Abstract
Now a day’s heavy metal pollution has become a serious environmental problem. The presence of heavy metal ions is a major problem due to their toxicity to many life forms on this planet. Therefore the removal of heavy metals from the environment is of special concern due to their persistence. During these days natural adsorbents are most frequently studied and widely applied for the metal contaminated water. Adsorption processes are being widely used by various researchers for the removal of heavy metals from the waste streams. The need for the safe and economical methods for the elimination of heavy metals from contaminated waters has developed the interest of researchers towards the production of low cost adsorbents. Therefore there is an urgent need that all possible sources of agro-based inexpensive adsorbents should be explored and their role for the removal of heavy metals should be studied in detail.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
Most of the chromatographic methods employed for the separation and analysis of components form mixtures are based on adsorption. We know that the particles on the surface of a solid or a liquid experience a strong inward pull because of unbalanced attractive interaction with other particles which surround them only one side and not all sides. At the same time when a new solid surface is created by breaking a solid, some interatomic bonds are broken and some valencies of the surface atoms are left unsatisfied. As a result the surface of a solid has a tendency to attract and hold molecules of a gas, a liquid, a dissolved solute or other particles with which it comes in contact. By holding them on the surface, the surface energy of the solid is decreased and consequently a more stable state is acquired by the solid. The molecules or other particles held by the solid, however, remain only at the surface of the solid and don’t penetrate into the bulk of the solid. This phenomenon of adhesion of other substances on the surface of a solid without any penetration into the bulk phase in known as adsorption. The solid on the surface of which adsorption takes place is called an adsorbent and the substance which gets adsorbed is called adsorbate. Activated charcoal, silica gel, alumina etc. are some of the well-known adsorbents. Since adsorption is a surface phenomenon, the greater the surface area of the adsorbent, the greater is adsorption. The following simple experiments illustrate the phenomenon of adsorption of a gas and a dissolved solute respectively.
-
(i)
Take some ammonia gas in a gas tube over mercury and note the level of mercury. Now introduce a piece of activated charcoal in the tube. The level of mercury starts rising. This shows the disappearance of ammonia due to its adsorption on the surface of charcoal.
-
(ii)
Dissolve a small quantity of some organic dye in water. Add some animal charcoal to it and filter, the filtrate is found to be colorless because the dye gets adsorbed by charcoal.
The term adsorption should be clearly distinguished from absorption. Adsorption involves the concentration of a substance at the surface while absorption involves the penetration of substance into the bulk of a solid or a liquid. Adsorption is a fast process while absorption is a slow process. This is because absorption involves the penetration into the interior of mater.
Sometimes a substance may undergo adsorption as well as absorption such process is termed as sorption. For example, if ammonia gas is passed through water containing some charcoal ammonia first dissolves in water (i.e. absorption takes place) and is then adsorbed by charcoal.
11.2 Types of Adsorption
11.2.1 Physical Adsorption
This type of adsorption is due to van der waals’ forces between the gases and solids. These are weak forces but in case of polar gases these are relatively stronger. This type of adsorption is not permanent and can be decreased by decreasing pressure or increasing temperature.
11.2.2 Chemical Adsorption
In some solids there exist some unsatisfied valencies on the surface. There is a possibility of adsorbate forming chemical bond with these free valencies of adsorbent. These types of adsorption are called chemical adsorption or chemisorption. Chemisorption is much stronger than physical adsorption. Chemisorption may or may not decrease by raising temperature or reducing pressure.
The extent to which a gas is adsorbed on a solid adsorbent depends upon the following factors:
-
(i)
Nature of the solid and of the gas.
-
(ii)
Temperature
-
(iii)
Pressure
11.2.3 Nature of the Gas and the Solid
Adsorption of gases on solids generally involves physical adsorption. Therefore, it is non-specific in nature and as such every gas is adsorbed to a small or large extent on every solid surface. However, it has been found that gases such as NH3, HCl & SO2 which are non-soluble and more easily liquefiable are adsorbed to a large extent then gases like N2 & H2. The reason for greater adsorption of easily liquefiable gasses is that van dar Waals’ forces are more predominant in their case.
As far as solid adsorbents are concerned, adsorbents having large surface area such as charcoal & silica gel acts as a good adsorbent.
11.2.4 Temperature
The adsorption of a gas is always accompanied by evolution of heat. Therefore, according to La Chatelier’s Principle, extent of adsorption decreases with the rise in the temperature.
However, chemisorptions of a gas may be endothermic in nature far a small initial range of temperature.
