Abstract
Knee dislocation of any type can be associated with major concomitant vascular injury. Serious vascular injury resulting in ischemia demands prompt recognition and efficient management to prevent devastating long-term sequelae such as ischemic nerve injury, and in the worst-case scenario, major amputation. In this chapter, the mechanism by which knee dislocation results in popliteal vascular injury will be reviewed. Physical exam findings indicative of vascular injury will be described. Diagnostic modalities used to evaluate the extent of vascular injury will be individually discussed. Appropriate initial management of vascular injuries is crucial, and an algorithm for diagnosis and management will be reviewed. Finally, the salient points of vascular reconstruction in the context of the dislocated knee including surgical approach, conduct of the procedure, and adjunctive maneuvers will be discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Major popliteal artery or vein injury can accompany multi-ligamentous dislocation of the knee, and accounts for one of the most common reasons for medicolegal litigation with this entity. Only with a high clinical index of suspicion, rapid diagnosis, evaluation, and treatment can complications from vascular injury be avoided. Always present in the mind of the vascular surgeon is the very real possibility of a major vascular injury leading to irreversible ischemic injury and limb loss.
Knee dislocation itself is an infrequent injury. It accounts for less than 1% of all extremity injuries. Its rarity adds to the danger of underappreciation of the possibility of associated vascular injury. Modern diagnostic imaging, particularly MRI, has increased the ability to diagnose the ligamentous injuries. Knee dislocation was previously diagnosed on clinical grounds which were often unreliable. Two important practical diagnostic considerations must be appreciated. There is a high incidence of spontaneous reduction of the dislocation by the time orthopedic evaluation is performed which reduces the likelihood of recognizing the injury clinically as a dislocation. Second, knee MRI is generally not obtained at the time of initial injury and is therefore not a factor in the initial diagnostic algorithm.
Vascular injury is associated with knee dislocation in a significant minority of cases. Popliteal artery injury rates range from 7 to 100% in multiple series of knee dislocations [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18]. In studies published since 1992, the range is 7–32% (Table 23.1) [9,10,11,12,13,14,15,16,17,18]. A frequently quoted average is 30%. Many injuries are minor and heal spontaneously without sequelae. Some are significant and present with ischemia or, less frequently, hemorrhage and require immediate treatment for a successful outcome. It is this subgroup with significant vascular injury that accounts for a disproportionate percentage of the serious morbidity, limb loss, and medicolegal exposure.
Recognition of the association of vascular injury with knee dislocation is a prerequisite to successful application of the management strategy. In this publication, the authors will review the mechanics of injury, vascular evaluation, vascular repair, and adjunctive measures as they apply to knee dislocation.
2 Mechanics of Knee Dislocations and the Causation of Vascular Injury
Multi-ligamentous disruption of the knee results in injury to the soft tissues in the region. Depending on the magnitude and mechanics of the disruption, neurovascular injury may occur. The mechanism of neurovascular injury is predominantly excessive stretching with some component of mechanical blunt force trauma is also possible. Due to an intrinsically poor collateral pathway bridging the popliteal region, severe ischemia is most often the result of acute popliteal artery occlusion. Without immediate recognition and rapid correction of perfusion, muscle and tissue necrosis occurs within hours, and above-knee amputation is the most likely outcome. A delay in correction of ischemia in excess of 8 h nearly always results in amputation. Better salvage results are seen with more rapid revascularization.
In the modern era, the majority of knee dislocations result from high energy trauma predominantly involving motor vehicles. Trauma to the legs may result from dashboard contact for vehicle occupants, vehicle contact for pedestrians, and environmental contact for motorcycle and all-terrain-vehicle riders. These mechanisms most commonly result in posterior dislocations. The next largest group of knee dislocations results from medium energy trauma, most commonly sporting events such as football, gymnastics, and trampoline activities. Some result from low energy trauma which includes falls and missteps particularly in the obese. Even low energy-induced knee dislocations are associated with a 15% incidence of vascular injury [19]. This incidence rises with the energy involved in the traumatic event and the severity of the orthopedic derangement.
Anatomically, the popliteal artery is secured superiorly at the tendinous attachment of the adductor magnus muscle within the adductor hiatus. Within the popliteal space, it has paired superior genicular arteries, an unpaired middle genicular artery, and then paired inferior genicular arteries. As it traverses the popliteal space, it has relatively little structural attachment before passing behind the fibrous arch of the soleus muscle where it is tightly bound to the posterior aspect of the tibia. Its tibial branches anchor it in place inferiorly as they penetrate the fascial planes in three distinct directions. When the dislocation injury occurs, the linear distance across the popliteal space acutely increases and stretch injury occurs to the vessels and nerves which span this region. Direct contusion of the vessels by the adjacent bone is also possible during the injury process. Contusion is presumed to be most likely to occur in posterior knee dislocations.
