Abstract
For many years asthma has been described as a single disease. However, asthma is a heterogeneous syndrome with complex pathophysiology contributing to numerous clinical phenotypes. Despite various treatments a large proportion of patients remain uncontrolled or poorly controlled. Understanding the underlying inflammatory process in asthma is key for stratification of patients toward personalized therapy. Recently described inflammatory pathways include T2-high and T2-low or non-T2 inflammation. Clinically, T2-high inflammation is associated with atopic/allergic disease with increased evidence of eosinophils in the airway and the peripheral blood, whereas T2-low inflammation is correlated with neutrophilic or paucigranulocytic cells in the airways. Several biomarkers have been identified for T2-high inflammation; however, their utility is limited. Linking the clinical phenotypes to the underlying molecular biology will enhance the successful development of personalized therapies for asthma in the future.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
1 Introduction
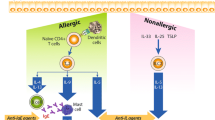
Asthma is a chronic disease that affects 5–10% of children and adults in many developed countries. In the United States, 21.8 million people live with asthma, and 46.9% of those report having one or more asthma attack annually. In 2013, 1.6 million emergency room visits displayed asthma as the primary diagnosis. The average length of hospitalization for patients with asthma is 3.6 days. An estimated US$19.7 billion dollars annually makes asthma one of the top ten conditions impacting healthcare costs. Despite therapeutic advancement it is unclear why patients remain uncontrolled or poorly controlled (Centers for Disease Control and Prevention 2015). Although lack of adherence to the prescribed medications is a significant factor, asthma is a heterogeneous disease with variability in clinical presentation. Various attempts have been made to define phenotypes and evolution of asthma. Traditional classification of asthma has been associated with common triggers such as allergens, aspirin, obesity, exposure to cigarette smoke, viruses, and exercise. Several studies have taken an unbiased approach in analyzing variables in asthma that provide insight into the complexity of persistent asthma; however, it is difficult to ascertain the clinical value. Linking the observable characteristics to underlying molecular inflammation in the lungs, often referred to as the endotype, is a shift in asthma management toward individualized therapy. In this chapter, we will discuss the clinical asthma phenotypes and different mechanisms of inflammation intrinsic to the current endotypes of asthma defined (Fig. 1). Furthermore, we will address the utility of point-of-care biomarkers available for optimization of targeted therapy.
Various inflammatory patterns in the airway contribute to different underlying molecular mechanism among various cells. In T2-low pattern, a predominant neutrophilic and paucigranulocytic inflammation is consistent with patients who are less responsive to corticosteroid therapy. An increase in TNF-α, IL-17, IL-23, and IL-8 is seen at the molecular level. Innate lymphoid cells groups 1 and 3 are more predominate in T2-low disease. Eosinophilic inflammation correlates with phenotype of patients who are more likely to respond to corticosteroids and the various biologics currently available on the market that are FDA approved for persistent asthma. In T2-high asthma, type 2 cytokines including IL-4, IL-5, and IL-13 play a central role in the underlying inflammation. IgE produced by B cells and innate cytokines TSLP, IL-25, and IL-33 produced by the epithelial cells are present in T2-high inflammation. PGD2, prostaglandin D2; IFN-γ, interferon gamma; TNF-α, tumor necrosis factor-alpha; ROS, reactive oxygen species; ILC1, type 1 innate lymphoid cells; ILC2, group 2 innate lymphoid cells; ILC3, group 3 innate lymphoid cells; NKT, natural killer cells; IgE, immunoglobulin E; TSLP, thymic stromal lymphopoietin. (Adapted from Sonnenberg et al. Nature Immunology and Muroro et al. Journal of Allergy and Immunology)
2 Asthma Phenotypes: Cluster Analysis and Clinical Subgroups
Phenotype is defined as “observable characteristics of an organism that are produced by the interactions of the genotype and the environment” (Phenotype). Several groups have taken a less biased approach using cluster analysis in grouping important variables to identify asthma phenotypes (Table 1) (Haldar et al. 2008; Moore et al. 2007). Taking into account steroid bursts, emergency room visits, hospitalization, measurement of airway obstruction via forced expiratory volume in 1 s (FEV1), sputum eosinophils, and bronchodilator responsiveness, several similarities were identified in patients (Wenzel 2012). In the National Institutes of Health-sponsored Severe Asthma Research Program (SARP) data, early-onset asthma was associated with atopy and allergic disease (Moore et al. 2007, 2010). Interestingly, the severity of disease did not correlate to the degree of allergen skin test reactivity, higher IgE, or higher exhaled nitric oxide (FeNO) which are markers of atopic disease. Rather severity was closely linked to duration of disease, medication use, and lung function (Fitzpatrick et al. 2011). The children in the study had normal weights with increased prevalence in boys before pubescence. The correlation of early-onset asthma to other atopic disease including allergic rhinitis and atopic dermatitis has been confirmed by multiple other cluster analyses. In fact, 40% of patients with early-onset asthma have a history of atopic dermatitis compared to only 4% of people with adult-onset asthma (Miranda et al. 2004).
