Abstract
In nuclear medicine practice, there have been many diagnostic tools developed for primary detection, staging, and evaluation of treatment response in breast cancer. Although recent developments in breast imaging have been achieved, especially in positron emission tomography (PET) systems, conventional nuclear medicine imaging methods, including bone scintigraphy and sentinel lymph node (SLN) scintigraphy, still have important roles in the management of breast cancer. Radionuclide therapies, which have constituted a large part of nuclear medicine practice in recent decades, also offer both palliation and longer survival in breast cancer patients. This chapter outlines the role of nuclear medicine both in imaging and the treatment of patients with breast cancer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Nuclear medicine
- SPECT
- SPECT/CT
- PET/CT
- Scintimammography
- Sentinel lymph node scintigraphy
- Bone scintigraphy
- Positron emission tomography
- Positron emission mammography
- Magnetic resonance imaging
- Radionuclide therapies
- Osteoblastic skeletal metastases
- Radium-223
- Sm-13 EDTMP
- Sr-89
- Radioembolization
Introduction
In nuclear medicine, radioactive substances (radiopharmaceuticals) are used for the diagnosis and treatment of diseases. A radiopharmaceutical has two parts: a chemical part for targeting and a radioactive part for either imaging or therapy. Nuclear medicine imaging systems that convert gamma rays emitted from the patient as a result of a previously administered radiopharmaceutical to diagnostic images are mainly designed for whole-body imaging. In radionuclide therapies, radiopharmaceuticals that have either beta or alpha ray-emitting radioactive parts are given to patients. The chemical parts of the radiopharmaceuticals enable localization to and internal radiotherapy in diseased tissues.
Nuclear medicine imaging systems capable of single photon emission computed tomography (SPECT) and positron emission tomography (PET) as a special function of the device are able to measure the in vivo cellular, molecular, and biochemical properties of neoplasms and normal tissues. Hybrid imaging systems, such as PET/CT, PET/MR, and SPECT/CT devices, combine the functional information provided by the use of a radiopharmaceutical with anatomical information provided either by the computerized tomography (CT) or magnetic resonance imaging (MRI) unit of the same machine in a single acquisition.
SPECT imaging devices mostly use radiopharmaceuticals with technetium-99 m (Tc-99m), among other radionuclides, which decays with a single gamma ray at a time. The energy of the gamma ray differs for different radionuclides, such as Tc-99m, iodine-123 (I-123), iodine-131 (I-131), indium-111 (In-111), and gallium-67 (Ga-67). In contrast to SPECT agents, PET agents use pharmaceuticals labeled with positron-emitting radionuclides, such as fluorine-18 (F-18), carbon-11 (C-11), nitrogen-13 (N-13), oxygen-15 (O-15), and gallium-68 (Ga-68), which emit two gamma photons per each decay with an energy of 511 keV.
In PET/CT devices, radiopharmaceuticals are most commonly used to target cancer cells. In current oncology practice, imaging with PET/CT is an essential component of staging and monitoring treatment for numerous types of cancer. In recent years, there has been technological advancement of PET equipment through the development of new detectors and equipment designed specifically for breast imaging, such as positron emission mammography (PEM) devices. In addition, the development of more specific PET radiopharmaceuticals that target different biological processes of breast cancer will enable personalized therapy for patients with breast cancer. Although molecular imaging with PET is a rapidly emerging approach in breast cancer, conventional single photon nuclear medicine imaging, including bone scintigraphy and sentinel lymph node scintigraphy, still has an important role in the management of breast cancer. For several decades, systemic radionuclide treatment of painful bone metastases has been performed in breast cancer patients. New radiopharmaceuticals not only palliate pain but also prolong survival in patients with bone and liver metastases.
In this chapter, we will review diagnostic and therapeutic applications of nuclear medicine for breast cancer, starting from conventional single photon nuclear medicine techniques and then moving to PET applications and radionuclide treatment options for breast cancer patients.
