Abstract
Skull base fungal pathologies present with headache, nasal symptoms, orbital symptoms, cranial nerve palsy and paresis. Isolated skull base involvement is rare; commonly, skull base is involved by the lesion spreading from the adjacent paranasal sinuses. Vital structures like the orbit, optic nerve, internal carotid artery, pituitary gland, cavernous sinus and cranial nerves may often be involved. This chapter gives the overview of various types of skull base fungal diseases and their management principles.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Fungal infection of the skull base is not uncommon in clinical practice. In the recent times, the incidence of this entity has been increasing due to increase in the prevalence of immunocompromised conditions like diabetes mellitus and haematological malignancies (Dubey et al. 2005). Rarely, it can be present in immunocompetent individuals also (Shah et al. 2017). Fungal infection of the central nervous system can manifest as meningitis, meningoencephalitis, vasculitis, abscess formation and granuloma formation (Mohindra et al. 2008). Skull base fungal pathologies present with headache, nasal symptoms, orbital symptoms, cranial nerve palsy and paresis. Isolated skull base involvement is rare; commonly, skull base is involved by the lesion spreading from the adjacent paranasal sinuses. The paranasal sinuses are closely related to the anterior and middle cranial base and also to vital structures like the orbit, optic nerve, internal carotid artery, pituitary gland, cavernous sinus and cranial nerves. Fungal diseases have a wide spectrum of acute-to-chronic clinical presentation. There are various fungal organisms involved in causing lesions of the skull base, like Aspergillus, Scedosporium, Alternaria, Curvularia and Mucor, and these are more abundant in the air and soil (Süslü et al. 2009; Tarkan et al. 2012; Zuniga and Turner 2014).
de Shazo et al. (1997) classified fungal diseases into two major categories, non-invasive and invasive, based on the histopathology. The non-invasive fungal diseases are further classified as allergic fungal rhinosinusitis (AFRS) and chronic non-invasive diseases (fungal ball). The invasive fungal disease has a spectrum that is classified as acute fulminant invasive, chronic invasive and granulomatous invasive.
The prognosis depends more on the type of infection and the host immunity and to a lesser extent on the type of the fungal species, but identification of the fungal species by culture does aid in the antifungal medication selection and the therapy administered.
2 Invasive Fungal Disease
Invasive fungal disease involving the skull base is classified based on the onset, duration and progression of the disease as chronic invasive and acute invasive.
2.1 Chronic Invasive Skull Base Fungal Disease (CISBFD)
Chronic or indolent fungal pathologies involving the skull base occur in both immunocompetent and immunocompromised individuals. The usual clinical course of these lesions is slowly progressive in nature, developing over weeks to months, in contrast to their acute counterparts. Any immunocompromised patient presenting with fever of unknown origin, headache, facial swelling, facial pain, nasal obstruction, nasal discharge, nasal bleed, nasal crusting, proptosis, diplopia and cranial nerve deficits should be suspected of having an invasive fungal pathology. In immunocompetent individuals, however, diplopia, painless proptosis and orbital complaints are the commonest presentations (Shah et al. 2017). All the suspected patients should undergo a complete cranial nerve examination followed by nasal endoscopy and oral cavity examination to look for a change in the colour of the mucosa or the presence of any mucosal ulceration or necrosis. A biopsy from the suspected site should be taken for histopathologic confirmation. Skull base involvement is usually secondary to the sinonasal involvement (Figs. 21.1, 21.2, and 21.3). Moreover, an isolated sphenoidal sinus (Fig. 21.4) involvement is more aggressive in nature because of its anatomical proximity to the orbital apex, optic nerve, carotid artery, cavernous sinus, pituitary gland and cranial nerves (Dhiwakar et al. 2003b). Rarely do we encounter isolated skull base involvement without sinonasal pathology (Figs. 21.5 and 21.6). In isolated skull base fungal pathology, it is difficult to differentiate the lesion from other pathologies like a meningioma and a tuberculoma (Mohindra et al. 2008).