The arterial wall consists of three layers which differ in their elastic properties. The single cell layer of the intima is inelastic and requires relatively little linear stretching to disrupt its confluent monolayer structure. Intimal injury then exposes the subendothelial layer, which naturally activates its procoagulant properties. The multilayered smooth muscle cell and elastic fiber zone in the middle of the vessel wall is called the media. It has modest resistance to stretch injury. It is disrupted only after the endothelial layer, and therefore, medial injury exposes the intravascular contents to collagen and other components of the media which are potent thrombogenic substances (Figs. 23.1 and 23.2). The adventitia is the most resistant to stretch injury of the three layers of the arterial wall. It is often the only layer remaining intact following the injury event and maintains vascular integrity. If of extreme magnitude, the stretch injury can cause complete disruption of arterial continuity and the obvious symptoms of ischemia in addition to the consequences of hemorrhage. The latter is nearly always contained within the popliteal space unless the injury is of such severity that skin disruption occurs.
Knee dislocations are characterized by the relationship of the tibia to the femur in the dislocated position. Five categories are commonly described: anterior, posterior, lateral, medial, and rotatory. In 1963, Kennedy described, in the largest series at the time, 22 clinical cases of complete knee dislocation [2]. In addition, he performed and reported the results of an experimental design using human cadaver knee specimens mounted on a static stress machine capable of reproducing traumatic knee dislocation and measuring the forces involved. He observed that only anterior dislocations could be reproducibly induced. The mechanism of induction involved hyperextension of the knee. This mechanism invariably resulted in stretching of the posterior capsule and cruciate ligaments until rupture occurred. The accompanying stretch injury to the popliteal artery resulted in complete disruption at 50° of hyperextension. He clinically postulated that popliteal injury occurs at a lesser degree of hyperextension, but his model was not sufficient to make a more precise estimate. His model was unable to satisfactorily reproduce posterior dislocations which required disruption of the patellar tendon in each of the few instances produced. Medial and lateral dislocations were generally associated with fracture of the tibial plateau and supracondylar femur and thus dissimilar to the injury seen clinically.
In his clinical series, anterior dislocations predominated (14 of 22) with only one posterior and one posterolateral dislocation. Eight of 22 experienced popliteal artery injury with 5 resulting in above-knee amputations. Three underwent immediate vascular repair with one uneventful recovery, one amputation, and the other developing claudication. In the presence of anatomic arterial injury, delayed exploration universally resulted in amputation. Two patients had popliteal spasm and recovered uneventfully from a vascular perspective. Anterior dislocation accounted for six of the eight vascular injuries. Anterior dislocation is the most commonly reported type of dislocation in older series [3]. Modern series report a predominance of posterior dislocations, thought to be due to the increasing frequency of dashboard trauma from motor vehicle accidents.
More recently, the Wascher modification of the Schenck Classification has been devised. It uses the abbreviation KD for knee dislocation. Increasing anatomic degree of injury is represented by five roman numeral classifications as seen below:
-
KD-I multi-ligamentous injury without injuring both cruciates
-
KD-II bicruciate injury only
-
KD-III bicruciate injury + either posteromedial or posterolateral corner
-
KD-IV bicruciate injury + both posteromedial and posterolateral corners
-
KD-V multi-ligamentous injury with periarticular fracture
Advanced imaging modalities have allowed this newer classification scheme where the older system preceded this technological advent and was based on mechanism and clinical exam primarily.
3 Vascular Evaluation
Since the majority of these injuries involve high energy trauma, the likelihood of life-threatening-associated injuries is high. Trauma protocols should be universally followed in every case of major traumatic injury. Knee dislocation is most often seen in association with additional serious injuries particularly when a popliteal vascular injury is present. Essentially, there is no systematic data reported for vein injuries in any of these series. In a study from the National Trauma Data Bank, the combination of arterial and venous injury as a result of blunt trauma resulted in the highest amputation rate of 27% [20].
The injured extremity mandates a careful and accurate clinical vascular examination in all situations. Because of the known association of knee dislocation with vascular injury, it is of even greater imperative when multiple ligament injuries are suspected, in particular, avulsion of the posterior cruciate ligament. Nonrecognition or delayed recognition of a significant vascular injury invariably results in an unfavorable outcome. The key to making the diagnosis is a high index of suspicion. Because the minority of multi-ligamentous injured knee dislocations will involve a significant vascular injury, it is important to follow a rigid protocol in their evaluation. A review of such an algorithm which includes a careful and accurate vascular physical exam, ankle–brachial index (ABI) testing, followed by selective duplex evaluation and, when necessary, catheter-based arteriography, will be presented.