Therefore, chemisorption shows an initial increase in extent of adsorption with increase in temperature and then a regular decrease takes place.
The effect of temperature on the amount of gas adsorbed, X /m, where x is the amount of gas adsorbed and m is the weight of the adsorbent is shown is in Figs. 11.1 and 11.2 for physical and chemical adsorption respectively. There curves are known as adsorption isobars.
11.2.5 Pressure
The adsorption of a gas by an adsorbent depends upon the pressure of the gas. Initially the amount of gas adsorbed from a given amount of adsorbent increases rapidly with increase in pressure. However, as the pressure becomes high and almost the enter surface of the adsorbent gets saturated with the gas, the effect of pressure becomes very small. Ultimately a stage is reached when no more gas is adsorbed even if the pressure is increased. This stage known as saturation stage and the pressure applied is known as saturation pressure.
The relationship between the magnitude of the adsorption and pressure can be expressed mathematically by an empirical equation called Freundlich Isotherm which may be written as:
Where x is the amount gas adsorbed, m is the weight of the adsorbent, p is the pressure & k and n are constants which depend upon the nature of gas & adsorbent.
The effect of pressure on the amount of gas adsorbed, \( \raisebox{1ex}{$x$}\!\left/ \!\raisebox{-1ex}{$m$}\right. \), is shown graphically in Fig. 11.3. This curve is known as adsorption isotherm.
We know that water is the main source of energy and important for life. But due to some ignorance it has got polluted to a large extent, mainly due to the heavy metal ions. The contamination of water due to heavy metals is a challenging problem due to their toxic effects. These metals like Zn, Hg, Cu, Pb, Cd etc. are responsible for the damage of liver and nerves and can block functional group of vital enzymes and bones (Awwad et al. 2013). These metal ions can be present in water naturally form anthropogenic sources or from leaching of ore deposits, which may include solid waste disposal and industrial effluents. The metal ions are being added to water sources at a much higher concentration, hence leading to health hazards and environmental degradation.
-
Mercury: it is not present naturally in living organisms. It is a toxic substance. The major natural sources of Hg are degassing of earth’s crust, emissions from volcanoes and evaporation from natural bodies of water. The Hg is commonly used in industrial processes and in different products. It is mostly present in a relatively uncreative form as a gaseous element. The methylated forms of Hg are bioaccumulated over a million fold and concentrated in living beings especially fish these forms of Hg are highly toxic causing neurotoxicological disorders. Monomethyl Hg causes damage to central nervous system, while foetal and postnatal exposures have given rise to abortion, congenital malformations and development changes in young children.
-
Cadmium: The cadmium derives it toxicological properties from its chemical similarity to Zn. As an impurity, Cd is present is several products like phosphate fertilizers, detergents and refined petroleum products. As reported average daily intake of cadmium for humans is 0.15 μg from air and 1 μg from water. Cadmium effects kidney if exposed for a long time. Its high exposure may cause obstructive pulmonary disease and lung cancer. In humans and animals it is reported that cadmium. Causes bone defects. It can also cause increased blood pressure and myocardial disease in animal.
-
Lead: children are more sensitive towards lead then the adults. The Pb exposure can be through drinking water, food, air, soil & dust from old paint. The high levels of Pb may results in toxic effects in humans which in turn cause problems in the synthesis of hemoglobin, effects kidneys, reproductive system, joints and damage to nervous system.
-
Selenium: it is needed in small amounts by humans & animals. But if it level increases, it can damage to nervous system & causes fatigue and irritability, it accumulates in living tissues. Its high content in fish and other animals can cause health problems in human beings over a lifetime of overexposure. There may be damage to nervous system, circulating tissues & liver tissues and may be hair and fingernail loss.
-
Nickel: it is needed in small amounts to produce red blood cells, but in excess quantity, it becomes toxic and results in decrease in body weights. It damages heart and liver. It can also cause skin irritation.
-
Copper: if copper is present in high doses anemia may occur. It damages kidney liver and stomach if its level gets increased. During Wilson’s disease, it effects greatly.