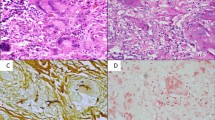
Biopsy from the suspected lesion should be subjected to microscopy, fungal culture and histopathology. For fungal microscopy, the commonest strain used is potassium hydroxide (Fig. 21.7) and the calcofluor white (a special fluorescent stain that binds strongly to structures containing cellulose and chitin) method using fluorescence microscopy. For histopathology, the commonest stain used for fungal identification is Gomori methenamine silver staining (Schell 2000) (Fig. 21.8). The histopathological examination of the tissue establishes the presence and nature of the fungal hyphae (Fig. 21.9). In mucormycosis, the fungal elements are broad, ribbon-like, irregular and rarely septate, whereas aspergillosis shows narrow, regular septae with 45° branching patterns (Ferguson 2000; Gillespie and O’Malley 2000). Fungal elements should also be looked for in the submucosa. The presence of angio-invasion, tissue necrosis and recruitment of inflammatory cells should also be assessed (de Shazo 1998). One of the most striking differences between aspergillosis and mucormycosis is that the former causes angio-invasion but does not lead to vaso-occlusion, whereas mucormycosis is almost always associated with vaso-obliteration (Epstein and Kern 2008). Fungal culture is also important to initiate species-specific antifungal therapy and to identify the additional rare fungal species causing skull base pathologies like the Fusarium, Alternaria and Pseudallescheria species (Kalkanci et al. 2006) (Figs. 21.10 and 21.11). Sometimes, the routine evaluation does not show any fungal infection; in these conditions, the newer modalities like identification of fungal cell wall markers in the serum like galactomannan (Chen et al. 2011; Schwartz et al. 2005), beta-D-glucan and mannan enzyme immunoassay may be done. These tests are more specific for detection of aspergillosis infection. Molecular testing method that uses the oligonucleotide probe and gene sequencing can be used for rapid identification of the fungal species. In case of frank intracranial involvement by invasive aspergillosis, the sensitivity of cerebrospinal fluid (CSF) polymerase chain reaction (PCR) is 100% as compared to the sensitivity of galactomannan, which is 80% (Chen et al. 2011).
To evaluate the extent of the lesion is as important as the clinical and laboratory confirmation of the fungal infection. The computed tomographic (CT) scan is the primary imaging modality in all the suspected cases harbouring a fungal pathology to assess for abnormalities in the paranasal sinuses and the skull base. The common findings are partial or complete sinus opacification with or without destruction of the bony wall and the sclerotic thickening of the sinus wall (Hoon et al. 2014). On contrast-enhanced CT scan images of the skull base, aspergilloma (Fig. 21.12) is present as an enhancing mass with irregular border with surrounding cerebral oedema (Jain et al. 2007). Contrast-enhanced magnetic resonance imaging (MRI) may be a better tool for establishing the diagnosis and to determine the extent of disease (Dubey et al. 2005; Yamada et al. 2002). On MRI, fungal lesions are isointense on T1-weighted images (WI) with a hypo-to-isointense signal intensity on T2WI. There is a uniform enhancement on contrast T1W1 (Hoon et al. 2014; Jain et al. 2007) (Fig. 21.13).
After confirming the diagnosis and the extent of pathology, the basic principles of management include correcting the immunocompromised condition, the surgical debridement (Selvam et al. 2010) (Figs. 21.14 and 21.15) and the starting of the appropriate antifungal therapy. Among these, the most important issue is to take steps to cause reversal of the underlying co-morbid condition. In some cases, even an infusion of granulocytes is used to reverse the immune status of the patients (Martinez et al. 2013).
An aggressive surgical debridement is essential to reduce the disease load and for gaining a better outcome; however, due to the presence of vital structures in and around the skull base, like the optic nerve, carotid artery, cranial base dura, cavernous sinus and cranial nerves, radical debridement is often not possible. Lesions involving the orbital apex (Fig. 21.16) and the retrobulbar region warrant an orbital exenteration, but this is not required during the anterior and inferomedial involvement of the orbit (Dhiwakar et al. 2003a). Some studies have shown that the extent of debridement was not a significant factor in influencing the patient survival (Hoon et al. 2014). To tackle the residual disease, initiation of antifungal therapy is mandatory.
