activity (Zhao et al. 2010). Developments in screening technologies have received much attention; thus, fungal endophytes are an outstanding source of biologically active compounds with applications in medicine and agriculture (Aly et al. 2011). A large number of bioactive compounds produced by fungal endophytes are alkaloids, steroids, terpenoids, peptides, polyketones, flavonoids, quinols, phenols, xanthones, chinones, isocoumarins, benzopyranones, tetralones, cytochalasines, perylene derivatives, furandiones, depsipeptides, and enniatins (Elfita et al. 2011) Tenguria et al. (2011) reported that endophytic fungi of Tinospora crispa (L.) was a probable candidate for the synthesis of bioactive compounds. Plasmodium species cause the most acute diseases in human beings, and even death. Hypericin isolated from fungal endophytes of medicinal plants possess antimicrobial activity against Staphylococcus sp., Klebsiella pneumoniae, Pseudomonas aeroginosa, Salmonella enteric, and Escherichia coli (Kusari and Spiteller 2012).
Khan et al. (2012a) reported five fungal endophytes isolated from Capsicum annuum, Cucumis sativus, and G. max roots. Using phylogenetic analysis, the isolate was found to belong to Paraconiothyrium sp. and produce the phytotoxic compound ascotoxin characterized using gas chromatography-mass spectrometry and the nuclear magnetic resonance technique. On seed germination of Echinochloa crus-galli and Lactuca sativa, 100% inhibitory effects were shown by ascotoxin. The buds and leaf of Malabar Embelia, found in peninsular India, were subjected to the isolation of fungal endophytes. Four different fungal endophytes were identified, Cladosporium cladosporiodes, Penicillium sp., Aspergillus niger, and Alternaria sp., and were characterized for phytochemical analysis and antibacterial activity against Pseudomonas aeuroginosa, Bacillus subtilis, and Shigella flexneri. The four different fungal endophytes exhibited the presence of phytochemicals at different concentrations: cardiac glycoside, flavonoids, phenols, tannins, terpenoids, cardenolides, and saponins. Endophytic microbes are a great source of bioactive compounds to satisfy the demands of the pharmaceutical and medical industries (Chandrappa et al. 2013). Pinellia ternata is used as a traditional medicine for anti-emetic and sedative effects, and as an antitussive and analgesic. Su et al. (2014) isolated 193 endophytic microbes from Chinese medicinal plants, Camptotheca cuminata Decne, Gastrodia elata Blume, and Pinellia ternata. On the basis of morphological and rDNA sequences, the fungal isolates belong to Ascomycota, Basidiomycota, and Mucoromycotina. Endophytes produce various types of compounds, for instance, essential oils, azadirachtins, terpenes, flavonoids, lignins, cytochalasins, steroids, and alkaloids (Nicoletti and Fiorentino 2015).
1.3.4 Lignocellulosic Biorefineries: Biofuel Production
One of the main renewable materials on earth is wood. The cell walls of wood are composed of cellulose microfibrils covered with hemicelluloses and lignin hemicellulose matrices (Higuchi 2012). In 1813, Swiss botanist, A. P. de Candolle, mentioned lignin for the first time. About 20–30% of the dry weight of wood is made up of lignin (Abdel-Raheem and Shearer 2002). It is covalently linked to hemicellulose and confers mechanical strength to the cell wall (Chabannes et al. 2001). Owing to the chemical complexity and recalcitrant properties of lignin, very few microbes are able to degrade it (Guillén et al. 2005). In biorefinery processes, such as the production of ethanol and cellulose-based papers, the degradation of lignin is a central issue (Cañas and Camarero 2010).
An array of extracellular oxidative enzymes are produced by white-rot fungi (basidiomycetes), as they are the main wood rotters that synergistically and proficiently degrade lignin. Ligninolytic enzymes include lignin peroxidases (LiPs), manganese peroxidases (MnPs), versatile peroxidases, and laccases (Wong 2009). On the basis of macroscopic features, wood-rotting basidiomycetes are categorized into white-rot and brown-rot fungi (Schwarze et al. 2000). In the mid-1980s, LiP and MnP were discovered in P. chrysosporium and termed true ligninases because of their high redox potential (Evans et al. 1994). Pleurotus eryngii were reported to produce versatile peroxidase that showed catalytic properties similar to LiP and MnP (Ruiz-Dueñas et al. 1999). Other extracellular enzymes involved in wood lignin degradation are oxidases generating H2O2, aryl-alcohol oxidase (AAO ), glyoxal oxidase, aryl-alcohol dehydrogenases (AAD ), and quinone reductases (QR ) (Guillén et al. 1997; Gutierrez et al. 1994).
Laccases have been known for many years to play a variety of roles, including production of pigments, fruit body morphogenesis, lignification of cell walls, and detoxification in plants, fungi, and insects (Mayer and Staples 2002). The preliminary steps in the biodegradation of lignin must be extracellular. LiP is also called a ligninase . First discovered in Phanerochaete chrysosporium, this enzyme is a heme peroxidase with a remarkably high redox potential and low optimal pH (Tien 1987). Laccase enzymes are copper-containing oxidases that mostly oxidize only those lignin model compounds with a free phenolic group, forming phenoxy radicals (Bourbonnais and Paice 1990). The most common laccase-producing endophytic fungi are Chaetomium sp., C. globosum, Podospora anserina, Botryosphaeria sp., and Neofusicoccum austral (Fillat et al. 2016; Sara et al. 2016).
Laccase enzymes produced by endophytic fungi have extensive substrate specificity and generally act on small organic substrates, such as polyphenols, methoxy-substituted phenols, and aromatic amines. Fungal laccases are used in paper manufacture for delignification, bioremediation of phenolic compounds, and biobleaching (Kunamneni et al. 2008). Exoglucanases, endoglucanases, β-glycosidase, exoxylanases and endoxylanases, and β-xylosidases are the main hydrolytic enzymes involved in lignocellulose degradation (Van Dyk and Pletschke 2012). For complete degradation of lignocellulose materials, laccases, MnP and LiP (oxidative enzymes), and additional hemicelluloses (e.g., acetyl esterase, b-glucuronidase, endo-1, 4-β-mannanase, and α-galactosidase) and oxidoreductases (aryl-alcohol oxidase, glucose-1-oxidase, glyoxal oxidase, and pyranose-2-oxidase) are necessary (Correa et al. 2014).
1.3.5 Endophytic Fungi in Bioremediation
Bioremediation is a process used to treat contaminated media, including water, soil, and subsurface material, by varying the conditions of the environment to stimulate the growth of microorganisms (fungi or bacteria) and degrade the target pollutants into simpler compounds. Biological treatment of the contaminated site is the least expensive method (Barranco et al. 2012). To optimize the conditions for the microorganisms, additional nutrients, vitamins, minerals, and pH buffers are added. The prime goal of bioremediation is to create an optimal environment for the microbes to degrade pollutants. Although it is a cost-effective option, it is a very slow process, sometimes taking weeks to months for results to appear. Technologies can be generally classified as in situ or ex situ. In situ bioremediation involves treating the contaminated material at the site, whereas ex situ involves the removal of the contaminated material to be treated elsewhere.
To restrain the growth of endophytes, the plant synthesizes a range of toxic metabolites and endophytes over a period of co-evolution, progressively establishing a genetic system as a tolerant mechanism by generating exoenzymes and mycotoxins (Mucciarelli et al. 2007; Pinto et al. 2000). Fungal endophytes synthesizing the enzymes degrade the macromolecules into simpler compounds, including amylases, lipases, pectinase, cellulase, proteinase, phenol oxidase, and lignin catabolic enzymes (Oses et al. 2006; Tan and Zou 2001; Zikmundova et al. 2002). In general, fungal endophytes have been stated to have the ability to use various organic compounds, such as glucose, oligosaccharides, cellulose, hemicelluloses, lignin, keratin, pectin, lipids, and proteins, allowing the degradation of structural components into simpler forms (Kudanga and Mwenje 2005; Tomita 2003; Urairuj et al. 2003).
One of the methodologies in which green plants are used for the process of bioremediation is referred to as phytoremediation . It has been documented to be a promising technology for the in situ remediation of contaminated soils. Numerous studies have demonstrated that endophytes produce various enzymes for the degradation of organic contaminants and reduce both the phytotoxicity and evapotranspiration of volatile contaminants (Li et al. 2012b). Soleimani et al. (2010) reported the infection of Festuca pratensis and Festuca arundinacea, two grass species, by two endophytic fungi, Neotyphodium coenophialum and Neotyphodium uncinatum, increasing the ability of the plants to accumulate more Cd in roots and shoots and decreasing stress in the plants in addition to increasing the production of biomass. Rabie (2005) reported the phytoremediation efficiency of wheat, mung beans, and eggplant grown in soil contaminated with hydrocarbons. He concluded that the plants provided a larger sink for the contaminants, because they were better able to survive and grow, leading to the significance of treatment with arbuscular mycorrhizal fungi.
Since the industrial revolution, there has been a widespread rise in the discharge of waste into the environment, which is mostly collected in soil and water, comprises heavy metals, and generates distressing conditions for human life and aquatic biota. Heavy metals are metals with relatively high densities, atomic weights, or atomic numbers. Some heavy metals are either vital nutrients, such as iron, cobalt, and zinc, or comparatively harmless, such as ruthenium, silver, and indium, but in higher amounts or definite forms they can be toxic. Cadmium, mercury, and lead are reported to be highly poisonous heavy metals. Salem et al. (2000) reported that arsenic, cadmium, chromium, copper, lead, mercury, nickel, selenium, silver, zinc, etc., are not only cytotoxic, but also carcinogenic and mutagenic (Ahluwalia and Goyal 2007). In heavy metal-polluted habitats, microorganisms are known to change different detoxifying mechanisms, such as biosorption, bioaccumulation, biotransformation, and biomineralization (Gadd 2000; Lim et al. 2003; Malik 2004).
One of the biological processes in which chemical changes on compounds take place is referred to as biotransformation. The endophytic fungus Phomopsis sp. (VA-35), obtained from Viguiera arenaria, was reported to biotransform the tetrahydrofuran lignan, (-)-grandisin, into a new compound, 3,4-dimethyl-2-(4'-hydroxy-3',5'-dimethoxyphenyl)-5-methoxy-tetrahydrofuran (Verza et al. 2009). In another study, endophytic fungi Fusarium sambucinum, Plectosporium tabacinum, Gliocladium cibotii, and Chaetosphaeria sp., isolated from the roots and shoots of Aphelandra tetragona, were capable of transforming the benzoxazinones 2-benzoxazolinone (BOA ) and 2-hydroxy-1,4-benzoxazin-3-one (HBOA ). Aminophenol was formed as a key intermediate during the metabolic pathway for HBOA and BOA degradation (Zikmundova et al. 2002). On the basis of 18S rRNA gene sequencing, Lasiodiplodia theobromae isolated from the leaves of Boswellia ovalifoliolata, an endemic medicinal plant from the Tirumala Hills, was reported to show resistance to all four heavy metals, Co, Cd, Cu, and Zn, up to 600 ppm (Sani et al. 2017).
1.3.6 Endophytic Fungi in Agriculture
A lot of research into fungal endophytes is underway, which signifies that they are the most important source of biocontrol agents. They have a considerable effect on the physiological actions of their host plants. Further, various environmental factors, including rainfall and humidity, may have an influence on the occurrence of some fungal endophytic species (Khiralla et al. 2017; Petrini 1991; Selvanathan et al. 2011). According to Schaechter (2012), endophytic fungi have frequently been categorized into two major groups, including clavicipitaceous endophytes, which are known to infect some grasses, and nonclavicipitaceous endophytes. The Clavicipitaceae family of fungi include free-living and symbiotic species in association with insects and fungi or grasses, rushes, and sedges (Bacon and White 2000; Khiralla et al. 2017). Many of its members produce alkaloids, which are toxic to animals and humans, whereas nonclavicipitaceous endophytic fungi, mainly in association with leaves of tropical trees, have been discovered to play an important role in defending the host from abiotic stress, fungal pathogens, and an increase in the biomass (Fröhlich and Hyde 1999; Gamboa and Bayman 2001; Khiralla et al. 2017; Yadav and Yadav 2018; Yadav 2019)
Endophytic fungi play vital roles in host plants, protecting them from stress conditions, making nutrients, such as phosphorus, potassium, and many more, available, producing auxins, cytokinins, gibberellins, siderophores, ammonia, HCN, and diverse hydrolytic enzymes, and ultimately promoting the growth of host plants. A number of studies suggest that inoculating crops with endophytic fungi might improve growth by diverse plant growth-promoting traits and might also mitigate the effect of stress conditions. Khan et al. (2011b) demonstrated the role of a newly isolated endophytic fungus, Penicillium funiculosum, with diverse plant growth-promoting attributes in G. max growing under salinity stress. The study revealed that the fungus ameliorated the effect of salinity stress. Kedar et al. (2014) studied the growth promotion potential of Phoma sp. isolated from Tinospora cordifolia and Calotropis procera for maize. The fungal endophytes were found to enhance growth in inoculated maize plants compared with non-inoculated plants. In the study by Rinu et al. (2014), Trichoderma gamsii isolated from the lateral roots of lentil with multifarious plant growth-promoting attributes showed its potential in plant growth promotion conducted under greenhouse conditions using two cereals and two legumes, suggesting its potential to be developed as a bioformulation for application under a mountain ecosystem. Yuan et al. (2017) studied the effect of Penicillium simplicissimum, Leptosphaeria sp., Talaromyces flavus, and Acremonium sp. isolated from cotton roots with wilt disease caused by the defoliating Verticillium dahliae (Vd080). The study demonstrated that all treatments considerably reduced disease incidence and the disease index. The results clearly signified that these endophytes not only delayed, but also led to a reduction in, wilt symptoms in cotton.
In the study by Asaf et al. (2018), Aspergillus flavus CHS1, an endophytic fungus, isolated from the roots of Chenopodium album with multiple growth-promoting activities, was assayed for its ability to promote the growth of mutant Waito-C rice. The results revealed an increase in chlorophyll content, root–shoot length, and biomass production. Furthermore, the strain was used to evaluate its potential to improve the growth of soybean under salinity stress. Dastogeer et al. (2018) evaluated whether the colonization of two fungal endophytes isolated from wild Nicotiana species from areas of drought-prone northern Australia, and a plant virus, yellowtail flower mild mottle virus, could improve water stress tolerance in N. benthamiana plants. Inoculation with the fungal strains and the virus considerably increased the tolerance of the plants to water stress. Inoculation with the fungal strains alone resulted in an increase in the relative water content, soluble sugar, soluble protein, proline content, plant biomass, and enzymatic activity, and a decrease in the production of reactive oxygen species and electrical conductivity. Furthermore, there was noteworthy upregulation of numerous genes that had previously been identified as drought-induced. The influence of the virus was similar to that of the fungi in terms of increasing the plant osmolytes, antioxidant enzyme activity, and gene expression. Fungal endophytic communities associated with plants play a vital role in balancing the ecosystem and in enhancing the growth of hosts. They have been shown to be potent biocontrol agents; furthermore, they produce a large number of fungal metabolites that could protect the host from disease, insects, and mammalian herbivores . They have been known to increase the tolerance of their host to abiotic stress. Thus, fungal endophytes are gaining greater attention and are of greater interest to chemists, ecologists, and microbiologists as a treasure of biological resources, because of their diverse vital roles in the ecosystem.
