Abstract
A specifically designed reference electrode was developed for analyzing the electrochemical behaviors of alloy materials in supercritical water (SCW) environments and identifying the associated electrochemical parameters. The internal Zr/ZrO2 reference electrodes constructed for high-temperature conditions were manufactured and adopted to measure the electrochemical corrosion potential (ECP) of the sample in SCW environments. Before the electrochemical analysis, the oxidation behaviour of zirconium would be investigated in SCW environment. The mass gain of zirconium is assumed due to formation of ZrO2 and there was only 78% of the original thickness of zirconium existed after the 1300 h immersion test in SCW environments with 8.3 ppm dissolved oxygen. In deaerated SCW environments, the thickness of zirconium is about 88% of the original one. The outcome indicated that the laboratory-made Zr/ZrO2 reference electrode was able to continuously operate for several months and delivered consistent and steady ECP data of the sample in SCW environments.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
In spite of the nuclear accident in Japan in 2011, nuclear energy is still expected to play an essential role in meeting a safe, environmentally clean and affordable supply of energy for many countries, especially given the increasing energy demands worldwide. Nuclear power technology has evolved through three generations of system designs. Generation IV systems that are under development in conjunction with the Generation IV International Forum have shed light on the inherent safety and reliability of advanced nuclear technologies [1]. The technologies adopted in these systems are aimed at improving the performance of reactor operations and fuel cycles compared with the current nuclear systems. Several types of Generation IV nuclear reactors have been proposed, and the supercritical water reactor (SCWR) is one of them.
A SCWR adopts supercritical light water as the reactor coolant. While current in-service light water reactors (LWRs) have an average thermal efficiency of 33%, the thermal efficiency of an SCWR could be exceeding 44% [1, 2]. SCWRs are similar to LWRs in concept but operate at comparatively higher pressure and temperature over a supercritical point of 22.1 MPa and 374 °C. Above this supercritical point, the properties are very different from ambient liquid water. The density will become lower and there will be only one single phase. The reactor coolant does not boil but changes into the supercritical phase instead. Upon leaving the reactor core, the entire coolant has only one phase, and the reactor could therefore be spared from having a recirculation system, a pressurizer, a steam generator, a separator, and a steam dryer, as commonly seen in LWRs. Accordingly, the size of an SCWR is about one half of that of an LWR, the construction cost can thus be reduced by 30–40%. In boiling water reactors (BWRs), the reactor coolant is generally oxidizing due to the presence of hydrogen peroxide (H2O2) and oxygen (O2), which are generated by water radiolysis and the decomposition of H2O, respectively. The oxidizing coolant ultimately become one of the reasons lead to the degradation of structural materials, mainly stress corrosion cracking. Unlike the limited solubility in BWR coolants, oxygen is completely soluble in supercritical water . Furthermore, the degree of water radiolysis would become more intense, and higher concentrations of the oxidizing species can be expected, due to the relatively higher power density of an SCWR. Accordingly, the greater oxidizing power and a relatively high coolant temperature are likely to cause worse degradation phenomena in the structural and core components of an SCWR than in those of a BWR [3, 4].
Fundamental knowledge about various physicochemical and corrosion processes in an SCWR environment is essential but is insufficient in the literature. The potentiometric measurement is a powerful tool for exploring the electrochemical characteristics of alloys exposed to high temperature aqueous solutions. However, reliable measurements of important parameters, such as corrosion potential and pH values, are difficult to achieve in supercritical water environments, due to the lack of appropriate reference electrodes . The purpose of this study is to make sure a zirconium-zirconium dioxide (Zr/ZrO2) reference electrode is suitable for measuring the electrochemical corrosion potentials (ECPs) of alloy materials in supercritical water . The mass change and surface analyses were taken in order to understand the oxidation behavior of Zr at 400, 450, 500 °C and whether Zr is suitable to use as a reference electrode or not. After the experiment we found that Zr is stable at 400 °C in SCW environment, which is suitable to use as a reference electrode.
