Abstract
Salmonella is a major threat to human public health since it causes several foodborne outbreaks in the USA and worldwide. Poultry harbor Salmonella in their gastrointestinal tract and excrete the pathogen through the feces contaminating the environment. In addition, there are several sources of the pathogen at the farms and processing facilities. Until humans contract the infection by consuming contaminated poultry products, Salmonella must overcome several hurdles, including methods of control applied at the farm and processing facilities. Even after the adoption of intervention techniques at multiple levels, Salmonella still contaminates the poultry meat supply. In this chapter, we discuss Salmonella as a major pathogen transmitted through live poultry and poultry meat, a brief overview of different poultry-associated Salmonella serovars, including the familiar and the emerging, and various sources of contamination on farm and processing facilities. This information will help the industry to design and/or revisit the plans before appropriate intervention strategies are applied to control Salmonella in the poultry farms and processing environment in a highly automated vertically integrated production system.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1.1 Salmonella: A Major Foodborne Pathogen in Poultry
Foodborne illness caused by various pathogens represents a major public health concern that results in significant loss to the U.S. economy (Marder et al. 2017; Scharff 2012). In 2016, the Centers for Disease Control and Prevention (CDC) Foodborne Diseases Active Surveillance Network (FoodNet) identified 24,029 infections, 5512 hospitalizations, and 98 deaths caused by pathogens such as Campylobacter, Cryptosporidium, Cyclospora, Listeria, Salmonella, Shiga toxin-producing E. coli, Shigella, Vibrio, and Yersinia (Marder et al. 2017). Among the bacterial pathogens associated with foodborne illness, non-typhoidal Salmonella (NTS) caused the second largest number of confirmed and culture-independent diagnostic test (CIDT)-positive infections (8172 cases) in the USA, second only to Campylobacter that caused 8547 illness cases (Marder et al. 2017).
Salmonella is a major foodborne pathogen implicated in outbreaks causing human illness for over a century (Bean and Griffin 1990; CDC 2000, 2013; Chalker and Blaser 1988). The organism is historically considered as the causative agent of the “meat poisoning” outbreak reported in Germany in 1888 and was first isolated by A. Gärtner, naming it as Bacillus enteritidis. In the USA, salmonellosis was designated as a notifiable disease in 1943, and since then, a steady increase in the reported incidence of Salmonella has been noted (Angulo and Swerdlow 1999; Tauxe et al. 1989). Since the mid-1980s, the pathogen gained tremendous importance due to its association with foodborne illnesses worldwide (Rodrigue et al. 1990; Tirado and Schmidt 2001). Currently, many serotypes of Salmonella are prevalent, and others are emerging as health threats to humans who contract the infection by consuming Salmonella-contaminated food products, the major animal-derived foods being poultry meat and eggs.
Salmonella is a Gram-negative, non-spore-forming, and motile bacillus belonging to the Enterobacteriaceae family. It is a facultative anaerobe that grows between 8 oC and 45 oC and at a pH range of 4–8. The pathogen is broadly classified into typhoidal and NTS based on host adaptability and infectious nature. The NTS has a wide range of vertebrate hosts, whereas host range of typhoidal Salmonella is limited to humans (Feasey et al. 2012; Winter et al. 2010).
Since chickens serve as natural hosts for many NTS serovars, the pathogens are frequently isolated from poultry and poultry products, with meat and shell eggs being the most commonly implicated vehicles in outbreaks. Most serovars of Salmonella colonize almost every part of the chicken intestinal tract, with highest predilection potential noticed in the paired blind sacs at the hind end of the tract called the ceca. Once colonized, the pathogen can be excreted through the feces without chickens showing any obvious clinical signs of infection. This eventually leads to the horizontal transmission of infection to other healthy birds and flocks, contamination of carcasses during slaughter, contamination of eggs with feces, and the retrograde transmission of infection via the transovarian route by major serovars such as S. Enteritidis and S. Heidelberg (Gantois et al. 2008; De Reu et al. 2006).
Although zoonotic in nature, NTS often causes self-limiting gastroenteritis in healthy humans. However, the infection process is more severe in immunocompromised individuals, children, and older adults, and the infectious dose can be low. The incubation period of the disease typically ranges 12–72 h with the illness lasting for 2–7 days. Patients usually recover within a week without any antibiotic treatment except in cases of severe diarrhea, where intravenous fluid therapy is warranted (Feasey et al. 2012). However, the severe illness caused by antibiotic-resistant strains of Salmonella may result in longer periods of stay in the hospital (Lee et al. 1994). The infection often proceeds to bacteremia and invasive form in immunocompromised individuals (Antunes et al. 2016; Chen et al. 2013). The fecal excretion of the pathogen from infected humans leads to the transmission of the pathogen among different vertebrate hosts (Dhanoa and Fatt 2009).
1.2 Salmonella in Poultry Production
1.2.1 S. Pullorum and S. Gallinarum
Diarrheal diseases have been a serious problem in poultry rearing/production systems that resulted in significant economic loss to the producers/industry, historically. Salmonella serovars such as S. Gallinarum and S. Pullorum were commonly isolated from poultry intestinal contents, droppings, and internal organs ever since poultry rearing was considered a financial enterprise (Stafseth and Mallmann 1928). The industry was aware of the importance of hygienic practices in poultry production to avoid diarrheal diseases from their valuable flocks. During that time, the major focus of the poultry sector was selection of superior breeds for improved egg production. Poor hatchability and smaller eggs were significant concerns, and the market trend was more toward producing eggs with superior hatchability. To aid this process, selective breeding and progeny testing were made common practices.
Although methods such as sanitation, immunization, and elimination of carriers and birds that showed signs of disease were practiced to control fowl pox and pullorum disease in hopes of saving the production strains (Hutt 1938), numerous outbreaks of S. Pullorum were reported in poultry in the early 1900s, and the carrier status of chicken for pullorum disease had been established. S. Pullorum emerged as a significant pathogen in poultry production. The egg-borne transmission of the disease was reported in 1909, and the septicemic nature of the pathogen was first reported in 1913. The young birds were mainly susceptible to S. Pullorum, and the disease was known as “fatal septicemia of young chicks” or “bacillary white diarrhea” or “pullorum disease” (Bullis 1977; Tittsler 1930). The bacterium was isolated from the liver, heart, lungs, and ovaries. A severe economic loss was reported due to the loss of egg production and mortality. The serum agglutination test and pullorum test were commonly employed to detect the disease in the flock (Tittsler 1930).
Commercial hatcheries became the source of infection, and the use of disinfectants was practiced in hatcheries. The dedicated incubators and use of formaldehyde for fumigation of eggs to control S. Pullorum became a common practice (Bullis 1977; Bushnell and Payne 1932). Responding to the situation, the National Poultry Improvement Plan (NPIP) was introduced in the USA in 1935 to control the pullorum disease. As a part of the NPIP, screening tests such as whole blood tests, tube agglutination tests, and rapid serum tests were used to detect S. Pullorum in the poultry flocks to eradicate and limit the disease. Based on the test results, the flocks were categorized into pullorum-tested flocks, pullorum-passed flocks, and pullorum-cleaned flocks (Bullis 1977).
However, S. Gallinarum caused fowl typhoid in adult chickens and was recognized as early as 1888. The tests that were used to screen pullorum disease were also used to detect S. Gallinarum. With the introduction of NPIP, the establishment of pullorum-free flocks also resulted in reduced incidence of fowl typhoid. In addition, the breeds such as White Leghorn were inherently resistant to these diseases, and the rearing of breeds resistant to infection became a common mitigation practice. Later, in 1954, screening of S. Gallinarum was also included as a part of NPIP. Moreover, antibiotics were used in poultry production to control these pathogens, resulting in tremendous improvement (Bullis 1977). Pullorum disease and fowl typhoid have been currently eradicated from the commercial flocks of developed countries such as the USA and Canada (Shivaprasad 2000). Although non-zoonotic, these pathogens still cause major economic problems in developing countries since they are highly adapted to poultry (Barrow and Freitas Neto 2011).
1.2.2 Non-typhoidal Salmonella
Though the eradication of S. Pullorum and S. Gallinarum could be achieved, the emergence of NTS resulted in significant safety concerns over the production of poultry meat and eggs for human consumption. The NTS Salmonella serovars caused 28% illness associated with foodborne outbreaks during 1973–1987 (Bean and Griffin 1990). On a later time-frame, 40% Salmonella-associated foodborne illness were reported in the USA from 1993–1998 (CDC 2000). The proportion of foodborne salmonellosis by poultry meat and eggs increased significantly from 1993 to 1998 compared to that occurred in the preceding decade. In the following decade (1998–2008), NTS Salmonella contributed 18% of the total illness associated with foodborne outbreaks in the USA (CDC 2013), underscoring a constant presence of NTS as the etiological agent in those outbreaks. Salmonella remains a major foodborne bacterial pathogen in the USA over a period of 50 or more years (Bean and Griffin 1990; CDC 2000, 2013; Chalker and Blaser 1988).
Two major epidemiological events that occurred in relation to the Salmonella serovars in the previous century were the emergence of S. Enteritidis as a major pathogen in poultry and the emergence of antibiotic-resistant strains of Salmonella (Rabsch et al. 2001). Although the poultry-adapted serovars of Salmonella such as S. Pullorum and S. Gallinarum were eradicated from commercial flocks in the USA by 1950, this successful event, however, created an environmental niche to be occupied by S. Enteritidis which was abundant in the rodent population. Since S. Gallinarum possessed cross-immunity against S. Enteritidis infection, it is reasonable to believe that the eradication of one resulted in the emergence of the other. In addition, higher bird density and vertical integration of poultry production system also facilitated the transmission of S. Enteritidis among poultry flocks (Foley et al. 2008, 2011).
1.2.3 S. Enteritidis: A Major Serovar
S. Enteritidis is the most genetically homogenous serotype of all Salmonella (Porwollik et al. 2005). Although limited in genomic diversity, the field isolates of the serotype vary in their capabilities to form biofilms, growth characteristics, production of high molecular mass lipopolysaccharides, and survival within the egg albumen (Clavijo et al. 2006; Jain and Chen 2007; Yim et al. 2010). In chickens, the pathogen varies in its virulence potential to cause mortality or to colonize the intestinal tract and invade the spleen and liver (Gast and Benson 1995, 1996). On-farm investigations indicate that once chickens are exposed to the pathogen, the entire flock can become colonized rapidly (Berrang et al. 2009; Foley et al. 2008). This could be attributed to the ability of the pathogen to proliferate in the gastrointestinal tract of chicken (Poppe 2000) and the multitude of sources in farms contributing to pathogen spread in birds.