-
Chromium: The low level Cr can irritate skin and can produce ulcer. Its chronic exposure can damage liver and kidney. It also damages circulatory and nervous tissues. It is accumulated in aquatic animals and can cause toxicity to eating fish. Chromium occurs in several oxidation states from (+2) to (+6) in environment. The most abundantly occurring forms of chromium are +4 and + 6, which are being toxic to plants, animals and humans. Chromium occurs naturally by burning coal, petroleum, fertilizers, oil mill drilling and metal plating tenures. Fertilizers and sewages are the common sources of release of chromium in environment anthropogenically. Most of the industries using chromium play important role in environmental pollution and causing the adverse effects on the biological and ecological species with chromium. Agricultural and industrial practices on chromium increase the level of toxicity in surrounding of living being. Due to the presence of oxygen in the environment, the forms of chromium are oxidized to very harmful and poisonous substances, which are highly soluble in water. Chromate manufacturing process causes series pollution to farm land. Chromium also causes toxicity in plants. Consumption of toxic plant effects the biological factors. Phytotoxicity due to chromium is the common feature in reduction of root growth, inhibition of seeds, germination, suppresses biomass and chlorosis of leaf. It is reported that hexavalent chromium is considered group 1 in bone cancer causing.
-
Iron: Iron is important for growth and survival of human beings. Many people exposed to iron through drinking water that was collected from ground. Fishes are effected by iron contamination, causing disturbances is respiration. Rice, the main food item, is also effected by iron toxicity. Iron stops the rice production and causes zinc deficiency. When iron fails to bind with protein, they form harmful free radical. This harmful red radical which effects the level of iron in human cells, damages digestive track, they also penetrate into the cells of vital organs like liver, brain & heart. Over intake of iron can increase the risk of these free radicals to cause further DNA damage. As reported these free radicals are also responsible for mutations and transformations.
-
Aluminum: We are exposed to aluminum by water, food and drugs containing aluminum. Symptoms of high level of aluminum are vomiting, ulcer, skin rashes and bone pain. Aluminum effects brain and causes loss of memory and imbalance in human posture.
-
Arsenic: It is toxic in nature and cancer causing. It is found in different forms, mainly in the form of oxide and sulphide. It is also found in salt form with sodium, copper and iron. Arsenate and its compounds are dangerous to human beings and also to the surroundings. Arsenic causes cell respiration malfunctioning. Inorganic methylated arsenic compounds are transformed biologically into harmful forms due to bacteria, fungi, humans and algae to provide mono and dimethyl arsenic acids. This biological process of transformation converts inorganic arsenic species enzymatically into methylated arsenic which shows the severe exposure of arsenic. Arsenic minerals are the causes of ground water contamination. So higher amounts of arsenic exposure to environment are due to fertilizers and certain pesticides and animal feeding operation. Arsenide and arsenate are cancer causing to lungs, liver, skin & bladder. Arsenic exposure to humans is mainly by air, food and water.
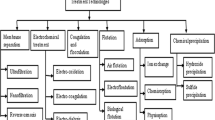
For the removal of these metal ions from water various methods like ion-exchange, reverse osmosis, chemical precipitation, chemical oxidation or reduction, electrodialysis and ultrafiltration are used. But these techniques have their own inherent limitations such as sensitive operating conditions, less efficiency and production of secondary sludge and further the disposal is a costly affair (Fu and Wang 2011; Ahluwalia and Goyal 2005). On the other hand, adsorption technique has gained high attention due to its advantages. These days’ natural absorbents have received much attention for the removal of heavy metal ions. Untreated plant wastes e.g. peanut hull pellets (Johnson et al. 2002), Papaya stem (Saeed et al. 2005a), rise husk ash and neem bark (Bhattacharya et al. 2006) Saltbush (Atriplex canescens) leaves have received a wide attention of adsorption studies.
The various adsorbents used for this purpose are:
11.3 Agricultural Waste Adsorbents
These are different forms of inexpensive and non-living plant material such as black gram husk (Saeed et al. 2005b), eggs hell (Park et al. 2007) Sugar-beet pectin gels (Mata et al. 2009) and citrus peels (Schiewer and Patil 2008) are considered as potential adsorbents. Annaduri et al. (2003) performed the adsorption of heavy metal ions like Cu2+, Zn2+, Co2+, Ni2+ & Pb2+ onto acid and alkali treated banana and orange peels. It has been reported that residues of banana & orange peels are cellulose-based wastes. Hence, can be processed and converted to be adsorbents as they have large surface areas, high swelling capacities, and good mechanical strength, are convenient to use, and have great potential to adsorb harmful contaminants like heavy metals. Based on regeneration studies, it was reported that the peels could be used for two regenerations for removal and recovery of heavy metal ions.