4 Physical Examination
Pulse examination is the critical element of the physical exam. Reliability is of paramount importance. There is a well-described phenomenon whereby the examiner expects a pulse and therefore reports its detection when it is not actually present. This is most likely to occur when the examiner is inexperienced in vascular evaluation and results from feeling one’s own pulse. Confirmation of the presence of a palpable pulse by the additional presence of a normal Doppler signal will reduce the frequency of this mistake. Other clues of vascular injury on the physical exam include coolness, delayed capillary refill, and pale, cyanotic, or mottled discoloration. None of these are entirely specific to vascular injury and may reflect systemic issues such as shock or hypothermia. Associated neurologic abnormalities indicate an increased but not certain risk of vascular injury. These are some of the soft signs of vascular injury and are less frequently absolute indicators.
The hard signs of vascular injury include absent or diminished distal pulses; a visible or expanding hematoma, usually in the popliteal fossa; palpable thrill; audible bruit; or visible pulsatile hemorrhage. The association of these signs with substantial vascular injury requiring repair is high enough to mandate surgical exploration based upon these findings alone. Thus, if on physical exam any hard signs of vascular injury are present after relocation of the knee, immediate surgical exploration or on-table arteriography should be performed. If no hard signs of vascular injury are present, many authorities recommend no further testing beyond a period of observation for 24 h with serial examinations at 4–6-h intervals [18]. One study of 134 knee dislocations in 126 patients resulted in 10 abnormal physical exam findings, and arteriography confirmed 9 arterial injuries and 1 false positive physical exam. No patient with a normal exam developed clinical findings of arterial injury in follow-up. Seventeen normal physical exam patients underwent arteriography due to surgeon preference, and none had an arterial injury. Another study of 35 knee dislocations revealed 6 arterial injuries, and all were identified by physical exam findings which selectively lead to arteriography [16]. Six retrospective studies with a total of 283 knee dislocations involving protocols of selective arteriography for abnormal physical exam have resulted in no reports to date of missed significant arterial injury [9,10,11,12, 14, 15]. Anecdotal reports of vascular complications despite a reportedly normal vascular physical exam do exist [6, 9, 20] and continue to fuel this decades-old controversy concerning mandatory versus selective use of arteriography. The majority opinion is that selective arteriography is the most appropriate protocol. Noninvasive arteriographic substitutes such as duplex ultrasound, computed tomographic arteriography (CTA), and magnetic resonance arteriography (MRA) have promise but have not been adequately studied to date to form a conclusive opinion.
5 Diagnostic Tests
5.1 Ankle–Brachial Index (Fig. 23.3)
ABI testing requires a continuous-wave handheld Doppler and appropriately sized blood pressure cuffs. Using the Doppler to detect resumption of arterial flow after blood pressure cuff inflation, the highest systolic blood pressure is recorded in all four extremities in the supine position. For arm pressures, the cuff is placed in the typical location, and Doppler interrogation is at the brachial artery at the antecubital fossa. In the lower extremities, cuffs are placed as close to the ankles as possible and pressures assessed in both the dorsalis pedis and posterior tibial locations bilaterally. The highest ankle pressure in each leg is then divided by the highest of the arm pressures. Many vascular laboratories today will also assess the peroneal arteries during this exam.
Lynch and Johansen pioneered the use of ABI in the evaluation of penetrating and blunt extremity trauma and compared its results to the findings on arteriography in a prospective study [21]. An ABI less than 0.90 had an 87% sensitivity and 97% specificity for arterial injury compared to arteriography. In a prospective study specific to knee dislocation, an ABI greater than 0.90 has been found to have a negative predictive value of 100% [17]. An ABI of less than 0.90 has reported sensitivities of 95–100% and specificities of 80–100% in detecting arterial injuries requiring operative management [15, 17, 21].
The combination of a careful vascular physical exam with ABI calculation is a standard tenet in the vascular clinic for evaluation of peripheral vascular disease. Many argue that it is mandatory in the evaluation of knee dislocation patients as well. In the study by Miranda et al., the combination of the physical exam with the ABI would not have identified any additional vascular injuries or avoided any complications, but it would have further reduced unnecessary (negative) arteriographic evaluations by an additional 25% [16]. The reduced exposure to iatrogenic risk from the invasive procedure and monetary expenditures would seem a worthy and worthwhile goal.