S. Enteritidis is invasive in both young and adult chickens (Shah et al. 2011). Young chickens develop systemic disease with varying degrees of mortality (Duchet-Suchaux et al. 1995; Velge et al. 2005). The affected chicks may show all or some signs such as anorexia, depression, ruffled feathers, huddling together in groups, reluctance to move, drowsiness, dehydration, white diarrhea, stained and pasted vents, and stunted growth (McIlroy et al. 1989). However, adult chickens, once colonized with the pathogen, may remain as asymptomatic carriers, shedding the pathogen to the environment continuously or intermittently (Golden et al. 2008; Velge et al. 2005). Chickens infected with high doses of S. Enteritidis can subsequently develop clinical salmonellosis with high mortality, whereas infection with low doses will result in clinically healthy carrier birds (Desmidt et al. 1997; Gast and Benson 1995; Van Immerseel et al. 2004a, b). Currently, improvement in the vaccination strategies and the development of targeted interventions to control S. Enteritidis in/on eggs and meat have tremendously improved the situation. However, the emergence of other NTS serovars, such as S. Heidelberg, S. Oranienburg, S. Infantis, S. Hadar, S. Kentucky, and others, have raised serious concerns for the industry (Dutil et al. 2010; Foley et al. 2011; Wong et al. 2014; CDC, 2016; Hindermann et al., 2017).
1.2.4 Antibiotic-Resistant Salmonella
The development of antibiotic resistance in NTS serovars, including the most prevalent serovars such as S. Heidelberg and S. Kentucky, is an increasing concern for the U.S. poultry industry (Dutil et al. 2010; Foley et al. 2011; White et al. 2001). For example, the outbreak isolates of S. Heidelberg in the recent foodborne outbreaks were resistant to many clinically relevant drugs such as streptomycin, ampicillin, gentamicin, tetracycline, sulfamethoxazole, chloramphenicol, and trimethoprim-sulfamethoxazole. In addition, the isolates were resistant to the drug of choice to treat human salmonellosis—ceftriaxone (Medeiros et al. 2011; Foley et al. 2011; Hoffmann et al. 2014). Ceftriaxone-resistant S. Heidelberg was isolated from the retail meat sold in the USA (White et al. 2001) and Canada (Dutil et al. 2010). The resistant genes are encoded on plasmids in S. Heidelberg. S. Kentucky also possesses plasmids that encode genes for antibiotic resistance, resistance to disinfectants, iron acquisition, and bacteriocin production that enhance the survival of the pathogen in poultry flocks (Han et al. 2012).
Isolation of antibiotic-resistant strains of the Salmonella is not restricted to the U.S. poultry market. Jørgensen et al. (2002) reported that 70% of Salmonella isolated from 241 whole carcasses collected from retail stores in England were resistant to least one antibiotic, and 46% were resistant to more than one antibiotic. In a Portugal study, Antunes et al. (2003) detected 10 different serotypes of Salmonella from 60% of chicken samples, of which 50% were resistant to nalidixic acid and enrofloxacin. In a U.S. study, Cui et al. (2005) reported that all S. Typhimurium isolates obtained from retail chicken were resistant to more than five antimicrobials, whereas those isolated from organic chicken were resistant to more than 17 antimicrobials. Out of the 569 samples positive for Salmonella (N = 4745), Roy et al. (2002) reported 92 samples collected from various environmental sources had isolates having resistance to erythromycin, lincomycin, and penicillin antibiotics, whereas all were susceptible to sarafloxacin and ceftiofur. In a different study, Parveen et al. (2007) found high levels of Salmonella from pre- and post-chilled poultry carcasses and water samples collected at the entrance of the chiller. Among the serovars isolated, 79.8% were resistant to at least one antibiotic, whereas 53.4% were resistant to more than one antibiotic, including tetracycline, ampicillin, amoxicillin-clavulanic acid, ceftiofur, streptomycin, and sulfisoxazole.
1.2.5 Salmonella Serotypes in Poultry Meat Products
It is well evidenced by the literature that poultry meat plays a major role in causing Salmonella-associated foodborne outbreaks since the 1950s (Bean and Griffin 1990; CDC 2000, 2013; Chalker and Blaser 1988). Poultry meat is the cheapest source of protein, and a large majority of U.S. population likes to have it in their diet (NCC 2017). Since poultry are the natural reservoirs of Salmonella, unhygienic processing and abused storage conditions of poultry meat can contribute to the incidence of salmonellosis in humans (CDC 2013).
Poultry meat, including the whole carcass, cut-up parts, and processed meats, are significant sources of several Salmonella serotypes that can cause disease in humans. In an early Canadian study, Salmonella was detected from 73.7% turkey carcasses and 38.2% chicken carcasses (Lammerding et al. 1988). Later, Logue et al. (2003) studied the incidence of Salmonella in two turkey processing plants in the Midwestern USA. Surface swabs were collected from poultry carcasses pre-chill and post-chill. Samples were also collected from the chill water. The overall incidence of Salmonella was found to be 16.7% after enrichment, and more positive samples were observed in pre-chill than post-chill. Major serotypes recovered were S. Senftenberg, S. Agona, S. Heidelberg, and S. Hadar. Jørgensen et al. (2002) studied the prevalence of Salmonella in 241 whole raw chicken samples purchased from retail shops in the UK at two different winter seasons of 1998/1999 and 1999/2000. The study found that Salmonella were present in 25% of the chicken samples. Among these, 19% of Salmonella was detected from both inside and outside of the chicken packages. The predominant serotypes detected were S. Indiana, S. Enteritidis, and S. Hadar (Jørgensen et al. 2002). Roy et al. (2002) detected Salmonella in 569 samples (11.99%) among 4745 samples collected from poultry liver and yolk sac, chicken ground meat, rinse water from spent hens and broilers, hatchery fluff, and drag samples from poultry environment during 1999/2000 in the Pacific Northwest. Out of the 97 positive samples serotyped, S. Heidelberg (25.77%), S. Kentucky (21.64%), S. Montevideo (11.34%), S. Hadar (5.15%), and S. Enteritidis (5.15%) were the major serotypes isolated. Likewise, the incidence of Salmonella in several poultry products obtained from a local butcher shop in Belgium revealed that 60% of the samples were contaminated with Salmonella consisting of ten different serotypes. The most prominent serotypes isolated in the study were S. Enteritidis and S. Hadar (Antunes et al. 2003). In a study conducted in Spain to isolate Salmonella from 198 samples of chicken meat for sale in retail outlets, it was reported that the pathogen was isolated from 35.83% of the samples where the predominant serovars were S. Enteritidis (47.88%), S. Hadar (25.35%), and serotype 4, 12: b:-(II) (19.71%) (Domınguez et al. 2002). In yet another study conducted in Maryland, USA, Cui et al. (2005) reported 61% of organic and 44% of conventional chickens were contaminated with Salmonella. Between the years 2002 and 2006, Salmonella was isolated from 59.7% ground turkey, 36.9% chicken breast, and 3.4% pork chops among retail meat outlets in the USA (Zhao et al. 2008).
Frozen chicken nuggets, strips, and eggs were the main poultry foods implicated in the causation of human S. Heidelberg infections in Canada (Currie et al. 2005). Bohaychuk et al. (2006) detected Salmonella in 30% of raw chicken legs and meat and poultry products collected from a retail market in Alberta, Canada. In a Portugal study, Antunes et al. (2003) found Salmonella in 60 samples of poultry products obtained from local shops and canteens and detected ten different serotypes of Salmonella in 60% of samples and identified S. Enteritidis and S. Hadar as more prevalent. Jackson et al. (2013) studied the link between different Salmonella serotypes and various foods, including poultry, by analyzing outbreaks that occurred between 1998 and 2008. The study found that eggs and poultry meat were vehicles in more than 80% cases of Salmonella outbreaks caused by S. Enteritidis, S. Heidelberg, and S. Hadar. In another epidemiological study, Chittick et al. (2006) analyzed the national foodborne outbreak data from 1973 to 2001 and found that among 6633 outbreaks of known etiology, 184 (3%) were contributed by S. Heidelberg. Among these, 3 outbreaks were due to egg consumption, 17 cases were related to consumption of foods prepared using eggs, 25 cases were related to poultry, and 8 cases were due to consumption of food containing both poultry and eggs.
Foley et al. (2008) had observed that serovars S. Senftenberg and S. Hadar have become more prevalent in poultry, compared to S. Enteritidis, and S. Typhimurium. S. Heidelberg was reported to be more isolated from clinical cases and suggested to be more virulent than other serovars. The study concluded that among the top ten serovars of Salmonella associated with human infections, the majority were from swine and poultry, including S. Heidelberg. In a different study, Parveen et al. (2007) reported high Salmonella contamination in processed poultry products. In this study, 480 pre-chill and post-chill poultry carcasses and the chill water from entry and exit point were enriched and analyzed using an automated BAX system and culture methods to detect Salmonella. Approximately, 88.4% of pre-chill and 84.1% post-chill carcasses were found to be positive for the pathogen. In addition, 92% of the samples collected from entry points were found to be positive for Salmonella, whereas none were identified at the exit point. The predominant serotypes isolated were S. Kentucky (59.5%) and S. Typhimurium (17.8%) (Parveen et al. 2007). In yet another study, Lestari et al. (2009) studied the prevalence of Salmonella isolated from 141 conventionally raised and 53 organically raised chicken carcasses from 27 retail stores located in Baton Rouge, Louisiana. Recovery rates were similar. Twenty-two percent of the conventionally raised chicken was found to be positive for Salmonella, whereas 20.8% organic chicken was found to be positive for Salmonella. Out of the eight serotypes isolated, predominant ones were S. Kentucky, S. Hadar, and S. Enteritidis (Lestari et al. 2009).
1.3 Salmonella in Vertically Integrated Production Systems
1.3.1 Breeders
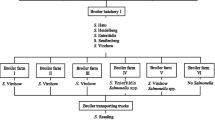
Salmonella has multiple routes of entry in a poultry production system. Once the pathogen is introduced in poultry, the infected birds act as a constant source of infection through horizontal and vertical transmission of the pathogen in large poultry grow-out houses. Salmonella colonizes the reproductive organs such as ovary and oviduct, and during egg formation, the pathogen may enter internal contents such as the vitelline membrane and albumen (Gast et al. 2004, 2007; Heyndrickx et al. 2002). Subsequently, the chicks hatching from the contaminated eggs will serve as a source of infection to the flock. This is the common process involved in vertical transmission (Cason et al. 1994; Cox et al. 2000; Gast 1994). Therefore, the breeder stocks harboring Salmonella in the vertically integrated system have an imperative role in the prevalence and persistence of Salmonella in broiler meat production.