Scintimammography
Scintimammography is a functional imaging method that enables differentiation of malignant from benign processes when mainstay anatomic modalities, such as mammography, ultrasound, and MRI, are limited [1]. In recent years, SPECT and hybrid SPECT/CT imaging have enhanced conventional planar scintimammography along with dedicated small field-of-view (FOV) breast-specific gamma imaging (BSGI) devices. Tc-99 m methoxyisobutylisonitrile (MIBI) is the radiopharmaceutical of choice for SPECT studies in breast imaging [2]. Tc-99m MIBI is localized in mitochondria, which are abundant in malignant cells. The uptake of Tc-99m MIBI depends on regional blood flow, tumor angiogenesis, and increased metabolism and is driven by plasma and mitochondrial membrane potentials [3, 4]. Studies have shown that the early uptake of Tc-99m MIBI reflects mitochondrial status, which is affected by both apoptosis and proliferation, but the clearance of the tracer reflects the activity of drug transporters, such as P-glycoprotein [5, 6]. Both proliferative activity and the apoptotic index have been shown to be directly correlated with Tc-99m MIBI uptake [7, 8].
A recent meta-analysis that evaluated the diagnostic value of BSGI and MRI in the same patient cohort with breast cancer showed that BSGI had similar sensitivity as MRI (84% vs 89%) but higher specificity (82% vs 39%) and diagnostic efficacy (AUC 0.93 vs 0.72), indicating excellent diagnostic performance [9]. The high specificity of scintimammography allows a positive scintigraphic finding to be supported by an invasive evaluation. Tumor types, such as poorly differentiated DCIS and lobular and tubulolobular carcinomas, and tumors with a size <1 cm and diminished cellularity, blood supply, and cell viability can cause a false-negative result on scintimammography [10, 11]. Benign hyperplasia lesions, such as fibrocystic changes and fibroadenomas, can also cause false-positive results in scintimammography.
The inability to detect axillary lymph nodes and delineate adjacent lesions are other limitations of scintimammography . SPECT/CT hybrid imaging, which combines functional and morphological information, enables an increase in the noninvasive diagnosis of axillary lymph node invasion by breast cancer. In a study of 60 patients, the addition of SPECT/CT evaluation increased sensitivity by 1.4 times (from 55% to 75%) compared with that of CT, with excellent specificity (97% and 89%) and comparable overall accuracy (82% and 84%) [12]. An effective radiation dose was estimated to be 5.9–9.4 mSv compared to 0.44 mSv for digital mammography [13].
Tc-99m MIBI scintimammography can also be used to monitor the treatment response to neoadjuvant chemotherapy. In a recent meta-analysis that include 14 studies, pooled sensitivity was 86% (95% CI, 0.78–0.92), and pooled specificity was 69% (95% CI, 0.64–0.74) for Tc-99m MIBI scintimammography in the prediction of neoadjuvant chemotherapy response in breast cancer [14]. This analysis suggested that negative scintimammography could not fully exclude the presence of a residual tumor, especially remaining ductal carcinoma in situ or a residual tumor of less than 1 cm in size. Subgroup analysis also showed that performing early mid-treatment Tc-99m MIBI scintimammography (using the reduction rate of one or two cycles or within the first half-course of chemotherapy compared with the baseline) was superior to later treatment (after three courses or more) or posttreatment scintimammography in the prediction of neoadjuvant chemotherapy response. In a study by Lee et al., although the direct comparison between MRI and scintimammography was statistically insignificant, MRI added value to scintimammography in the detection of residual tumor after neoadjuvant chemotherapy, and scintimammography also helped to locate tumors after therapy that were false negative on MRI. Thus, the authors concluded that a combination of scintimammography and MRI would be more accurate in the prediction of treatment response [15].
Sentinel Lymph Node Scintigraphy
Axillary lymph node status is a major prognostic factor in early-stage breast cancer. Sentinel lymph node (SLN) biopsy is the standard surgical procedure for staging clinically tumor-free regional nodes in patients with early-stage breast cancer. In this patient group, axillary lymph node dissection is no longer recommended, as it only adds to limb morbidity without providing any prognostic or staging benefit [16].