A second phenotype described is the late-onset persistent eosinophilic asthma, characterized by a higher degree of eosinophils in the sputum and peripheral blood. Some individuals did have a mixture of eosinophils and neutrophils in the sputum (Hastie et al. 2010). This phenotype lacks clinical allergy with a much less degree of family history of asthma as observed in early-onset disease (Wenzel 2012). Adults show greater airflow obstruction with a decrease in bronchodilator response. This cluster of patients displays difficult-to-control disease and more frequent asthma exacerbation (Teague et al. 2018). A subtype of this phenotype is aspirin-exacerbated respiratory disease (AERD) with severe eosinophilic asthma, concurrent sinusitis, and nasal polyposis with severe non-IgE-mediated reaction to aspirin and other cyclooxygenase-1 inhibitors (Rodriguez-Jimenez et al. 2018).
Obesity plays a role in asthma with regard to control and severity of disease. Several studies support the increased expression of pro-inflammatory cytokines like TNF-α, interleukin-6 (IL-6), and leptins in obesity (Leiria et al. 2015). Obesity-related phenotype has an increased prevalence in women with later-onset disease and minimal allergic/atopic burden (Miranda et al. 2004; Teague et al. 2018). Patients also have fewer eosinophils in the sputum with diminished response to corticosteroid and higher burden of symptoms overall (Wenzel 2012). A separate adult-onset phenotype in the SARP cluster included individuals with neutrophilic asthma. Affected individuals had increased air trapping, lower lung function, and thicker airway as measured by computed tomography scans. Generally, the degree of obstruction was not reversible. Many of the patients were on systemic steroids with a high-intensity usage of healthcare and economic burden (Moore et al. 2007, 2010; Teague et al. 2018).
Exercise-induced asthma (EIA) is a phenotype that has been described for many years, typically associated with reactive bronchoconstriction after sustained exercise despite baseline mild asthma. Symptoms are exacerbated by cold or dry air. This phenotype is more common among atopic athletes; however, no distinct genetic factors or biomarkers have been identified. Histamine, prostaglandins, and cysteinyl leukotrienes secreted by mast cells are key players in EIA (Hastie et al. 2010; Caggiano et al. 2017).
While the various phenotypes have provided insight into patient population with asthma, the prognostic value in therapeutic decision is not clear. In linking the clinical characteristics to the underlying molecular pathway, several immunomodulatory biologic therapies have emerged. Better understanding the underlying pathophysiologic mechanisms of the different phenotypes, known as the endotype, will further advance and guide therapeutic decisions in treating asthma.
3 Asthma Endotypes: The Inflammatory Pathways in Asthma
Two main endotypes have been described in the asthma literature including T2 low (Th2-low) and T2 high (Th2-high) (Wenzel 2012; Fahy 2015). In T2 high there is an increase in eosinophils in the sputum. On the other hand, T2-low asthma is associated with neutrophilic or paucigranulocytic inflammation in the sputum and airways.
3.1 T2-Low Asthma or Non-T2 Asthma
Neutrophilic inflammation has long been associated with refractory asthma (Alam et al. 2017). Clinically these patients have adult-onset disease that is less responsive to corticosteroids (Wenzel 2012). In this type of inflammation, the expression of type 2 cytokines is absent (Liu et al. 2017; Lambrecht and Hammad 2015). Instead, a predominance of Th1 and Th17 cells is noted with an increased production of interleukin-8 (IL-8), a potent neutrophil chemoattractant. Several studies illustrate the role of IL-17 in inducing the production of IL-8 and airway remodeling (Lambrecht and Hammad 2015; Bellini et al. 2012). However, clinically the inhibition of IL-17 receptor A antagonist is of little benefit in patients with mild-to-moderate asthma (Busse et al. 2013a). In a small preliminary study of 12 patients on CXCR2 antagonist blocking IL-8, the sputum neutrophils decreased; however, there was no statistical improvement of FEV1 or symptom scores (Barnes 2015). Hence, direct targeting of IL-8 through its chemokine receptor CXCR2 is of insignificant clinical value. In recent studies, immunophenotyping patients with Th2-/Th17-predominant asthma and Th2-/Th17-low asthma illustrated increased expression of pro-inflammatory cytokines including IL-1, IL-6, and C3 (Alam et al. 2017; Liu et al. 2017). Furthermore, the presence of subclinical infection led to a pronounced infection cytokine profile. Targeted therapy with antimicrobial agents and IL-1 receptor antagonist are potential therapeutic interventions (Alam et al. 2017; Liu et al. 2017). Further studies are needed to demonstrate clinical efficacy.
Paucigranulocytic inflammation is another subtype of T2-low endotype. In this inflammatory process, a normal number of eosinophils and neutrophils are found in the sputum with no evidence of IL-8 or type 2 cytokines (Alam et al. 2017). Clinically, patients are resistant to corticosteroid therapy presumably due to decreased levels of airway inflammation. The use of long-acting muscarinic receptor antagonist and long-acting beta-receptor agonists is of some benefit. Many patients in this subgroup ultimately may be candidates for bronchial thermoplasty to reduce airflow obstruction (Wilhelm and Chipps 2016). Unfortunately, a significant challenge in both neutrophilic and paucigranulocytic inflammation is the lack of reliable biomarkers. To date many of the targeted therapies have not proven effective.