The constant presence of Salmonella in the poultry houses is mainly due to the vertical transmission of the pathogen from breeder flocks and horizontal transmission occurring in the housing facilities. Salmonella testing conducted in processing facilities of seven consecutive flocks of two vertically integrated broiler production systems in Georgia revealed a high prevalence Salmonella serovars such as S. Typhimurium, S. Montevideo, S. Kentucky, and S. Enteritidis. In addition, the carcass isolates of S. Enteritidis and S. Typhimurium showed indistinguishable PFGE patterns with the serovars isolated from the breeder flocks indicating the likelihood of Salmonella originating from the breeder flocks, subsequently contaminating the carcasses (Liljebjelke et al. 2005). Another retrospective study conducted by Crespo et al. (2004) also reported the continuum of S. Arizona from breeder flocks to eggs and meat.
1.3.2 Hatchery
In a vertically integrated broiler production system, hatcheries could be reservoirs of the pathogen, and the serovars of Salmonella present in processing environment are often traced back to hatcheries. Hatcheries harboring Salmonella could contaminate the eggs and eventually lead to the colonization in chicks (Bailey et al. 1994). Salmonella serovars can survive as an endemic population in hatcheries and can act as a source of infection to the subsequent flocks (Bailey et al. 2002). Salmonella colonization in day-old chicks is of critical importance since the chicks are susceptible to the low infectious dose of Salmonella. In addition, less microbial diversity and an unstable gut microbiome will make the flocks susceptible to Salmonella (Oakley et al. 2014).
1.3.3 Farmed and Wild Animals, Rodents, and Other Vectors
Salmonella has wide host range and is distributed all over the environment. Domestic animals such as cattle, small ruminants, and pigs harbor NTS and act as a source of infection, especially in organic production or free-range settings (Davies and Wray 1996; Hoelzer et al. 2011). Wild birds such as raptors, vultures, crows, and gulls also serve as potential carriers of Salmonella. In addition, domestic pigeons, passerines, colonial water birds, finches, and house sparrows carry Salmonella in their intestines (Tizard 2004). Salmonella has been isolated from a wide variety of wild animals including squirrels, raccoons, foxes, mink, tigers, wild boars, rhinoceroses, seals, hedgehogs, and white-tailed deers. The transmission of Salmonella happens when infringement of wild and captive animals occurs (Hoelzer et al. 2011).
The carrier status of rodents for Salmonella serovars such as S. Typhimurium and S. Enteritidis often warrants pest control programs in poultry farms. It could be the direct transmission of the pathogen from the birds to the pests or vice versa (Wales et al. 2007). The rodents amplify the pathogen load in the environment and transmit those to the food animals, especially in the organic production system. Then the pathogen constantly circulates in the food chain (Meerburg and Kijlstra 2007). Salmonella prevalence in the farm premises due to rodents was estimated at 5.2% (Skov et al. 2008). Studies also revealed the genotypic and serological similarity between samples isolated from rodents and chicks (Liebana et al. 2003).
1.3.4 Human Traffic and Related Activities
Movement of people in and out of the farms is a major Salmonella introduction process in a poultry farm. Salmonella can be introduced into the farm through cages, feeders, drinkers, clothes, and boots (Wales et al. 2007). The movement of employees between different farms and contact with different species of animals are also potential threats to the safety. Therefore, proper physical barriers, disinfection procedures, dedicated clothes, and boots could be useful to reduce the introduction of the pathogens into the flock (Newell and Fearnley 2003).
The crates used for transportation of birds to the farms and processing plants carry Salmonella. Salmonella survives on crates even after washing them using quaternary ammonium compounds with an exposure time of 10 or 20 s. The flocks that were previously Salmonella-negative became positive from the contamination of the crates (Slader et al. 2002). Therefore, the movement of portable equipment, including the transport crates could be an immediate source of Salmonella infection to the processing facility or poultry farms (Heyndrickx et al. 2002; Slader et al. 2002).
1.3.5 Feed, Litter, and Water
Contaminated feed is one of the main sources for contraction of Salmonella infection by poultry. Most of the time, the traditional techniques would not allow the recovery of a low level of Salmonella from the feed, although Salmonella numbers as low as ten cells can colonize in day-old chicks (Maciorowski et al. 2006; Park et al. 2011). Also, less than one Salmonella per gram feed is sufficient to cause colonization in 1- to 7-day-old chicks (Schleifer et al. 1984).
Salmonella survives in poultry feed in a strain-dependent manner. Most of the virulence genes are downregulated during its survival in a low water activity environment such as poultry feed (Andino et al. 2014). Salmonella serovars such as S. Typhimurium can persist in feed for months and act as a source of infection to the chicks or adult chickens. S. Typhimurium survives in feed for 40 days, 16 months, or 18 months at 38 °C, 25 °C, and 11 °C, respectively (Williams and Benson 1978). Therefore, hurdle technologies and intervention strategies are recommended during feed manufacturing, transportation, and storage (Maciorowski et al. 2004)
Salmonella persists in poultry litter and acts as a major source for intestinal colonization by the pathogen in chicks (Fanelli et al. 1970). Similar to the survivability in feed, serovars such as S. Typhimurium survives in the litter for months and acts as a source of infection to the chicks or broiler chickens. S. Typhimurium survives in the litter for 13 days at 38 °C and 18 months at 25 °C or 11 °C (Williams and Benson 1978).
Poultry drinking water can be contaminated with feed, litter, droppings, or dust carrying Salmonella. The residual organic contamination reduces the free available chlorine (FAC) in the water and changes the pH of the water which in turn reduces the efficacy of chlorination (Poppe et al. 1986). Salmonella at a level of 4–5 log CFU/ml has been recovered from the poultry drinking water. The main source of Salmonella contamination to the poultry drinking water is from the Salmonella attached to the trough drinkers and plastic bell drinkers (Renwick et al. 1992). Nipple drinkers are less likely to be contaminated with Salmonella because of their closed nature (Poppe et al. 1986). Salmonella forms biofilms in pipes and drinkers and acts as a persistent source of infection to the poultry (Poppe et al. 1986).
1.3.6 Aerosols
Salmonella survives in the aerosols, dust particles, and droplets. Salmonella persists in the dust particles for years and serves as a constant source for pathogen colonization (Davies and Wray 1996). The pathogen is often found in air inlets or fans and can be a recontamination source (Higgins et al. 1982). Studies conducted in a controlled environment by regulating the air flow of the cabinet between challenged and non-challenged birds revealed that Salmonella could be transmitted from infected to non-infected birds via aerosols. Also, 33% of the non-challenged birds became infected with S. Enteritidis. Moreover, S. Enteritidis was isolated from the feathers of 77% non-challenged birds (Gast et al. 1998).
Aerosolizing of S. Enteritidis causes systemic infections through nasal and conjunctival routes (Baskerville et al. 1992; Humphrey et al. 1992) and elicits varying degrees of immune response in a dose-dependent manner. A low infectious dose of 103 CFU S. Enteritidis can cause lung infection and systemic infection in the liver, spleen, kidney, ovary, and oviduct in 2-day-old chicks. Also, the pathogen can be excreted through feces for 28 days (Cooper et al. 1996).
Currently different antibacterial interventions are practiced at the farm level to control NTS serovars. The interventions include prebiotics, probiotics, organic acids, short-chain fatty acids, vaccines, bacteriophages, and essential oils and are being supplemented through feed or drinking water (Atterbury et al. 2007; Callaway et al. 2008; Donalson et al. 2007, 2008; Higgins et al. 2008; Van Immerseel et al. 2006; Kollanoor Johny et al. 2009, 2012; Nair et al. 2016; Patterson and Burkholder 2003; Tellez et al. 2012; Zhang-Barber et al. 1999). These interventions will be explained in detail in a following chapter.
1.3.7 Processing Environment
Salmonella-colonized flocks excrete the pathogen through the feces that transmit the infection to the other birds in the flock, contaminating the poultry farm. The sharing of the common equipment also causes the introduction of the pathogen to the processing facility (Heyndrickx et al. 2002). Among the different stages of poultry processing, scalding, picking, evisceration, and chilling reduce the total microbial load on the carcass. In addition, cross contamination of carcasses is possible during these stages, if a single carcass is contaminated with Salmonella (Heyndrickx et al. 2002). Therefore, these are considered as critical operations in poultry processing in terms of reducing the prevalence of Salmonella on poultry carcasses (Svobodová et al. 2012). However, other steps of poultry processing are also important. For example, inappropriate stunning causes wing flapping and quivering which lead to soiling of the carcass with feces and transfer of Salmonella from inside to the outside of the body (Gregory 2005). Therefore, poultry processing is considered as a complicated and delicate procedure where a breach in the hygiene and sanitation affects public health that ultimately leads to billions worth product recalls in the industry.
1.3.7.1 Scalding
Scalding is the process in which broiler carcass is immersed at 59–64 °C for 30–75 s (hard scald) or 51–54 °C for 90–120 s (soft scald) to loosen up the skin for facilitating further picking (FSIS 2015). This is the first step in poultry processing where the carcasses are immersed in water, and there is a high possibility of cross contamination with pathogens, including Salmonella (Carrasco et al. 2012; Russell 2008). A study conducted by Nde et al. (2007) revealed that Salmonella survived the scalding process, and the same isolates were identified before and after defeathering and from the rubber fingers of the defeathering equipment. Salmonella can be attached to the skin during the scalding process, evades the action of common antimicrobial agents, and acts as a source of infection in the subsequent stages of processing (Kim et al. 1996; Lillard 1990; Nchez et al. 2002; Yang et al. 2001). Also, a higher concentration of antimicrobial agent is necessary to kill Salmonella if it is attached to the skin surfaces (Yang et al. 2001).
1.3.7.2 Defeathering
Defeathering is another step in poultry processing where the possibility of cross contamination is high if contaminated water is used along with improper disinfection of rubber picking fingers (Nde et al. 2007). Salmonella that are attached to the skin on a carcass cross-contaminates other carcasses. A high prevalence of 47% and 63% Salmonella before and after defeathering, respectively, was noticed in this study (Nde et al. 2007). Other studies also reported significantly high Salmonella-positive carcasses after defeathering (71%) compared to that of pre-defeathering (21%) in the conventional defeathering method (Clouser et al. 1995a, b). Rubber fingers/picking fingers can cause peristaltic movements which also lead to the expulsion of feces (Berrang et al. 2001). Since the picking fingers are not changed between the carcasses, there is a high likelihood of carcass cross contamination (Nde et al. 2007). Therefore, sanitation using appropriate disinfectants is recommended during the defeathering process.