Predominantly the few reported missed injuries of clinical importance after following a non-imaging algorithm have consisted of pseudoaneurysms. These have presented in delayed fashion (weeks to months) with rupture and have been successfully repaired without limb loss [22]. Though delayed recognition of a missed injury is not ideal, it is likely acceptable considering the potential for an equal or greater number of iatrogenic complications with a more liberal invasive arteriographic policy where the vast majority of the arteriograms would be normal or reveal clinically insignificant findings. We would propose that early imaging by noninvasive modalities would identify these lesions and facilitate their repair before the patient’s dramatic representation with a delayed diagnosis of a vascular injury.
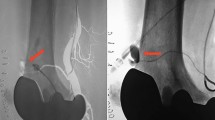
5.2 Catheter-Based Arteriography (Fig. 23.4)
Arteriography is still considered the gold standard for evaluation of arterial injury. The controversy as it applies in evaluation of knee dislocation involves whether to apply it in selective or routine fashion. The incidence of identifying a significant vascular injury in the absence of hard signs is very low. Thus, in the majority of patients, angiography will not provide information that will alter the overall management of the patient. Furthermore, angiography is not without risk, as potential complications such as iatrogenic arterial injury, access site complications, contrast-induced nephropathy, and radiation injury can complicate an already challenging case. Therefore, when hard signs of vascular injury are present, arteriography should be used to identify the exact location and nature of the injury and to help formulate an expeditious operative approach to correct the injury in a timely fashion.
Outcome is best when confirmation and correction of vascular injury are made rapidly [23]. Delays in excess of 6–8 h often end in tissue loss and/or amputation. To facilitate management, arteriography should ideally be performed in the operating room with the patient under anesthesia, with the affected extremity prepped and draped and ready for operative intervention. When arteriography is performed outside of the operating room, unnecessary delay is introduced into the treatment algorithm, and is best avoided altogether.
Depending on the severity and certainty of the clinical indicators of vascular injury, the arteriogram will obviate the need for vascular exploration and on occasion can provide correction of the abnormality. These are generally infrequent outcomes and are predictably more common as the clinical indicators are less certain and severe. Most of these patients could have been managed without arteriography with no clinically important complications. It must be reiterated that when hard signs of vascular injury occur, the patient should be taken to the operating room for vascular evaluation and management. An excellent and detailed review of this algorithm is presented by Nicandri et al. [24].
5.3 Duplex Ultrasound
Duplex evaluation of extremity arterial anatomy and hemodynamics is another standard vascular practice. Extremity duplex ultrasound is an ideal application of the technology because the vessels are in close proximity to the skin and the soft tissues transmit ultrasound frequencies well. In one series, arterial duplex identified all arterial injuries in a series of penetrating extremity trauma patients [25]. In anecdotal cases, it has been used in the evaluation of vascular injury after knee dislocation, but no randomized studies have been published. Despite not being rigorously studied in the trauma setting, there is an expectation that duplex technology would have excellent sensitivity in the detection or exclusion of significant vascular injury. Benefits of duplex imaging include its noninvasive nature, its portability, safety, and low expense compared to catheter-based arteriography, CT arteriography, or MR arteriography. One disadvantage is that an experienced sonographer may not always be readily available around the clock and on weekends, even in level 1 Trauma Centers. Duplex evaluation is also technician-dependent, which adds another limitation.
5.3.1 Computed Tomographic Arteriography (Fig. 23.5)
Advanced CT imaging is available 24/7 in nearly every facility and certainly at major trauma centers in the current era. Using modern CT scanners and intravenous contrast injection, CT arteriography (CTA) images with excellent resolution are rapidly acquired. Radiologic interpretation is frequently available instantaneously or with minimal delay, and most vascular surgeons are facile with CTA interpretation even in the absence of radiologist support. Prompt evaluation of the concomitant orthopedic and vascular injuries can be made with a single examination. In a general study of extremity trauma patients with suspected vascular injury, CTA demonstrated sensitivity and specificity in excess of 90% [26]. CTA is routinely used to plan vascular surgical procedures for a wide range of pathologies and is readily used by vascular surgeons in the modern era. Its beneficial application to vascular injury after knee dislocation has been demonstrated and does not represent a major departure from its current routine use in the standard vascular patient. The requirement for a relatively large contrast bolus, expense, and radiation exposure are the main drawbacks.