Antifungals like triazoles (fluconazole, itraconazole, voriconazole and posaconazole), echinocandidins (caspofungin, micafungin, anidulafungin), polyenes (amphotericin B) and flucytosine are available for the medical treatment of invasive fungal disease (Herbrecht et al. 2002; Kontoyiannis 2012; Redmond et al. 2007). Amphotericin B is the broad-spectrum fungicidal agent, but its toxicity limits its widespread and prolonged usage. The newer liposomal formulations are comparatively less toxic and can be used in higher dosages. Voriconazole, approved by the US Food and Drug Administration (FDA) in 2002, is more effective than amphotericin in invasive aspergillosis (Gillespie and O’Malley 2000). The duration of therapy is based on clinical and radiological response and varies from 3 to 6 months. Posaconazole has shown its efficacy as a salvage therapy in patients with end-stage renal disease caused due to diabetes (Mehta and Langston 2009).
Shah et al. (2017) and Mohindra et al. (2008) have described the protocol for the management of skull base invasive aspergillosis. Patient with skull base involvement with minimal invasion of the basal frontal lobe, cavernous sinus and the infratemporal fossa should be treated with extradural debridement followed by systemic antifungal therapy. Stable patients with massive intracranial invasion of the frontal or temporal lobes with cerebral oedema should be preloaded with liposomal amphotericin B of 2 g followed by debridement; and after debridement, they should be given a cumulative dose of up to 6 g. Patients who are not hemodynamically stable because of reasons like uncal herniation need immediate debridement without the requirement for preloading of antifungal medications. The debridement may followed by administration of 6–8 g of amphotericin B. After completion of the desired dose of amphotericin B, the patients should be started on azole group of drugs. The medicines are continued for 3–6 months (Fig. 21.17).
2.2 Acute Invasive Skull Base Fungal Disease (AISBFD)
Acute invasive fungal disease is also called as fulminant fungal disease. AISBFD results from rapid progression of the fungus into the paranasal sinus, orbit, vessels and nerves, the musculoskeletal system surrounding the skull base and the brain parenchyma. The time course of less than 4 weeks’ duration differentiates this entity from the CISBFD. AISBFD is almost always common in individuals suffering from an immunocompromised condition like a haematological malignancy, uncontrolled diabetes mellitus, prolonged steroid use, organ transplantation or autoimmune deficiency syndrome (Abu El-Naaj et al. 2013; Kasapoglu et al. 2010). Although rare, this condition is also reported in immunocompetent individuals (Chopra et al. 2006; Gillespie and O’Malley 2000; Marple 2001; Saravanan et al. 2006). AISBFD is a condition that requires immediate management; otherwise the mortality can be as high as 50–80% (Gillespie et al. 1998; Kennedy et al. 1997). The most common organisms responsible are the Aspergillus species and the Zygomycetes species (Süslü et al. 2009; Tarkan et al. 2012). Patients with uncontrolled diabetes mellitus are more prone to developing mucormycosis infection because of their altered transferrin binding capacity (Spellberg et al. 2012). Any patient in an immunocompromised status with facial swelling, facial pain, headache, prolonged fever, orbital symptoms and cranial nerve palsies should be evaluated clinically, radiologically and pathologically to confirm the diagnosis. These patients require steps to revert the immunocompromised status, the surgical debridement and the administration of appropriate systemic antifungal therapy (Fig. 21.18).
2.3 Chronic Invasive Granulomatous Fungal Disease (CIGFD)
CIGFD is seen in immunocompetent individuals, and it is most commonly caused by Aspergillus flavus (Stringer and Ryan 2000). The most common presentation is unilateral proptosis. Other symptoms include nasal congestion, nasal obstruction, facial pain, headache and facial numbness (Stringer and Ryan 2000). On a CT scan, it presents as a unilateral isodense or hypodense lesion, whereas on MRI, it is isointense on T1WI and hypointense on T2WI (Reddy et al. 2010). On histopathology, CIGFD may be differentiated from chronic invasive fungal disease (CIFD) by the presence of non-caseating granulomas with fungal hyphae within the giant cells of the granuloma, with occasional invasion of blood vessels and adjoining tissues (Stringer and Ryan 2000). The treatment for CIGFD is still under debate, with the most accepted treatment being surgical debridement followed by oral antifungal agents (Halderman et al. 2014; Kim et al. 2012). Voriconazole is a very effective oral agent when there is involvement of skull base as there is good penetration of the CSF (Black and Baden 2007). There is no consensus on the duration for which oral antifungal treatment needs to be continued, but to prevent a relapse, most authors recommend treatment until complete remission is achieved (Black and Baden 2007; Halderman et al. 2014; Stringer and Ryan 2000).