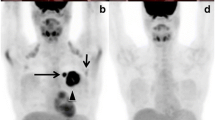
Tumors drain in an orderly manner through the lymphatic system. The SLN is the first to be affected by metastasis if the tumor has spread. A tumor-free SLN makes it highly unlikely for other nodes to be affected. SLN scintigraphy (lymphoscintigraphy) using radiolabeled colloids can accurately localize the sentinel nodes and can show atypical drainage patterns preoperatively (Fig. 6.1). Although lymphoscintigraphy and SLN biopsy (SLNB) have been used to stage many solid cancers, these procedures are most commonly performed in patients with breast cancer and melanoma. In the SLNB procedure, lymphoscintigraphy can improve accuracy, especially in extra-axillary lymph nodes, and can also reduce surgical morbidity [17]. The SPECT/CT procedure may improve the localization of SLNs during the acquisition of lymphoscintigraphy images. Intraoperative detection of SLNs is managed by a gamma probe. Recently, several portable gamma cameras have been developed to provide real-time image guidance for the detection of SLNs during the operation. The most recent developments include the combination of conventional gamma probes with position- and orientation-tracking systems, which permits virtual reconstruction in a three-dimensional environment.
A 52-year-old woman with a newly diagnosed left breast cancer was scanned for preoperative sentinel lymph node evaluation with Tc-99m nanocolloid lymphoscintigraphy. The Tc-99m nanocolloid was injected intramammary in the region of the tumor and periareolar subcutaneously. Dynamic, planar, and SPECT/CT images were recorded after the injections. Planar (c) and SPECT/CT images (a, CT image; b, fusion image; d, SPECT image) showed increased radiotracer uptake in the left axillary lymph node suggestive of the sentinel lymph node. The patient underwent a left mastectomy and left axillary sentinel lymph node biopsy. The surgical pathology report of the left axilla was negative for lymph node metastasis
Currently, the radioactive SLNB technique is combined with a dye technique to improve the detection rate. Recently, near-infrared fluorescence imaging using indocyanine green (ICG) has been applied to SLN procedures, and experience is growing in breast cancer [18,19,20]. Investigations have shown comparable results for radioactive and fluorescence techniques and that ICG fluorescence imaging can be a helpful tool for institutions without radioactive equipment. ICG fluorescence guidance has also been investigated for the excision of nonpalpable breast cancer lesions, and the first results are encouraging [21]. Clinical trials that are underway for ICG fluorescence guidance both for SLN procedures and for nonpalpable lesions in breast cancer will give more solid results (NCT02875626 and NCT01796041).
Identification of the SLN is crucial to the success of SLNB, and with a detection rate between 94% and 100%, preoperative SLN imaging is ideally suited for this purpose [22,23,24,25]. Recent multi-institutional studies have revealed SLNB false-negative rates ranging from 5.5% to 16.7%, higher than the target set by the 2005 ASCO guidelines (<5%) [26, 27]. Unfortunately, SLNB remains an unstandardized procedure with many unresolved controversies concerning the technique itself. The radiopharmaceuticals that are routinely used for SLNB are Tc-99m sulfur colloid (particle size, 15–5000 nm), Tc-99m nanocolloid (5–100 nm), and Tc-99m antimony trisulfide (3–30 nm). The radiocolloid measuring 100–200 nm is considered the best compromise between fast lymphatic drainage and optimal retention in SLNs [28]. The use of small volumes (0.3–0.4 ml) with high specific activity improves SLN detection. The standard procedure for SLN detection is based on the use of radiocolloid alone or in combination with blue dye, especially when the SLN is suspected to be diffusely metastatic [29]. Currently, no clinical consensus exists on the optimal site of injection of the radiocolloid or blue dye. Superficial (periareolar, subareolar, intradermal, subdermal) and deep (peritumoral, intratumoral) injections within the breast have been reported widely for radiocolloid administration [26, 30]. A recent meta-analysis comparing superficial and deep injections of radiocolloid demonstrated no significant difference in the SLN detection rate on lymphoscintigraphy or during intraoperative SLNB [31]. The rate of extra-axillary SLN identification was significantly greater when deep rather than superficial injection was used (OR: 3.00; 1.92–4.67).