3.2 T2-High Asthma
T2-high inflammation is central to allergic disease. As described earlier, childhood- or early-onset asthma is associated with atopic disease with increased eosinophils in the sputum and airway. Clinically this phenotype of patients is corticosteroid responsive. However, the degree of response may be variable (Woodruff et al. 2007, 2009). Haldar and colleagues used the epithelial brushings of asthma patients who were corticosteroid naïve to illustrate an increased level of IL-5 and IL-13 messenger RNA in subjects with increased atopy suggestive of T2-high asthma compared to those with T2-low asthma (Haldar et al. 2008). T2-high inflammation is a complex pathway between innate and adaptive immune response. This inflammatory process begins with the differentiation of uncommitted naive T cells toward Th2 cells under the stimulation of local cytokines and co-stimulatory molecules on dendritic cells (Fig. 2). The initial activation of Th2 cells and innate lymphoid cell type 2 (ILC2) is via innate cytokines IL-33, IL-25, and thymic stromal lymphopoietin (TLSP) that are secreted by the airway epithelial cells induced by external stimuli. The master regulator of T2 inflammation is the transcription factor GATA-3 which is required for the development and function of Th2 and ILC2. Th2 cells contribute to the production of type 2 cytokines which include IL-4, IL-5, and IL-13. ILC2s are an alternative source of IL-5 and IL-13. ILC2s do not express any phenotypic markers of dendritic or conventional lymphocytes. The continuous accumulation of type 2 cytokines is key for stimulation of eosinophils, mast cells, and basophils. Type 2 cytokines also cause mucous cell hyperplasia and fibrosis leading to airway remodeling. IL-4 and IL-13 are both involved in class switching of naïve B cells toward synthesis of immunoglobulin E (IgE). IL-5 is important for the survival of eosinophils and chemotaxis from blood vessels into the airway (Fahy 2015; Tabatabaian et al. 2017).
A very complex interplay of various cytokines and inflammatory cells is central in T2-high inflammation. Airway epithelial cells activated by environmental stimuli produce innate cytokines TSLP, IL-25, and IL-33. These innate cytokines contribute to the expression of GATA-3, a master regulator and transcription factor, in both Th2 and ICL2, subsequently enhancing the production of type 2 cytokines. Secretion of IL-5 stimulates the production of eosinophils in the bone marrow and elicits the migration of eosinophils to the area of inflammation. IL-5 and IL-13 contribute to smooth muscle changes and remodeling changes. IL-4 contributes to IgE class switching in B cells. TSLP, thymic stromal lymphopoietin; PGD2, prostaglandin D2; CRTH2, chemoattractant receptor-homologous molecule expressed on TH2 cells; GATA-3, transcription factor
Several targets have been examined for downregulation of T2-high inflammation. These mediators and cytokines include IgE, IL-5, IL-4, IL-13, IL-4 receptor alpha, TSLP, and chemoattractant receptor-homologous molecules on T2 cells (CRTH2) (Tabatabaian et al. 2017). Antagonists targeting IgE, IL-5, and IL-5 receptors are FDA approved for use in severe persistent asthma (Table 2). The challenge remains in identifying the right patient for these therapeutic interventions. Hanania et al. in a retrospective study used biomarkers to identify possible responders to an anti-IgE monoclonal antibody (Hanania et al. 2013). This was one of the first studies that separated patients to T2-high versus T2-low inflammation. Identification of biomarkers in T2-high inflammation can help guide the choice of therapy and assess responsiveness.
3.3 Biomarkers in T2-High Inflammation
Biomarkers have long been used as a surrogate for diagnosis and to assess disease progression as well as responsiveness to therapy. Examples of biomarkers include hemoglobin A1C (HgA1C) which is used to diagnose diabetes. In T2-low asthma, the development of biomarkers is much needed and is currently underway. A recent publication evaluated the role of sputum-to-serum hydrogen sulfide ratio in neutrophilic airway inflammation and association with asthma exacerbations (Suzuki et al. 2018). On the other hand, in T2-high asthma, a few biomarkers have been identified to help facilitate selection of patients that would likely respond therapeutically to FDA-approved biologics for severe persistent asthma (Busse et al. 2013b). These include blood and sputum eosinophils, periostin, IgE, and fractional exhaled nitric oxide (FeNO). Unfortunately, these biomarkers are not adequate for identification of early-onset asthma, nor are they all available at bedside for clinical use. Nevertheless, they do provide some insight to the type of inflammation that might be involved.
3.3.1 Eosinophils
Several studies have illustrated that persistent or poorly controlled asthma with increased exacerbation is associated with increased blood or sputum eosinophils (Pavord et al. 2012; Berry and Busse 2016). Clinically it is difficult to measure eosinophils in the sputum; however, obtaining peripheral blood eosinophils is relatively easy. While peripheral blood eosinophilia is not an optimal surrogate for airway eosinophils, it is suggestive of T2-high inflammation. In a large UK cohort, patients with peripheral blood eosinophil counts of 400 cell/μl or greater had poor asthma control and experienced worse asthma exacerbation compared to those patients with blood eosinophil counts less than 400 cells/μl (Price et al. 2015). Current research is underway for other markers of eosinophils that might be useful. Eosinophil peroxidase (EPX), an eosinophil granule protein in the sputum, seems to correlate with respiratory disease activity. Measurement of nasal and pharyngeal EPX using a bioactive paper strip is a promising tool to use at bedside to measure the burden of eosinophils in the lungs (Tabatabaian et al. 2017; Rank et al. 2016).