1.3.7.3 Evisceration
Evisceration is a critical step in poultry processing where an effective application of antimicrobial agents is recommended to prevent contamination of carcasses with intestinal contents. A faulty evisceration can lead to contamination of carcasses with fecal material and intestinal contents. Therefore, proper feed withdrawal before slaughtering, antimicrobial rinses such as chlorine, proper maintenance of evisceration machinery, and removal of ceca and crop without tear are recommended (FSIS 2015).
1.3.7.4 Chilling
Poultry carcasses are immersed in cold water during the chilling process to reduce the carcass temperature to 40 °F (4.4 °C) or below within 4–8 h of slaughtering to prevent the growth of pathogenic bacteria (FSIS 2014). The carcasses leaving the chillers often carry Salmonella (Lillard 1990; Nchez et al. 2002). Under natural conditions, the carcasses exiting the chillers contains 1–30 CFU Salmonella per carcass (Waldroup 1996). The possibility of cross contamination is high in chillers compared to other steps in processing. Lillard (1990) showed 37% incidence of Salmonella on carcasses exiting the chillers, whereas in all other stages of processing the incidence was 10–20%.
In addition, Lillard (1990) reported that immersion chilling has washing effects and reduces the aerobic Enterobacteriaceae members. However, the incidence of Salmonella on post-chill carcasses was high indicating cross contamination of carcasses in chilling tanks and converting Salmonella-negative carcasses to positive. The same study observed a 15% and 28% increase in the incidence of Salmonella on post-chill carcasses compared to the pre-chill carcasses. The chilling process alone had no effect on reducing the pathogen numbers (Yang et al. 2001). The chilling process and associated water uptake also aid pathogen attachment on the skin since the process exposes deep channels and crevices on the skin (Kim et al. 1996). Along with these, aging of chilling water and increase in organic load in water reduce the efficacy of common antimicrobial agents, including chlorine and pose a significant threat for carcass contamination (Kim et al. 1996; Lillard 1990; Nagel et al. 2013; Nchez et al. 2002; Yang et al. 2001).
Currently, different antimicrobial interventions including chlorine, organic acids, essential oils, sodium hypochlorite, acetic acid, trisodium phosphate, sodium metabisulfite, and per acetic acid are applied or studied to control/eliminate NTS in poultry processing (Bucher et al. 2012; Burt 2004; Milillo and Ricke 2010; Nagel et al. 2013; Nair et al. 2014, 2015; Tamblyn et al. 1997; Tamblyn and Conner 1997; Venkitanarayanan et al. 2013). Among the different antimicrobial agents, USDA-approved safe and suitable antimicrobial agents for the application of meat, poultry, and egg products are described in the FSIS Directive (FSIS 2017). Those interventions will be dealt in detail in the subsequent chapters.
1.4 Conclusions
Intensive production of poultry in a vertically integrated system and the high consumption rate and demand for poultry meat in the USA make poultry meat a potentially important vehicle for foodborne outbreaks. Live poultry and poultry meat are commonly encountered in human salmonellosis as epidemiological links between them are understood. Salmonella colonization in the gastrointestinal tract of poultry and the excretion of the pathogen through droppings result in environmental contamination and contamination of poultry carcasses during processing. In a vertically integrated production system, Salmonella that are potentially present in the breeder flocks can be found on poultry carcasses if the intervention strategies are not effective to control the pathogen during production and processing steps. The persistence of Salmonella is often worsened by horizontal transmission of the pathogen by different carriers in and out of the farm and processing facilities. Therefore, vector control programs, proper biosafety measures, accurate disinfection, and intervention strategies are necessary to control Salmonella in poultry production systems.
References
Andino, A., Pendleton, S., Zhang, N., Chen, W., Critzer, F., & Hanning, I. (2014). Survival of Salmonella enterica in poultry feed is strain dependent. Poultry Science, 93(2), 441–447. [cited 2017 Sep 5]. Retrieved from https://academic.oup.com/ps/article-lookup/doi/10.3382/ps.2013-03401
Angulo, F. J., & Swerdlow, D. L. (1999). Epidemiology of human Salmonella enterica serovar enteritidis infections in the United States. In A. M. Saeed, R. K. Gast, M. E. Potter, & P. G. Wall (Eds.), Salmonella enterica serovar enteritidis in humans and animals: Epidemiology, pathogenesis and control. Ames, IA: Iowa State University Press. [cited 2017 Sep 6]. Retrieved from https://www.cabdirect.org/cabdirect/abstract/19992205149
Antunes, P., Mourão, J., Campos, J., & Peixe, L. (2016). Salmonellosis: The role of poultry meat. Clinical Microbiology and Infection, 22, 110. [cited 2017 Sep 5]. Retrieved from http://ac.els-cdn.com/S1198743X15010307/1-s2.0-S1198743X15010307-main.pdf?_tid=09c36dc4-9262-11e7-8fd5-00000aab0f01&acdnat=1504633722_6265da3f1319622904b415c62d0ce5ba
Antunes, P., Réu, C., Sousa, J. C., Peixe, L., & Pestana, N. (2003). Incidence of Salmonella from poultry products and their susceptibility to antimicrobial agents. International Journal of Food Microbiology, 82(2), 97–103. [cited 2017 Sep 5]. Retrieved from http://linkinghub.elsevier.com/retrieve/pii/S0168160502002519
Atterbury, R. J., Van Bergen, M. A. P., Ortiz, F., Lovell, M. A., Harris, J. A., De Boer, A., et al. (2007). Bacteriophage therapy to reduce Salmonella colonization of broiler chickens. Applied and Environmental Microbiology, 73(14), 4543–4549. [cited 2017 Sep 6]. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/17526794
Bailey, J. S., Cox, N. A., & Berrang, M. E. (1994). Hatchery-acquired Salmonellae in broiler chicks. Poultry Science, 73(7), 1153–1157. [cited 2017 Sep 5]. Retrieved from https://academic.oup.com/ps/article-lookup/doi/10.3382/ps.0731153
Bailey, J. S., Cox, N. A., Craven, S. E., & Cosby, D. E. (2002). Serotype tracking of Salmonella through integrated broiler chicken operations. Journal of Food Protection, 65(5), 742–745. [cited 2017 Sep 5]. Retrieved from http://www.jfoodprotection.org/doi/pdf/10.4315/0362-028X-65.5.742
Barrow, P. A., & Freitas Neto, O. C. (2011). Pullorum disease and fowl typhoid—New thoughts on old diseases: A review. Avian Pathology, 40(1), 1–13. [cited 2017 Sep 5]. Retrieved from http://www.tandfonline.com/doi/abs/10.1080/03079457.2010.542575
Baskerville, A., Humphrey, T. J., Fitzgeorge, R. B., Cook, R. W., Chart, H., Rowe, B., et al. (1992). Airborne infection of laying hens with Salmonella enteritidis phage type 4. The Veterinary Record, 130(18), 395–398. [cited 2017 Sep 5]. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1609467
Bean, N. H., & Griffin, P. M. (1990). Foodborne disease outbreaks in the United States, 1973-1987: Pathogens, vehicles, and trends. Journal of Food Protection, 53(9), 804–817. [cited 2017 Sep 5]. Retrieved from http://jfoodprotection.org/doi/pdf/10.4315/0362-028X-53.9.804
Berrang, M. E., Bailey, J. S., Altekruse, S. F., Shaw, W. K., Patel, B. L., Meinersmann, R. J., et al. (2009). Prevalence, serotype, and antimicrobial resistance of Salmonella on broiler carcasses postpick and postchill in 20 U.S. processing plants. Journal of Food Protection, 72(8), 1610–1615. [cited 2017 Sep 5]. Retrieved from http://www.jfoodprotection.org/doi/pdf/10.4315/0362-028X-72.8.1610
Berrang, M. E., Buhr, R. J., Cason, J. A., & Dickens, J. A. (2001). Broiler carcass contamination with Campylobacter from feces during defeathering. Journal of Food Protection, 64(12), 2063–2066. [cited 2017 Sep 5]. Retrieved from http://www.jfoodprotection.org/doi/pdf/10.4315/0362-028X-64.12.2063
Bohaychuk, V. M., Gensler, G. E., King, R. K., Manninen, K. I., Sorensen, O., Wu, J. T., et al. (2006). Occurrence of pathogens in raw and ready-to-eat meat and poultry products collected from the retail marketplace in Edmonton, Alberta, Canada. Journal of Food Protection, 69(9), 2176–2182. [cited 2017 Sep 5]. Retrieved from http://jfoodprotection.org/doi/pdf/10.4315/0362-028X-69.9.2176
Bucher, O., Farrar, A. M., Totton, S. C., Wilkins, W., Waddell, L. A., Wilhelm, B. J., et al. (2012). A systematic review-meta-analysis of chilling interventions and a meta-regression of various processing interventions for Salmonella contamination of chicken. Preventive Veterinary Medicine, 103(1), 1–15.
Bullis, K. L. (1977). The history of avian medicine in the U.S. II. Pullorum disease and fowl typhoid. Avian Diseases, 21(3), 422–429. [cited 2017 Sep 5]. Retrieved from http://www.jstor.org/stable/1589326
Burt, S. (2004). Essential oils: Their antibacterial properties and potential applications in foods—A review. International Journal of Food Microbiology, 94(3), 223–253.