5.3.2 Magnetic Resonance Arteriography
Magnetic resonance arteriography (MRA) has some disadvantages compared with CTA in the trauma population, including its lack of widespread acceptance, variable access around the clock in some centers, and its longtime of image acquisition. From an imaging perspective, MR technology provides exceptional orthopedic evaluation and is the study of choice for many orthopedic pathologies. It can offer vascular evaluation and images that are comparable to CTA and catheter-based arteriography, but in the severely injured patient, it has practical limitations and safety concerns because of the need to exclude metallic equipment from the scanner region and longer image acquisition times. It is best reserved for the elective orthopedic evaluation in the stable patient, a time when the vascular considerations have already declared themselves to be of no major clinical consequence. It may detect more subtle delayed vascular complications from incomplete healing of the initial injury such as pseudoaneurysm, intimal flap, hematoma, or deep vein thrombosis. Vascular imaging can be obtained without contrast administration but resolution suffers. Renal insufficiency (GFR <30 mg/dl) precludes the administration of current MRI contrast agents, but in the general trauma population, this concern is often not an issue (Fig. 23.6).
6 Treatment
The injured patient with suspected popliteal arterial injury and an acutely ischemic lower extremity must first be thoroughly evaluated using standard Advanced Trauma Life Support protocols. Concomitant life-threatening injuries, if present, must be addressed first. Once satisfactory stabilization of the patient is achieved, expeditious management of the popliteal artery injury is indicated.
Systemic anticoagulation using 100 units/kg of unfractionated heparin should be performed, provided there are no contraindications such as intracranial hemorrhage or evidence of bleeding into pericardium, peritoneal cavity, retroperitoneum, or extremities. Early anticoagulation serves to mitigate secondary thrombosis of the microcirculation of the distal extremity, which otherwise may cause revascularization efforts to fail. When systemic use of heparin is contraindicated, regional use of heparinized saline is generally adequate once vessels are exposed and controlled, but obviously, there is a time delay in getting to this part of the care.
Unless there is a significant delay in the availability of orthopedic expertise, immobilization of the injured knee if it is substantially unstable using external fixation should ideally precede definitive arterial repair. In most scenarios, stabilization can be accomplished rapidly and will safeguard against limb instability consequences jeopardizing the integrity of the vascular repair. When feasible, the vascular surgeon should be present to assist with planning the placement of external fixation, as improperly located hardware may hamper surgical exposure during revascularization. The importance of clear physician-to-physician communication cannot be overemphasized in this situation.
There are two open surgical approaches to popliteal artery injuries, medial and posterior. In the medial approach, the patient is placed in the supine position with the injured lower extremity externally rotated and supported under the knee (the presence of external fixation limits the ability to simultaneously flex hip and knee). Exposure of the proximal popliteal vessels is obtained through a longitudinal medial distal thigh incision [27]. Once the subfascial plane is entered, the sartorius is retracted posteriorly, the popliteal fat pad is entered, and the vessels readily exposed. The distal popliteal artery is exposed through a separate longitudinal medial calf incision parallel and one fingerbreadth posterior to the proximal tibia. The medial head of the gastrocnemius muscle is retracted posteriorly. The popliteal fossa is entered and the distal popliteal vessels exposed. Care must be taken to avoid injury to the tibial nerve which is in intimate approximation to the vessels in this location.
The posterior approach requires prone positioning of the patient and for this reason is often not the preferred approach in the setting of concomitant orthopedic and vascular injuries, especially when external fixation is required to stabilize the orthopedic injury prior to vascular repair. It is imperative to first provide adequate cushioning for both lower extremities if this approach must be utilized. The presence of external fixation usually mandates a liberal stack of pillows or blankets to maintain a position that not only facilitates exposure but also prevents unintended injury. A “lazy S” incision is made medially along the distal thigh, horizontally across the skin crease, and laterally along the proximal calf. Subcutaneous flaps are created, and fascia is incised. Depending on the extent of soft tissue injury, the lesser saphenous vein may or may not be readily identified. Care is taken to avoid inadvertent neural injury while dissection of the neurovascular bundle is performed [28]. The mid portion of the popliteal artery is very nicely exposed through this approach, but often in the setting of a traumatic injury, local soft tissue edema and hematoma distorts the otherwise pristine anatomy that is appreciated only in the elective setting.