1.4 Future Prospects and Conclusion
For the previous two and half decades, the scientific community has been aware of the effective role of fungal endophytes in agriculture, ecology, biotechnology, and industry. Fungal endophytes are also an alternative to existing industrial processes of transformation of lignocellulosic biomass, possessing great potential for application in the lignocellulosic industry. The ability of hydrolytic enzymes to synthesize can be employed in enzyme fermentation industries. New techniques with advanced sensitivity are required for enzyme quantification, such as fluorescence spectrophotometry, near-infrared-, and Fourier-transform infrared-based methods. The consequences of enzymes generating endophytes with distinctive consideration of remediating environmental pollutants , such as metals, polyaromatic hydrocarbons, and polychlorinated hydrocarbons, have been understated at the very minimum. Production of secondary metabolites of interest to the pharmaceutical industry is a very attractive field of research using biotechnological methods. The integration of genetic manipulation technology to progress the research to recognize the regulatory gene/s of numerous biosynthesis pathways of metabolite construction can lead to an increase in growth production of the compounds to be used for human welfare. The participation of fungal endophytes in the cycling of nutrients has significant consequences for living organisms and human health. For future research, there are still many areas that need to be explored, including new technologies and new crops with endophytes. Modern techniques of molecular biology, involving metagenomes, proteomes, and transcriptomes, will help to define the characteristics of endophytes and to find novel products for industrial development. The future of research into endophytes is bright, as demand for pharmaceutical products and agricultural produce is increasing day by day with an ever-increasing population.
References
Abdel-Raheem A, Shearer C (2002) Extracellular enzyme production by freshwater ascomycetes. Fungal Div 11:1–19
Abrunhosa L, Oliveira F, Dantas D, Gonçalves C, Belo I (2013) Lipase production by Aspergillus ibericus using olive mill wastewater. Bioprocess Biosyst Eng 36:285–291
Adav SS, Sze SK (2014) Trichoderma secretome: an overview. In: Gupta VK, Schmoll M, Herrera-Estrella A, Upadhyay RS, Druzhinina I, Tuohy MG (eds) Biotechnology and biology of Trichoderma. Elsevier, Amsterdam, pp 103–114
Adnan M, Alshammari E, Ashraf SA, Patel K, Lad K, Patel M (2018) Physiological and molecular characterization of biosurfactant producing endophytic fungi Xylaria regalis from the cones of Thuja plicata as a potent plant growth promoter with its potential application. BioMed Res Int. https://doi.org/10.1155/2018/7362148
Ahluwalia SS, Goyal D (2007) Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresour Technol 98:2243–2257
Ahmad N, Hamayun M, Khan SA, Khan AL, Lee I-J, Shin D-H (2010) Gibberellin-producing endophytic fungi isolated from Monochoria vaginalis. J Microbiol Biotechnol 20:1744–1749
Alshammari AM, Adnan FM, Mustafa H, Hammad N (2011) Bioethanol fuel production from rotten banana as an environmental waste management and sustainable energy. Afr. J Microbiol Res 5:586–598
Aly AH, Edrada-Ebel R, Wray V, Müller WE, Kozytska S, Hentschel U, Proksch P, Ebel R (2008) Bioactive metabolites from the endophytic fungus Ampelomyces sp. isolated from the medicinal plant Urospermum picroides. Phytochemistry 69:1716–1725
Aly AH, Debbab A, Proksch P (2011) Fungal endophytes: unique plant inhabitants with great promises. Appl microbiol Biotechnol 90:1829–1845
Amin N (2013) Diversity of endophytic fungi from root of Maize var. Pulut (waxy corn local variety of South Sulawesi, Indonesia). Int J Curr Microbiol App Sci 2:148–154
Amirita A, Sindhu P, Swetha J, Vasanthi N, Kannan K (2012) Enumeration of endophytic fungi from medicinal plants and screening of extracellular enzymes. World J Sci Technol 2:13–19
Anbu P, Noh M-J, Kim D-H, Seo J-S, Hur B-K, Min KH (2011) Screening and optimization of extracellular lipases by Acinetobacter species isolated from oil-contaminated soil in South Korea. Afr J Biotechnol 10:4147–4156
Anderson AJ, Kwon S-I, Carnicero A, Falcón MA (2005) Two isolates of Fusarium proliferatum from different habitats and global locations have similar abilities to degrade lignin. FEMS Microbiol Lett 249:149–155
Aranda FJ, Teruel JA, Ortiz A (2005) Further aspects on the hemolytic activity of the antibiotic lipopeptide iturin A. Biochim Biophys Acta Biomembr 1713:51–56
Aravindan R, Anbumathi P, Viruthagiri T (2007) Lipase applications in food industry. Indian J Biotechnol 6:141–158
Arivudainambi UE, Anand TD, Shanmugaiah V, Karunakaran C, Rajendran A (2011) Novel bioactive metabolites producing endophytic fungus Colletotrichum gloeosporioides against multidrug-resistant Staphylococcus aureus. FEMS Immunol Med Microbiol 61:340–345
Arora J, Ramawat KG (2017) An introduction to endophytes. In: Maheshwari D (ed) Endophytes: biology and biotechnology. Sustainable development and biodiversity, vol 15. Springer, Cham. https://doi.org/10.1007/978-3-319-66541-2_1
Asaf S, Hamayun M, Khan AL, Waqas M, Khan MA, Jan R, Lee I-J, Hussain A (2018) Salt tolerance of Glycine max. L induced by endophytic fungus Aspergillus flavus CSH1, via regulating its endogenous hormones and antioxidative system. Plant Physiol Biochem 128:13–23
Ayob FW, Simarani K (2016) Endophytic filamentous fungi from a Catharanthus roseus: identification and its hydrolytic enzymes. Saudi Pharm J 24:273–278
Azevedo JL, Maccheroni W Jr, Pereira JO, de Araújo WL (2000) Endophytic microorganisms: a review on insect control and recent advances on tropical plants. Electron J Biotech 3:15–16
Bacon CW, White JF (2000) Physiological adaptations in the evolution of endophytism in the Clavicipitaceae. In: Redlin SC, Carris LM (eds) Microbial endophytes. Marcel Dekker, New York, pp 237–261
Bal HB, Das S, Dangar TK, Adhya TK (2013) ACC deaminase and IAA producing growth promoting bacteria from the rhizosphere soil of tropical rice plants. J Basic Microbiol 53:972–984
Barr DP, Aust SD (1994) Mechanisms white rot fungi use to degrade pollutants. Environ Sci Technol 28:78A–87A
Barranco FT, Saalfield SL, Tenbus FJ, Shedd BP (2012) Subsurface fate and transport of chemicals. In: Gulliver J (ed) Transport and fate of chemicals in the environment. Springer, New York. https://doi.org/10.1007/978-1-4614-5731-2_13
Barrasa J, Martínez A, Martínez M (2009) Isolation and selection of novel basidiomycetes for decolorization of recalcitrant dyes. Folia Microbiol 54(1):59. https://doi.org/10.1007/s12223-009-0009-6
Bashyal BP, Wijeratne EK, Faeth SH, Gunatilaka AL (2005) Globosumones A−C, cytotoxic orsellinic acid esters from the Sonoran desert endophytic fungus Chaetomium globosum. J Nat Prod 68:724–728
Battestin V, Macedo GA (2007) Effects of temperature, pH and additives on the activity of tannase produced by Paecilomyces variotii. Electron J Biotechnol 10:191–199
Bezerra J, Santos M, Svedese V, Lima D, Fernandes M, Paiva L, Souza-Motta C (2012) Richness of endophytic fungi isolated from Opuntia ficus-indica Mill.(Cactaceae) and preliminary screening for enzyme production. World J Microbiol Biotechnol 28:1989–1995
Bezerra JD, Nascimento CC, Barbosa RN, da Silva DC, Svedese VM, Silva-Nogueira EB, Gomes BS, Paiva LM, Souza-Motta CM (2015) Endophytic fungi from medicinal plant Bauhinia forficata: diversity and biotechnological potential. Braz J Microbiol 46:49–57
Bhagobaty R, Joshi S (2009) Promotion of seed germination of Green gram and Chick pea by Penicillium verruculosum RS7PF, a root endophytic fungus of Potentilla fulgens L. Advanced Biotech 8:16–18
Bhat M, Bhat S (1997) Cellulose degrading enzymes and their potential industrial applications. Biotechnology Adv 15:583–620
Bilal L, Asaf S, Hamayun M, Gul H, Iqbal A, Ullah I, Lee I-J, Hussain A (2018) Plant growth promoting endophytic fungi Aspergillus fumigatus TS1 and Fusarium proliferatum BRL1 produce gibberellins and regulates plant endogenous hormones. Symbiosis 97:1–11. https://doi.org/10.1007/s13199-018-0545-4
Biswas S, Kundu DK, Mazumdar SP, Saha AR, Majumdar B, Ghorai AK, Ghosh D, Yadav AN, Saxena AK (2018) Study on the activity and diversity of bacteria in a New Gangetic alluvial soil (Eutrocrept) under rice-wheatjute cropping system. Journal of Environmental Biology 39(3):379–386
Biz A, Farias FC, Motter FA, de Paula DH, Richard P, Krieger N, Mitchell DA (2014) Pectinase activity determination: an early deceleration in the release of reducing sugars throws a spanner in the works. PLoS One 9:e109529. https://doi.org/10.1371/journal.pone.0109529
Bogner CW, Kariuki GM, Elashry A, Sichtermann G, Buch A-K, Mishra B, Thines M, Grundler FM, Schouten A (2016) Fungal root endophytes of tomato from Kenya and their nematode biocontrol potential. Mycol Progress. https://doi.org/10.1007/s11557-016-1169-9
Bogner CW, Kamdem RS, Sichtermann G, Matthäus C, Hölscher D, Popp J, Proksch P, Grundler FM, Schouten A (2017) Bioactive secondary metabolites with multiple activities from a fungal endophyte. Microbial Biotechnol 10:175–188
Bömke C, Tudzynski B (2009) Diversity, regulation, and evolution of the gibberellin biosynthetic pathway in fungi compared to plants and bacteria. Phytochemistry 70:1876–1893
Bourbonnais R, Paice MG (1990) Oxidation of non-phenolic substrates: an expanded role for laccase in lignin biodegradation. FEBS Lett 267:99–102
Braddock RJ (1981) Pectinase treatment of raw orange juice and subsequent quality changes in 60o Brix concentrate. P Fl St Hortic Soc 94:270–273
Breen J (1994) Acremonium endophyte interactions with enhanced plant resistance to insects. Annu Rev Entomol 39:401–423
Brem D, Leuchtmann A (2001) Epichloë grass endophytes increase herbivore resistance in the woodland grass Brachypodium sylvaticum. Oecologia 126:522–530
Butt MS, Tahir-Nadeem M, Ahmad Z, Sultan MT (2008) Xylanases and Their Applications in Baking Industry. Food Technol Biotechnol 46:22–31
Butterworth J, Morgan E, Percy G (1972) The structure of azadirachtin; the functional groups. J Chem Soc Perkin Trans 1:2445–2450
Cabezas L, Calderon C, Medina LM, Bahamon I, Cardenas M, Bernal AJ, Gonzalez A, Restrepo S (2012) Characterization of cellulases of fungal endophytes isolated from Espeletia spp. J Microbiol 50:1009–1013
Cañas AI, Camarero S (2010) Laccases and their natural mediators: biotechnological tools for sustainable eco-friendly processes. Biotechnol Adv 28:694–705
Carvalho CR, Gonçalves VN, Pereira CB, Johann S, Galliza IV, Alves TM, Rabello A, Sobral ME, Zani CL, Rosa CA (2012) The diversity, antimicrobial and anticancer activity of endophytic fungi associated with the medicinal plant Stryphnodendron adstringens (Mart.) Coville (Fabaceae) from the Brazilian savannah. Symbiosis 57:95–107
Cavalcanti RMF, Ornela P, Jorge JA, Guimarães L (2017) Screening, selection and optimization of the culture conditions for tannase production by endophytic fungi isolated from caatinga. J Appl Biol Biotechnol 5:1–9
Chabannes M, Ruel K, Yoshinaga A, Chabbert B, Jauneau A, Joseleau JP, Boudet AM (2001) In situ analysis of lignins in transgenic tobacco reveals a differential impact of individual transformations on the spatial patterns of lignin deposition at the cellular and subcellular levels. Plant J 28:271–282
Chadha N, Prasad R, Varma A (2015) Plant promoting activities of fungal endophytes associated with tomato roots from central Himalaya, India and their interaction with Piriformosporaindica. Int J Pharm Bio Sci 6:333–343
Chandrappa C, Anitha R, Jyothi P, Rajalakshmi K, Seema Mahammadi H, Govindappa M (2013) Phytochemical analysis and antibacterial activity of Endophytes of Embelia Tsjeriam cottam Linn. Int J Pharma Bio Sci 3:201–203
Chaudhri A, Suneetha V (2012) Microbially derived pectinases: a review. IOSR J Pharm Biol Sci 2:1–5
Chen XM, Dong HL, Hu KX, Sun ZR, Chen J, Guo SX (2010) Diversity and antimicrobial and plant-growth-promoting activities of endophytic fungi in Dendrobium loddigesii Rolfe. J Plant Growth Regul 29:328–337
Chen Z, Song Y, Chen Y, Huang H, Zhang W, Ju J (2012) Cyclic heptapeptides, cordyheptapeptides C–E, from the marine-derived fungus Acremonium persicinum SCSIO 115 and their cytotoxic activities. J Nat Prod 75:1215–1219
Chen G-D, Chen Y, Gao H, Shen L-Q, Wu Y, Li XX, Li Y, Guo LD, Cen YZ, Yao X-S (2013) Xanthoquinodins from the endolichenic fungal strain Chaetomium elatum. J Nat Prod 76:702–709
Choi W-Y, Rim S-O, Lee J-H, Lee J-M, Lee I-J, Cho K-J, Rhee I-K, Kwon J-B, Kim J-G (2005) Isolation of gibberellins-producing fungi from the root of several Sesamum indicum plants. J Microbiol Biotechnol 15:22–28
Chow Y, Ting AS (2015) Endophytic L-asparaginase-producing fungi from plants associated with anticancer properties. J Adv Res 6:869–876
Clark R, Lee SH (2016) Anticancer properties of capsaicin against human cancer. Anticancer Res 36:837–843
Clay K, Holah J (1999) Fungal endophyte symbiosis and plant diversity in successional fields. Science 285:1742–1744
Colla G, Rouphael Y, Bonini P, Cardarelli M (2015) Coating seeds with endophytic fungi enhances growth, nutrient uptake, yield and grain quality of winter wheat. Int J Plant Prot 9:171–189