3.3.2 Fractional Exhaled of Nitric Oxide (FeNO)
In the lung,the oxidation of amino acid L-arginine via nitric oxide synthase produces nitric oxide (NO). A variety of cells including epithelial cells, macrophages, mast cells, neutrophils, and endothelial cells produce various forms of nitric oxide synthase. In particular, the epithelial cells lining the airway and alveoli express a high quantity of inducible nitric oxide synthase (iNOS). Both IL-4 and IL-13, prominent in T2-high inflammation, contribute to increased expression of iNOS leading to production of NO. Hence, the measurement of FeNO is a noninvasive biomarker reflective of T2-high asthma that is easily obtainable (Hanania et al. 2013; Tabatabaian and Ledford 2018). Current available analyzers for the measurement of NO concentration in the lungs include NIOX MINO, NIOX VERO (Aerocrine, Stockholm, Sweden), and NO Breath (Bedfront Scientific LtD, Kent, UK).
For clinical use, guidelines by the American Thoracic Society propose FeNO <25 ppb in adults and <20 ppb in children as normal (Dweik et al. 2011). In adults, a FeNO >50 ppb is more responsive to inhaled corticosteroids (ICS). A decrease in FeNO is observed within 1 week of therapy (Mehta et al. 2009). Non-compliance or decreased corticosteroid responsiveness should be considered if FeNO remains >50 ppb in adults (>35 ppb in children) despite ICS use (Dweik et al. 2011). In children, a FeNO >49 ppb within 4 weeks of ICS discontinuation is associated with an increase in asthma exacerbations (Pijnenburg et al. 2005). In a Cochrane review of adjustment of asthma medication based on FeNO levels in both adult and children, a reduction in FeNO was not associated with improvement of daily symptoms but rather a reduction in asthma exacerbations (Petsky et al. 2016). Several studies have demonstrated that patients with severe persistent asthma with higher FeNO had greater reduction in asthma exacerbation with treatment of anti-IgE monoclonal antibody (omalizumab) compared to those with lower FeNO levels (Hanania et al. 2013; Mansur et al. 2017). In a recent study observing a biologic inhibiting IL-4 and IL-13, suppression of FeNO was observed in the treated group compared to placebo by week 2 of therapy (Rabe et al. 2018). Interestingly, the anti-IL-5 biologics have not demonstrated much effect on FeNO (Haldar et al. 2009). A host of environmental factors impact the level of FeNO measured. Spirometry and exercise prior to measuring FeNO contribute to transiently lower levels. Use of ICS, systemic steroids, leukotriene receptor antagonist, smoking, and obesity are associated with lower FeNO. High-nitrate foods falsely increase FeNO. In adults, males have higher FeNO compared to females. In children, FeNO increases at a rate of 5% per year attributed to height increase (Berry and Busse 2016). Despite the various factors that affect the measurement of FeNO, it serves as a clinical biomarker in T2-high inflammation and potentially predicts response to targeted T2-high asthma biologics.
3.3.3 Periostin
Periostin is another marker of T2-high inflammation secreted by airway epithelial cells and fibroblast in response to IL-13. Periostin gene expression is increased in the airway of those with asthma (Corren et al. 2011). Hanania et al. demonstrated a 30% reduction in asthma exacerbation in the high-periostin group (>50 ng/ml at baseline) compared to 3% reduction in low-periostin group (<50 ng/ml at baseline) of those treated with anti-IgE monoclonal antibody (Hanania et al. 2013). Treatment with IL-13 antagonist showed greater improvement in FEV1 in subjects with higher baseline periostin compared to those with lower periostin (Corren et al. 2011). Serum periostin is a good biomarker of T2 inflammation; however, the assay to measure it at bedside is not commercially available.
3.3.4 Serum IgE
Sensitization to aeroallergens and increased serum total IgE is a risk factor for allergic asthma. In pediatric cohorts, children with severe asthma had higher serum IgE and increased aeroallergen sensitization (Fitzpatrick et al. 2011). The processing of antigens by dendritic cells and presentation to naïve T cells shift the inflammatory pathway toward T2-high inflammation (Fig. 2). Th2 shift contributes to class switching of B cells and production of specific IgE. IgE bound FcεRI, the high-affinity IgE receptor, and cross-links the receptors initiating a signaling cascade of mast cell degranulation releasing histamine, leukotrienes, and other inflammatory factors. IgE also activates eosinophils, basophils, macrophages, and airway smooth muscles via FcεRI receptor to produce pro-inflammatory cytokines involved in tissue remodeling (Pelaia et al. 2017). Anti-IgE monoclonal antibodies decrease blood eosinophils and asthma exacerbations.