Each surgical approach has practical advantages and limitations. The medial approach permits supine position for the entire procedure (fixation, vein harvest, and reconstruction). Provided, there is a suitable length of autologous conduit, the medial approach may allow for expedient exposure in areas with less soft tissue injury and distortion by hematoma. The locations of incisions, inherently with less tension than the prone approach, facilitate successful wound healing. Additionally, fasciotomies of the superficial and deep compartments of the lower leg can be easily performed by mere extension of the distal incision. The posterior approach requires staging of the procedure: supine positioning for harvest of the contralateral thigh saphenous vein which is usually the best size match, followed by prone positioning for repair. The ischemic time, therefore, should be considered. Wound healing may be more problematic, owing to soft tissue swelling related to injuries as well as reperfusion. The posterior approach does provide excellent visualization of involved vessels, however. Through this approach, occasionally the popliteal artery can be repaired primarily after initial debridement; otherwise, posterior exposure allows for the minimal necessary length of arterial (and venous) reconstruction. In the setting where saphenous veins are not satisfactory and exhaustive search for other venous conduit is prohibited by time, a much shorter synthetic graft can be placed from the posterior approach, which improves patency relative to the medial approach.
Provided that it is of acceptable caliber (>3.5 mm), greater saphenous vein (GSV) remains the conduit of choice. With an endothelium that naturally elaborates a variety of antithrombotic factors, patency is strongly favored over synthetic conduit such as Dacron or expanded polytetrafluoroethylene (PTFE). Furthermore, the risk of infection in a traumatic operative field is reduced when using autologous vein. The most appropriately sized saphenous vein is most often located in the proximal thigh, and is exposed through an incision beginning 2 cm laterally and inferior to the pubic tubercle and carried distally as far on the thigh as necessary to obtain adequate length for reconstruction. Of paramount importance, both legs should be prepared for vein harvest. The contralateral leg should be explored for vein first, as preexisting deep venous injuries or subsequent deep vein thrombosis due to swelling and instrumentation may make the superficial venous system of the injured leg a critical collateral pathway for venous outflow that influences the durability of arterial repair.
An exception to proceeding in this order is if the ischemic period has already been long and the degree of ischemia severe. In this situation, repair should be done in the supine position with proximal and distal arterial exposure beyond the region of injury performed first. A temporary shunt is then placed and checked to confirm patency and distal flow. Once reperfusion is established, appropriate conduit for bypass can be harvested.
From a posterior approach, the popliteal artery is exposed, and proximal and distal control is obtained. The level of injury is identified and the popliteal artery is opened lengthwise and inspected; injured areas should be debrided and resected back to healthy artery. Antegrade and retrograde flow are next established, and commonly a Fogarty balloon catheter is helpful for this purpose. Instillation of heparinized saline prevents thrombosis while the repair is performed.
Infrequently, the injury is limited to an intimal flap or short arterial segment. Judicious use of tacking sutures and vein patch angioplasty may be all that is required to manage an intimal flap. A direct, end-to-end repair of the popliteal artery can occasionally be performed if the involved segment is short (~1 cm), but often this approach will require extensive mobilization of the popliteal artery with ligation and division of geniculate collateral vessels, making it less appealing. In any case, primary repairs are ill-advised if the remaining arterial ends cannot be brought together without tension or if tension-free repair could only be obtained by failing to debride injured artery.
Most often, an interposition graft using GSV is required. The choice of reversed or non-reversed vein is less important than an appropriate size match; if a non-reversed configuration is selected, valve lysis is mandatory, either initially or before completion of the distal anastomosis. Vessel ends are spatulated to prevent stenosis at the suture line. Intima-to-intima re-approximation is performed using 5–0 or 6–0 nonabsorbable monofilament suture in standard fashion (Fig. 23.7).
From a medial approach, the conduct of the operation differs in that the actual area of injury is often not visualized. Instead, the goal is to construct a bypass from the healthy above-knee popliteal artery to the healthy below-knee popliteal artery, thus bypassing the injured segment. If the injured segment was completely thrombosed, no effort is made to surgically remove the thrombus. Some authors even recommend suture ligating the popliteal artery on either side of the injury/thrombosis in order to prevent the possibility of distal embolization. In cases where there is clear evidence of complete popliteal artery transection, the popliteal artery should be suture ligated on either side of the injury to prevent hemorrhage following arterial reconstruction.
On completion of the reconstruction, whether it be from a posterior or a medial approach, adequacy of the distal circulation must be ascertained. Some surgeons routinely use angiography at this point, whereas others will only use it selectively when pedal pulses are not immediately palpable following repair. Either a small gauge (#20) butterfly needle or small caliber arterial catheter may be utilized for contrast injection. Digital subtraction angiography is preferred, and imaging should include not only the repair site but also the runoff vessels. If there is inadequate runoff to the foot, additional adjunctive surgical maneuvers may need to be employed by the vascular surgeon, the details of which are outside of the scope of this chapter, but the goal is to not leave the operating room until flow to the foot has been unequivocally reestablished.