The HCl treated carrot residues can remove the heavy metals like Cr3+, Cu2+, Zn2+ from waste water. The adsorption of metal ions onto carrot residues was possible due to cation exchange properties of these residues which was attributed to the presence of carboxylic and phenolic functional groups, which exist in either the cellulosic matrix or in the materials associated with cellulose, such as hemicelluloses and lignin (Nasernejad et al. 2005).
11.3.1 Biomass Adsorbents
Many biomass source adsorbents have seen widely investigated as potential biosorbents for heavy metals. Algae, a renewable natural biomass which proliferates ubiquitously and abundantly in the littoral zones of the world, have attracted the attention of many investigators as organisms to be tested and used as new adsorbents to adsorb metal ions. The biosorption of Cu2+ and Zn2+ by dried marine green macroalga (c.linum) investigated by Ajjabi and chouba (2009).
11.3.2 Byproduct Adsorbents
-
Sawdust: It is obtained from wood industry as a byproduct. It has been found that sawdust contains several organic compounds (e.g. cellulose, lignin and hemicelluloses) with polyphenolic groups that could bind heavy metal ions through different mechanisms. Sawdust was used by Sciban et al. (2006) for the removal of heavy metals.
-
Lignin: Lignin is also used as an adsorbent for the metal ions. The high adsorption capacity of lignin is in part due to polyhydric phenols and other functional groups on the surface. Ion-exchange may also play a role in the adsorption of metals by lignin
-
Rice Husk and Rice Husk Ash: Rice husk is insoluble in water. It has high mechanical strength and has good chemical stability. It has a granular structure. It is a good adsorbent material for the removal of heavy metals from waste water. Rice husk has been extensively reviewed by Chuah et al. (2005) for the removal of heavy metal. It has been used for the removal of heavy metals either in untreated form or modified form by different modification methods. The common chemical treatment methods of rice husk are sodium carbonate and hydrochloric acid (Kumar and Bandyopadhyay 2006), epichlordhydrin & sodium hydroxide (Bhattacharya et al. 2006) and tartaric acid. Rick husk’s pretreatment can remove hemicelluloses and lignin, reduce cellulose crystallinity and increase the surface area or porosity. It was reported by Kumar and Bandyopadhyay (2006) that rice husk treated with sodium carbonate, sodium hydroxide and epichlorihydrin enhanced the adsorption capacity of heavy metals. Rice husk ash is used for the removal of Zn2+ (Bhattacharya et al. 2006). Rice bran was evaluated for its potential as an adsorbent for Cd2+, Cu2+, Pb2+ & Zn2+ (Montanher et al. 2005). Rice bran adsorbent is able to adsorb metal ions from aqueous solutions.
-
Fly Ash: Since the industrial revolution, a wide scale coal burning for power generation started. Although that were many millions of tons of ash that have been generated. It was estimated that the current annual production of coal ash (worldwide) is around 600 million tons, with fly ash forming about 500 million tons at 75–80% of the total ash produced. Thus the amount of fly ash as coal waste released by thermal power plants and factories has been increasing throughout the world.
The utilization of fly ash for the removal of heavy metals from industrial waste waters was reported by Gangoli et al. (1975).
As a law-cost adsorbent, fly ash has been widely used for use removal of heavy metals. As early as 1975, the use of fly ash for the removal of heavy metals was reported. Bayat (2002a) investigated the removal of heavy metal ions (Bayat 2002b), using lignite-based fly ash and activated carbon and discovered that fly ash was effective as activated carbon.
A series of investigations was conducted on the adsorption of heavy metals, using bagasse fly ash adsorbent bagasse fly ash from sugar industries was used for the removal of zinc and copper form aqueous solution (Gupta and Ali 2000; Gupta and Sharma 2003).
After the adsorption process, fly ash can be regenerated using certain reagents. Batabyal et al. (1995) reported that saturated fly ash can be regenerated using 20% of aqueous H2O2 solution.
11.4 Conclusion
Overall adsorption processes are most conviniest method for removal of heavy metals from the solvent and waste water. Biomaterials based biosorbents are safe and economical to eliminate heavy metals from contaminated waters. The gain the interest of researchers towards the production of low cost adsorbents for more application and sustainable development of ecosystem. Therefore there is an urgent need that all possible sources of agro-based inexpensive adsorbents should be explored and their role for the removal of heavy metals.