3 The Non-invasive Fungal Diseases
Non-invasive fungal disease of the paranasal sinuses can involve the skull base, but these are usually extradural lesions that can cause symptoms based on the type and its location. Allergic fungal rhinosinusitis and fungal ball are the two types of non-invasive fungal diseases. AFRS is considered to be the sinonasal form of allergic bronchopulmonary aspergillosis (ABPA) (Marple 2001). Type I hypersensitivity to the fungal antigen is the proposed pathophysiology. The commonest fungi that are attributed to this disease are the Alternaria, Bipolaris, Curvularia and Aspergillus species (Kim et al. 2012). Manning and Holman analysed the serum from patients with AFRS and found 82% IgE antibodies (Halderman et al. 2014). In extensive disease with orbital involvement, patients present with proptosis, telecanthus as well as visual disturbance and cranial nerve palsy. When the anterior skull base and cavernous sinus are involved, these manifestations are mainly caused by the pressure effect of the expanding fungal tissue (Fig. 21.19). Studies by Saravan and colleagues and Diwakar and colleagues found considerably increased incidence of bony erosion and sinus expansion on the CT scan (Black and Baden 2007; Kennedy et al. 1997; Marple 2001; Saravanan et al. 2006; Stringer and Ryan 2000). Ghegan and colleagues showed that 56% of AFRS had skull base erosion (Ryan 2011; Süslü et al. 2009). Though this is not an invasive condition, surgery followed by intranasal steroid is the treatment modality of choice (Manning and Holman 1998). The role of systemic antifungal therapy (itraconazole) is controversial (Khalil et al. 2011; Seiberling and Wormald 2009). Fungal ball is a non-invasive lesion most commonly involving the maxillary sinus. It occurs in both immunocompetent and immunocompromised hosts. Sphenoid sinus fungal ball comprises of 13–25% of all fungal balls, and of that, about 50% of patients have visual complaints. The visual symptoms are caused because of neuritis, ischemic infiltrates or compression due to the fungal ball (Fig. 21.20). Surgical removal of the fungal ball is the definitive management. There is no role of antifungal therapy (Kim et al. 2016).
4 Conclusion
Fungal disease of the skull base covers the entire spectrum of diseases ranging from diseases with a lower morbidity status to extremely fatal conditions. In many cases, clinical suspicion and an adequate workup can make a huge impact on the outcome of the disease. Though surgery plays a very important role in the initial management, antifungal therapy and a serial follow-up are more important for a better long-term morbidity control.
Abbreviations
- AFRS:
-
Allergic fungal rhinosinusitis
- AISBFD:
-
Acute invasive skull base fungal disease
- CIGFD:
-
Chronic invasive granulomatous fungal disease
- CISBFD:
-
Chronic invasive skull base fungal disease
- CSF:
-
Cerebrospinal fluid
- CT scan:
-
Computed tomographic scan
- FDA:
-
Food and Drug Administration
- Ig E:
-
Immunoglobulin E
- MRI:
-
Magnetic resonance imaging
- PCR:
-
Polymerase chain reaction
- WI:
-
Weighted images
References
Abu El-Naaj I, Leiser Y, Wolff A, Peled M. The surgical management of rhinocerebral mucormycosis. J Craniomaxillofac Surg. 2013;41(4):291–5.
Black KE, Baden LR. Fungal infections of the CNS: treatment strategies for the immunocompromised patient. CNS Drugs. 2007;21(4):293–318.
Chen S, Pu J-L, Yu J, Zhang J-M. Multiple Aspergillus cerebellar abscesses in a middle-aged female: case report and literature review. Int J Med Sci. 2011;8(7):635–9.
Chopra H, Dua K, Malhotra V, Gupta RP, Puri H. Invasive fungal sinusitis of isolated sphenoid sinus in immunocompetent subjects. Mycoses. 2006;49(1):30–6.
de Shazo RD. Fungal sinusitis. Am J Med Sci. 1998;316(1):39–45.
de Shazo RD, O’Brien M, Chapin K, Soto-Aguilar M, Gardner L, Swain R. A new classification and diagnostic criteria for invasive fungal sinusitis. Arch Otolaryngol Head Neck Surg. 1997;123(11):1181–8.