Primary contraindications for SLNB include grossly palpable lymph nodes and inflammatory breast cancer. Healthy lymphatic tissue is necessary for the localization and retention of radiocolloids in lymph nodes. A metastatic lymph node that is enlarged with no healthy lymphatic tissue can lead to a false-negative SLNB procedure. Investigations of inflammatory breast cancer have also reported an SLN identification rate of only 80–85% with a relatively high false-negative rate (6.18%) [32]. Since the updated ASCO guidelines were published in 2017, no new data have become available to support the benefit of SLNB in women with large or locally advanced invasive breast cancers (T3/T4) and inflammatory breast cancer [33]. SLNB is also not recommended for women who have DCIS and for whom breast-conserving surgery is planned. SLNB is instead recommended for smaller tumors (T1 and T2), multiple tumors, and DCIS when mastectomy is planned, for older or obese patients, in male patients with breast cancer, and in patients with prior breast or axillary surgery. SLNB may be offered before or after neoadjuvant systemic therapy, but the procedure appears to be less accurate after neoadjuvant systemic therapy.
Today, the prognostic relevance of isolated tumor cells and micrometastases is negligible. Two multi-institutional randomized studies demonstrated an SLNB detection rate of 98% in cN0 stage I/II breast cancer patients [34, 35]. Thus, SLNB could prevent axillary lymph node dissection for SLN-negative women. In the ACOSOG Z0010 trial, occult metastases were detected in 9% of cases, but no difference was observed in disease-free survival and overall survival [36]. The 10-year follow-up data of the NSABP B-32 trial, which reported a prevalence of occult metastases of 15.9% of patients, revealed small differences in disease-free survival and overall survival that were statistically but not clinically significant. Therefore, complete axillary lymph node dissection in cases of SLN micrometastases is no longer recommended [37].
Bone Scintigraphy
The skeleton is the most common site for metastases from breast cancer. In approximately 50–70% of recurrent patients, skeleton metastases are detected, and it is the only metastatic site of disease in 28–44% of patients [38]. It is important to detect bone metastases at an early stage to minimize skeleton-related events. In patients who are receiving treatments, it is also important to determine the response to therapy as early as possible to limit toxicity and accelerate the therapeutic transition in nonresponding patients. Imaging has always played a key role in the diagnosis of bone metastases in breast cancer, and planar Tc-99m diphosphonate bone scanning remains widely used. The sensitivity of bone scintigraphy is high, and its lack of specificity has been improved with the addition of SPECT and SPECT/CT imaging to the acquisitions (Fig. 6.2). Despite improved accuracy in staging of the skeleton, effective monitoring of the treatment response is lacking. Although radiographs have been used historically to determine a response by lesion resolution or sclerosis, this method has been recognized as insensitive and may take at least 6 months to yield a confident assessment of response. Abnormal accumulation of Tc-99m diphosphonates is related to changes in local blood flow and osteoblastic activity. The mechanism of accumulation indicates that the uptake of Tc-99m diphosphonate is not specific for metastatic disease. Increased reparative osteoblastic activity resembles unresponsive progressive disease. The problem of the flare phenomenon (a temporary osteoblastic response to successful therapy), which makes the differentiation of progression from healing difficult for 3–6 months, has been described after chemotherapy and endocrine therapy in breast cancer [39]. Limitations of bone scintigraphy are reported when evaluating treatment response, with only 52% of responders showing scintigraphic improvement and 62% of nonresponders showing scintigraphic deterioration at 6–8 months in breast cancer [40].
A 67-year-old female patient with breast cancer had a mastectomy and received chemoradiotherapy. Due to new onset of back pain, she underwent bone scintigraphy. On whole-body images, pathologic Tc-99m MDP uptake in the vertebrae and pelvis was seen. On SPECT/CT images of the lumbar and pelvic regions, sclerotic metastatic lesions, indicated by arrows, on lumbar 1 and 2 vertebral bodies (upper row) and right iliac bone (middle row) were observed with pathologic Tc-99m MDP uptake. In addition, a pathologic fracture on the right ischium with increased Tc-99m MDP uptake (lower row) was detected
Positron Emission Tomography/Computed Tomography
PET/CT with F-18 fluoro-2-deoxy-D-glucose (FDG) has been established as an effective modality for different stages of evaluation of various types of cancer: making the diagnosis, determining the stage, evaluating the response to therapy, and follow-up.