Collins T, Gerday C, Feller G (2005) Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol Rev 29:3–23
Comby M, Gacoin M, Robineau M, Rabenoelina F, Ptas S, Dupont J, Profizi C, Baillieul F (2017) Screening of wheat endophytes as biological control agents against Fusarium head blight using two different in vitro tests. Microbiol Res 202:11–20
Correa A, Pacheco S, Mechaly AE, Obal G, Béhar G, Mouratou B, Oppezzo P, Alzari PM, Pecorari F (2014) Potent and specific inhibition of glycosidases by small artificial binding proteins (Affitins). PLoS One 9:e97438
Costa JM, Loper JE (1994) Characterization of siderophore production by the biological control agent Enterobacter cloacae. MPMI 7:440–448
Costa-Silva TA, Nogueira MA, Fernandes Souza CR, Oliveira WP, Said S (2011) Lipase production by endophytic fungus Cercospora kikuchii: stability of enzymatic activity after spray drying in the presence of carbohydrates. Drying Technol 29:1112–1119
Cui J-L, Guo S-X, Xiao P-G (2011) Antitumor and antimicrobial activities of endophytic fungi from medicinal parts of Aquilaria sinensis. J Zhejiang Univ Sci B 12:385–392
Dai C-C, Tian L-S, Zhao Y-T, Chen Y, Xie H (2010) Degradation of phenanthrene by the endophytic fungus Ceratobasidum stevensii found in Bischofia polycarpa. Biodegradation 21:245–255
Dastogeer KM, Li H, Sivasithamparam K, Jones MG, Wylie SJ (2018) Fungal endophytes and a virus confer drought tolerance to Nicotiana benthamiana plants through modulating osmolytes, antioxidant enzymes and expression of host drought responsive genes. Environ Exper Bot 149:95–108
De Bary A (1866) Morpholodie und Physiologie del Pilze. Flechten und Myxomyceten, Engelmann, Leipzig
De Siqueira VM, Conti R, de Araújo JM, Souza-Motta CM (2011) Endophytic fungi from the medicinal plant Lippia sidoides Cham. and their antimicrobial activity. Symbiosis 53:89–95
De Souza Leite T, Cnossen-Fassoni A, Pereira OL, Mizubuti ESG, de Araújo EF, de Queiroz MV (2013) Novel and highly diverse fungal endophytes in soybean revealed by the consortium of two different techniques. J Microbiol 51:56–69
Del Giudice L, Massardo DR, Pontieri P, Bertea CM, Mombello D, Carata E, Tredici SM, Talà A, Mucciarelli M, Groudeva VI (2008) The microbial community of Vetiver root and its involvement into essential oil biogenesis. Environ Microbiol 10:2824–2841
Demain AL, Sanchez S (2009) Microbial drug discovery: 80 years of progress. J Antibiot 62:5–16
Dhouib A, Hamza M, Zouari H, Mechichi T, Hmidi R, Labat M, Martinez MJ, Sayadi S (2005) Screening for ligninolytic enzyme production by diverse fungi from Tunisia. World J Microbiol Biotechnol 21:1415–1423
Ding G, Li Y, Fu S, Liu S, Wei J, Che Y (2008) Ambuic acid and torreyanic acid derivatives from the endolichenic fungus Pestalotiopsis sp. J Nat Prod 72:182–186
Dissanayake RK, Ratnaweera PB, Williams DE, Wijayarathne CD, Wijesundera RL, Andersen RJ, de Silva ED (2016) Antimicrobial activities of endophytic fungi of the Sri Lankan aquatic plant Nymphaea nouchali and chaetoglobosin A and C, produced by the endophytic fungus Chaetomium globosum. Mycology 7:1–8
Dos Santos Souza B, dos Santos TT (2017) Endophytic fungi in economically important plants: ecological aspects, diversity and potential biotechnological applications. J Bio Food Sci 4:113–126
Dos Santos TT, de Souza Leite T, de Queiroz CB, de Araújo EF, Pereira OL, de Queiroz MV (2016) High genetic variability in endophytic fungi from the genus Diaporthe isolated from common bean (Phaseolus vulgaris L.) in Brazil. J App Microbiol 120:388–401
Dou Y, Wang X, Jiang D, Wang H, Jiao Y, Lou H, Wang X (2014) Metabolites from Aspergillus versicolor, an endolichenic fungus from the lichen Lobaria retigera. Drug Discov Ther 8:84–88
Durand F, Gounel S, Mano N (2013) Purification and characterization of a new laccase from the filamentous fungus Podospora anserina. Prot Expr Purif 88:61–66
Dutta S, Mishra A, Kumar BD (2008) Induction of systemic resistance against fusarial wilt in pigeon pea through interaction of plant growth promoting rhizobacteria and rhizobia. Soil Biol Biochem 40:452–461
Elfita E, Muharni M, Munawar M, Legasari L, Darwati D (2011) Antimalarial compounds from endophytic fungi of Brotowali (Tinaspora crispa L). Indones J Chemis 11:53–58
El-Zayat S (2008) Preliminary studies on laccase production by Chaetomium globosum an endophytic fungus in Glinus lotoides. Am Eurasian J Agric Environ Sci 3:86–90
Erbert C, Lopes AA, Yokoya NS, Furtado NA, Conti R, Pupo MT, Lopes JLC, Debonsi HM (2012) Antibacterial compound from the endophytic fungus Phomopsis longicolla isolated from the tropical red seaweed Bostrychia radicans. Bot Mar 55:435–440
Escudero N, Ferreira SR, Lopez-Moya F, Naranjo-Ortiz MA, Marin-Ortiz AI, Thornton CR, Lopez-Llorca LV (2016) Chitosan enhances parasitism of Meloidogyne javanica eggs by the nematophagous fungus Pochonia chlamydosporia. Fungal Biol 120:572–585
Evans CS, Dutton MV, Guillén F, Veness RG (1994) Enzymes and small molecular mass agents involved with lignocellulose degradation. FEMS Microbiol Rev 13:235–239
Eyberger AL, Dondapati R, Porter JR (2006) Endophyte fungal isolates from Podophyllum peltatum produce podophyllotoxin. J Nat Prod 69:1121–1124
Fareed S, Jadoon UN, Ullah I, Ayub M, Jadoon MUR, Bibi Z, Waqas M, Nisa S (2017) Isolation and biological evaluation of endophytic fungus from Ziziphus nummularia. J Entomol Zool Stud 5(3):32–38
Fernandes EG, Pereira OL, da Silva CC, Bento CBP, de Queiroz MV (2015) Diversity of endophytic fungi in Glycine max. Microbiol Res 181:84–92
Fillat Ú, Martín-Sampedro R, Macaya-Sanz D, Martín JA, Ibarra D, Martínez MJ, Eugenio ME (2016) Screening of eucalyptus wood endophytes for laccase activity. Process Biochem 51:589–598
Fisher P, Petrini O (1992) Fungal saprobes and pathogens as endophytes of rice (Oryza sativa L.). New Phytol 120:137–143
Fisher P, Graf F, Petrini L, Sutton B, Wookey P (1995) Fungal endophytes of Dryas octopetala from a high arctic polar semidesert and from the Swiss Alps. Mycologia 87:319–323
Fouda AH, Hassan SE-D, Eid AM, Ewais EE-D (2015) Biotechnological applications of fungal endophytes associated with medicinal plant Asclepias sinaica (Bioss.). Ann Agric Sci 60:95–104
Freeman EM (1904) I.—The seed-fungus of Lolium temulentum, L., the darnel. Phil Trans R Soc Lond B 196:1–27
Fröhlich J, Hyde KD (1999) Biodiversity of palm fungi in the tropics: are global fungal diversity estimates realistic? Biodivers Conserv 8:977–1004
Fu J, Zhou Y, Li H-F, Ye Y-H, Guo J-H (2011) Antifungal metabolites from Phomopsis sp. By254, an endophytic fungus in Gossypium hirsutum. A J Microbiol Res 5:1231–1236
Gadd GM (2000) Bioremedial potential of microbial mechanisms of metal mobilization and immobilization. Curr Opin Biotechnol 11:271–279
Gamboa MA, Bayman P (2001) Communities of endophytic fungi in leaves of a tropical timber tree (Guarea guidonia: Meliaceae) 1. Biotropica 33:352–360
Gangadevi V, Muthumary J (2008) Taxol, an anticancer drug produced by an endophytic fungus Bartalinia robillardoides Tassi, isolated from a medicinal plant, Aegle marmelos Correa ex Roxb. W J Microbiol Biotechnol 24:717
Gao Y, Zhao JT, Zu YG, Fu YJ, Wang W, Luo M, Efferth T (2011) Characterization of five fungal endophytes producing Cajaninstilbene acid isolated from pigeon pea [Cajanus cajan (L.) Millsp.]. PLoS One 6:e27589
Gao Y, Zhao J, Zu Y, Fu Y, Liang L, Luo M, Wang W, Efferth T (2012) Antioxidant properties, superoxide dismutase and glutathione reductase activities in HepG2 cells with a fungal endophyte producing apigenin from pigeon pea [Cajanus cajan (L.) Millsp.]. Food Res Int 49:147–152
García A, Rhoden SA, Rubin Filho CJ, Nakamura CV, Pamphile JA (2012) Diversity of foliar endophytic fungi from the medicinal plant Sapindus saponaria L. and their localization by scanning electron microscopy. Biol Res 45:139–148
Garg G, Singh A, Kaur A, Singh R, Kaur J, Mahajan R (2016) Microbial pectinases: an ecofriendly tool of nature for industries. 3 Biotech 6:47. https://doi.org/10.1007/s13205-016-0371-4
Ginalska G, Bancerz R, Korniłłowicz-Kowalska T (2004) A thermostable lipase produced by a newly isolated Geotrichum-like strain, R59. J Ind Microbiol Biotechnol 31:177–182
Gond S, Verma V, Kumar A, Kumar V, Kharwar R (2007) Study of endophytic fungal community from different parts of Aegle marmelos Correae (Rutaceae) from Varanasi (India). World J Microbiol Biotechnol 23:1371–1375
Gond SK, Mishra A, Sharma VK, Verma SK, Kumar J, Kharwar RN, Kumar A (2012) Diversity and antimicrobial activity of endophytic fungi isolated from Nyctanthes arbor-tristis, a well-known medicinal plant of India. Mycoscience 53:113–121
Gong L, Guo S (2009) Endophytic fungi from Dracaena cambodiana and Aquilaria sinensis and their antimicrobial activity. Afr J Biotechnol 8(5):731–736
Gontia-Mishra I, Tiwari S (2013) Molecular characterization and comparative phylogenetic analysis of phytases from fungi with their prospective applications. Food Technol Biotechnol 51:313–326
Gonzaga L, Costa L, Santos T, Araújo E, Queiroz M (2015) Endophytic fungi from the genus Colletotrichum are abundant in the Phaseolus vulgaris and have high genetic diversity. J Appl Microbiol 118:485–496
González MC, Buenrostro-Figueroa J, Durán LR, Zárate P, Rodríguez R, Rodríguez-Jasso RM, Ruiz HA, Aguilar CN (2017) Tannases. In: Current developments in biotechnology and bioengineering. Elsevier, Amsterdam/Boston, pp 471–489
Gopinath SC, Anbu P, Lakshmipriya T, Hilda A (2013) Strategies to characterize fungal lipases for applications in medicine and dairy industry. Biomed Res Int. https://doi.org/10.1155/2013/154549
Govindachari T, Viswanathan N (1972) 9-Methoxycamptothecin. A new alkaloid from Mappia foetida Miers. Ind J Chem 10:453–454
Goyal S, Ramawat K, Mérillon J (2016) Different shades of fungal metabolites: an overview. In: Merillon JM, Ramawat K (eds) Fungal metabolites. Reference series in phytochemistry. Springer, Cham, pp 1–29
Gray J, Bemiller J (2003) Bread staling: molecular basis and control. Compr Rev Food Sci Food Saf 2:1–21
Guillén F, Martınez Ma J, Muñoz C, Martınez AT (1997) Quinone redox cycling in the ligninolytic fungus Pleurotus eryngii leading to extracellular production of superoxide anion radical. Arch Biochem Biophys 339:190–199
Guillén F, Martínez MJ, Gutiérrez A, Del Rio J (2005) Biodegradation of lignocellulosics: microbial, chemical, and enzymatic aspects of the fungal attack of lignin. Int Microbiol 8:195–204
Guo B, Li H, Zhang L (1998) Isolation of a fungus producing vinblastine. J Yunnan Uni (Nat Sci) 20:214–215
Guo B, Dai J-R, Ng S, Huang Y, Leong C, Ong W, Carté BK (2000) Cytonic acids A and B: novel tridepside inhibitors of hCMV protease from the endophytic fungus Cytonaema species. J Nat Prod 63:602–604
Guo B, Wang Y, Sun X, Tang K (2008) Bioactive natural products from endophytes: a review. App Biochem Microbiol 44:136–142
Gutierrez A, Caramelo L, Prieto A, Martínez MJ, Martinez AT (1994) Anisaldehyde production and aryl-alcohol oxidase and dehydrogenase activities in ligninolytic fungi of the genus Pleurotus. App Environ Microbiol 60:1783–1788
Hallmann J, Quadt-Hallmann A, Mahaffee W, Kloepper J (1997) Bacterial endophytes in agricultural crops. Can J Microbiol 43:895–914
Hamayun M, Khan SA, Ahmad N, Tang D-S, Kang S-M, Na C-I, Sohn E-Y, Hwang Y-H, Shin D-H, Lee B-H (2009a) Cladosporium sphaerospermum as a new plant growth-promoting endophyte from the roots of Glycine max (L.) Merr. World J Microbiol Biotechnol 25:627–632
Hamayun M, Khan SA, Khan AL, Rehman G, Sohn E-Y, Shah AA, Kim S-K, Joo G-J, Lee I-J (2009b) Phoma herbarum as a new gibberellin-producing and plant growth-promoting fungus. J Microbiol Biotechnol 19:1244–1249
Hamayun M, Khan SA, Khan MA, Khan AL, Kang S-M, Kim S-K, Joo G-J, Lee I-J (2009c) Gibberellin production by pure cultures of a new strain of Aspergillus fumigatus. World J Microbiol Biotechnol 25:1785–1792
Hamayun M, Khan SA, Khan AL, Rehman G, Kim Y-H, Iqbal I, Hussain J, Sohn E-Y, Lee I-J (2010) Gibberellin production and plant growth promotion from pure cultures of Cladosporium sp. MH-6 isolated from cucumber (Cucumis sativus L.). Mycologia 102:989–995
Hamayun M, Hussain A, Khan SA, Kim H-Y, Khan AL, Waqas M, Irshad M, Iqbal A, Rehman G, Jan S (2017) Gibberellins producing endophytic fungus Porostereum spadiceum AGH786 rescues growth of salt affected soybean. Front Microbiol. https://doi.org/10.3389/fmicb.2017.00686
Hanada RE, Pomella AWV, Costa HS, Bezerra JL, Loguercio LL, Pereira JO (2010) Endophytic fungal diversity in Theobroma cacao (cacao) and T. grandiflorum (cupuaçu) trees and their potential for growth promotion and biocontrol of black-pod disease. Fungal Biol 114:901–910
Harish S, Kavino M, Kumar N, Balasubramanian P, Samiyappan R (2009) Induction of defense-related proteins by mixtures of plant growth promoting endophytic bacteria against Banana bunchy top virus. Biol Control 51:16–25
Harnpicharnchai P, Champreda V, Sornlake W, Eurwilaichitr L (2009) A thermotolerant β-glucosidase isolated from an endophytic fungi, Periconia sp., with a possible use for biomass conversion to sugars. Prot Expr Purif 67:61–69
Haros M, Rosell CM, Benedito C (2001) Fungal phytase as a potential breadmaking additive. Eur Food Res Technol 213:317–322