Dhiwakar M, Thakar A, Bahadur S. Invasive sino-orbital aspergillosis: surgical decisions and dilemmas. J Laryngol Otol. 2003a;117(4):280–5.
Dhiwakar M, Thakar A, Bahadur S, Sarkar C, Banerji U, Handa KK, et al. Preoperative diagnosis of allergic fungal sinusitis. Laryngoscope. 2003b;113(4):688–94.
Dubey A, Patwardhan RV, Sampath S, Santosh V, Kolluri S, Nanda A. Intracranial fungal granuloma: analysis of 40 patients and review of the literature. Surg Neurol. 2005;63(3):254–60.
Epstein VA, Kern RC. Invasive fungal sinusitis and complications of rhinosinusitis. Otolaryngol Clin North Am. 2008;41(3):497–524.
Ferguson BJ. Definitions of fungal rhinosinusitis. Otolaryngol Clin North Am. 2000;33(2):227–35.
Gillespie MB, O’Malley BW. An algorithmic approach to the diagnosis and management of invasive fungal rhinosinusitis in the immunocompromised patient. Otolaryngol Clin North Am. 2000;33(2):323–34.
Gillespie MB, O’Malley BW, Francis HW. An approach to fulminant invasive fungal rhinosinusitis in the immunocompromised host. Arch Otolaryngol Head Neck Surg. 1998;124(5):520–6.
Halderman A, Shrestha R, Sindwani R. Chronic granulomatous invasive fungal sinusitis: an evolving approach to management. Int Forum Allergy Rhinol. 2014;4(4):280–3.
Herbrecht R, Denning DW, Patterson TF, Bennett JE, Greene RE, Oestmann J-W, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347(6):408–15.
Hoon D, Tae L, Yoon M, Kyoo J, Young L, Joo E, et al. Invasive fungal sinusitis of the sphenoid sinus. Clin Exp Otorhinolaryngol. 2014;7(3):181–7.
Jain KK, Mittal SK, Kumar S, Gupta RK. Imaging features of central nervous system fungal infections. Neurol India. 2007;55(3):241–50.
Kalkanci A, Kustimur S, Turkoz Sucak G, Senol E, Sugita T, Adams G, et al. Fulminating fungal sinusitis caused by Valsa sordida, a plant pathogen, in a patient immunocompromised by acute myeloid leukemia. Med Mycol. 2006;44(6):531–9.
Kasapoglu F, Coskun H, Ozmen OA, Akalin H, Ener B. Acute invasive fungal rhinosinusitis: evaluation of 26 patients treated with endonasal or open surgical procedures. Otolaryngol Head Neck Surg. 2010;143(5):614–20.
Kennedy CA, Adams GL, Neglia JP, Giebink GS. Impact of surgical treatment on paranasal fungal infections in bone marrow transplant patients. Otolaryngol Head Neck Surg. 1997;116(6 Pt 1):610–6.
Khalil Y, Tharwat A, Abdou AG, Essa E, Elsawy AH, Essawy AH, et al. The role of antifungal therapy in the prevention of recurrent allergic fungal rhinosinusitis after functional endoscopic sinus surgery: a randomized, controlled study. Ear Nose Throat J. 2011;90(8):E1–7.
Kim JS, Kim BK, Hong SD, Kim HJ, Kim HY. Clinical characteristics of sphenoid sinus fungal ball patients with visual disturbance. Clin Exp Otorhinolaryngol. 2016;9(4):326–31.
Kim TH, Jang HU, Jung YY, Kim JS. Granulomatous invasive fungal rhinosinusitis extending into the pterygopalatine fossa and orbital floor: a case report. Med Mycol Case Rep. 2012;1(1):107–11.
Kontoyiannis DP. Invasive mycoses: strategies for effective management. Am J Med. 2012;125:S25–38.
Manning SC, Holman M. Further evidence for allergic pathophysiology in allergic fungal sinusitis. Laryngoscope. 1998;108(10):1485–96.
Marple BF. Allergic fungal rhinosinusitis: current theories and management strategies. Laryngoscope. 2001;111(6):1006–19.