Bushnell, L. D., & Payne, L. F. (1932). Dissemination of pullorum disease in the incubator. In B. J. C. Te Hennepe (Ed.), International review of poultry science. Fifth World’s Poultry Congress and Exhibition, Rome (pp. 46–48). Holland, The Netherlands: Rotterdam. Retrieved from https://naldc.nal.usda.gov/naldc/download.xhtml?id=CAT40000897&content=PDF
Callaway, T. R., Edrington, T. S., Anderson, R. C., Harvey, R. B., Genovese, K. J., Kennedy, C. N., et al. (2008). Probiotics, prebiotics and competitive exclusion for prophylaxis against bacterial disease. Animal Health Research Reviews, 9(2), 217–225. [cited 2017 Sep 6]. Retrieved from https://www.cambridge.org/core/services/aop-cambridge-core/content/view/69E86594270E6E333D68F6DF88D9CF9D/S1466252308001540a.pdf/probiotics_prebiotics_and_competitive_exclusion_for_prophylaxis_against_bacterial_disease.pdf
Carrasco, E., Morales-Rueda, A., & García-Gimeno, R. M. (2012). Cross-contamination and recontamination by Salmonella in foods: A review. Food Research International, 45(2), 545–556. [cited 2017 Mar 11]. Retrieved from http://linkinghub.elsevier.com/retrieve/pii/S0963996911006260
Cason, J. A., Cox, N. A., & Bailey, J. S. (1994). Transmission of Salmonella Typhimurium during hatching of broiler chicks. Avian Diseases, 38(3), 583. [cited 2017 Sep 5]. Retrieved from http://www.jstor.org/stable/1592082?origin=crossref
CDC. (2000). Surveillance for foodborne disease outbreaks—United States, 1993-1997. [cited 2017 Sep 5]. Retrieved from https://www.cdc.gov/MMWr/preview/mmwrhtml/ss4901a1.htm
CDC. (2013). Surveillance for foodborne disease outbreaks—United States, 1998–2008. [cited 2017 Jan 15]. Retrieved from https://www.cdc.gov/mmwr/preview/mmwrhtml/ss6202a1.htm
CDC. (2016). Multi-state outbreak of Salmonella Oranienburg infections linked to shell eggs. [cited 2017 Sep 17]. Retrieved from https://www.cdc.gov/salmonella/oranienburg-10-16/
Chalker, R. B., & Blaser, M. J. (1988). A review of human salmonellosis: Magnitude of Salmonella infection in the United States. Reviews of Infectious Diseases, 10(1), 111–124. [cited 2017 Sep 5]. Retrieved from http://www.jstor.org/stable/4454281
Chen, H.-M., Wang, Y., Su, L.-H., & Chiu, C.-H. (2013). Nontyphoid Salmonella infection: Microbiology, clinical features, and antimicrobial therapy. Pediatrics and Neonatology, 54(3), 147–152. [cited 2017 Sep 5]. Retrieved from http://linkinghub.elsevier.com/retrieve/pii/S1875957213000119
Chittick, P., Sulka, A., Tauxe, R. V., & Fry, A. M. (2006). A summary of national reports of foodborne outbreaks of Salmonella Heidelberg infections in the United States: Clues for disease prevention. Journal of Food Protection, 69(5), 1150–1153. [cited 2017 Sep 5]. Retrieved from http://jfoodprotection.org/doi/pdf/10.4315/0362-028X-69.5.1150
Clavijo, R. I., Loui, C., Andersen, G. L., Riley, L. W., & Lu, S. (2006). Identification of genes associated with survival of Salmonella enterica serovar Enteritidis in chicken egg albumen. Applied and Environmental Microbiology, 72(2), 1055–1064. [cited 2017 Sep 5]. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16461649
Clouser, C. S., Doores, S., Mast, M. G., & Knabel, S. J. (1995a). The role of defeathering in the contamination of turkey skin by Salmonella species and Listeria monocytogenes. Poultry Science, 74(4), 723–731. [cited 2017 Sep 6]. Retrieved from https://watermark.silverchair.com/api/watermark?token=AQECAHi208BE49Ooan9kkhW_Ercy7Dm3ZL_9Cf3qfKAc485ysgAAAgEwggH9BgkqhkiG9w0BBwagggHuMIIB6gIBADCCAeMGCSqGSIb3DQEHATAeBglghkgBZQMEAS4wEQQMPP8pXrAo5qEytwnOAgEQgIIBtAfzUrNnJTQuxEYb7yOd2_jVLuZDk2rL1gqjt3HJEPxJ0
Clouser, C. S., Knabel, S. J., Mast, M. G., & Doores, S. (1995b). Effect of type of defeathering system on Salmonella cross-contamination during commercial processing. Poultry Science, 74(4), 732–741. [cited 2017 Sep 6]. Retrieved from https://academic.oup.com/ps/article-lookup/doi/10.3382/ps.0740732
Cooper, G. L., Venables, L. M., & Lever, M. S. (1996). Airborne challenge of chickens vaccinated orally with the genetically-defined Salmonella enteritidis aroA strain CVL30. The Veterinary Record, 139(18), 447–448. [cited 2017 Sep 5]. Retrieved from http://veterinaryrecord.bmj.com/cgi/doi/10.1136/vr.139.18.447
Cox, N. A., Berrang, M. E., & Cason, J. A. (2000). Salmonella penetration of egg shells and proliferation in broiler hatching eggs—A review. Poultry Science, 79(11), 1571–1574. [cited 2017 Sep 5]. Retrieved from https://academic.oup.com/ps/article-lookup/doi/10.1093/ps/79.11.1571
Crespo, R., Jeffrey, J. S., Chin, R. P., Sentíes-Cué, G., & Shivaprasad, H. L. (2004). Phenotypic and genotypic characterization of Salmonella arizonae from an integrated turkey operation. Avian Diseases, 48(2), 344–350. [cited 2017 Sep 5]. Retrieved from http://www.bioone.org/doi/abs/10.1637/7116
Cui, S., Ge, B., Zheng, J., & Meng, J. (2005). Prevalence and antimicrobial resistance of Campylobacter spp. and Salmonella serovars in organic chickens from Maryland retail stores. Applied and Environmental Microbiology, 71(7), 4108–4111. [cited 2017 Sep 5]. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16000828
Currie, A., MaCdougall, L., Aramini, J., Gaulin, C., Ahmed, R., & Issacs, S. (2005). Frozen chicken nuggets and strips and eggs are leading risk factors for Salmonella Heidelberg infections in Canada. Epidemiology and Infection, 133(5), 809. [cited 2017 Sep 5]. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/16181499
Davies, R. H., & Wray, C. (1996). Persistence of Salmonella enteritidis in poultry units and poultry food. British Poultry Science, 37(3), 589–596. [cited 2017 Sep 5]. Retrieved from http://www.tandfonline.com/doi/abs/10.1080/00071669608417889
De Reu, K., Grijspeerdt, K., Messens, W., Heyndrickx, M., Uyttendaele, M., Debevere, J., et al. (2006). Eggshell factors influencing eggshell penetration and whole egg contamination by different bacteria, including Salmonella Enteritidis. International Journal of Food Microbiology, 112(3), 253–260. [cited 2017 Sep 6]. Retrieved from http://linkinghub.elsevier.com/retrieve/pii/S0168160506002303
Desmidt, M., Ducatelle, R., & Haesebrouck, F. (1997). Pathogenesis of Salmonella Enteritidis phage type four after experimental infection of young chickens. Veterinary Microbiology, 56((1-2)), 99–109. [cited 2017 Sep 5]. Retrieved from http://linkinghub.elsevier.com/retrieve/pii/S0378113596013508
Dhanoa, A., & Fatt, Q. K. (2009). Non-typhoidal Salmonella bacteraemia: Epidemiology, clinical characteristics and its’ association with severe immunosuppression. Annals of Clinical Microbiology and Antimicrobials, 8(1), 15. [cited 2017 Sep 5]. Retrieved from https://ann-clinmicrob.biomedcentral.com/track/pdf/10.1186/1476-0711-8-15?site=ann-clinmicrob.biomedcentral.com
Domınguez, C., Gómez, I., & Zumalacárregui, J. (2002). Prevalence of Salmonella and Campylobacter in retail chicken meat in Spain. International Journal of Food Microbiology, 72(1-2), 165–168. [cited 2017 Sep 6]. Retrieved from http://linkinghub.elsevier.com/retrieve/pii/S0168160501006389
Donalson, L. M., Kim, W.-K., Chalova, V. I., Herrera, P., Woodward, C. L., McReynolds, J. L., et al. (2007). In vitro anaerobic incubation of Salmonella enterica serotype Typhimurium and laying hen cecal bacteria in poultry feed substrates and a fructooligosaccharide prebiotic. Anaerobe, 13(5-6), 208–214. [cited 2017 Sep 6]. Retrieved from http://linkinghub.elsevier.com/retrieve/pii/S1075996407000303
Donalson, L. M., McReynolds, J. L., Kim, W. K., Chalova, V. I., Woodward, C. L., Kubena, L. F., et al. (2008). The influence of a fructooligosaccharide prebiotic combined with alfalfa molt diets on the gastrointestinal tract fermentation, Salmonella Enteritidis infection, and intestinal shedding in laying hens. Poultry Science, 87(7), 1253–1262. [cited 2017 Sep 6]. Retrieved from https://academic.oup.com/ps/article-lookup/doi/10.3382/ps.2007-00166
Duchet-Suchaux, M., Léchopier, P., Marly, J., Bernardet, P., & Delaunay, R. (1995). Quantification of experimental Salmonella Enteritidis carrier state in B13 leghorn chicks. Avian Diseases, 39(4), 796–803. [cited 2017 Sep 5]. Retrieved from http://www.jstor.org/stable/1592416
Dutil, L., Irwin, R., Finley, R., Ng, L. K., Avery, B., Boerlin, P., et al. (2010). Ceftiofur resistance in Salmonella enterica serovar Heidelberg from chicken meat and humans, Canada. Emerging Infectious Diseases, 16(1), 48–54. [cited 2017 Sep 5]. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/20031042
Fanelli, M. J., Sadler, W. W., & Brownell, J. R. (1970). Preliminary studies on persistence of Salmonellae in poultry litter. Avian Diseases, 14(1), 131. [cited 2017 Sep 5]. Retrieved from http://www.jstor.org/stable/1588564?origin=crossref
Feasey, N. A., Dougan, G., Kingsley, R. A., Heyderman, R. S., & Gordon, M. A. (2012). Invasive non-typhoidal Salmonella disease: An emerging and neglected tropical disease in Africa. Lancet, 379, 2489–2499. [cited 2017 Sep 5]. Retrieved from http://ac.els-cdn.com/S0140673611617522/1-s2.0-S0140673611617522-main.pdf?_tid=45bdafda-9260-11e7-a723-00000aacb362&acdnat=1504632964_3f1afdbdc5cb208c61499dde7731a8b5
Foley, S. L., Lynne, A. M., & Nayak, R. (2008). Salmonella challenges: Prevalence in swine and poultry and potential pathogenicity of such isolates. Journal of Animal Science, 86(14 Suppl), E149–E162. [cited 2017 Sep 5]. Retrieved from http://www.animalsciencepublications.org/publications/jas/abstracts/86/14/14
Foley, S. L., Nayak, R., Hanning, I. B., Johnson, T. J., Han, J., & Ricke, S. C. (2011). Population dynamics of Salmonella enterica serotypes in commercial egg and poultry production. Applied and Environmental Microbiology, 77(13), 4273–4279.