Harper JK, Arif AM, Ford EJ, Strobel GA, Porco JA, Tomer DP, Oneill KL, Heider EM, Grant DM (2003) Pestacin: a 1, 3-dihydro isobenzofuran from Pestalotiopsis microspora possessing antioxidant and antimycotic activities. Tetrahedron 59:2471–2476
Harris AD, Ramalingam C (2010) Xylanases and its application in food industry: a review. J Exp Sci 1:1–11
Hasan H (2002) Gibberellin and auxin-indole production by plant root-fungi and their biosynthesis under salinity-calcium interaction. Acta Microbiol Immunol Hung 49:105–118
Hassan SED, Liu A, Bittman S, Forge TA, Hunt DE, Hijri M, St-Arnaud M (2013) Impact of 12-year field treatments with organic and inorganic fertilizers on crop productivity and mycorrhizal community structure. Biol Fert Soils 49:1109–1121
Hazalin NAMN, Ramasamy K, Lim SM, Cole AL, Majeed ABA (2012) Induction of apoptosis against cancer cell lines by four ascomycetes (endophytes) from Malaysian rainforest. Phytomedicine 19:609–617
Heidarizadeh M, Rezaei PF, Shahabivand S (2018) Novel pectinase from Piriformospora indica, optimization of growth parameters and enzyme production in submerged culture condition. Turk J Biochem 43:289–295
Higuchi T (2012) Biochemistry and molecular biology of wood. Springer, London
Hoffman AM, Mayer SG, Strobel GA, Hess WM, Sovocool GW, Grange AH, Harper JK, Arif AM, Grant DM, Kelley-Swift EG (2008) Purification, identification and activity of phomodione, a furandione from an endophytic Phoma species. Phytochemistry 69:1049–1056
Huang H, She Z, Lin Y, Vrijmoed L, Lin W (2007) Cyclic peptides from an endophytic fungus obtained from a mangrove leaf (Kandelia candel). J Nat Prod 70:1696–1699
Huang W, Cai Y, Hyde K, Corke H, Sun M (2008) Biodiversity of endophytic fungi associated with 29 traditional Chinese medicinal plants. Fungal Divers 33:61–75
Huang W, Cai Y, Surveswaran S, Hyde K, Corke H, Sun M (2009) Molecular phylogenetic identification of endophytic fungi isolated from three Artemisia species. Fungal Divers 36:69–88
Huang Y-L, Zimmerman NB, Arnold AE (2018) Observations on the early establishment of foliar endophytic fungi in leaf discs and living leaves of a model woody angiosperm, Populus trichocarpa (Salicaceae). J Fungi (Basel, Switzerland). https://doi.org/10.3390/jof4020058
Hung PQ, Kumar SM, Govindsamy V, Annapurna K (2007) Isolation and characterization of endophytic bacteria from wild and cultivated soybean varieties. Biol Fert Soils 44:155–162
Impullitti A, Malvick D (2013) Fungal endophyte diversity in soybean. J Appl Microbiol 114:1500–1506
Jacobsen T, Olsen J, Allermann K (1990) Substrate specificity of Geotrichum candidum lipase preparations. Biotechnol Lett 12:121–126
Jalgaonwala RE, Mahajan RT (2014) Production of anticancer enzyme asparaginase from endophytic Eurotium sp. isolated from rhizomes of Curcuma longa. Euro J Exp Biol 4:36–43
Jalgaonwala RE, Mohite BV, Mahajan RT (2017) A review: natural products from plant associated endophytic fungi. J Microbiol Biotechnol Research 1:21–32
Jarvis AP, Morgan ED, Van Der Esch SA, Vitali F, Ley SV, Pape A (1997) Identification of azadirachtin in tissue-cultured cells of neem (Azadirachta indica). Nat Prod Lett 10:95–98
Jennifer Mordue A, Simmonds MS, Ley SV, Blaney WM, Mordue W, Nasiruddin M, Nisbet AJ (1998) Actions of azadirachtin, a plant allelochemical, against insects. Pes Sci 54:277–284
Jerry B (1994) A role of endophytic fungi in regulating nutrients and energy in plants within a desert ecosystem. International symposium and workshop on desertification in developed countries. Accessed on 2011/10/25
Jha PN, Gupta G, Jha P, Mehrotra R (2013) Association of rhizospheric/endophytic bacteria with plants: a potential gateway to sustainable agriculture. Greener J Agr Sci 3:73–84
Jin H, Yan Z, Liu Q, Yang X, Chen J, Qin B (2013) Diversity and dynamics of fungal endophytes in leaves, stems and roots of Stellera chamaejasme L. in northwestern China. Antonie Van Leeuwenhoek 104:949–963
Kalyanasundaram I, Nagamuthu J, Srinivasan B, Pachayappan A, Muthukumarasamy S (2015) Production, purification and characterisation of extracellular L-asparaginase from salt marsh fungal endophytes. World J Pharm Sci 4:663–677
Kaul S, Ahmed M, Zargar K, Sharma P, Dhar MK (2013) Prospecting endophytic fungal assemblage of Digitalis lanata Ehrh. (foxglove) as a novel source of digoxin: a cardiac glycoside. 3 Biotech 3:335–677
Kaur R, Saxena A, Sangwan P, Yadav AN, Kumar V, Dhaliwal HS (2017) Production and characterization of a neutral phytase of Penicillium oxalicum EUFR-3 isolated from Himalayan region. Nus Biosci 9:68–76
Kawaide H (2006) Biochemical and molecular analyses of gibberellin biosynthesis in fungi. Biosci Biotechnol Biochem 70:583–590
Kedar A, Rathod D, Yadav A, Agarkar G, Rai M (2014) Endophytic Phoma sp. isolated from medicinal plants promote the growth of Zea mays. Nus Biosci 6:132–139
Keyser CA, Jensen B, Meyling NV (2016) Dual effects of Metarhizium spp. and Clonostachys rosea against an insect and a seed-borne pathogen in wheat. Pest Manag Sci 72:517–526
Khan SA, Hamayun M, Yoon H, Kim H-Y, Suh S-J, Hwang S-K, Kim J-M, Lee I-J, Choo Y-S, Yoon U-H (2008) Plant growth promotion and Penicillium citrinum. BMC Microbiol. https://doi.org/10.1186/1471-2180-8-231
Khan SA, Hamayun M, Kim H-Y, Yoon H-J, Lee I-J, Kim J-G (2009a) Gibberellin production and plant growth promotion by a newly isolated strain of Gliomastix murorum. W J Microbiol Biotechnol 25:829–833
Khan SA, Hamayun M, Kim H-Y, Yoon H-J, Seo J-C, Choo Y-S, Lee I-J, Rhee I-K, Kim J-G (2009b) A new strain of Arthrinium phaeospermum isolated from Carex kobomugi Ohwi is capable of gibberellin production. Biotechnol Lett 31:283–287
Khan AL, Hamayun M, Ahmad N, Waqas M, Kang SM, Kim YH, Lee IJ (2011a) Exophiala sp. LHL08 reprograms Cucumis sativus to higher growth under abiotic stresses. Physiol Plant 143:329–343
Khan AL, Hamayun M, Kim Y-H, Kang S-M, Lee I-J (2011b) Ameliorative symbiosis of endophyte (Penicillium funiculosum LHL06) under salt stress elevated plant growth of Glycine max L. Plant Physiol Biochem 49:852–861
Khan AL, Hamayun M, Hussain J, Kang S-M, Lee I-J (2012a) The newly isolated endophytic fungus Paraconiothyrium sp. LK1 produces ascotoxin. Molecules 17:1103–1112
Khan AL, Hamayun M, Khan SA, Kang S-M, Shinwari ZK, Kamran M, ur Rehman S, Kim JG, Lee IJ (2012b) Pure culture of Metarhizium anisopliae LHL07 reprograms soybean to higher growth and mitigates salt stress. World J Microbiol Biotechnol 28:1483–1494
Khan AL, Hussain J, Al-Harrasi A, Al-Rawahi A, Lee I-J (2015) Endophytic fungi: resource for gibberellins and crop abiotic stress resistance. Crit Rev Biotechnol 35:62–74
Khan AL, Shahzad R, Al-Harrasi A, Lee IJ (2017) Endophytic microbes: a resource for producing extracellular enzymes. In: Maheshwari D, Annapurna K (eds) Endophytes: crop productivity and protection. Sustainable development and biodiversity, vol 16. Springer, Cham
Kharwar RN, Verma VC, Kumar A, Gond SK, Harper JK, Hess WM, Lobkovosky E, Ma C, Ren Y, Strobel GA (2009) Javanicin, an antibacterial naphthaquinone from an endophytic fungus of neem, Chloridium sp. Curr Microbiol 58:233–238
Kharwar R, Maurya A, Verma V, Kumar A, Gond S, Mishra A (2012) Diversity and antimicrobial activity of endophytic fungal community isolated from medicinal plant Cinnamomum camphora. Proc Natl Acad Sci India Section B Biol Sci 82:557–565
Khiralla A, Spina R, Yagi S, Mohamed I, Laurain-Mattar D (2017) Endophytic fungi: occurrence, classification, function and natural products. In: Hughes E (ed) Endophytic fungi: diversity, characterization and biocontrol. Nova Science Publishers, New York, pp 1–38
Kim S, Shin D-S, Lee T, Oh K-B (2004) Periconicins, two new fusicoccane diterpenes produced by an endophytic fungus Periconia sp. with antibacterial activity. J Nat Prod 67:448–450
Kim JS, Gao J, Daniel G (2015) Cytochemical and immunocytochemical characterization of wood decayed by the white rot fungus Pycnoporus sanguineus I. preferential lignin degradation prior to hemicelluloses in Norway spruce wood. Int Biodeterior Biodegradation 105:30–40
Kirti S, Reddy M (2013) Characterization of thermostable and alkalophilic lipase enzyme from endophytic fungus Leptosphaerulina sp. Ph.D. Thesis. http://hdl.handle.net/10266/2541
Kjer J, Wray V, Edrada-Ebel R, Ebel R, Pretsch A, Lin W, Proksch P (2009) Xanalteric acids I and II and related phenolic compounds from an endophytic Alternaria sp. isolated from the mangrove plant Sonneratia alba. J Nat Prod 72:2053–2057
Klenk A, Bokel M, Kraus W (1986) 3-Tigloylazadirachtol (tigloyl= 2-methylcrotonoyl), an insect growth regulating constituent of Azadirachta indica. J Chem Soc Chem Commun 0:523–524
Köhl J, Lombaers C, Moretti A, Bandyopadhyay R, Somma S, Kastelein P (2015) Analysis of microbial taxonomical groups present in maize stalks suppressive to colonization by toxigenic Fusarium spp.: a strategy for the identification of potential antagonists. Biol Control 83:20–28
Koukol O, Kolařík M, Kolářová Z, Baldrian P (2012) Diversity of foliar endophytes in wind-fallen Picea abies trees. Fungal Divers 54:69–77
Kraus W, Bokel M, Klenk A, Pöhn H (1985) The structure of azadirachtin and 22, 23-dihydro-23β-methoxyazadirachtin. Tetrahedron Lett 26:6435–6438
Krishnamurthy YL, Naik BS (2017) Endophytic fungi bioremediation. In: Maheshwari D, Annapurna K (eds) Endophytes: crop productivity and protection. Sustainable development and biodiversity, vol 16. Springer, Cham
Kudalkar P, Strobel G, Riyaz-Ul-Hassan S, Geary B, Sears J (2012) Muscodor sutura, a novel endophytic fungus with volatile antibiotic activities. Mycoscience 53:319–325
Kudanga T, Mwenje E (2005) Extracellular cellulase production by tropical isolates of Aureobasidium pullulans. Can J Microbiol 51:773–776
Kumar S, Kaushik N (2013) Endophytic fungi isolated from oil-seed crop Jatropha curcas produces oil and exhibit antifungal activity. PLoS One 8:e56202
Kumar A, Patil D, Rajamohanan PR, Ahmad A (2013) Isolation, purification and characterization of vinblastine and vincristine from endophytic fungus Fusarium oxysporum isolated from Catharanthus roseus. PLoS One 8:e71805
Kumar M, Yadav AN, Tiwari R, Prasanna R, Saxena AK (2014) Evaluating the diversity of culturable thermotolerant bacteria from four hot springs of India. J Biodivers Biopros Dev 1:1–9
Kumar V, Yadav AN, Saxena A, Sangwan P, Dhaliwal HS (2016) Unravelling rhizospheric diversity and potential of phytase producing microbes. SM J Biol 2:1009
Kumar K, Yadav AN, Kumar V, Vyas P, Dhaliwal HS (2017a) Food waste: a potential bioresource for extraction of nutraceuticals and bioactive compounds. Biores Bioprocess. https://doi.org/10.1186/s40643-017-0148-6
Kumar V, Yadav AN, Verema P, Sangwan P, Abhishake S, Singh B (2017b) β-Propeller phytases: diversity, catalytic attributes, current developments and potential biotechnological applications. Int J Biol Macromol 98:595–609
Kumara PM, Shweta S, Vasanthakumari M, Sachin N, Manjunatha B, Jadhav SS, Ravikanth G, Ganeshaiah K, Shaanker RU (2014) Endophytes and plant secondary metabolite synthesis: molecular and evolutionary perspective. In: Advances in endophytic research. Springer, New Delhi, pp 177–190
Kunamneni A, Camarero S, García-Burgos C, Plou FJ, Ballesteros A, Alcalde M (2008) Engineering and applications of fungal laccases for organic synthesis. Microbial Cell Factories. https://doi.org/10.1186/1475-2859-7-32
Kurose D, Furuya N, Tsuchiya K, Tsushima S, Evans HC (2012) Endophytic fungi associated with Fallopia japonica (Polygonaceae) in Japan and their interactions with Puccinia polygoni-amphibii var. tovariae, a candidate for classical biological control. Fungal Biol 116:785–791
Kusari S, Spiteller M (2011) Are we ready for industrial production of bioactive plant secondary metabolites utilizing endophytes? Nat Prod Rep 28:1203–1207
Kusari S, Spiteller M (2012) Metabolomics of endophytic fungi producing associated plant secondary metabolites: progress, challenges and opportunities. In: Roessner U (ed) Metabolomics. InTech, Rijeka, pp 241–66
Kusari S, Lamshöft M, Spiteller M (2009) Aspergillus fumigatus Fresenius, an endophytic fungus from Juniperus communis L. Horstmann as a novel source of the anticancer pro-drug deoxypodophyllotoxin. J App Microbiol 107:1019–1030
Kusari S, Zuhlke S, Spiteller M (2011) Effect of artificial reconstitution of the interaction between the plant Camptotheca acuminata and the fungal endophyte Fusarium solani on camptothecin biosynthesis. J Nat Prod 74:764–775
Kusari S, Verma VC, Lamshoeft M, Spiteller M (2012) An endophytic fungus from Azadirachta indica A. Juss. that produces azadirachtin. World J Microbiol Biotechnol 28:1287–1294
Kusari P, Kusari S, Spiteller M, Kayser O (2013a) Endophytic fungi harbored in Cannabis sativa L.:diversity and potential as biocontrol agents against host plant-specific phytopathogens. Fungal Diversity 60:137–151
Kusari S, Pandey SP, Spiteller M (2013b) Untapped mutualistic paradigms linking host plant and endophytic fungal production of similar bioactive secondary metabolites. Phytochemistry 91:81–87
Kusari S, Singh S, Jayabaskaran C (2014) Rethinking production of Taxol®(paclitaxel) using endophyte biotechnology. Trends Biotechnol 32:304–311