Martinez M, Chen V, Tong AJ, Hamilton K, Clemons KV, Stevens DA. Experimental evidence that granulocyte transfusions are efficacious in treatment of neutropenic hosts with pulmonary aspergillosis. Antimicrob Agents Chemother. 2013;57(4):1882–7.
Mehta AK, Langston AA. Use of posaconazole in the treatment of invasive fungal infections. Expert Rev Hematol. 2009;2(6):619–30.
Mohindra S, Mukherjee KK, Chhabra R, Gupta SK, Gupta R, Khosla VK. Invasive intracranial aspergillosis: the management dilemmas. Surg Neurol. 2008;69(5):496–505.
Reddy CEE, Gupta AK, Singh P, Mann SBS. Imaging of granulomatous and chronic invasive fungal sinusitis: comparison with allergic fungal sinusitis. Otolaryngol Neck Surg. 2010;143(2):294–300.
Redmond A, Dancer C, Woods ML. Fungal infections of the central nervous system: a review of fungal pathogens and treatment. Neurol India. 2007;55(3):251–9.
Ryan MW. Allergic fungal rhinosinusitis. Otolaryngol Clin North Am. 2011;44(3):697–710.
Saravanan K, Panda NK, Chakrabarti A, Das A, Bapuraj RJ. Allergic fungal rhinosinusitis: an attempt to resolve the diagnostic dilemma. Arch Otolaryngol Head Neck Surg. 2006;132(2):173–8.
Schell WA. Histopathology of fungal rhinosinusitis. Otolaryngol Clin North Am. 2000;33(2):251–76.
Schwartz S, Ruhnke M, Ribaud P, Corey L, Driscoll T, Cornely OA, et al. Improved outcome in central nervous system aspergillosis, using voriconazole treatment. Blood. 2005;106(8):2641–5.
Seiberling K, Wormald P-J. The role of itraconazole in recalcitrant fungal sinusitis. Am J Rhinol Allergy. 2009;23(3):303–6.
Selvam M, Pande A, Chakravarthy V, Ramamurthi R. Invasive rhino-cerebral fungal granuloma. Neurol India. 2010;58(2):270.
Shah SR, Keshri A, Patadia S, Marak RSK, Behari S. Invasive aspergillosis of anterior skull base in the immunocompetent host: outcomes with a combined treatment modality—an institutional experience. Orig Artic. 2017;78:89–95.
Spellberg B, Kontoyiannis DP, Fredricks D, Morris MI, Perfect JR, Chin-Hong PV, et al. Risk factors for mortality in patients with mucormycosis. Med Mycol. 2012;50(6):611–8.
Stringer SP, Ryan MW. Chronic invasive fungal rhinosinusitis. Otolaryngol Clin North Am. 2000;33(2):375–87.
Süslü AE, Oğretmenoğlu O, Süslü N, Yücel OT, Onerci TM. Acute invasive fungal rhinosinusitis: our experience with 19 patients. Eur Arch Otorhinolaryngol. 2009;266(1):77–82.
Tarkan O, Karagün B, Ozdemir S, Tuncer U, Sürmelioğlu O, Cekiç E, et al. Endonasal treatment of acute invasive fungal rhinosinusitis in immunocompromised pediatric hematology-oncology patients. Int J Pediatr Otorhinolaryngol. 2012;76(10):1458–64.
Yamada K, Shrier DA, Rubio A, Shan Y, Zoarski GH, Yoshiura T, et al. Imaging findings in intracranial aspergillosis. Acad Radiol. 2002;9(2):163–71.
Zuniga MG, Turner JH. Treatment outcomes in acute invasive fungal rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2014;22(3):242–8.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ravi Sankar, M., Arulalan, M., Keshri, A.K., Srivastava, A.K., Jaiswal, A.K., Behari, S. (2019). Invasive Fungal Diseases of the Skull Base. In: Turgut, M., Challa, S., Akhaddar, A. (eds) Fungal Infections of the Central Nervous System. Springer, Cham. https://doi.org/10.1007/978-3-030-06088-6_21
Download citation
DOI: https://doi.org/10.1007/978-3-030-06088-6_21
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-06087-9
Online ISBN: 978-3-030-06088-6
eBook Packages: MedicineMedicine (R0)