FSIS. (2014). FSIS. Compliance guide: Modernization of poultry slaughter inspection: Amendments to chilling requirements. [cited 2017 Sep 6]. Retrieved from https://www.fsis.usda.gov/wps/wcm/connect/7a0a728e-3b29-49e9-9c1b-ec55f2f04887/Chilling-Requirements-1014.pdf?MOD=AJPERES
FSIS. (2015). FSIS - Compliance guideline for controlling Salmonella and Campylobacter in raw poultry. [cited 2017 Sep 6]. Retrieved from https://www.fsis.usda.gov/wps/wcm/connect/6732c082-af40-415e-9b57-90533ea4c252/Controlling-Salmonella-Campylobacter-Poultry-2015.pdf?MOD=AJPERES
FSIS. (2017). Table of safe and suitable ingredients: Antimicrobial update 5/25/2017. [cited 2017 Sep 6]. Retrieved from http://www.fsis.usda.gov/wps/wcm/connect/bab10e09-aefa-483b-8be8-809a1f051d4c/7120
Gantois, I., Ducatelle, R., Pasmans, F., Haesebrouck, F., & Van Immerseel, F. (2008). Salmonella enterica serovar Enteritidis genes induced during oviduct colonization and egg contamination in laying hens. Applied and Environmental Microbiology, 74(21), 6616–6622. [cited 2017 Sep 5]. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/18776023
Gast, R. K. (1994). Understanding Salmonella Enteritidis in laying chickens: The contributions of experimental infections. International Journal of Food Microbiology, 21(1-2), 107–116. [cited 2017 Sep 5]. Retrieved from http://linkinghub.elsevier.com/retrieve/pii/0168160594902046
Gast, R. K., & Benson, S. T. (1995). The comparative virulence for chicks of Salmonella Enteritidis phage type 4 isolates and isolates of phage types commonly found in poultry in the United States. Avian Diseases, 39(3), 567–574. [cited 2017 Sep 5]. Retrieved from http://www.jstor.org/stable/1591810
Gast, R. K., & Benson, S. T. (1996). Intestinal colonization and organ invasion in chicks experimentally infected with Salmonella Enteritidis phage type 4 and other phage types isolated from poultry in the intestinal colonization and organ invasion in chicks experimentally infected with Salmonella. Avian Diseases, 40(4), 853–857. [cited 2017 Sep 5]. Retrieved from http://www.jstor.org/stable/1592309
Gast, R. K., Guard-Bouldin, J., & Holt, P. S. (2004). Colonization of reproductive organs and internal contamination of eggs after experimental infection of laying hens with Salmonella Heidelberg and Salmonella Enteritidis. Avian Diseases, 48(4), 863–869. [cited 2017 Sep 5]. Retrieved from https://naldc.nal.usda.gov/download/36855/PDF
Gast, R. K., Guraya, R., Guard-Bouldin, J., Holt, P. S., & Moore, R. W. (2007). Colonization of specific regions of the reproductive tract and deposition at different locations inside eggs laid by hens infected with Salmonella Enteritidis or Salmonella Heidelberg. Avian Diseases, 51(1), 40–44. [cited 2017 Sep 5]. Retrieved from https://pubag.nal.usda.gov/pubag/downloadPDF.xhtml?id=20217&content=PDF
Gast, R. K., Mitchell, B. W., & Holt, P. S. (1998). Airborne transmission of Salmonella Enteritidis infection between groups of chicks in controlled-environment isolation cabinets. Avian Diseases, 42(2), 315. [cited 2017 Sep 5]. Retrieved from http://www.jstor.org/stable/1592482?origin=crossref
Golden, N. J., Marks, H. H., Coleman, M. E., Schroeder, C. M., Bauer, N. E., & Schlosser, W. D. (2008). Review of induced molting by feed removal and contamination of eggs with Salmonella enterica serovar Enteritidis. Veterinary Microbiology, 131(3-4), 215–228. [cited 2017 Sep 5]. Retrieved from http://linkinghub.elsevier.com/retrieve/pii/S0378113508001053
Gregory, N. G. (2005). Recent concerns about stunning and slaughter. Meat Science, 70(3), 481–491. [cited 2017 Sep 5]. Retrieved from http://linkinghub.elsevier.com/retrieve/pii/S0309174005000458
Han, J., Lynne, A. M., David, D. E., Tang, H., Xu, J., Nayak, R., et al. (2012). DNA sequence analysis of plasmids from multidrug resistant Salmonella enterica serotype Heidelberg isolates. PLoS One, 7(12), e51160. [cited 2017 Sep 5]. Retrieved from http://dx.plos.org/10.1371/journal.pone.0051160
Heyndrickx, M., Vandekerchove, D., Herman, L., Rollier, I., Grijspeerdt, K., & De Zutter, L. (2002). Routes for Salmonella contamination of poultry meat: Epidemiological study from hatchery to slaughterhouse. Epidemiology and Infection, 129, 253–265. [cited 2017 Sep 5]. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2869884/pdf/12403101.pdf
Higgins, S. E., Higgins, J. P., Wolfenden, A. D., Henderson, S. N., Torres-Rodriguez, A., Tellez, G., et al. (2008). Evaluation of a Lactobacillus-based probiotic culture for the reduction of Salmonella Enteritidis in neonatal broiler chicks. Poultry Science, 87(1), 27–31. [cited 2017 Sep 6]. Retrieved from https://academic.oup.com/ps/article-lookup/doi/10.3382/ps.2007-00210
Higgins, R., Malo, R., Rene-Roberge, E., & Gauthier, R. (1982). Studies on the dissemination of Salmonella in nine broiler-chicken flocks. Avian Diseases, 26(1), 26. [cited 2017 Sep 5]. Retrieved from http://www.jstor.org/stable/1590021?origin=crossref
Hindermann, D., Gopinath, G., Chase, H., Negrete, F., Althaus, D., Zurfluh, K., et al. (2017). Salmonella enterica serovar Infantis from food and human infections, Switzerland, 2010–2015: Poultry-related multi-drug resistant clones and an emerging ESBL producing clonal lineage. Frontiers in Microbiology, 8, 1322. [cited 2017 Sep 17]. Retrieved from http://journal.frontiersin.org/article/10.3389/fmicb.2017.01322/full
Hoelzer, K., Moreno Switt, A., Wiedmann, M., Werker, D., Signs, K., Davis, C., et al. (2011). Animal contact as a source of human non-typhoidal salmonellosis. Veterinary Research, 42(1), 34. [cited 2017 Sep 5]. Retrieved from http://www.veterinaryresearch.org/content/42/1/34
Hoffmann, M., Zhao, S., Pettengill, J., Luo, Y., Monday, S. R., Abbott, J., et al. (2014). Comparative genomic analysis and virulence differences in closely related Salmonella enterica serotype Heidelberg isolates from humans, retail meats, and animals. Genome Biology and Evolution, 6(5), 1046–1068.
Humphrey, T. J., Baskerville, A., Chart, H., Rowe, B., & Whitehead, A. (1992). Infection of laying hens with Salmonella Enteritidis PT4 by conjunctival challenge. The Veterinary Record, 131(17), 386–388. [cited 2017 Sep 5]. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1455584
Hutt, F. B. (1938). The geneticist’s objectives in poultry improvement. The American Naturalist, 72(740), 268–284. [cited 2017 Sep 5]. Retrieved from http://www.jstor.org/stable/2457554
Jackson, B. R., Griffin, P. M., Cole, D., Walsh, K. A., & Chai, S. J. (2013). Outbreak-associated Salmonella enterica serotypes and food commodities, United States, 1998-2008. Emerging Infectious Diseases, 19(8), 1239–1244. [cited 2017 Sep 6]. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/23876503
Jain, S., & Chen, J. (2007). Attachment and biofilm formation by various serotypes of Salmonella as influenced by cellulose production and thin aggregative fimbriae biosynthesis. Journal of Food Protection, 70(11), 2473–2479. [cited 2017 Sep 5]. Retrieved from http://www.jfoodprotection.org/doi/pdf/10.4315/0362-028X-70.11.2473
Jørgensen, F., Bailey, R., Williams, S., Henderson, P., Wareing, D. R. A., Bolton, F. J., et al. (2002). Prevalence and numbers of Salmonella and Campylobacter spp. on raw, whole chickens in relation to sampling methods. International Journal of Food Microbiology, 76(1-2), 151–164. [cited 2017 Sep 6]. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12038572
Kim, K. Y., Frank, J. F., & Craven, S. E. (1996). Three-dimensional visualization of Salmonella attachment to poultry skin using confocal scanning laser microscopy. Letters in Applied Microbiology, 22(4), 280–282. [cited 2016 Nov 18]. Retrieved from http://doi.wiley.com/10.1111/j.1472-765X.1996.tb01161.x
Kollanoor Johny, A., Baskaran, S. A., Charles, A. S., Amalaradjou, M. A., Darre, M. J., Khan, M. I., et al. (2009). Prophylactic supplementation of caprylic acid in feed reduces Salmonella Enteritidis colonization in commercial broiler chicks. Journal of Food Protection, 72(4), 722–727. [cited 2017 Mar 26]. Retrieved from http://jfoodprotection.org/doi/pdf/10.4315/0362-028X-72.4.722
Kollanoor Johny, A., Mattson, T., Baskaran, S. A., Amalaradjou, M. A., Babapoor, S., March, B., et al. (2012). Reduction of Salmonella enterica serovar Enteritidis colonization in 20-day-old broiler chickens by the plant-derived compounds trans-cinnamaldehyde and eugenol. Applied and Environmental Microbiology, 78(8), 2981–2987. [cited 2017 Mar 11]. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/22327574
Lammerding, A. M., Garcia, M. M., Mann, E. D., Robinson, Y., Ward, W. J. D., Truscott, R. B., et al. (1988). Prevalence of Salmonella and thermophilic Campylobacter in fresh pork, beef, veal and poultry in Canada. Journal of Food Protection, 51(1), 47–52. [cited 2017 Sep 5]. Retrieved from http://jfoodprotection.org/doi/pdf/10.4315/0362-028X-51.1.47?code=fopr-site
Lee, L. A., Puhr, N. D., Maloney, E. K., Bean, N. H., & Tauxe, R. V. (1994). Increase in antimicrobial-resistant Salmonella infections in the United States, 1989-1990. The Journal of Infectious Diseases, 170(1), 128–134. [cited 2017 Sep 6]. Retrieved from https://academic.oup.com/jid/article-lookup/doi/10.1093/infdis/170.1.128
Lestari, S. I., Han, F., Wang, F., & Ge, B. (2009). Prevalence and antimicrobial resistance of Salmonella serovars in conventional and organic chickens from Louisiana retail stores. Journal of Food Protection, 72(6), 1165–1172. [cited 2017 Sep 6]. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/19610326
Liebana, E., Garcia-Migura, L., Clouting, C., Clifton-Hadley, F. A., Breslin, M., & Davies, R. H. (2003). Molecular fingerprinting evidence of the contribution of wildlife vectors in the maintenance of Salmonella Enteritidis infection in layer farms. Journal of Applied Microbiology, 94(6), 1024–1029. [cited 2017 Sep 5]. Retrieved from http://doi.wiley.com/10.1046/j.1365-2672.2003.01924.x
Liljebjelke, K. A., Hofacre, C. L., Liu, T., White, D. G., Ayers, S., Young, S., et al. (2005). Vertical and horizontal transmission of Salmonella within integrated broiler production system. Foodborne Pathogens and Disease, 2(1), 90–102. [cited 2017 Sep 5]. Retrieved from http://www.liebertonline.com/doi/abs/10.1089/fpd.2005.2.90
Lillard, H. S. (1990). The impact of commercial processing procedures on the bacterial contamination and cross-contamination of broiler carcasses. Journal of Food Protection, 53(3), 202–207.