Larran S, Monaco C, Alippi H (2001) Endophytic fungi in leaves of Lycopersicon esculentum Mill. World J Microbiol Biotechnol 17:181–184
Larran S, Perello A, Simon M, Moreno V (2002) Isolation and analysis of endophytic microorganisms in wheat (Triticum aestivum L.) leaves. World J Microbiol Biotechnol 18:683–686
Larran S, Perelló A, Simón MR, Moreno V (2007) The endophytic fungi from wheat (Triticum aestivum L.). World J Microbiol Biotechnol 23:565–572
Larran S, Siurana MPS, Caselles JR, Simón MR, Perelló A (2018) Fusarium sudanense, endophytic fungus causing typical symptoms of seedling blight and seed rot on wheat. J King Saud Uni-Sci. https://doi.org/10.1016/j.jksus.2018.07.005
Lee JC, Lobkovsky E, Pliam NB, Strobel G, Clardy J (1995) Subglutinols A and B: immunosuppressive compounds from the endophytic fungus Fusarium subglutinans. J Org Chem 60:7076–7077
Lee JC, Strobel GA, Lobkovsky E, Clardy J (1996) Torreyanic acid: a selectively cytotoxic quinone dimer from the endophytic fungus Pestalotiopsis microspora. J Org Chem 61:3232–3233
Ley S, Denholm A, Wood A (1993) The chemistry of azadirachtin. Nat Prod Rep 10:109–157
Li JY, Strobel GA (2001) Jesterone and hydroxy-jesterone antioomycete cyclohexenone epoxides from the endophytic fungus Pestalotiopsis jesteri. Phytochemistry 57:261–265
Li J, Harper JK, Grant DM, Tombe BO, Bashyal B, Hess W, Strobel GA (2001) Ambuic acid, a highly functionalized cyclohexenone with antifungal activity from Pestalotiopsis spp. and Monochaetia sp. Phytochemistry 56:463–468
Li GH, Yu ZF, Li X, Wang XB, Zheng LJ, Zhang KQ (2007a) Nematicidal metabolites produced by the endophytic fungus Geotrichum sp. AL4. Chem Biodivers 4:1520–1524
Li WC, Zhou J, Guo SY, Guo LD (2007b) Endophytic fungi associated with lichens in Baihua mountain of Beijing, China. Fungal Divers 25:69–80
Li M, Wu Y, Jiang F, Yu X, Tang K, Miao Z (2009) Isolation, identification and anticancer activity of an endophytic fungi from Juglans mandshurica. Zhongguo Zhong Yao Za Zhi 34:1623–1627
Li H-Q, Li X-J, Wang Y-L, Zhang Q, Zhang A-L, Gao J-M, Zhang X-C (2011) Antifungal metabolites from Chaetomium globosum, an endophytic fungus in Ginkgo biloba. Biochem Sys Eco 39:876–879
Li G, Wang H, Zhu R, Sun L, Wang L, Li M, Li Y, Liu Y, Zhao Z, Lou H (2012a) Phaeosphaerins A–F, cytotoxic perylenequinones from an endolichenic fungus, Phaeosphaeria sp. J Nat Prod 75:142–147
Li H-Y, Wei D-Q, Shen M, Zhou Z-P (2012b) Endophytes and their role in phytoremediation. Fungal Divers 54:11–18
Li G, Kusari S, Lamshöft M, Schüffler A, Laatsch H, Spiteller M (2014) Antibacterial secondary metabolites from an endophytic fungus, Eupenicillium sp. LG41. J Nat Prod 77:2335–2341
Li Y, Yang J, Zhou X, Zhao W, Jian Z (2015) Isolation and identification of a 10-deacetyl baccatin-III-producing endophyte from Taxus wallichiana. App Biochem Biotechnol 175:2224–2231
Lim PE, Mak K, Mohamed N, Noor AM (2003) Removal and speciation of heavy metals along the treatment path of wastewater in subsurface-flow constructed wetlands. Wat Sci Technol 48:307–313
Lin Z-J, Lu Z-Y, Zhu T-J, Fang Y-C, Gu Q-Q, Zhu W-M (2008a) Penicillenols from Penicillium sp. GQ-7, an endophytic fungus associated with Aegiceras corniculatum. Chem Pharmaceu Bull 56:217–221
Lin Z, Zhu T, Fang Y, Gu Q, Zhu W (2008b) Polyketides from Penicillium sp. JP-1, an endophytic fungus associated with the mangrove plant Aegiceras corniculatum. Phytochemistry 69:1273–1278
Liu Y, Yang Q, Xia G, Huang H, Li H, Ma L, Lu Y, He L, Xia X, She Z (2015) Polyketides with α-glucosidase inhibitory activity from a mangrove endophytic fungus, Penicillium sp. HN29-3B1. J Nat Prod 78:1816–1822
Liu Y, Nan L, Liu J, Yan H, Zhang D, Han X (2016) Isolation and identification of resveratrol-producing endophytes from wine grape Cabernet Sauvignon. SpringerPlus 5:1–13
Maheshwari DK (2011) Bacteria in agrobiology: plant growth responses. Springer, Berlin
Maheswari S, Rajagopal K (2013) Biodiversity of endophytic fungi in Kigelia pinnata during two different seasons. Curr Sci 104:515–518
Makky EA, Yusoff MM (2015) Bioeconomy: pectinases purification and application of fermented waste from Thermomyces lanuginosus. J Med Bioeng 4(1):76–80
Malfanova N, Kamilova F, Validov S, Shcherbakov A, Chebotar V, Tikhonovich I, Lugtenberg B (2011) Characterization of Bacillus subtilis HC8, a novel plant-beneficial endophytic strain from giant hogweed. Microbial Biotechnol 4:523–532
Malik A (2004) Metal bioremediation through growing cells. Environ Int 30:261–278
Mandels M (1985) Applications of cellulases. Portland Press Limited, Biochem Soc Trans 13:414–415. https://doi.org/10.1042/bst0130414
Marcenaro D, Valkonen JP (2016) Seedborne pathogenic fungi in common bean (Phaseolus vulgaris cv. INTA Rojo) in Nicaragua. PLoS One 11:e0168662
Maria G, Sridhar K, Raviraja N (2005) Antimicrobial and enzyme activity of mangrove endophytic fungi of southwest coast of India. J Agri Technol 1:67–80
Marinho AM, Rodrigues-Filho E, Moitinho MLR, Santos LS (2005) Biologically active polyketides produced by Penicillium janthinellum isolated as an endophytic fungus from fruits of Melia azedarach. J Braz Chem Soc 16:280–283
Marlida Y, Delfita R, Gusmanizar N, Ciptaan G (2010) Identification characterization and production of phytase from endophytic fungi. World Acad Sci Eng Technol 65:1043–1046
Marques NP, de Cassia Pereira J, Gomes E, da Silva R, Araújo AR, Ferreira H, Rodrigues A, Dussán KJ, Bocchini DA (2018) Cellulases and xylanases production by endophytic fungi by solid state fermentation using lignocellulosic substrates and enzymatic saccharification of pretreated sugarcane bagasse. Ind Crop Prod 122:66–75
Martin-Sampedro R, Miranda J, Villar JC, Eugenio ME (2013) Laccase from Trametes sp. I-62: production, characterization, and application as a new laccase for eucalyptus globulus kraft pulp biobleaching. Ind Eng Chem Res 52:15533–15540
Mayer AM, Staples RC (2002) Laccase: new functions for an old enzyme. Phytochemistry 60:551–565
Mayerhofer MS, Fraser E, Kernaghan G (2015) Acid protease production in fungal root endophytes. Mycologia 107:1–11
Mehdipour-Moghaddam M, Emtiazi G, Bouzari M, Mostajeran A, Salehi Z (2010) Novel phytase and cellulase activities in endophytic Azospirilla. W Appl Sci J 10:1129–1135
Mercado-Blanco J, Alós E, Rey MD, Prieto P (2016) Pseudomonas fluorescens PICF7 displays an endophytic lifestyle in cultivated cereals and enhances yield in barley. FEMS Microbiol 92(8):fiw092. https://doi.org/10.1093/femsec/fiw092
Mishra A, Gond SK, Kumar A, Sharma VK, Verma SK, Kharwar RN, Sieber TN (2012) Season and tissue type affect fungal endophyte communities of the Indian medicinal plant Tinospora cordifolia more strongly than geographic location. Microbial Ecol 64:388–398
Mitchell DB, Vogel K, Weimann BJ, Pasamontes L, van Loon AP (1997) The phytase subfamily of histidine acid phosphatases: isolation of genes for two novel phytases from the fungi Aspergillus terreus and Myceliophthora thermophila. Microbiology 143:245–252
Mohali S, Burgess T, Wingfield M (2005) Diversity and host association of the tropical tree endophyte Lasiodiplodia theobromae revealed using simple sequence repeat markers. Forest Pathol 35:385–396
Mucciarelli M, Camusso W, Maffei M, Panicco P, Bicchi C (2007) Volatile terpenoids of endophyte-free and infected peppermint (Mentha piperita L.): chemical partitioning of a symbiosis. Microbial Ecol. https://doi.org/10.1007/s00248-007-9227-0
Muthezhilan R, Vinoth S, Gopi K, JaffarHussain A (2014) Dye degrading potential of immobilized laccase from endophytic fungi of coastal sand dune plants. Int J Chem Tech Res 6:4154–4160
Naik BS, Krishnamurthy Y (2010) Endophytes: the real untapped high energy biofuel resource. Curr Sci 98:883
Naik BS, Shashikala J, Krishnamurthy Y (2009) Study on the diversity of endophytic communities from rice (Oryza sativa L.) and their antagonistic activities in vitro. Microbiol Res 164:290–296
Narayan OP, Verma N, Singh AK, Oelmüller R, Kumar M, Prasad D, Kapoor R, Dua M, Johri AK (2017) Antioxidant enzymes in chickpea colonized by Piriformospora indica participate in defense against the pathogen Botrytis cinerea. Scientific Rep 7(1):13553
Nascimento T, Oki Y, Lima D, Almeida-Cortez J, Fernandes GW, Souza-Motta C (2015) Biodiversity of endophytic fungi in different leaf ages of Calotropis procera and their antimicrobial activity. Fungal Ecol 14:79–86
Nassar AH, El-Tarabily KA, Sivasithamparam K (2005) Promotion of plant growth by an auxin-producing isolate of the yeast Williopsis saturnus endophytic in maize (Zea mays L.) roots. Biol Fert Soils 42:97–108
Nayini N (1984) The phytase of yeast. Lebens Wiss Technol 17:24–26
Ngamau C, Matiru V, Tani A, Muthuri C (2014) Potential use of endophytic bacteria as biofertilizer for sustainable banana (Musa spp.) Production. African J Hortic Sci 8(1):1–11
Nicoletti R, Fiorentino A (2015) Plant bioactive metabolites and drugs produced by endophytic fungi of Spermatophyta. Agriculture 5:918–970
Nisbet AJ (2000) Azadirachtin from the neem tree Azadirachta indica: its action against insects. An Soc Entomol Bras 29:615–632
Nuyens F, Verachtert H, Michiels C (2001) Evaluation of a recombinant Saccharomyces cerevisiae strain secreting a Bacillus pumilus endo-beta-xylanase for use in bread-making. In: Meeting of the Benelux Yeast Research Groups, Leuven, Belgium
Nygren CM, Edqvist J, Elfstrand M, Heller G, Taylor AF (2007) Detection of extracellular protease activity in different species and genera of ectomycorrhizal fungi. Mycorrhiza 17:241–248
Ofek-Lalzar M, Gur Y, Ben-Moshe S, Sharon O, Kosman E, Mochli E, Sharon A (2016) Diversity of fungal endophytes in recent and ancient wheat ancestors Triticum dicoccoides and Aegilops sharonensis. FEMS Microbiol Ecol. https://doi.org/10.1093/femsec/fiw152
Ondeyka JG, Helms GL, Hensens OD, Goetz MA, Zink DL, Tsipouras A, Shoop WL, Slayton L, Dombrowski AW, Polishook JD (1997) Nodulisporic acid A, a novel and potent insecticide from a Nodulisporium sp. Isolation, structure determination, and chemical transformations. J Am Chem Soc 119:8809–8816
Onofre SB, Mattiello SP, da Silva GC, Groth D, Malagi I (2013) Production of cellulases by the endophytic fungus Fusarium oxysporum. J Microbiol Res 3:131–134
Orlandelli R, Alberto R, Rubin Filho C, Pamphile J (2012) Diversity of endophytic fungal community associated with Piper hispidum (Piperaceae) leaves. Genet Mol Res 11:1575–1585
Oses R, Valenzuela S, Freer J, Baeza J, Rodríguez J (2006) Evaluation of fungal endophytes for lignocellulolytic enzyme production and wood biodegradation. Int Biodeterior Biodegradation 57:129–135
Pan JJ, Baumgarten AM, May G (2008) Effects of host plant environment and Ustilago maydis infection on the fungal endophyte community of maize (Zea mays). New Phytol 178:147–156
Pancher M, Ceol M, Corneo PE, Longa CMO, Yousaf S, Pertot I, Campisano A (2012) Fungal endophytic communities in grapevines (Vitis vinifera L.) respond to crop management. App Environ Microbiol. https://doi.org/10.1128/AEM.07655-11
Panjiar N, Mishra S, Yadav AN, Verma P (2017) Functional foods from cyanobacteria: an emerging source for functional food products of pharmaceutical importance. In: Gupta VK, Treichel H, Shapaval VO, Oliveira LA, Tuohy MG (eds) Microbial functional foods and nutraceuticals. Wiley, Hoboken, pp 21–37. https://doi.org/10.1002/9781119048961.ch2
Panuthai T, Sihanonth P, Piapukiew J, Sooksai S, Sangvanich P, Karnchanatat A (2012) An extracellular lipase from the endophytic fungi Fusarium oxysporum isolated from the Thai medicinal plant, Croton oblongifolius Roxb. Afr J Microbiol Res 6:2622–2638
Parsa S, García-Lemos AM, Castillo K, Ortiz V, López-Lavalle LAB, Braun J, Vega FE (2016) Fungal endophytes in germinated seeds of the common bean, Phaseolus vulgaris. Fungal Biol 120:783–790
Patil MG, Pagare J, Patil SN, Sidhu AK (2015) Extracellular enzymatic activities of endophytic fungi isolated from various medicinal plants. Int J Curr Microbiol App Sci 4:1035–1042
Peng X-W, Chen H-Z (2007) Microbial oil accumulation and cellulase secretion of the endophytic fungi from oleaginous plants. Ann Microbiol. https://doi.org/10.1007/BF03175213
Petrini O (1991) Fungal endophytes of tree leaves. In: Andrews JH, Hirano SS (eds) Microbial ecology of leaves. Brock/Springer series in contemporary bioscience. Springer, New York. https://doi.org/10.1007/978-1-4612-3168-4_9
Petrović S, Škrinjar M, Bećarević A, Vujičić I, Banka L (1990) Effect of various carbon sources on microbial lipases biosynthesis. Biotechnol Lett 12:299–304
Phongpaichit S, Rungjindamai N, Rukachaisirikul V, Sakayaroj J (2006) Antimicrobial activity in cultures of endophytic fungi isolated from Garcinia species. FEMS Immunol Med Microbiol 48:367–372
Pierre E, Louise NW, Marie TKR, Valere T, Arc-en-ce J, Fekam B (2016) Integrated assessment of phytostimulation and biocontrol potential of endophytic Trichoderma spp against common bean (Phaseolus vulgaris L.) root rot fungi complex in centre region, Cameroon. Int J Pure App Biosci 4:50–68
Pieterse Z, Aveling TA, Jacobs A, Cowan DA (2018) Seasonal variability in fungal endophytes from Aizoaceae plants in the Succulent Karoo biodiversity hotspot, South Africa. J Arid Environ. https://doi.org/10.1016/j.jaridenv.2018.05.004