3.4 Biologics Targeting T2-High Inflammation
Early-onset asthma is a phenotype linked to atopy and allergic sensitization. In fact, 70% of patients with asthma have an allergic phenotype. IgE is an integral part of allergic asthma. The first biologic approved for asthma in the United states was omalizumab (Xolair; Genentech USA, Inc. and Novartis Pharmaceuticals Corporation). Omalizumab is a humanized monoclonal antibody with specificity for the IgE molecule. This drug also downregulates the high-affinity IgE receptor (FCεRI) on eosinophils, basophils, circulating dendritic cells, and mast cells (Fig. 3) (Humbert et al. 2014). Treatment with omalizumab reduces asthma exacerbation, use of ICS, and overall symptoms (Table 2). In clinical trials, improvement of lung function is less evident with the use of omalizumab (Humbert et al. 2014). Subjects with elevated T2-high biomarkers, including blood eosinophils and FeNO, seem to benefit most from this therapy (Hanania et al. 2013). In one study, peripheral eosinophil count of 300 cells/μl or more predicts a favorable response to omalizumab with a 60% drop in asthma exacerbations (Busse et al. 2013b). In the United States, omalizumab is approved as add-on therapy for moderate-to-severe persistent allergic asthma in children 6 years and older (XOLAIR).
Omalizumab is humanized monoclonal antibody that binds to IgE and decreases serum level of IgE. Omalizumab also downregulates the IgE high-affinity receptor (FcεR1) on mast cells, basophils, and dendritic cells. (Adapted from Tabatabaian and Ledford 2018)
As described above, patients with elevated peripheral and sputum eosinophils have increased asthma exacerbations and overall poorly controlled asthma. A key cytokine in T2-high inflammation is IL-5. Eosinophils require the presence of IL-5 for growth, differentiation, and migration into the airways. To date, three monoclonal antibodies have been FDA approved that effect IL-5 which include mepolizumab, reslizumab, and benralizumab (Table 2). Mepolizumab is a humanized monoclonal antibody that binds to IL-5. Flood-Page and colleagues, in an initial double-blind, placebo-controlled study, evaluated patients with uncontrolled moderate-to-severe asthma despite an inhaled corticosteroid treatment (Flood-Page et al. 2007). Those treated with mepolizumab showed significant improvement in rate of exacerbations, lung function, and overall quality of life. The authors also found a drop in the number of blood and sputum eosinophils in the mepolizumab group. Ortega and colleagues, in a randomized double-blind study, compared mepolizumab 75 mg IV or 100 mg sub-q to placebo administered every 4 weeks for a total of 32 weeks in subjects with recurrent asthma exacerbations and eosinophilic inflammation. Initial entry did require subjects to have peripheral eosinophil count of 150 cells/μl or greater than 300 cells/μl in the previous year. Compared to placebo both active groups had an overall 50% reduction in asthma exacerbation, 100 ml improvement in FEV1, better asthma quality of life scores, and a decrease in both peripheral blood and sputum eosinophils (Ortega et al. 2014). Mepolizumab is approved in the United States as an add-on therapy for severe persistent asthma given as a 100 mg sub-q injection every 4 weeks in patients 12 years and older. Individuals with higher levels of peripheral blood eosinophils have the greatest benefit. Most recently mepolizumab was approved for eosinophilic granulomatosis with polyangiitis at a higher dose of 300 mg sub-q every 4 weeks (Raffray and Guillevin 2018; NUCALA). Reslizumab is also a humanized anti-IL-5 monoclonal antibody that was FDA approved in 2016 as add-on therapy for severe eosinophilic asthma. Castro and colleagues, in two double-blind multicenter studies with patients between the ages of 12 and 75 and eosinophil count of 400 cells/μl or greater, illustrated a decrease in asthma exacerbations and significant improvement in FEV1 in those treated with IV reslizumab 3 mg/kg compared to placebo. All of the patients enrolled were on ICS plus another controller therapy and had reversibility on spirometry with use of short-acting beta-agonist (Castro et al. 2015). Several other studies confirmed the clinical benefit of reslizumab in a similar patient population (Bjermer et al. 2016; Corren et al. 2016). Compared to the other IL-5 blocking agents, reslizumab may elicit the greatest improvement in FEV1 (Castro et al. 2015; Bjermer et al. 2016; Corren et al. 2016). Reslizumab is administered IV at 3.0 mg/kg over a 20- to 50-min infusion. It does have a black box warning for a small risk of anaphylaxis (CINQAIR). The latest biologic approved that targets IL-5 is benralizumab. This drug binds to the alpha (α) chain of IL-5 receptor, enhancing the antibody-dependent cell-mediated cytotoxicity leading to apoptosis of eosinophils, basophils, and eosinophil progenitors in the bone marrow (Laviolette et al. 2013). Eosinophils can enter tissue independent of IL-5, making the direct effect of benralizumab more attractive. In a phase 2b trial, Castro et al. evaluated the impact of variable doses of benralizumab compared to placebo in subjects with uncontrolled eosinophilic asthma. Benralizumab was administered every 4 weeks for the first three doses and subsequently every 8 weeks. A decrease in exacerbation occurred in treated group compared to placebo, and those subjects with eosinophil count greater than 300 cells/μl had the greatest improvement in FEV1 (Castro et al. 2014). A subsequent small study illustrated a decrease of 50% in asthma exacerbation with one dose of benralizumab administered during an acute ER visit over the next 12 weeks (Nowak et al. 2015). This opens the door for a novel use of biologics in the ER to prevent readmission rates and subsequent associated cost. Benralizumab is approved as an add-on therapy for severe persistent asthma eosinophilic phenotype in 12 years and older. It is a sub-q injection of 30 mg every 4 weeks for the first three injections and subsequently every 8 weeks (Fasenra). Although direct comparisons of the biologics targeting IL-5 do not exist, no clear superiority was elicited among the three therapies in an indirect meta-analysis (Cabon et al. 2017). All three biologics reduce asthma exacerbations and improve quality of life scores. To date, the ability to clearly identify the best therapeutic choice among the three available IL-5 inhibitors is lacking.