Repair of popliteal venous injuries is less straightforward. Options include simple ligation, venorrhaphy, and interposition grafting. In the 1960s, the paradigm was to reconstruct all major venous injuries along with the arterial injuries. Proponents argued that the ensuing venous hypertension from venous ligation would compromise the patency of the arterial repair; furthermore, impaired venous outflow compounds the edema in the postoperative period, which already may be considerable due to reperfusion injury. However, several more contemporary series demonstrate similar long-term morbidity and outcomes between ligation and repair. Additionally, the majority of venous repairs or reconstructions ultimately go on to thrombose in the post-op period. Overall, the combined arterial and venous injury does have a worse outcome than arterial injury alone [20].
Accordingly, venous repairs should be undertaken selectively. In a hemodynamically stable patient with injuries amenable to lateral venorrhaphy or simple re-approximation, this approach is prudent. If the anatomic distribution of injury would require more extensive repair—synthetic or composite interposition grafts—or if the patient is unstable, simple ligation is preferable [29, 30]. The topic of vein repair versus ligation remains controversial, and available published data include penetrating traumatic mechanisms and dislocations associated with fractures. No definitive conclusions can be made; thus, it remains an unresolved issue. If ligation is chosen as the most prudent maneuver given the particular situation, effort should be made to ensure the ipsilateral greater saphenous vein is uninjured as it will serve as the primary collateral pathway for venous drainage of the leg. Postoperative leg compression and elevation help with management.
Four-compartment fasciotomy should be strongly considered at the time of arterial reconstruction based on the nuances of the case. Prophylactic fasciotomy is advised in the following circumstances: confirmation of compartment syndrome by direct pressure measurements, concomitant venous repair or ligation, prolonged ischemia, extensive injury or swelling, concomitant disabling neurologic injury in which physical assessment may be confounded, and institutions where rapid return to the operating room is compromised. In settings where the patient has multiple other injuries that require separate time-consuming diagnostic or therapeutic interventions, prophylactic fasciotomy should likewise be considered. Proponents of prophylactic fasciotomy cite avoidance of a second ischemic event as a critical determinant of limb salvage.
If fasciotomy is not performed at the time of reconstruction, bedside clinical assessment by experienced personnel is crucial to detect the development of compartment syndrome. Direct transduction of compartment pressures remains the most reliable method and in fact may be the only reliable method in sedated, intubated, or neurologically impaired patients. Otherwise, a complete neurovascular assessment, coupled with limb circumference measurements, is imperative. Caution is advised when using a palpable pulse as a determinant of compartment syndrome; loss of pulses is a late finding in the sequence of progressive tissue injury.
Primary amputations are not often indicated on a presentation based on ischemia alone. However, devastating injury of the tibial nerve, extensive associated crush or mangling injuries, and prolonged warm ischemic time (associated with rigor or capillary extravasation) forecast a dismal prognosis. In these selected circumstances, limb salvage efforts may not only be futile but place the patient at unnecessary risk of death or renal failure from myoglobinuria, and primary amputation may be indicated. It should be a unanimous decision by the vascular surgeon, orthopedic surgeon, and trauma surgeon to proceed with primary amputation, and compelling reasons for this approach should be well documented in the medical record.
Optimal outcomes after knee dislocation with arterial and venous injuries can be accomplished in the majority of cases. With a high index of suspicion for the presence of a vascular injury and a clear understanding of the management strategy, the disastrous consequences of the missed vascular injury can be avoided. It is our hope that the information provided in this review will be used to facilitate management and improve outcomes in patients who present with combined knee dislocations and vascular injuries.
References
Hoover NW. Injuries of the popliteal artery associated with fractures and dislocations. Surg Clin North Am. 1961;41:1099–112.
Kennedy JC. Complete dislocation of the knee joint. J Bone Joint Surg Am. 1963;45:889–904.
Green NE, Allen BL. Vascular injuries associated with dislocation of the knee. J Bone Joint Surg Am. 1977;59:236–9.
ODonnell Jr TF, Brewster DC, Darling RC, et al. Arterial injuries associated with fractures and/or dislocations of the knee. J Trauma. 1977;17:775–84.
Jones RE, Smith EC, Bone GE. Vascular and orthopaedic complications of knee dislocation. Surg Gynecol Obstet. 1979;149:554–8.
Sisto DJ, Warren RF. Complete knee dislocation. Clin Orthop Relat Res. 1985;198:94–101.