Pimentel MR, Molina G, Dionísio AP, Maróstica Junior MR, Pastore GM (2011) The use of endophytes to obtain bioactive compounds and their application in biotransformation process. Biotechnol Res Int. https://doi.org/10.4061/2011/576286
Pinto LSRC, Azevedo JL, Pereira JO, Vieira MLC, Labate CA (2000) Symptomless infection of banana and maize by endophytic fungi impairs photosynthetic efficiency. New Phytol 147:609–615
Polizeli M, Rizzatti A, Monti R, Terenzi H, Jorge JA, Amorim D (2005) Xylanases from fungi: properties and industrial applications. App Microbiol Biotechnol 67:577–591
Potshangbam M, Devi SI, Sahoo D, Strobel GA (2017) Functional characterization of endophytic fungal community associated with Oryza sativa L. and Zea mays L. Front Microbiol. https://doi.org/10.3389/fmicb.2017.00325
Prade RA (1996) Xylanases: from biology to biotechnology. Biotechnol Genet Eng Rev 13:101–132
Prakash G, Bhojwani SS, Srivastava AK (2002) Production of azadirachtin from plant tissue culture: state of the art and future prospects. Biotechnol Bioprocess Eng 7:185–193
Premjanu N, Jayanthy C (2012) Endophytic fungi a repository of bioactive compounds – a review. Intl J Inst Phar Life Sci 2:135–162
Puri SC, Verma V, Amna T, Qazi GN, Spiteller M (2005) An Endophytic Fungus from Nothapodytes foetida that Produces Camptothecin. J Nat Prod 68:1717–1719
Qadri M, Rajput R, Abdin MZ, Vishwakarma RA, Riyaz-Ul-Hassan S (2014) Diversity, molecular phylogeny, and bioactive potential of fungal endophytes associated with the Himalayan blue pine (Pinus wallichiana). Microbial Ecol 67:877–887
Qi F, Jing T, Zhan Y (2012) Characterization of endophytic fungi from Acer ginnala Maxim. in an artificial plantation: media effect and tissue-dependent variation. PLoS One 7:e46785
Rabha AJ, Naglot A, Sharma GD, Gogoi HK, Veer V (2014) In vitro evaluation of antagonism of endophytic Colletotrichum gloeosporioides against potent fungal pathogens of Camellia sinensis. Indian J Microbiol 54:302–309
Rabie GH (2005) Role of arbuscular mycorrhizal fungi in phytoremediation of soil rhizosphere spiked with poly aromatic hydrocarbons. Mycobiology 33:41–50
Rademacher W (1994) Gibberellin formation in microorganisms. Plant Growth Regul 15:303–314
Rafiq M, Dahot MU (2010) Callus and azadirachtin related limonoids production through in vitro culture of neem (Azadirachta indica A. Juss). Afr J Biotechnol 9(4):449–453
Ramawat K, Dass S, Mathur M (2009) The chemical diversity of bioactive molecules and therapeutic potential of medicinal plants. In: Ramawat K (ed) Herbal drugs: ethnomedicine to modern medicine. Springer, Berlin/Heidelberg. https://doi.org/10.1007/978-3-540-79116-4_2
Ramírez MC, Rivera-Ríos J, Téllez-Jurado A, Gálvez AM, Mercado-Flores Y, Arana-Cuenca A (2012) Screening for thermotolerant ligninolytic fungi with laccase, lipase, and protease activity isolated in Mexico. J Environ Manage 95:S256–S259
Rana KL, Kour D, Yadav AN, Kumar V, Dhaliwal HS (2016a) Biotechnological applications of endophytic microbes associated with barley (Hordeum vulgare L.) growing in Indian Himalayan regions. In: Proceeding of 86th annual session of NASI & symposium on “science, technology and entrepreneurship for human welfare in the Himalayan Region”, p 80
Rana KL, Kour D, Yadav AN, Kumar V, Dhaliwal HS (2016b) Endophytic microbes from wheat: diversity and biotechnological applications for sustainable agriculture. In: Proceeding of 57th association of microbiologist of India & International symposium on “Microbes and biosphere: what’s new what’s next”. p 453
Rana KL, Kour D, Verma P, Yadav AN, Kumar V, Singh DH (2017) Diversity and biotechnological applications of endophytic microbes associated with maize (Zea mays L.) growing in Indian Himalayan regions. In: Proceeding of national conference on advances in food science and technology, pp 41
Ratnaweera PB, Williams DE, de Silva ED, Wijesundera RL, Dalisay DS, Andersen RJ (2014) Helvolic acid, an antibacterial nortriterpenoid from a fungal endophyte, Xylaria sp. of orchid Anoectochilus setaceus endemic to Sri Lanka. Mycology 5:23–28
Ratnaweera PB, de Silva ED, Williams DE, Andersen RJ (2015a) Antimicrobial activities of endophytic fungi obtained from the arid zone invasive plant Opuntia dillenii and the isolation of equisetin, from endophytic Fusarium sp. BMC Complem Altern Med. https://doi.org/10.1186/s12906-015-0722-4
Ratnaweera PB, Williams DE, Patrick BO, de Silva ED, Andersen RJ (2015b) Solanioic acid, an antibacterial degraded steroid produced in culture by the fungus Rhizoctonia solani isolated from tubers of the medicinal plant Cyperus rotundus. Org Lett 17:2074–2077
Ratnaweera P, de Silva ED, Wijesundera RL, Andersen RJ (2016) Antimicrobial constituents of Hypocrea virens, an endophyte of the mangrove-associate plant Premna serratifolia L. J Natl Sci Found Sri Lanka 44(1):43–51
Ray A (2012) Application of lipase in industry. Asian J Pharm Technol 2(2):33–37
Reddy NR, Pierson MD, Sathe SK, Salunkhe DK (1989) Phytates in cereals and legumes. CRC Press, Boca Raton
Redman RS, Sheehan KB, Stout RG, Rodriguez RJ, Henson JM (2002) Thermotolerance generated by plant/fungal symbiosis. Science 298:1581–1581
Reimerdes EH, Franke K, Sell M (2004) Influencing functional properties of egg yolk by using phospholipases, paper presented at Conf on Food Structure and Food Quality, held on 3–7 October 2004
Renuka S, Ramanujam B (2016) Fungal endophytes from maize (Zea mays L.): isolation, identification and screening against maize stem borer, Chilo partellus (Swinhoe). J Pure Appl Microbiol 10:523–529
Rhoden S, Garcia A, Rubin Filho C, Azevedo J, Pamphile J (2012) Phylogenetic diversity of endophytic leaf fungus isolates from the medicinal tree Trichilia elegans (Meliaceae). Genet Mol Res 11:2513–2522
Rinu K, Sati P, Pandey A (2014) Trichoderma gamsii (NFCCI 2177): a newly isolated endophytic, psychrotolerant, plant growth promoting, and antagonistic fungal strain. J Basic Microbiol 54:408–417
Rivera-Orduña FN, Suarez-Sanchez RA, Flores-Bustamante ZR, Gracida-Rodriguez JN, Flores-Cotera LB (2011) Diversity of endophytic fungi of Taxus globosa (Mexican yew). Fungal Divers 47:65–74
Rodriguez R, White J Jr, Arnold A, Redman RA (2009) Fungal endophytes: diversity and functional roles. New phytol 182:314–330
Rosa LH, Vaz AB, Caligiorne RB, Campolina S, Rosa CA (2009) Endophytic fungi associated with the Antarctic grass Deschampsia antarctica Desv. (Poaceae). Polar Biol 32:161–167
Rosa LH, Almeida Vieira ML, Santiago IF, Rosa CA (2010) Endophytic fungi community associated with the dicotyledonous plant Colobanthus quitensis (Kunth) Bartl. (Caryophyllaceae) in Antarctica. FEMS Microbiol Ecol 73:178–189
Rosa LH, Tabanca N, Techen N, Wedge DE, Pan Z, Bernier UR, Becnel JJ, Agramonte NM, Walker LA, Moraes RM (2012) Diversity and biological activities of endophytic fungi associated with micropropagated medicinal plant Echinacea purpurea (L.) Moench. Am J Plant Sci 3:1105–1114
Rothen C, Miranda V, Aranda-Rickert A, Fracchia S, Rodríguez M (2017) Characterization of dark septate endophyte fungi associated with cultivated soybean at two growth stages. App Soil Ecol 120:62–69
Ruiz-Dueñas FJ, Martínez MJ, Martínez AT (1999) Molecular characterization of a novel peroxidase isolated from the ligninolytic fungus Pleurotus eryngii. Mol Microbiol 31:223–235
Ruma K, Kumar S, Prakash H (2013) Antioxidant, anti-inflammatory, antimicrobial and cytotoxic properties of fungal endophytes from Garcinia species. Int J Pharm Pharm Sci 5:889–897
Ryu DD, Mandels M (1980) Cellulases: biosynthesis and applications. Enzyme Microbial Technol 2:91–102
Sahay H, Yadav AN, Singh AK, Singh S, Kaushik R, Saxena AK (2017) Hot springs of Indian Himalayas: Potential sources of microbial diversity and thermostable hydrolytic enzymes. 3 Biotech 7:1–11
Salem HM, Eweida EA, Farag A (2000) Heavy metals in drinking water and their environmental impact on human health. ICEHM 2000:542–556
Sani A, Nagam V, Netala VR, Tartte V (2017) Characterization of heavy metal resitant endophytic fungi from Boswellia Ovalifoliolata. Imp J Int Res 3(2):1072–1076
Sansinenea E, Ortiz A (2011) Secondary metabolites of soil Bacillus spp. Biotechnol Lett 33:1523–1538
Sara B, Noreddine KC, Jacqueline D (2016) Production of laccase without inducer by Chaetomium species isolated from Chettaba forest situated in the East of Algeria. Afr J Biotechnol 15:207–213
Satdive RK, Fulzele DP, Eapen S (2007) Enhanced production of azadirachtin by hairy root cultures of Azadirachta indica A. Juss by elicitation and media optimization. J Biotechnol 128:281–289
Saunders M, Kohn LM (2008) Host-synthesized secondary compounds influence the in vitro interactions between fungal endophytes of maize. Appl Environ Microbiol 74:136–142
Savitha J, Srividya S, Jagat R, Payal P, Priyanki S, Rashmi G, Roshini K, Shantala Y (2007) Identification of potential fungal strain (s) for the production of inducible, extracellular and alkalophilic lipase. Afr J Biotechnol 6(5):564–568
Saxena AK, Yadav AN, Kaushik R, Tyagi SP, Shukla L (2015a) Biotechnological applications of microbes isolated from cold environments in agriculture and allied sectors. In: International conference on “low temperature science and biotechnological advances”, society of low temperature biology. p 104. https://doi.org/10.13140/RG.2.1.2853.5202
Saxena S, Meshram V, Kapoor N (2015b) Muscodor tigerii sp. nov.-Volatile antibiotic producing endophytic fungus from the Northeastern Himalayas. Ann Microbiol 65:47–57
Saxena AK, Yadav AN, Rajawat M, Kaushik R, Kumar R, Kumar M, Prasanna R, Shukla L (2016) Microbial diversity of extreme regions: an unseen heritage and wealth. Indian J Plant Genet Resour 29:246–248
Schaechter M (2012) Eukaryotic Microbes. Elsevier, San Diego
Schulz B, Boyle C, Draeger S, Römmert A-K, Krohn K (2002) Endophytic fungi: a source of novel biologically active secondary metabolites. Mycol Res 106:996–1004
Schwarze FW, Engels J, Mattheck C (2000) Fundamental aspects. In: Fungal strategies of wood decay in trees. Springer, Heidelberg, pp 5–31
Selim KA, Nagia MM, Ghwas DEE (2017) Endophytic fungi are multifunctional biosynthesizers: ecological role and chemical diversity. In: Endophytic fungi: diversity, characterization and Biocontrol, nova publishers, New York, pp 39–92
Selvanathan S, Indrakumar I, Johnpaul M (2011) Biodiversity of the endophytic fungi isolated from Calotropis gigantea (L.) R. Br. Recent Res Sci Technol 3(4):94–100
Sharma D, Pramanik A, Agrawal PK (2016) Evaluation of bioactive secondary metabolites from endophytic fungus Pestalotiopsis neglectaBAB-5510 isolated from leaves of Cupressus torulosa D. Don. 3 Biotech. https://doi.org/10.1007/s13205-016-0518-3
Shi Y, Dai C, Wu Y, Yuan Z (2004) Study on the degradation of wheat straw by endophytic fungi. ACTA Scientiae Circumstantiae 1:27
Shweta S, Zuehlke S, Ramesha B, Priti V, Kumar PM, Ravikanth G, Spiteller M, Vasudeva R, Shaanker RU (2010) Endophytic fungal strains of Fusarium solani, from Apodytes dimidiata E. Mey. ex Arn (Icacinaceae) produce camptothecin, 10-hydroxycamptothecin and 9-methoxycamptothecin. Phytochemistry 71:117–122
Sieber T, Riesen T, Müller E, Fried P (1988) Endophytic fungi in four winter wheat cultivars (Triticum aestivum L.) differing in resistance against Stagonospora nodorum (Berk.) Cast. & Germ.= Septoria nodorum (Berk.) Berk. J Phytopathol 122:289–306
Silva GH, de Oliveira CM, Teles HL, Pauletti PM, Castro-Gamboa I, Silva DH, Bolzani VS, Young MC, Costa-Neto CM, Pfenning LH (2010) Sesquiterpenes from Xylaria sp., an endophytic fungus associated with Piper aduncum (Piperaceae). Phytochem Lett 3:164–167
Singh H (2006) Mycoremediation: fungal bioremediation. Wiley, Hoboken
Singh SP, Gaur R (2017) Endophytic Streptomyces spp. underscore induction of defense regulatory genes and confers resistance against Sclerotium rolfsii in chickpea. Biol Control 104:44–56
Singh AK, Mukhopadhyay M (2012) Overview of fungal lipase: a review. Appl Biochem Biotechnol 166:486–520
Singh RN, Gaba S, Yadav AN, Gaur P, Gulati S, Kaushik R, Saxena AK (2016) First, high quality draft genome sequence of a plant growth promoting and Cold Active Enzymes producing psychrotrophic Arthrobacter agilis strain L77. Stand Genomic Sci 11:54. https://doi.org/10.1186/s40793-016-0176-4
Soleimani M, Hajabbasi MA, Afyuni M, Mirlohi A, Borggaard OK, Holm PE (2010) Effect of endophytic fungi on cadmium tolerance and bioaccumulation by Festuca arundinacea and Festuca pratensis. Int J Phytoremediat 12:535–549
Sommart U, Rukachaisirikul V, Tadpetch K, Sukpondma Y, Phongpaichit S, Hutadilok-Towatana N, Sakayaroj J (2012) Modiolin and phthalide derivatives from the endophytic fungus Microsphaeropsis arundinis PSU-G18. Tetrahedron 68:10005–10010
Sorgatto M, Guimarães N, Zanoelo F, Marques M, Peixoto-Nogueira S, Giannesi G (2012) Purification and characterization of an extracellular xylanase produced by the endophytic fungus, Aspergillus terreus, grown in submerged fermentation. Afr J Biotechnol 11:8076–8084
Spaepen S, Vanderleyden J (2011) Auxin and plant-microbe interactions. Cold Spring Harb Perspect Biol 3:a001438
Spaepen S, Vanderleyden J, Remans R (2007) Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev 31:425–448