Experimental
Sample Preparation
Pure zirconium rods were used in this study. For the oxidation test, the Zr rods were fabricated into the sample with a length of 31 mm and a diameter of 2 mm. The samples were polished using abrasive paper to 50 grit. After polishing, each sample was cleaned with acetone (CH3COCH3), and then dried.
Test Facility
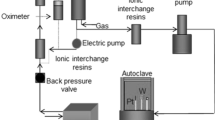
The oxidation tests were performed in a high-temperature (400, 450, 500 °C), different dissolved oxygen concentration and high-pressure (25 MPa) dynamic loop system that simulated the SCWR environment. The schematic representation of the test loop is shown in Fig. 1. The test facility consists of a primary loop for SCW environment simulation and a secondary loop for water chemistry monitoring. The high purity water is pressurized and heated up to the test condition before it flows into a 0.5–l autoclave (made from Alloy 625) through a high-pressure liquid chromatography (HPLC) pump. Then the coolant flows back from the autoclave and is cooled down to ambient temperature via a heat exchanger before it reaches the water reservoir. The pressure is reduced when coolant passes the back pressure regulator located ahead of the reservoir. The flow rate of the coolant in the loop system was controlled at approximately 5 mL/min. The dissolved oxygen concentration and coolant conductivity were monitored at ambient temperature and atmospheric pressure in the secondary loop. A low conductivity of 0.06 μS/cm of the coolant was maintained via a water purification system.
Mass Change and Surface Analyses of Zr Reference Electrode
The weight gain and oxidation layer analyses were taken to find the suitable temperature range for Zr reference electrode in SCW environment. First, the pure Zr rod is machined into a 3.1 cm long and 2 mm in diameter sample. Second, 6 samples were placed on a special design holder (made from Alloy 625) and put into autoclave each time. The temperature was controlled at 400–500 °C for 100–1300 h in 3600 psi deaerated and 8.3 ppm dissolved oxygen environment. Finally, the samples were taken out to do the surface analysis and weight measurement. In this experiment, the dissolved oxygen concentration was also changed and the immersion time was extended to 1300 h to ensure the duration of Zr in different oxygen content environments. For the surface analysis, the samples were polished using abrasive paper starting from 50 grit up to 4000 grit. After polishing, each sample was ultrasonically cleaned in acetone (CH3COCH3), dried, and then the SEM analysis was conducted.
Results and Discussion
Zr Mass Change Test Under SCW Environment
Figure 2 show the results at 500 °C for 8.3 ppm dissolved oxygen and deaerated environment. All the samples were disintegrated after 100-h immersion, whether the dissolved oxygen content was high or not. Table 1 show the data of the three tests. We could justify that dissolved oxygen concentration was not the reason to cause the samples to disintegrate. As a result, we adjusted the temperature to 475, 450 and 400 °C. Table 2 shows the results. From Table 2 and the SEM morphology in Fig. 6, we discovered that the oxide layer was more complete and the weights of the samples were increased significantly. That indicates that the temperature is the key factor to influence the oxidation layer thickness. Further, we consider the effects of dissolved oxygen concentration to oxide layers. In Table 3, we compared the difference between deaerated and 8.3 ppm dissolved oxygen at 450–400 °C. We discovered that values of oxide layer thickness and weight gain (%) were close for deaerated and 8.3 ppm dissolved oxygen environment. The values were even higher under deaerated environment at 450 °C. Figure 3 shows the weight gain % versus temperature under 450, 400 °C and deaerated, 8.3 ppm SCW environments for 100-h immersion time. It indicated that the temperature was the main factor to influence the oxide layer thickness and the oxygen concentration did not have a positive correlation with oxide layer thickness or weight gain % in the short immersion time.
Duration Test for Zr
Besides having stable oxide layer, the durability also plays a very important role for a good reference electrode . In order to make sure the Zr could be used for a long time, we extended the immersion time for the tests to 1300 h at 450 and 400 °C under deaerated environment. Table 4 and Fig. 4 show the results of the duration tests. The data indicated that the weight gain in % was 3.44% in the first 100 to 200 h and then decreased to −0.67% from 200 to 300 h; the oxidation layer of 300 h sample was 10μm less than the 200 h one at 450 °C. This phenomenon may come from the peeling off of the oxide layers. After 400 h immersion time, the oxide layer thickness was not growing significantly anymore. The oxide layer reaches a stable state for more than 400 h at 450 °C in a deaerated environment.