Logue, C. M., Sherwood, J. S., Olah, P. A., Elijah, L. M., & Dockter, M. R. (2003). The incidence of antimicrobial-resistant Salmonella spp. on freshly processed poultry from US Midwestern processing plants. Journal of Applied Microbiology, 94(1), 16–24. [cited 2017 Sep 5]. Retrieved from http://doi.wiley.com/10.1046/j.1365-2672.2003.01815.x
Maciorowski, K. G., Herrera, P., Kundinger, M. M., & Ricke, S. C. (2006). Animal feed production and contamination by foodborne Salmonella. Journal für Verbraucherschutz und Lebensmittelsicherheit, 1(3), 197–209. [cited 2017 Sep 5]. Retrieved from http://springerlink.bibliotecabuap.elogim.com/10.1007/s00003-006-0036-z
Maciorowski, K. G., Jones, F. T., Pillai, S. D., & Ricke, S. C. (2004). Incidence, sources, and control of food-borne Salmonella spp. in poultry feeds. World’s Poultry Science Journal, 60(4), 446–457. [cited 2017 Sep 5]. Retrieved from https://www.cambridge.org/core/journals/world-s-poultry-science-journal/article/incidence-sources-and-control-of-food-borne-salmonella-spp-in-poultry-feeds/606CF89458709DD95ACBC1E48ECE67E0
Marder, E. P., Cieslak, P. R., Cronquist, A. B., Dunn, J., Lathrop, S., Rabatsky-Ehr, T., et al. (2017). Incidence and trends of infections with pathogens transmitted commonly through food and the effect of increasing use of culture-independent diagnostic tests on surveillance—Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2013–2016. MMWR. Morbidity and Mortality Weekly Report, 66(15), 397–403. [cited 2017 Sep 6]. Retrieved from http://www.cdc.gov/mmwr/volumes/66/wr/mm6615a1.htm
McIlroy, S. G., McCracken, R. M., Neill, S. D., & O’Brien, J. J. (1989). Control, prevention and eradication of Salmonella Enteritidis infection in broiler and broiler breeder flocks. The Veterinary Record, 125(22), 545–548. [cited 2017 Sep 6]. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2690452
Medeiros, M. A. N., Oliveira, D. C. N. D., Rodrigues, D. D. P., & Freitas, D. R. C. D. (2011). Prevalence and antimicrobial resistance of Salmonella in chicken carcasses at retail in 15 Brazilian cities. Revista Panamericana de Salud Pública, 30(6), 555. [cited 2017 Sep 5]. Retrieved from http://www.scielosp.org/pdf/rpsp/v30n6/a10v30n6.pdf
Meerburg, B. G., & Kijlstra, A. (2007). Role of rodents in transmission of Salmonella and Campylobacter. Journal of the Science of Food and Agriculture, 87(15), 2774–2781. [cited 2017 Sep 5]. Retrieved from http://doi.wiley.com/10.1002/jsfa.3004
Milillo, S. R., & Ricke, S. C. (2010). Synergistic reduction of Salmonella in a model raw chicken media using a combined thermal and acidified organic acid salt intervention treatment. Journal of Food Science, 75(2), M121–M125. [cited 2017 Sep 6].. https://doi.org/10.1111/j.1750-3841.2009.01510.x
Nagel, G. M., Bauermeister, L. J., Bratcher, C. L., Singh, M., & McKee, S. R. (2013). Salmonella and Campylobacter reduction and quality characteristics of poultry carcasses treated with various antimicrobials in a post-chill immersion tank. International Journal of Food Microbiology, 165(3), 281–286. https://doi.org/10.1016/j.ijfoodmicro.2013.05.016
Nair, D. V. T., Kiess, A., Nannapaneni, R., Schilling, W., & Sharma, C. S. (2015). The combined efficacy of carvacrol and modified atmosphere packaging on the survival of Salmonella, Campylobacter jejuni and lactic acid bacteria on Turkey breast cutlets. Food Microbiology, 49, 134.
Nair, D. V. T., Nannapaneni, R., Kiess, A., Schilling, W., & Sharma, C. S. (2014). Reduction of Salmonella on Turkey breast cutlets by plant-derived compounds. Foodborne Pathogens and Disease, 11(12), 981–987. [cited 2017 Mar 11]. Retrieved from http://online.liebertpub.com/doi/abs/10.1089/fpd.2014.1803
Nair, D., Thomas, J. V., & Kollanoor-Johny, A. (2016). Propionibacterium freudenreichii reduces cecal colonization of multidrug-resistant Salmonella Heidelberg in turkey poults. In Poultry Science Association 105th Annual Meeting Abstracts (pp. 21–22). Retrieved from http://www.poultryscience.org/psa16/abstracts/2016-PSA-Abstracts.pdf
NCC. (2017). Per capita consumption of poultry and livestock, 1965 to estimated 2018, in pounds - The National Chicken Council. [cited 2017 Sep 6]. Retrieved from http://www.nationalchickencouncil.org/about-the-industry/statistics/per-capita-consumption-of-poultry-and-livestock-1965-to-estimated-2012-in-pounds/
Nchez, M. X. S., Fluckey, W. M., Brashears, M. M., & McKee, S. R. (2002). Antibiotic susceptibility of Campylobacter spp. and Salmonella spp. in broilers processed in air-chilled and immersion-chilled environments. Journal of Food Protection, 65(6), 948–956.
Nde, C. W., McEvoy, J. M., Sherwood, J. S., & Logue, C. M. (2007). Cross contamination of Turkey carcasses by Salmonella species during defeathering. Poultry Science, 86(1), 162–167. [cited 2017 Sep 5]. Retrieved from https://academic.oup.com/ps/article-lookup/doi/10.1093/ps/86.1.162
Newell, D. G., & Fearnley, C. (2003). Sources of Campylobacter colonization in broiler chickens. Applied and Environmental Microbiology, 69(8), 4343–4351. [cited 2017 Sep 5]. Retrieved from http://aem.asm.org/content/69/8/4343.short
Oakley, B. B., Lillehoj, H. S., Kogut, M. H., Kim, W. K., Maurer, J. J., Pedroso, A., et al. (2014). The chicken gastrointestinal microbiome. FEMS Microbiology Letters, 360(2), 100–112. [cited 2017 Sep 5]. Retrieved from https://academic.oup.com/femsle/article-lookup/doi/10.1111/1574-6968.12608
Park, S. H., Jarquin, R., Hanning, I., Almeida, G., & Ricke, S. C. (2011). Detection of Salmonella spp. survival and virulence in poultry feed by targeting the hilA gene. Journal of Applied Microbiology, 111(2), 426–432. [cited 2017 Sep 5]. https://doi.org/10.1111/j.1365-2672.2011.05054.x
Parveen, S., Taabodi, M., Schwarz, J. G., Oscar, T. P., Harter-Dennis, J., & White, D. G. (2007). Prevalence and antimicrobial resistance of Salmonella recovered from processed poultry. Journal of Food Protection, 70(11), 2466–2472. [cited 2017 Sep 6]. Retrieved from https://www.ars.usda.gov/ARSUserFiles/80720500/Poultry/33.pdf
Patterson, J., & Burkholder, K. (2003). Application of prebiotics and probiotics in poultry production. Poultry Science, 82(4), 627–631. [cited 2017 Sep 6]. Retrieved from https://academic.oup.com/ps/article-lookup/doi/10.1093/ps/82.4.627
Poppe, C. (2000). In C. Wray & A. Wray (Eds.), Salmonella in domestic fowl (p. 194). Wallingford, UK: CABI Publishing. [cited 2017 Sep 5]. Retrieved from http://dl.dairycattlecenter.com/book/SalmonellainDomesticAnimals.pdf#page=117
Poppe, C., Barnum, D. A., & Mitchell, W. R. (1986). Effect of chlorination of drinking water on experimental Salmonella infection in poultry. Avian Diseases, 30(2), 362. [cited 2017 Sep 5]. Retrieved from: http://www.jstor.org/stable/1590543?origin=crossref
Porwollik, S., Santiviago, C. A., Cheng, P., Florea, L., Jackson, S., & McClelland, M. (2005). Differences in gene content between Salmonella enterica serovar Enteritidis isolates and comparison to closely related serovars Gallinarum and Dublin. Journal of Bacteriology, 187(18), 6545–6555. [cited 2017 Sep 5]. Retrieved from: http://www.ncbi.nlm.nih.gov/pubmed/16159788
Rabsch, W., Tschäpe, H., & Bäumler, A. J. (2001). Non-typhoidal salmonellosis: Emerging problems. Microbes and Infection, 3(3), 237–247. [cited 2017 Sep 5]. Retrieved from http://ac.els-cdn.com/S1286457901013752/1-s2.0-S1286457901013752-main.pdf?_tid=713b4854-9272-11e7-86c1-00000aacb35e&acdnat=1504640768_4c270667355101c02cd4681f69c5da33
Renwick, S. A., Irwin, R. J., Clarke, R. C., McNab, W. B., Poppe, C., & McEwen, S. A. (1992). Epidemiological associations between characteristics of registered broiler chicken flocks in Canada and the Salmonella culture status of floor litter and drinking water. The Canadian Veterinary Journal, 33(7), 449–458. [cited 2017 Sep 5]. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/17424037
Rodrigue, D. C., Tauxe, R. V., & Rowe, A. B. (1990). International increase in Salmonella Enteritidis: A new pandemic? Epidemiology and Infection, 105, 21–27. [cited 2017 Sep 5]. Retrieved from https://www.cambridge.org/core/services/aop-cambridge-core/content/view/A41CC08D3D6213170721E3C22F128140/S0950268800047609a.pdf/international_increase_in_salmonella_enteritidis_a_new_pandemic.pdf
Roy, P., Dhillon, A. S., Lauerman, L. H., Schaberg, D. M., Bandli, D., & Johnson, S. (2002). Results of Salmonella isolation from poultry products, poultry, poultry environment, and other characteristics. Avian Diseases, 46(1), 17–24. [cited 2017 Sep 5]. Retrieved from http://www.bioone.org/doi/abs/10.1637/0005-2086%282002%29046%5B0017%3AROSIFP%5D2.0.CO%3B2
Russell, S. M. (2008). Chemical residuals in the environment and on chicken carcasses associated with scalding chickens in an acidic, copper sulfate-based commercial sanitizer during poultry processing. Journal of Food Protection, 71(1), 226–230.