Spagnoletti F, Tobar N, Di Pardo AF, Chiocchio V, Lavado R (2017) Dark septate endophytes present different potential to solubilize calcium, iron and aluminum phosphates. Appl Soil Ecol 111:25–32
Šraj-Kržič N, Pongrac P, Klemenc M, Kladnik A, Regvar M, Gaberščik A (2006) Mycorrhizal colonisation in plants from intermittent aquatic habitats. Aquat Bot 85:331–336
Srivastava P, Andersen PC, Marois JJ, Wright DL, Srivastava M, Harmon PF (2013) Effect of phenolic compounds on growth and ligninolytic enzyme production in Botryosphaeria isolates. Crop Prot 43:146–156
Stierle A, Strobel G, Stierle D (1993) Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science 260:214–216
Strobel GA (2002) Rainforest endophytes and bioactive products. Crit Rev Biotechnol 22:315–333
Strobel G, Daisy B (2003) Bioprospecting for microbial endophytes and their natural products. Microbiol Mol Biol Rev 67:491–502
Strobel GA, Pliam NB (1997) Immuno suppressant diterpene compound. Google Patents
Strobel GA, Torczynski R, Bollon A (1997) Acremonium sp. – a leucinostatin A producing endophyte of European yew (Taxus baccata). Plant Sci 128:97–108
Strobel GA, Miller RV, Martinez-Miller C, Condron MM, Teplow DB, Hess W (1999) Cryptocandin, a potent antimycotic from the endophytic fungus Cryptosporiopsis cf. quercina. Microbiology 145:1919–1926
Strobel G, Ford E, Worapong J, Harper JK, Arif AM, Grant DM, Fung PC, Chau RMW (2002) Isopestacin, an isobenzofuranone from Pestalotiopsis microspora, possessing antifungal and antioxidant activities. Phytochemistry 60:179–183
Sturz A, Christie B, Matheson B, Nowak J (1997) Biodiversity of endophytic bacteria which colonize red clover nodules, roots, stems and foliage and their influence on host growth. Biol Fert Soils 25:13–19
Su H, Kang J, Cao J, Mo L, Hyde KD (2014) Medicinal plant endophytes produce analogous bioactive compounds. Chiang Mai J Sc 41:1–13
Sudhakar T, Dash S, Rao R, Srinivasan R, Zacharia S, Atmanand M, Subramaniam B, Nayak S (2013) Do endophytic fungi possess pathway genes for plant secondary metabolites? Curr Sci 104(2):178
Suffness M (1995) Taxol: science and applications, vol 22. CRC Press, Boca Raton
Sukumaran RK, Singhania RR, Pandey A (2005) Microbial cellulases-production, applications and challenges. J Sci Ind Res 64(11):832–844
Suman A, Verma P, Yadav AN, Saxena AK (2015) Bioprospecting for extracellular hydrolytic enzymes from culturable thermotolerant bacteria isolated from Manikaran thermal springs. Res J Biotechnol 10:33–42
Suman A, Yadav AN, Verma P (2016) Endophytic microbes in crops: diversity and beneficial impact for sustainable agriculture. In: Singh D, Abhilash P, Prabha R (eds) Microbial inoculants in sustainable agricultural productivity, research perspectives. Springer, New Delhi, pp 117–143. https://doi.org/10.1007/978-81-322-2647-5_7
Sun X, Guo LD, Hyde K (2011a) Community composition of endophytic fungi in Acer truncatum and their role in decomposition. Fungal Divers 47:85–95
Sun Y, Luo H, Li Y, Sun C, Song J, Niu Y, Zhu Y, Dong L, Lv A, Tramontano E (2011b) Pyrosequencing of the Camptotheca acuminata transcriptome reveals putative genes involved in camptothecin biosynthesis and transport. BMC Genomics. https://doi.org/10.1186/1471-2164-12-533
Sun J-F, Lin X, Zhou X-F, Wan J, Zhang T, Yang B, Yang X-W, Tu Z, Liu Y (2014) Pestalols A–E, new alkenyl phenol and benzaldehyde derivatives from endophytic fungus Pestalotiopsis sp. AcBC2 isolated from the Chinese mangrove plant Aegiceras corniculatum. J Antibiot 67:451–457
Sunitha V, Devi DN, Srinivas C (2013) Extracellular enzymatic activity of endophytic fungal strains isolated from medicinal plants. World J Agri Sci 9:01–09
Suryanarayanan T, Senthilarasu G, Muruganandam V (2000) Endophytic fungi from Cuscuta reflexa and its host plants. Fungal Divers 4:117–123
Suryanarayanan TS, Wittlinger SK, Faeth SH (2005) Endophytic fungi associated with cacti in Arizona. Mycol Res 109:635–639
Suto M, Takebayashi M, Saito K, Tanaka M, Yokota A, Tomita F (2002) Endophytes as producers of xylanase. J Biosci Bioeng 93:88–90
Syed S, Riyaz-Ul-Hassan S, Johri S (2013) A novel cellulase from an endophyte, Penicillium sp. NFCCI 2862. Am J Microbiol Res 1:84–91
Sztajer H, Maliszewska I (1989) The effect of culture conditions on lipolytic productivity of Penicillium citrinum. Biotechnol Lett 11:895–898
Taghavi S, Garafola C, Monchy S, Newman L, Hoffman A, Weyens N, Barac T, Vangronsveld J, van der Lelie D (2009) Genome survey and characterization of endophytic bacteria exhibiting a beneficial effect on growth and development of poplar trees. Appl Environ Microbiol 75:748–757
Tamehiro N, Okamoto-Hosoya Y, Okamoto S, Ubukata M, Hamada M, Naganawa H, Ochi K (2002) Bacilysocin, a novel phospholipid antibiotic produced by Bacillus subtilis 168. Antimicrob Agents Chemother 46:315–320
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. https://doi.org/10.1093/molbev/msr121
Tan RX, Zou WX (2001) Endophytes: a rich source of functional metabolites. Nat Prod Rep 18:448–459
Tasia W, Melliawati R (2017) Cellulase and xylanase production from three isolates of indigenous endophytic fungi. IOP Conf. Ser.: Earth Environ Sci 101:012035
Tejesvi MV, Kajula M, Mattila S, Pirttilä AM (2011) Bioactivity and genetic diversity of endophytic fungi in Rhododendron tomentosum Harmaja. Fungal Divers 47:97–107
Tekere M, Mswaka A, Zvauya R, Read J (2001) Growth, dye degradation and ligninolytic activity studies on Zimbabwean white rot fungi. Enzyme Microbial Technol 28:420–426
Tenguria RK, Firodiya A (2013) Diversity of endophytic fungi in leaves of Glycine max (L.) merr. from central region of Madhya Pradesh. World J Pharm Pharm Sci 2:5928–5934
Tenguria RK, Khan FN, Quereshi S (2011) Endophytes-mines of pharmacological therapeutics. World J Sci Technol 1:127–149
Thomas L, Joseph A, Singhania RR, Patel A, Pandey A (2017) Industrial enzymes: xylanases. In: Current developments in biotechnology and bioengineering. Elsevier, Amsterdam/Boston, pp 127–148
Thongsandee W, Matsuda Y, Ito S (2012) Temporal variations in endophytic fungal assemblages of Ginkgo biloba L. J Forest Res 17:213–218
Tian X, Cao L, Tan H, Zeng Q, Jia Y, Han W, Zhou S (2004) Study on the communities of endophytic fungi and endophytic actinomycetes from rice and their antipathogenic activities in vitro. World J Microbiol Biotechnol 20:303–309
Tian X, Yao Y, Chen G, Mao Z, Wang X, Xie B (2014) Suppression of Meloidogyne incognita by the endophytic fungus Acremonium implicatum from tomato root galls. Int J Pest Manage 60:239–245
Tian J, Fu L, Zhang Z, Dong X, Xu D, Mao Z, Liu Y, Lai D, Zhou L (2017) Dibenzo-α-pyrones from the endophytic fungus Alternaria sp. Samif01: isolation, structure elucidation, and their antibacterial and antioxidant activities. Nat Prod Res 31:387–396
Tien M (1987) Properties of ligninase from Phanerochaete chrysosporium and their possible applications. Crit Rev Microbiol 15:141–168
Toghueo R, Ejiya E, Sahal D, Yazdani S, Boyom F (2017) Production of cellulolytic enzymes by endophytic fungi isolated from Cameroonian medicinal plants. Int J Curr Microbiol App Sci 6:1264–1271
Tomita F (2003) Endophytes in Southeast Asia and Japan: their taxonomic diversity and potential applications. Fungal Divers 14:187–204
Torres M, Dolcet MM, Sala N, Canela R (2003) Endophytic fungi associated with Mediterranean plants as a source of mycelium-bound lipases. J Agri Food Chem 51:3328–3333
Traving SJ, Thygesen UH, Riemann L, Stedmon CA (2015) A model of extracellular enzymes in free-living microbes: which strategy pays off? Appl Environ Microbiol 81:7385–7393
Tsujisaka Y, Iwai M, Tominaga Y (1973) Purification, crystallization and some properties of lipase from Geotrichum candidum Link. Agric Biol Chem 37:1457–1464
Uhlig H (1998) Industrial enzymes and their applications. Wiley, New York
Undurraga D, Markovits A, Erazo S (2001) Cocoa butter equivalent through enzymic interesterification of palm oil midfraction. Process Biochem 36:933–939
Urairuj C, Khanongnuch C, Lumyong S (2003) Ligninolytic enzymes from tropical endophytic Xylariaceae. Fungal Divers 13:209–219
Uzma F, Konappa NM, Chowdappa S (2016) Diversity and extracellular enzyme activities of fungal endophytes isolated from medicinal plants of Western Ghats, Karnataka. Egyptian J Basic Appl Sci 3:335–342
Van Dyk J, Pletschke B (2012) A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes—factors affecting enzymes, conversion and synergy. Biotechnol Adv 30:1458–1480
Vandenbussche F, Fierro AC, Wiedemann G, Reski R, Van Der Straeten D (2007) Evolutionary conservation of plant gibberellin signalling pathway components. BMC Plant Biol. https://doi.org/10.1186/1471-2229-7-65
Vasundhara M, Kumar A, Reddy MS (2016) Molecular approaches to screen bioactive compounds from endophytic fungi. Front Microbiol. https://doi.org/10.3389/fmicb.2016.01774
Veitch GE, Beckmann E, Burke BJ, Boyer A, Ayats C, Ley SV (2007a) A relay route for the synthesis of azadirachtin. Angew Chem Int Ed 46:7633–7635
Veitch GE, Beckmann E, Burke BJ, Boyer A, Maslen SL, Ley SV (2007b) Synthesis of azadirachtin: a long but successful journey. Angew Chem Int Ed 46:7629–7632
Veitch GE, Beckmann E, Burke BJ, Boyer A, Maslen SL, Ley SV (2007c) Titelbild: synthesis of Azadirachtin: a long but successful journey (Angew. Chem. 40/2007). Angew Chemi 119:7663–7663
Venkatesagowda B, Ponugupaty E, Barbosa AM, Dekker RF (2012) Diversity of plant oil seed-associated fungi isolated from seven oil-bearing seeds and their potential for the production of lipolytic enzymes. World J Microbiol Biotechnol 28:71–80
Venugopalan A, Srivastava S (2015) Endophytes as in vitro production platforms of high value plant secondary metabolites. Biotechnol Adv 33:873–887
Verma P, Yadav AN, Kazy SK, Saxena AK, Suman A (2013) Elucidating the diversity and plant growth promoting attributes of wheat (Triticum aestivum) associated acidotolerant bacteria from southern hills zone of India. Natl J Life Sci 10:219–227
Verma P, Yadav AN, Kazy SK, Saxena AK, Suman A (2014a) Evaluating the diversity and phylogeny of plant growth promoting bacteria associated with wheat (Triticum aestivum) growing in central zone of India. Int J Curr Microbiol Appl Sci 3:432–447
Verma VC, Prakash S, Singh RG, Gange AC (2014b) Host-mimetic metabolomics of endophytes: looking back into the future. In: Advances in endophytic research. Springer, New Delhi, pp 203–218
Verma P, Yadav AN, Khannam KS, Panjiar N, Kumar S, Saxena AK, Suman A (2015a) Assessment of genetic diversity and plant growth promoting attributes of psychrotolerant bacteria allied with wheat (Triticum aestivum) from the northern hills zone of India. Ann Microbiol 65:1885–1899
Verma P, Yadav AN, Shukla L, Saxena AK, Suman A (2015b) Alleviation of cold stress in wheat seedlings by Bacillus amyloliquefaciens IARI-HHS2-30, an endophytic psychrotolerant K-solubilizing bacterium from NW Indian Himalayas. Natl J Life Sci 12:105–110
Verma P, Yadav AN, Shukla L, Saxena AK, Suman A (2015c) Hydrolytic enzymes production by thermotolerant Bacillus altitudinis IARI-MB-9 and Gulbenkiania mobilis IARI-MB-18 isolated from Manikaran hot springs. Int J Adv Res 3:1241–1250
Verma P, Yadav AN, Khannam KS, Kumar S, Saxena AK, Suman A (2016a) Molecular diversity and multifarious plant growth promoting attributes of Bacilli associated with wheat (Triticum aestivum L.) rhizosphere from six diverse agro-ecological zones of India. J Basic Microbiol 56:44–58
Verma P, Yadav AN, Khannam KS, Mishra S, Kumar S, Saxena AK, Suman A (2016b) Appraisal of diversity and functional attributes of thermotolerant wheat associated bacteria from the peninsular zone of India. Saudi J Biol Sci. https://doi.org/10.1016/j.sjbs.2016.01.042
Verma P, Yadav AN, Kumar V, Singh DP, Saxena AK (2017) Beneficial plant-microbes interactions: biodiversity of microbes from diverse extreme environments and its impact for crops improvement. In: Singh DP, Singh HB, Prabha R (eds) Plant-microbe interactions in agro-ecological perspectives. Springer Nature, Singapore, pp 543–580. https://doi.org/10.1007/978-981-10-6593-4_22
Verza M, Arakawa NS, Lopes NP, Kato MJ, Pupo MT, Said S, Carvalho I (2009) Biotransformation of a tetrahydrofuran lignan by the endophytic fungus Phomopsis Sp. J Braz Chem Soc 20:195–200
Vieira ML, Hughes AF, Gil VB, Alves TM, Vaz AB, Zani CL, Rosa CA, Rosa LH (2011) Diversity and antimicrobial activities of the fungal endophyte community associated with the traditional Brazilian medicinal plant Solanum cernuum Vell.(Solanaceae). Can J Microbiol 58:54–66
Wagenaar MM, Corwin J, Strobel G, Clardy J (2000) Three new cytochalasins produced by an endophytic fungus in the genus Rhinocladiella. J Nat Prod 63:1692–1695
Wakelin SA, Warren RA, Harvey PR, Ryder MH (2004) Phosphate solubilization by Penicillium spp. closely associated with wheat roots. Biol Fert Soils 40:36–43
Wall ME, Wani MC, Cook C, Palmer KH, McPhail AT, Sim G (1966) Plant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from Camptotheca acuminata. J Am Chem Soc 88:3888–3890
Wang Y, Dai C-C (2011) Endophytes: a potential resource for biosynthesis, biotransformation, and biodegradation. Ann Microbiol 61:207–215