An attractive target for T2-high asthma is an IL-4 and IL-13 inhibitor. IL-4 is important for class switching of B cells and production of IgE. IL-13 enhances mucus production in the airway, induces airway hyperresponsiveness, stimulates proliferation of bronchial fibroblast, and recruits eosinophils and basophils. Biologics targeting IL-13 initially showed some promise. IL-13 stimulates epithelial cells to produce dipeptidyl peptidase-4 (DPP-4) and periostin. Both periostin and DPP-4 serve as good biomarkers to predict response to the IL-13 antagonist (Corren et al. 2011). Unfortunately, phase 2b and 3 trials of the two drugs targeting IL-13 did not prove to be effective in reducing asthma exacerbation or improving asthma control (Hanania et al. 2015). Dupilumab, a fully humanized monoclonal antibody directed toward the α-subunit of IL-4 receptor, blocks both IL-4 and IL-13 (Wenzel et al. 2013, 2016). This drug is FDA approved in the United States for moderate-to-severe atopic dermatitis (DUPIXENT). It is efficacious in nasal polyposis. In phase 2b trials, subjects with moderate-to-severe persistent asthma on high-dose ICS plus a long-acting beta-agonist had a significant decrease in asthma exacerbation. Individuals with peripheral eosinophil counts of 300 cells/μl or greater showed the most benefit (Wenzel et al. 2016). Castro et al. in a phase 3 trial showed dupilumab as an add-on therapy in severe uncontrolled asthma contributes to a 65% reduction in asthma exacerbation in patients 12 years of age or older given as sub-q injection at home bi-weekly (Castro et al. 2018). In another study, Wenzel and colleagues show improvement of asthma exacerbation and pulmonary function regardless of pretreatment eosinophil count. However, individuals with higher peripheral eosinophils have the greatest benefit (Wenzel et al. 2016). Several other targets in T2-high inflammation are under investigational review. The therapeutic efficacies of these drugs still need to be established.
4 Conclusion
Asthma is a common medical condition seen routinely in the outpatient setting by physicians and healthcare providers. Current guidelines recommend a stepwise approach in management of asthma. Clearly, educating patients on the appropriate use of inhalers and ensuring compliance are key for optimal control. Many patients still utilize urgent care systems, the ER and hospitals for acute asthma symptoms, suggesting lack of control in this population. By obtaining a complete history and physical exam, providers are able to identify the phenotype of asthma and further define prognosis of disease. Furthermore, addressing comorbid conditions, smoking, obesity, GERD, and OSA all contribute to asthma control. Most importantly our advancement in understanding of the molecular inflammation or endotypes in asthma has paved a path toward personalized medicine. Two main endotypes T2 high and T2 low have been defined to date. T2-low asthma is phenotypically associated with neutrophils in the sputum, adult-onset disease, and less corticosteroid responsiveness. Patients with neutrophilic inflammation seem to benefit from macrolide therapy. Those with underlying paucigranulocytic inflammation show therapeutic relief with the use of a long-acting antimuscarinic antagonist. Biomarkers reflecting T2-low asthma are not available for use at the bedside. Despite early attempts, targeting cytokines in T2-low asthma therapeutic interventions remains limited and further investigation is needed.
On the other hand, eosinophils in the sputum, early-onset asthma, and prior history of atopic disease are the phenotype that correlates with T2-high asthma. Clinically accessible biomarkers reflecting T2-high inflammation include total serum IgE, FeNO, and peripheral blood eosinophils. In the United States and Europe, omalizumab, reslizumab, mepolizumab, and benralizumab are commercially available for use in uncontrolled asthma patients with T2-high inflammation. Unfortunately, the ability to predict better response to a specific T2-high targeted therapy is lacking. Current biomarkers are suggestive of T2 inflammation with clinical value, but they are limited in precision. Many uncertainties exist with the growing repertoire of biologics. None of them modify disease or induce remission. Furthermore, the optimal treatment duration or approach to discontinuation is not clearly defined. Nevertheless, emerging knowledge of asthma phenotypes, endotypes, and associated biomarkers is the first step toward new therapeutic intervention offering patients precision medicine.
References
Alam R, Good J, Rollins D, Verma M, Chu H, Pham T-H, et al. Airway and serum biochemical correlates of refractory neutrophilic asthma. J Allergy Clin Immunol. 2017;140(4):1004–14.e13.