Scharff, R. L. (2012). Economic burden from health losses due to foodborne illness in the United States. ProQuest. [cited 2017 Sep 5]. Retrieved from https://search.proquest.com/docview/913142449?pq-origsite=gscholar
Schleifer, J. H., Juven, B. J., Beard, C. W., & Cox, N. A. (1984). The susceptibility of chicks to Salmonella Montevideo in artificially contaminated poultry feed. Avian Diseases, 28(2), 497. [cited 2017 Sep 5]. Retrieved from http://www.jstor.org/stable/1590357?origin=crossref
Shah, D. H., Zhou, X., Addwebi, T., Davis, M. A., Orfe, L., Call, D. R., et al. (2011). Cell invasion of poultry-associated Salmonella enterica serovar Enteritidis isolates is associated with pathogenicity, motility and proteins secreted by the type III secretion system. Microbiology, 157(5), 1428–1445. [cited 2017 Sep 5]. Retrieved from http://www.microbiologyresearch.org/docserver/fulltext/micro/157/5/1428_mic044461.pdf?expires=1504644873&id=id&accname=guest&checksum=4EA85DB3BE33EA6144E037CB5C48641C
Shivaprasad, H. L. (2000). Fowl typhoid and pullorum disease. Revue Scientifique et Technique, 19(2), 405–424. [cited 2017 Sep 5]. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10935271
Skov, M. N., Madsen, J. J., Rahbek, C., Lodal, J., Jespersen, J. B., Jørgensen, J. C., et al. (2008). Transmission of Salmonella between wildlife and meat-production animals in Denmark. Journal of Applied Microbiology, 105(5), 1558–1568. [cited 2017 Sep 5]. https://doi.org/10.1111/j.1365-2672.2008.03914.x
Slader, J., Domingue, G., Jørgensen, F., McAlpine, K., Owen, R. J., Bolton, F. J., et al. (2002). Impact of transport crate reuse and of catching and processing on Campylobacter and Salmonella contamination of broiler chickens. Applied and Environmental Microbiology, 68(2), 713–719. [cited 2017 Sep 5]. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11823211
Stafseth, H. J., & Mallmann, W. L. (1928). Progress report on poultry disease investigation at the Michigan experiment station. Poultry Science, 8(1), 19–22. [cited 2017 Sep 5]. Retrieved from https://academic.oup.com/ps/article-lookup/doi/10.3382/ps.0080019
Svobodová, I., Bořilová, G., Hulánková, R., & Steinhauserová, I. (2012). Microbiological quality of broiler carcasses during slaughter processing. Acta Veterinaria, 81(1), 37–42. [cited 2017 Sep 6]. Retrieved from http://actavet.vfu.cz/81/1/0037/
Tamblyn, K. C., & Conner, D. E. (1997). Bactericidal activity of organic acids against Salmonella Typhimurium attached to broiler chicken skin. Journal of Food Protection, 60(6), 629–633.
Tamblyn, K. C., Conner, D. E., & Bilgili, S. F. (1997). Utilization of the skin attachment model to determine the antibacterial efficacy of potential carcass treatments. Poultry Science, 76(9), 1318–1323. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9276898
Tauxe, R. V., Cavanagh, T. R., & Cohen, M. L. (1989). Interspecies gene transfer in vivo producing an outbreak of multiply resistant shigellosis. The Journal of Infectious Diseases, 160, 1067–1070. [cited 2017 Sep 5]. Retrieved from https://watermark.silverchair.com/api/watermark?token=AQECAHi208BE49Ooan9kkhW_Ercy7Dm3ZL_9Cf3qfKAc485ysgAAAg0wggIJBgkqhkiG9w0BBwagggH6MIIB9gIBADCCAe8GCSqGSIb3DQEHATAeBglghkgBZQMEAS4wEQQMJHjVYc9MYCgAb14_AgEQgIIBwNPvoDvithlNDqVUjz3greFphY1_8SV692TTzjCWIIYG
Tellez, G., Pixley, C., Wolfenden, R. E., Layton, S. L., & Hargis, B. M. (2012). Probiotics/direct fed microbials for Salmonella control in poultry. Food Research International, 45(2), 628–633. [cited 2017 Sep 6]. Retrieved from http://linkinghub.elsevier.com/retrieve/pii/S0963996911002067
Tirado, C., & Schmidt, K. (2001). WHO surveillance programme for control of foodborne infections and intoxications: Preliminary results and trends across Greater Europe. The Journal of Infection, 43, 80. [cited 2017 Sep 5]. Retrieved from http://ac.els-cdn.com/S0163445301908618/1-s2.0-S0163445301908618-main.pdf?_tid=047937c4-925b-11e7-8e39-00000aab0f27&acdnat=1504630707_e9e8acdcf8bd25a720ce46c4b2556730
Tittsler, R. P. (1930). Septicemic Salmonella Pullorum infection in adult fowls. Poultry Science, 10(1), 17–23. [cited 2017 Sep 5]. Retrieved from https://academic.oup.com/ps/article-lookup/doi/10.3382/ps.0100017
Tizard, I. (2004). Salmonellosis in wild birds. Seminars in Avian and Exotic Pet Medicine, 13(2), 50–66. [cited 2017 Sep 5]. Retrieved from http://linkinghub.elsevier.com/retrieve/pii/S1055937X04000039
Van Immerseel, F., De Buck, J., Boyen, F., Bohez, L., Pasmans, F., Volf, J., et al. (2004b). Medium-chain fatty acids decrease colonization and invasion through hilA suppression shortly after infection of chickens with Salmonella enterica serovar Enteritidis. Applied and Environmental Microbiology, 70(6), 3582–3587. [cited 2017 Sep 5]. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15184160
Van Immerseel, F., De Buck, J., Pasmans, F., Bohez, L., Boyen, F., Haesebrouck, F., et al. (2004a). Intermittent long-term shedding and induction of carrier birds after infection of chickens early posthatch with a low or high dose of Salmonella Enteritidis. Poultry Science, 83(11), 1911–1916. [cited 2017 Sep 5]. Retrieved from https://academic.oup.com/ps/article-lookup/doi/10.1093/ps/83.11.1911
Van Immerseel, F., Russell, J. B., Flythe, M. D., Gantois, I., Timbermont, L., Pasmans, F., et al. (2006). The use of organic acids to combat Salmonella in poultry: A mechanistic explanation of the efficacy. Avian Pathology, 35(3), 182–188. [cited 2016 Oct 15]. Retrieved from http://www.tandfonline.com/doi/full/10.1080/03079450600711045
Velge, P., Cloeckaert, A., & Barrow, P. (2005). Emergence of Salmonella epidemics: The problems related to Salmonella enterica serotype Enteritidis and multiple antibiotic resistance in other major serotypes. Veterinary Research, 36, 267–288. [cited 2017 Sep 5]. Retrieved from https://www.vetres.org/articles/vetres/pdf/2005/03/v4058.pdf
Venkitanarayanan, K., Kollanoor-Johny, A., Darre, M. J., Donoghue, A. M., & Donoghue, D. J. (2013). Use of plant-derived antimicrobials for improving the safety of poultry products. Poultry Science, 92(2), 493–501.
Waldroup, A. L. (1996). Contamination of raw poultry with pathogens. World's Poultry Science Journal, 52(1), 20–25. Retrieved from http://www.scopus.com/inward/record.url?eid=2-s2.0-22944471495&partnerID=40&md5=aac074e5fa41a0e011d6ae897f349d62
Wales, A., Breslin, M., Carter, B., Sayers, R., & Davies, R. (2007). A longitudinal study of environmental Salmonella contamination in caged and free-range layer flocks. Avian Pathology, 36(3), 187–197. [cited 2017 Sep 5]. Retrieved from http://www.tandfonline.com/doi/full/10.1080/03079450701338755
White, D. G., Zhao, S., Sudler, R., Ayers, S., Friedman, S., Chen, S., et al. (2001). The isolation of antibiotic-resistant Salmonella from retail ground meats. The New England Journal of Medicine, 345(16), 1147–1154. [cited 2017 Sep 5]. Retrieved from http://www.nejm.org/doi/abs/10.1056/NEJMoa010315
Williams, J. E., & Benson, S. T. (1978). Survival of Salmonella Typhimurium in poultry feed and litter at three temperatures. Avian Diseases, 22(4), 742. [cited 2017 Sep 5]. Retrieved from http://www.jstor.org/stable/1589652?origin=crossref
Winter, S. E., Thiennimitr, P., Winter, M. G., Butler, B. P., Huseby, D. L., Crawford, R. W., et al. (2010). Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature, 467(7314), 426–429. [cited 2017 Sep 5]. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/20864996
Wong, M. H. Y., Yan, M., Chan, E. W. C., Biao, K., & Chen, S. (2014). Emergence of clinical Salmonella enterica serovar Typhimurium isolates with concurrent resistance to ciprofloxacin, ceftriaxone, and azithromycin. Antimicrobial Agents and Chemotherapy, 58(7), 3752–3756. [cited 2017 Sep 17]. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/24752251
Yang, H., Li, Y. B., & Johnson, M. G. (2001). Survival and death of Salmonella Typhimurium and Campylobacter jejuni in processing water and on chicken skin during poultry scalding and chilling. Journal of Food Protection, 64(6), 770–776.
Yim, L., Betancor, L., Martinez, A., Giossa, G., Bryant, C., Maskell, D., et al. (2010). Differential phenotypic diversity among epidemic-spanning Salmonella enterica serovar Enteritidis isolates from humans or animals. Applied and Environmental Microbiology, 76(20), 6812–6820. [cited 2017 Sep 5]. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/20802078
Zhang-Barber, L., Turner, A. K., & Barrow, P. A. (1999). Vaccination for control of Salmonella in poultry. Vaccine, 17(20-21), 2538–2545. [cited 2017 Sep 6]. Retrieved from http://linkinghub.elsevier.com/retrieve/pii/S0264410X99000602
Zhao, S., White, D. G., Friedman, S. L., Glenn, A., Blickenstaff, K., Ayers, S. L., et al. (2008). Antimicrobial resistance in Salmonella enterica serovar Heidelberg isolates from retail meats, including poultry, from 2002 to 2006. Applied and Environmental Microbiology, 74(21), 6656–6662. [cited 2017 Sep 6]. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/18757574
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Nair, D.V.T., Kollanoor Johny, A. (2019). Salmonella in Poultry Meat Production. In: Venkitanarayanan, K., Thakur, S., Ricke, S. (eds) Food Safety in Poultry Meat Production. Food Microbiology and Food Safety(). Springer, Cham. https://doi.org/10.1007/978-3-030-05011-5_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-05011-5_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-05010-8
Online ISBN: 978-3-030-05011-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)