References
Ahluwalia SS, Goyal D (2005) Removal of heavy metals by waste tea leaves from aqueous solution. Eng Life Sci 5(2):158–162
Ajjabi LC, Chouba L (2009) Biosorption of Cu2+ and Zn2+ from aqueous solutions by dried marine green macroalga charctomorpha linum. J Environ Manag 90(11):3485–3489
Annaduroi G, Juang RS, Lee DJ (2003) Adsorption of heavy metals from water using banana & orange peels. Water Sci Technol 47(1):185–190
Awwad NS, El-Zahhar AA, Founda AM, Ibrahim HA (2013) Removal of heavy metal ions from ground water and surface water samples using carbons derived from date pits. J Environ Chem Eng 1(3):416–423
Batabyal D, Sahu A, Chaudhuri SK (1995) Kinetics and mechanism of removal of 2,4- dimethyl phenol from aqueous solutions with coal fly ash. Sep Technol 5(4):179–186
Bayat B (2002a) Combined removal of Zinc (II) and cadmium(II) from aqueous solutions by adsoption onto high-calcium Turkish fly ash. Water Air Soil Pollut 136(1–4):69–92
Bayat B (2002b) Comparative study of adsorption properties of Turkish fly ashes: I. the case of nickel (II), Copper (II) and Zinc (II). J Hazard Mater 95(3):251–273
Bhattacharya AK, Mandal SN, Das SK (2006) Adsorption of Zn (II) from aqueous solution by using different adsorbents. Chem Eng J 123(1–2):43–51
Chuoh TG, Jumosiah A, Azmi I, Katayan S, Thomas Choong SY (2005) Rice husk as a potential low-cost biosorbent for heavy metal and dye removal: an overview. Desalination 175(3):305–316
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manag 92(3):407–418
Gangoli N, Markey D, Thodas G (1975) Removal of heavy metal ions, from aqueous solutions with fly ash. In: Proceedings of the national conference on complete water use, Chicago, IL, USA, pp 270–275
Gupta VK, Ali I (2000) Utilization of bagasses fly ash (a sugar industry waste) for the removal of copper and zinc from waste water. Sep Purif Technol 18(2):131–140
Gupta VK, Sharma S (2003) Removal of Zinc from aqueous solutions using message fly ash a low cost adsorbent. Ind Eng Chem Res 42(25):6619–6624
Johnson PD, Watson MA, Brown J, Jefcoat IA (2002) Peanut hull pellets as a single use sorbent for the capture of cu (II) form wastewaer. Waste Manag 22(5):471–780
Kumar U, Bandyopadhyay M (2006) Sorption of cadmium from aqueous solution using pretreated rice husk. Bioresour Technol 97(1):104–109
Mata YN, Blazquez ML, Ballester A, Gonzalez F, Munoz JA (2009) Sugar-beet pulp pectin gels as biosorbent for heavy metals: preparation and determination of biosorption and desorption characteristics. Chem Eng J 150(2–3):289–301
Montanher SF, Oliveira EA, Rollemberg MC (2005) Removal of heavy metal ions from aqueous solutions by sorption onto rice bran. J Hazard Mater 117(2–3):207–211
Nasernejad B, Zadeh TE, Pour BB, Bygi ME, Zamani A (2005) Comparison for biosorption modeling of heavy metals from waste water by carrot residues. Process Biochem 40(3–4):1319–1322
Park HJ, Jeong SW, Yang JK, Kim BG, Lee SM (2007) Removal of heavy metals using waste egg shells. J Environ Sci 19(12):1436–1441
Saeed A, Akhtar MW, Iqbal M (2005a) Removal and recovery of heavy metals from aqueous solution using papaya wood as a new biosorbent. Sep Purif Technol 45(1):25–31
Saeed A, Iqbal M, Akhtar MW (2005b) Removal and recovery of lead (II) from single and multimetal (Cd, Cu, Ni, Zn) solutions by crop milling waste (black gram husk). J Hazard Mater 117(1):65–736
Schiewer S, Patil SB (2008) Modeling the effect of PH on biosorption of heavy metals by citrus peals. J Hazard Mater 157(1):8–17
Sciban M, Klasnja M, Skrbic B (2006) Modified hardwood sawdust as adsorbent of heavy metal ions from water. Wood Sci Technol 40(3):217–227
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Khan, A.M., Ganai, S.A. (2020). Removal and Recovery of Heavy Metal Ions Using Natural Adsorbents. In: Oves, M., Ansari, M., Zain Khan, M., Shahadat, M., M.I. Ismail, I. (eds) Modern Age Waste Water Problems . Springer, Cham. https://doi.org/10.1007/978-3-030-08283-3_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-08283-3_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-08282-6
Online ISBN: 978-3-030-08283-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)