Consequently, we adjusted the temperature to 400 °C. Table 4 shows that the weight gain % is about 0.6–1.49% higher and the oxide layer thickness become 36–60 μm thicker every 100 h in deaerated environment. On the other hand, the weight gain % is about 1.1–1.85% higher and the oxide layer thickness was 31–85 μm thicker every 100 h in 8.3 ppm dissolved oxygen environment
Surprisingly, the dissolved oxygen became an important factor of the growth of the oxide layer for longer immersion time. These results were different from those in Section “Zr Mass Change Test Under SCW Environment.” It can be inferred that the effect of dissolved oxygen concentration was not obvious at short immersion times. For longer immersion times, the oxygen would have enough time to diffuse into the metal, and then show the effect of extended immersion time.
Figure 5 shows the immersion time versus inner diameter and oxide film thickness in two dissolved oxygen conditions. In deaerated SCW environments, the thickness of zirconium was about 88% of the original one. The inner diameters were in an acceptable range and became more stable for both deaerated and 8.3 ppm environments. On account of that, we could make sure that even for long time operation, there was still pure Zr metal remaining to supply the oxidation reaction.
SEM Images Analysis
The SEM analyse were conducted after each tests. Figure 6. shows all the images. Comparing Fig. 6a–j, they show that the oxide layers were denser in a deaerated environment. It can be inferred that the stacking velocity of oxygen was slower in low dissolved oxygen content conditions. Figure 6b–d show that the oxide layer thickness increases with the temperature for 100 h immersion time. On the other hand, Fig. 6d–j show the duration tests results in the deaerated environment. Comparing Fig. 6b and f to Fig. 6d and e, the images show that the oxide layers were inward growing with the time and the morphology didn’t change a lot. It indicated that temperature have greater impact on the oxide layer than immersion time.
Conclusions
-
1.
Pure Zr was oxidized to form the ZrO2 layer at 450 °C in SCW deaerated environment. It can be inferred that the Zr–H2O reaction start at lower temperature than it usually does in atmospheric pressure.
-
2.
The thickness of the oxide layer is related to temperature in short immersion time and is less relevant to the dissolved oxygen content.
-
3.
The density of the oxide layer is dominated by the dissolved oxygen content. If the dissolved oxygen content is higher, the oxide layer will be less dense.
-
4.
Under deaerated condition at 450 °C, when the immersion time reaches 400 h, the oxide layer isn’t growing and some of the oxide layer will peel off. It can be resulting from that the oxidation layer has reach the saturation point at that temperature.
-
5.
When the immersion time is extended to 1300 h at 400 °C, the oxidation layer outside the pure Zr is looser and with some longitudinal cracks.
In summary, the Zr/ZrO2 reference electrode has been continuously operating for several months and remains in a stable state in the supercritical water environments.
References
GEN IV International Forum, GIF 2007 Annual Report, pp. 7–11 (2007)
A Technology Roadmap for Generation IV Nuclear Energy Systems, Report No. GIF002-00, 1 Dec 2002
G.S. Was, S. Teysseyre, Z. Jiao, Corrosion of austenitic alloys in supercritical water. Corrosion 62(11), 989–1005 (2006)
P. Kritzer, Corrosion in High-Temperature and Supercritical Water and Aqueous Solutions: Influence of Solution and Materials Parameters. SCR, Nov 6–8 (Tokyo, Japan, 2000)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Li, YH., Tung, YM., Yeh, TK., Wang, MY. (2019). Feasibility Study of the Internal Zr/ZrO2 Reference Electrodes in Supercritical Water Environments. In: Jackson, J., Paraventi, D., Wright, M. (eds) Proceedings of the 18th International Conference on Environmental Degradation of Materials in Nuclear Power Systems – Water Reactors. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-030-04639-2_70
Download citation
DOI: https://doi.org/10.1007/978-3-030-04639-2_70
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-04638-5
Online ISBN: 978-3-030-04639-2
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)