Roman PD, Hopson CN, Zenni EJ Jr. Traumatic dislocation of the knee: a report of 30 cases and literature review. Orthop Rev. 1987;16:917–24.
Varnell RM, Coldwell DM, Sangeorzan BJ, et al. Arterial injury complicating knee disruption. Am Surg. 1989;55:699–704.
Treiman GS, Yellin AE, Weaver FA, et al. Evaluation of the patient with a knee dislocation. Arch Surg. 1992;127:1056–63.
Kaufman SL, Martin LG. Arterial injuries associated with complete dislocation of the knee. Radiology. 1992;184:153–5.
Dennis JW, Jagger C, Buthcer JL, et al. Reassessing the role of arteriograms in the management of posterior knee dislocations. J Trauma. 1993;35:692–7.
Kendall RW, Taylor DC, Salvian AJ, et al. The role of arteriography in assessing vascular injuries associated with dislocations of the knee. J Trauma. 1993;35:875–8.
Wascher DC, Dvirnak PC, DeCoster TA. Knee dislocation: initial assessment and implications for treatment. J Orthop Trauma. 1997;11:525–9.
Martinez D, Sweatman K, Thompson ED. Popliteal artery injury associated with knee dislocations. Am Surg. 2001;67:165–7.
Abou-Sayed H, Berger DL. Blunt lower extremity trauma and arterial injuries. Arch Surg. 2002;137:585–9.
Miranda FE, Dennis JW, Veldenz HC, et al. Confirmation of the safety and accuracy of physical examination in the evaluation of knee dislocation for injury of the popliteal artery: a prospective study. J Trauma. 2002;52:247–52.
Mills WJ, Barei DP, McNair P. The value of the ankle-brachial index for diagnosing arterial injury after knee dislocation: a prospective study. J Trauma. 2004;56:1261–5.
Stannard JP, Sheils TM, Lopez-Ben RR, et al. Vascular injuries in knee dislocations: the role of physical examination in determining the need for arteriography. J Bone Joint Surg Am. 2004;86:910–5.
Johnson ME, Foster L, DeLee JC. Neurologic and vascular injuries associated with knee ligament injuries. Am J Sports Med. 2008;36:2448–62.
Mullenix PS, Steele SR, Anderson CA, et al. Limb salvage and outcomes among patients with traumatic popliteal vascular injury: an analysis of the national trauma data bank. J Vasc Surg. 2006;44:94–100.
Lynch K, Johansen K. Can Doppler pressure measurement replace “exclusion” arteriography in the diagnosis of occult extremity arterial trauma? Am Surg. 1991;214:737–41.
Gable DR, Allen JW, Richardson JR. Blunt popliteal artery injury is physical examination alone enough for evaluation? J Trauma. 1997;43:541–4.
Patterson BM, Agel J, Swiontkowski MF, et al. Knee dislocations with vascular injury: outcomes in the lower extremity assessment project (LEAP) study. J Trauma. 2007;63:855–8.
Nicandri GT, Chamberlain AM, Wahl CJ. Practical management of knee dislocations: a selective angiography protocol to detect limb-threatening vascular injuries. Clin J Sport Med. 2009;19:125–9.
Knudson MM, Lewis FR, Atkinson K, et al. The role of duplex ultrasound arterial imaging in patients with penetrating extremity trauma. Arch Surg. 1993;128:1033.
Soto JA, Múnera F, Cardoso N, et al. Diagnostic performance of helical CT angiography in trauma to large arteries of the extremities. J Comput Assist Tomogr. 1999;23:188.
Armstrong PJ, Franklin DP. Treatment of vascular injuries in the multiple-ligament-injured knee. Oper Tech Sports Med. 2003;11:199–207.
Armstrong PJ, Franklin DP. Management of arterial and venous injuries in the dislocated knee. New York: Springer; 2004. p. 151–66.
Bermudez KM, Knudson MM, Nelken NA, et al. Long term results of lower extremity venous injuries. Arch Surg. 1997;132:963–8.
Yelon JA, Scalea TM. Venous injuries of the lower extremities and pelvis: repair versus ligation. J Trauma. 1992;33(4):532–6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Garvin, R.P., Cindric, M.C. (2019). Management of Arterial and Venous Injuries in the Dislocated Knee. In: Fanelli, G. (eds) The Multiple Ligament Injured Knee. Springer, Cham. https://doi.org/10.1007/978-3-030-05396-3_23
Download citation
DOI: https://doi.org/10.1007/978-3-030-05396-3_23
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-05395-6
Online ISBN: 978-3-030-05396-3
eBook Packages: MedicineMedicine (R0)