Barnes PJ. Therapeutic approaches to asthma-chronic obstructive pulmonary disease overlap syndromes. J Allergy Clin Immunol. 2015;136(3):531–45.
Bellini A, Marini MA, Bianchetti L, Barczyk M, Schmidt M, Mattoli S. Interleukin (IL)-4, IL-13, and IL-17A differentially affect the profibrotic and proinflammatory functions of fibrocytes from asthmatic patients. Mucosal Immunol. 2012;5(2):140–9.
Berry A, Busse WW. Biomarkers in asthmatic patients: has their time come to direct treatment? J Allergy Clin Immunol. 2016;137(5):1317–24.
Bjermer L, Lemiere C, Maspero J, Weiss S, Zangrilli J, Germinaro M. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest. 2016;150(4):789–98.
Busse WW, Holgate S, Kerwin E, Chon Y, Feng J, Lin J, et al. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013a;188(11):1294–302.
Busse W, Spector S, Rosen K, Wang Y, Alpan O. High eosinophil count: a potential biomarker for assessing successful omalizumab treatment effects. J Allergy Clin Immunol. 2013b;132(2):485–6.e11.
Busse WW, Maspero JF, Rabe KF, Papi A, Wenzel SE, Ford LB, et al. Liberty asthma QUEST: phase 3 randomized, double-blind, placebo-controlled, parallel-group study to evaluate dupilumab efficacy/safety in patients with uncontrolled, moderate-to-severe asthma. Adv Ther. 2018;35:737.
Cabon Y, Molinari N, Marin G, Vachier I, Gamez AS, Chanez P, et al. Comparison of anti-interleukin-5 therapies in patients with severe asthma: global and indirect meta-analyses of randomized placebo-controlled trials. Clin Exp Allergy. 2017;47(1):129–38.
Caggiano S, Cutrera R, Di Marco A, Turchetta A. Exercise-induced bronchospasm and allergy. Front Pediatr. 2017;5:131.
Castro M, Wenzel SE, Bleecker ER, Pizzichini E, Kuna P, Busse WW, et al. Benralizumab, an anti-interleukin 5 receptor alpha monoclonal antibody, versus placebo for uncontrolled eosinophilic asthma: a phase 2b randomised dose-ranging study. Lancet Respir Med. 2014;2(11):879–90.
Castro M, Zangrilli J, Wechsler ME, Bateman ED, Brusselle GG, Bardin P, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3(5):355–66.
Castro M, Corren J, Pavord ID, Maspero J, Wenzel S, Rabe KF, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378:2486.
Centers for Disease Control and Prevention: Data, Statistics, and Surveillance. AsthmaStats. 2015. Available from http://www.cdc.gov/asthma/asthma_stats/default.htm.
CINQAIR (reslizumab) prescribing information. Available from http://www.cinqair.com/.
Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365(12): 1088–98.
Corren J, Weinstein S, Janka L, Zangrilli J, Garin M. Phase 3 study of reslizumab in patients with poorly controlled asthma: effects across a broad range of eosinophil counts. Chest. 2016;150:799.
DUPIXENT. Dupilumab prescribing information. Available from https://www.dupixent.com/.
Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–15.
Fahy JV. Type 2 inflammation in asthma–present in most, absent in many. Nat Rev Immunol. 2015;15(1):57–65.
Fasenra (benralizumab) prescribing information. Available from https://www.fasenrahcp.com/.
Fitzpatrick AM, Teague WG, Meyers DA, Peters SP, Li X, Li H, et al. Heterogeneity of severe asthma in childhood: confirmation by cluster analysis of children in the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. The J Allergy Clin Immunol 2011;127(2):382–9.e1–13.
Flood-Page P, Swenson C, Faiferman I, Matthews J, Williams M, Brannick L, et al. A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. Am J Respir Crit Care Med. 2007;176(11):1062–71.
Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178(3):218–24.
Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360(10):973–84.
Hanania NA, Wenzel S, Rosen K, Hsieh HJ, Mosesova S, Choy DF, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187(8):804–11.
Hanania NA, Noonan M, Corren J, Korenblat P, Zheng Y, Fischer SK, et al. Lebrikizumab in moderate-to-severe asthma: pooled data from two randomised placebo-controlled studies. Thorax. 2015;70(8):748–56.
Hastie AT, Moore WC, Meyers DA, Vestal PL, Li H, Peters SP, et al. Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. J Allergy Clin Immunol. 2010;125(5):1028–36.e13.
Humbert M, Busse W, Hanania NA, Lowe PJ, Canvin J, Erpenbeck VJ, et al. Omalizumab in asthma: an update on recent developments. J Allergy Clin Immunol Pract. 2014;2(5):525–36.e1.
Lambrecht BN, Hammad H. The immunology of asthma. Nat Immunol. 2015;16(1):45–56.
Laviolette M, Gossage DL, Gauvreau G, Leigh R, Olivenstein R, Katial R, et al. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J Allergy Clin Immunol. 2013;132(5):1086–96.e5.
Leiria LOS, Martins MA, Saad MJA. Obesity and asthma: beyond TH2 inflammation. Metabolism. 2015;64(2): 172–81.