Wang J, Wu J, Huang W, Tan R (2006a) Laccase production by Monotospora sp., an endophytic fungus in Cynodon dactylon. Bioresour Technol 97:786–789
Wang S, Li X-M, Teuscher F, Li D-L, Diesel A, Ebel R, Proksch P, Wang B-G (2006b) Chaetopyranin, a benzaldehyde derivative, and other related metabolites from Chaetomium globosum, an endophytic fungus derived from the marine red alga Polysiphonia urceolata. J Nat Prod 69:1622–1625
Wang Y, Niu S, Liu S, Guo L, Che Y (2010a) The first naturally occurring thiepinols and thienol from an endolichenic fungus Coniochaeta sp. Org Lett 12:5081–5083
Wang Y, Zheng Z, Liu S, Zhang H, Li E, Guo L, Che Y (2010b) Oxepinochromenones, furochromenone, and their putative precursors from the endolichenic fungus Coniochaeta sp. J Nat Prod 73:920–924
Wang L-W, Xu BG, Wang J-Y, Su Z-Z, Lin F-C, Zhang C-L, Kubicek CP (2012) Bioactive metabolites from Phoma species, an endophytic fungus from the Chinese medicinal plant Arisaema erubescens. Appl Microbiol Biotechnol 93:1231–1239
Wang Q-X, Bao L, Yang X-L, Liu D-L, Guo H, Dai H-Q, Song F-H, Zhang L-X, Guo L-D, Li S-J (2013) Ophiobolins P–T, five new cytotoxic and antibacterial sesterterpenes from the endolichenic fungus Ulocladium sp. Fitoterapia 90:220–227
Wang W, Zhai Y, Cao L, Tan H, Zhang R (2016) Endophytic bacterial and fungal microbiota in sprouts, roots and stems of rice (Oryza sativa L.). Microbiol Res 188:1–8
Waqas M, Khan AL, Kamran M, Hamayun M, Kang S-M, Kim Y-H, Lee I-J (2012) Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules 17:10754–10773
Weber D, Sterner O, Anke T, Gorzalczancy S, Martino V, Acevedo C (2004) Phomol, a new antiinflammatory metabolite from an endophyte of the medicinal plant Erythrina crista-galli. J Antibiot 57:559–563
Will M, Sylvia D (1990) Interaction of rhizosphere bacteria, fertilizer, and vesicular-arbuscular mycorrhizal fungi with sea oats. Appl Environ Microbiol 56:2073–2079
Wipusaree N, Sihanonth P, Piapukiew J, Sangvanich P, Karnchanatat A (2011) Purification and characterization of a xylanase from the endophytic fungus Alternaria alternata isolated from the Thai medicinal plant, Croton oblongifolius Roxb. African J Microbiol Res 5:5697–5712
Wong DW (2009) Structure and action mechanism of ligninolytic enzymes. Appl Biochem Biotechnol 157:174–209
Wu SH, Chen YW, Shao SC, Wang LD, Yu Y, Li ZY, Yang LY, Li SL, Huang R (2009) Two new solanapyrone analogues from the endophytic fungus Nigrospora sp. YB-141 of Azadirachta indica. Chem Biodivers 6:79–85
Wu H, Yang HY, You XL, Li YH (2013) Diversity of endophytic fungi from roots of Panax ginseng and their saponin yield capacities. SpringerPlus 2:107. https://doi.org/10.1186/2193-1801-2-107
Xie X-G, Dai C-C (2015) Biodegradation of a model allelochemical cinnamic acid by a novel endophytic fungus Phomopsis liquidambari. Int Biodeterior Biodegradation 104:498–507
Xing Y-M, Chen J, Cui J-L, Chen X-M, Guo S-X (2011) Antimicrobial activity and biodiversity of endophytic fungi in Dendrobium devonianum and Dendrobium thyrsiflorum from Vietnam. Curr Microbiol 62:1218–1224
Xing HQ, Ma JC, Xu BL, Zhang SW, Wang J, Cao L, Yang XM (2018) Mycobiota of maize seeds revealed by rDNA-ITS sequence analysis of samples with varying storage times. Microbiology Open 7(6):e00609
Xiong Z-Q, Yang Y-Y, Zhao N, Wang Y (2013) Diversity of endophytic fungi and screening of fungal paclitaxel producer from Anglojap yew, Taxus x media. BMC Microbiol. https://doi.org/10.1186/1471-2180-13-71
Xu QY, Huang YJ, Zheng ZH, Song SY (2005) Purification, elucidation and activities study of cytosporone. B J Xiamen Univ Nat Sci 44:425–428
Xu YM, Espinosa-Artiles P, Liu MX, Arnold AE, Gunatilaka AL (2013) Secoemestrin D, a Cytotoxic Epitetrathiodioxopiperizine, and Emericellenes A–E, Five Sesterterpenoids from Emericella sp. AST0036, a Fungal Endophyte of Astragalus lentiginosus 1. J Nat Prod 76:2330–2336
Yadav AN (2015) Bacterial diversity of cold deserts and mining of genes for low temperature tolerance. Ph.D. Thesis, IARI, New Delhi/BIT, Ranchi. p 234. https://doi.org/10.13140/RG.2.1.2948.1283/2
Yadav AN (2019) Endophytic fungi for plant growth promotion and adaptation under abiotic stress conditions. Acta Sci Agric 3:91–93
Yadav AN, Verma P, Sachan S, Kaushik R, Saxena A (2012) Diversity of culturable psychrotrophic bacteria from Leh Ladakh and bioprospecting for cold-active extracellular enzymes. In: Proceeding of national seminar on “biotechnological interventions for the benefit of mankind”, New Delhi, p 32
Yadav N, Yadav AN (2018) Biodiversity and biotechnological applications of novel plant growth promoting methylotrophs. J Appl Biotechnol Bioeng 5:342–344
Yadav R, Singh AV, Joshi S, Kumar M (2015) Antifungal and enzyme activity of endophytic fungi isolated from Ocimum sanctum and Aloe vera. Afr J Microbiol Res 9:1783–1788
Yadav AN, Sachan SG, Verma P, Saxena AK (2015a) Prospecting cold deserts of north western Himalayas for microbial diversity and plant growth promoting attributes. J Biosci Bioeng 119:683–693
Yadav AN, Sachan SG, Verma P, Tyagi SP, Kaushik R, Saxena AK (2015b) Culturable diversity and functional annotation of psychrotrophic bacteria from cold desert of Leh Ladakh (India). World J Microbiol Biotechnol 31:95–108
Yadav AN, Verma P, Kumar M, Pal KK, Dey R, Gupta A, Padaria JC, Gujar GT, Kumar S, Suman A, Prasanna R, Saxena AK (2015c) Diversity and phylogenetic profiling of niche-specific Bacilli from extreme environments of India. Ann Microbiol 65:611–629
Yadav AN, Sachan SG, Verma P, Kaushik R, Saxena AK (2016) Cold active hydrolytic enzymes production by psychrotrophic Bacilli isolated from three sub-glacial lakes of NW Indian Himalayas. J Basic Microbiol 56:294–307
Yadav AN, Kumar R, Kumar S, Kumar V, Sugitha T, Singh B, Chauhan VS, Dhaliwal HS, Saxena AK (2017a) Beneficial microbiomes: biodiversity and potential biotechnological applications for sustainable agriculture and human health. J Appl Biol Biotechnol 5:1–13
Yadav AN, Verma P, Kumar R, Kumar V, Kumar K (2017b) Current applications and future prospects of eco-friendly microbes. EU Voice 3:21–22
Yadav AN, Verma P, Kumar V, Sachan SG, Saxena AK (2017c) Extreme cold environments: a suitable niche for selection of novel psychrotrophic microbes for biotechnological applications. Adv Biotechnol Microbiol 2:1–4
Yadav AN, Verma P, Sachan SG, Saxena AK (2017d) Biodiversity and biotechnological applications of psychrotrophic microbes isolated from Indian Himalayan regions. EC Microbiol ECO. 01:48–54
Yadav AN, Kumar V, Prasad R, Saxena AK, Dhaliwal HS (2018a) Microbiome in crops: diversity, distribution and potential role in crops improvements. In: Prasad R, Gill SS, Tuteja N (eds) Crop improvement through microbial biotechnology. Elsevier, San Diego, pp 305–332
Yadav AN, Verma P, Kumar V, Sangwan P, Mishra S, Panjiar N, Gupta VK, Saxena AK (2018b) Biodiversity of the genus Penicillium in different habitats. In: Gupta VK, Rodriguez-Couto S (eds) New and future developments in microbial biotechnology and bioengineering, Penicillium system properties and applications. Elsevier, Amsterdam, pp 3–18. https://doi.org/10.1016/B978-0-444-63501-3.00001-6
Yamazaki Y, Sudo H, Yamazaki M, Aimi N, Saito K (2003) Camptothecin biosynthetic genes in hairy roots of Ophiorrhiza pumila: cloning, characterization and differential expression in tissues and by stress compounds. Plant Cell Physiol 44:395–403
Yamazaki Y, Kitajima M, Arita M, Takayama H, Sudo H, Yamazaki M, Aimi N, Saito K (2004) Biosynthesis of camptothecin. In silico and in vivo tracer study from [1-13C] glucose. Plant Physiol 134:161–170
Yang Y, Yan M, Hu B (2014) Endophytic fungal strains of soybean for lipid production. Bioenergy Res 7:353–361
Yang H, Ye W, Ma J, Zeng D, Rong Z, Xu M, Wang Y, Zheng X (2018) Endophytic fungal communities associated with field-grown soybean roots and seeds in the Huang-Huai region of China. Peer J 6:e4713
Yuan ZL, Zhang CL, Lin FC, Kubicek CP (2010) Identity, diversity, and molecular phylogeny of the endophytic mycobiota in the roots of rare wild rice (Oryza granulate) from a nature reserve in Yunnan, China. Appl Environ Microbiol 76:1642–1652
Yuan C, Wang H-Y, Wu C-S, Jiao Y, Li M, Wang Y-Y, Wang S-Q, Zhao Z-T, Lou H-X (2013) Austdiol, fulvic acid and citromycetin derivatives from an endolichenic fungus, Myxotrichum sp. Phytochem Lett 6:662–666
Yuan Y, Feng H, Wang L, Li Z, Shi Y, Zhao L, Feng Z, Zhu H (2017) Potential of endophytic fungi isolated from cotton roots for biological control against Verticillium Wilt disease. PloS One 12:e0170557
Zaferanloo B, Pepper SA, Coulthard SA, Redfern CP, Palombo EA (2018) Metabolites of endophytic fungi from Australian native plants as potential anticancer agents. FEMS Microbiol Lett. https://doi.org/10.1093/femsle/fny078
Zhang SS-QOM, Qi-Yong Z-DT (2007) Isolation and characterization of endophytic microorganisms in glycyrrhiza inflata Bat. from Xinjiang [J]. Microbiology 5:14
Zhang L, Guo B, Li H, Zeng S, Shao H, Gu S, Wei R (2000) Preliminary study on the isolation of endophytic fungus of Catharanthus roseus and its fermentation to produce products of therapeutic value. Chin Trad Herb Drugs 31:805–807
Zhang Y, Wang S, Li X-M, Cui C-M, Feng C, Wang B-G (2007) New sphingolipids with a previously unreported 9-methyl-C20-sphingosine moiety from a marine algous endophytic fungus Aspergillus niger EN-13. Lipids 42:759–764
Zhang HW, Huang WY, Chen JR, Yan WZ, Xie DQ, Tan RX (2008) Cephalosol: an antimicrobial metabolite with an unprecedented skeleton from endophytic Cephalosporium acremonium IFB-E007. Chem Eur J 14:10670–10674
Zhang F, Liu S, Lu X, Guo L, Zhang H, Che Y (2009a) Allenyl and alkynyl phenyl ethers from the endolichenic fungus Neurospora terricola. J Nat Prod 72:1782–1785
Zhang P, Zhou PP, Yu LJ (2009b) An endophytic taxol-producing fungus from Taxus x media, Aspergillus candidus MD3. FEMS Microbiol Lett 293:155–159
Zhang F, Li L, Niu S, Si Y, Guo L, Jiang X, Che Y (2012) A thiopyranchromenone and other chromone derivatives from an endolichenic fungus, Preussia africana. J Nat Prod 75:230–237
Zhang W, Xu L, Yang L, Huang Y, Li S, Shen Y (2014) Phomopsidone A, a novel depsidone metabolite from the mangrove endophytic fungus Phomopsis sp. A123. Fitoterapia 96:146–151
Zhao J, Mou Y, Shan T, Li Y, Zhou L, Wang M, Wang J (2010) Antimicrobial metabolites from the endophytic fungus Pichia guilliermondii isolated from Paris polyphylla var. yunnanensis. Molecules 15:7961–7970
Zhao J, Fu Y, Luo M, Zu Y, Wang W, Zhao C, Gu C (2012) Endophytic fungi from pigeon pea [Cajanus cajan (L.) Millsp.] produce antioxidant cajaninstilbene acid. J Agric Food Chem 60:4314–4319
Zhao J, Li C, Wang W, Zhao C, Luo M, Mu F, Fu Y, Zu Y, Yao M (2013) Hypocrea lixii, novel endophytic fungi producing anticancer agent cajanol, isolated from pigeon pea (C ajanuscajan [L.] Millsp.). J Appl Microbiol 115:102–113
Zhao J, Ma D, Luo M, Wang W, Zhao C, Zu Y, Fu Y, Wink M (2014) In vitro antioxidant activities and antioxidant enzyme activities in HepG2 cells and main active compounds of endophytic fungus from pigeon pea [Cajanus cajan (L.) Millsp.]. Food Res Int 56:243–251
Zhao L, Xu Y, Lai X (2018) Antagonistic endophytic bacteria associated with nodules of soybean (Glycine max L.) and plant growth-promoting properties. Braz J Microbiol 49:269–278
Zheng C-J, Li L, Zou J-P, Han T, Qin L-P (2012) Identification of a quinazoline alkaloid produced by Penicillium vinaceum, an endophytic fungus from Crocus sativus. Pharm Biol 50:129–133
Zheng C-J, Xu L-L, Li Y-Y, Han T, Zhang Q-Y, Ming Q-L, Rahman K, Qin L-P (2013) Cytotoxic metabolites from the cultures of endophytic fungi from Panax ginseng. Appl Microbiol Biotechnol 97:7617–7625
Zhou X, Zhu H, Liu L, Lin J, Tang K (2010) A review: recent advances and future prospects of taxol-producing endophytic fungi. Appl Microbiol Biotechnol 86:1707–1717
Zhou X-M, Zheng C-J, Song X-P, Han C-R, Chen W-H, Chen G-Y (2014) Antibacterial α-pyrone derivatives from a mangrove-derived fungus Stemphylium sp. 33231 from the South China Sea. J Antibiot 67:401–403
Zhou P, Wu Z, Tan D, Yang J, Zhou Q, Zeng F, Zhang M, Bie Q, Chen C, Xue Y (2017) Atrichodermones A–C, three new secondary metabolites from the solid culture of an endophytic fungal strain, Trichoderma atroviride. Fitoterapia 123:18–22
Zikmundova M, Drandarov K, Bigler L, Hesse M, Werner C (2002) Biotransformation of 2-benzoxazolinone and 2-hydroxy-1, 4-benzoxazin-3-one by endophytic fungi isolated from Aphelandra tetragona. Appl Environ Microbiol 68:4863–4870
Acknowledgements
The authors are grateful to Prof. Harcharan Singh Dhaliwal, Vice Chancellor, Eternal University, Baru Sahib, Himachal Pradesh, India, for providing infra-structural facilities and constant encouragement.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Rana, K.L. et al. (2019). Endophytic Fungi: Biodiversity, Ecological Significance, and Potential Industrial Applications. In: Yadav, A., Mishra, S., Singh, S., Gupta, A. (eds) Recent Advancement in White Biotechnology Through Fungi. Fungal Biology. Springer, Cham. https://doi.org/10.1007/978-3-030-10480-1_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-10480-1_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-10479-5
Online ISBN: 978-3-030-10480-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)