Liu W, Liu S, Verma M, Zafar I, Good JT, Rollins D, et al. Mechanism of TH2/TH17-predominant and neutrophilic TH2/TH17-low subtypes of asthma. J Allergy Clin Immunol. 2017;139(5):1548–58.e4.
Mansur AH, Srivastava S, Mitchell V, Sullivan J, Kasujee I. Longterm clinical outcomes of omalizumab therapy in severe allergic asthma: Study of efficacy and safety. Respir Med. 2017;124:36–43.
Mehta V, Stokes JR, Berro A, Romero FA, Casale TB. Time-dependent effects of inhaled corticosteroids on lung function, bronchial hyperresponsiveness, and airway inflammation in asthma. Ann Allergy Asthma Immunol. 2009;103(1):31–7.
Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE. Distinguishing severe asthma phenotypes: role of age at onset and eosinophilic inflammation. J Allergy Clin Immunol. 2004;113(1):101–8.
Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119(2): 405–13.
Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181(4): 315–23.
Nowak RM, Parker JM, Silverman RA, Rowe BH, Smithline H, Khan F, et al. A randomized trial of benralizumab, an antiinterleukin 5 receptor alpha monoclonal antibody, after acute asthma. Am J Emerg Med. 2015;33(1):14–20.
NUCALA (mepolizumab). Available from https://www.gsksource.com/pharma/content/gsk/source/us/en/brands/nucala/pi.html?cc=F736CF99B6F1&pid=.
Ortega HG, Liu MC, Pavord ID, Brusselle GG, FitzGerald JM, Chetta A, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371(13):1198–207.
Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–9.
Pelaia G, Canonica GW, Matucci A, Paolini R, Triggiani M, Paggiaro P. Targeted therapy in severe asthma today: focus on immunoglobulin E. Drug Des Devel Ther. 2017;11:1979–87.
Petsky HL, Kew KM, Turner C, Chang AB. Exhaled nitric oxide levels to guide treatment for adults with asthma. Cochrane Database Syst Rev. 2016;9:Cd011440.
Phenotype. Merriam-Webster.com. Available from https://www.merriam-webster.com/dictionary/phenotype.
Pijnenburg MW, Bakker EM, Lever S, Hop WC, De Jongste JC. High fractional concentration of nitric oxide in exhaled air despite steroid treatment in asthmatic children. Clin Exp Allergy. 2005;35(7):920–5.
Price DB, Rigazio A, Campbell JD, Bleecker ER, Corrigan CJ, Thomas M, et al. Blood eosinophil count and prospective annual asthma disease burden: a UK cohort study. Lancet Respir Med. 2015;3(11): 849–58.
Rabe KF, Nair P, Brusselle G, Maspero JF, Castro M, Sher L, et al. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med. 2018;378:2475.
Raffray L, Guillevin L. Treatment of eosinophilic granulomatosis with polyangiitis: a review. Drugs. 2018;78:809.
Rank MA, Ochkur SI, Lewis JC, Teaford HG 3rd, Wesselius LJ, Helmers RA, et al. Nasal and pharyngeal eosinophil peroxidase levels in adults with poorly controlled asthma correlate with sputum eosinophilia. Allergy. 2016;71(4):567–70.
Rodriguez-Jimenez JC, Moreno-Paz FJ, Teran LM, Guani-Guerra E. Aspirin exacerbated respiratory disease: current topics and trends. Respir Med. 2018;135:62–75.
Suzuki Y, Saito J, Kikuchi M, Uematsu M, Fukuhara A, Sato S, et al. Sputum-to-serum hydrogen sulfide ratio as a novel biomarker of predicting future risks of asthma exacerbation. Clin Exp Allergy. 2018;48:1155.
Tabatabaian F, Ledford DK. Omalizumab for severe asthma: toward personalized treatment based on biomarker profile and clinical history. J Asthma Allergy. 2018;11:53–61.
Tabatabaian F, Ledford DK, Casale TB. Biologic and new therapies in asthma. Immunol Allergy Clin N Am. 2017;37(2):329–43.
Teague WG, Phillips BR, Fahy JV, Wenzel SE, Fitzpatrick AM, Moore WC, et al. Baseline features of the severe asthma research program (SARP III) cohort: differences with age. J Allergy Clin Immunol Pract. 2018;6(2):545–54.e4.
Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18(5): 716–25.
Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368(26):2455–66.
Wenzel S, Castro M, Corren J, Maspero J, Wang L, Zhang B, et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting beta2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet. 2016;388(10039):31–44.
Wilhelm CP, Chipps BE. Bronchial thermoplasty: a review of the evidence. Ann Allergy Asthma Immunol. 2016;116(2):92–8.
Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci U S A. 2007;104(40):15858–63.
Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180(5):388–95.
XOLAIR (omalizumab) prescribing information. Available from http://www.xolair.com/allergic-asthma/hcp/.
Author information
Authors and Affiliations
Corresponding author
Section Editor information
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this entry
Cite this entry
Tabatabaian, F. (2019). Asthma Phenotypes and Biomarkers. In: Allergy and Asthma. Springer, Cham. https://doi.org/10.1007/978-3-030-05147-1_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-05147-1_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-05146-4
Online ISBN: 978-3-030-05147-1
eBook Packages: MedicineReference Module Medicine