Currently, FDG PET/CT is not used in breast cancer screening or diagnosing primary breast cancer mainly due to the high prevalence of false-negative results, particularly for tumors with a diameter smaller than 1 cm and tumors with low metabolic activity. The sensitivity of FDG PET/CT in primary breast cancer detection has been reported to be worse than that of ultrasonography, MRI, or mammography [41]. The metabolic activity of breast tumors is variable. For example, invasive lobular breast cancer has a considerably lower FDG uptake than invasive ductal cancer. Relatively high physiological glucose uptake in the surrounding mammary tissue is also another difficulty for the detection of tumors with low metabolic activity. The highest FDG uptake is observed for high-grade tumors, triple-negative tumors (ER-, PR-, HER2-), and inflammatory breast cancer [42, 43].
In early-stage breast cancer with clinically negative axilla, FDG PET/CT is not recommended due to its limited role in initial staging and treatment planning in most patients. In regional staging of these patients, FDG PET/CT is less sensitive than SLNB in assessing axillary lymph node involvement. In addition, the low prevalence of distant metastases in these patients and the probability of false-positive findings prevent the use of FDG PET/CT for distant staging [44]. By contrast, in patients with clinically positive axilla, especially in those with locally advanced breast cancer, FDG PET/CT can be useful prior to surgery or neoadjuvant chemotherapy, based on the high rate of detection of distant metastases, which ranges from 6% to 26% [45]. Extra-axillary lymph node involvement is detected by FDG PET/CT in 10–29% of patients with locally advanced breast cancer [46, 47]. FDG PET/CT changes the initial treatment in 1–8% of patients with early-stage breast cancer, in 7–13% of those with locally advanced breast cancer, and in up to 52% of those with more aggressive tumors, such as inflammatory breast cancer [48,49,50].
The level of FDG uptake by a primary tumor also has a prognostic value in many types of cancer. The prognostic impact of the glycolytic activity (SUVmax) of the primary breast tumor is controversial. Whereas some authors have found no correlation between FDG uptake by the tumor and the prognosis, others have reported that patients with high tumor uptake had worse outcomes [51,52,53,54]. Furthermore, the cutoff values for the SUVmax value ranged from 3 to 6. The evidence for the prognostic value of SUVmax in axillary lymph nodes is also limited, although higher values have been associated with higher recurrence rates [55, 56].
Changes in tumor metabolic activity have been shown to be an early indicator of effective treatment of breast cancer, mainly in the neoadjuvant setting. A decrease in tumor metabolic activity enables both assessment of the treatment response after the completion of therapy and early prediction of therapeutic effectiveness after the first or second cycle of chemotherapy. Identifying nonresponding patients on the basis of changes in tumor metabolic activity early during treatment can facilitate a change from an ineffective to a more effective treatment approach. In a study of 64 stage II and III breast cancer patients, Rousseau et al. observed a marked decrease in FDG uptake at multiple cycles during neoadjuvant chemotherapy in nearly all patients who had a therapeutic effect of more than 50% [57]. They determined that FDG PET after the second cycle of treatment potentially provided a more accurate prediction of treatment response. Using a 40% decrease of SUV as a cutoff value, Rousseau et al. found a negative predictive value of 68% for identifying nonresponders to therapy after the first cycle; this value increased to 85% after the second cycle. Schwarz-Dose et al. confirmed, in 104 patients, that the greater the reduction in tumor metabolic activity early during neoadjuvant treatment, the more likely that the patients would achieve a pathologic response [58]. In their study, they found that after the first cycle of chemotherapy, tumor metabolic activity decreased by 50% ± 18% in pathologic responders; by comparison, the decrease in pathologic nonresponders was 36% ± 20%. Of note, all breast carcinomas (23%) with a baseline SUV of less than 3.0 did not respond to chemotherapy. A recent meta-analysis of 19 studies with more than 900 patients found that the best cutoff value for decrease in FDG uptake for predicting response to therapy was 55–65% [59]. Although the sensitivity and specificity for identifying patients responding to treatment were limited (84% and 66%, respectively), the negative predictive value for identifying nonresponders was high (91%).
Changes in the sizes of bone metastases are particularly difficult to evaluate with conventional imaging as sclerotic lesions do not disappear and lytic lesions can show sclerotic changes as an indication of a treatment response. Two studies demonstrated a high sensitivity of FDG PET/CT for the detection of osseous metastases in patients with newly diagnosed metastatic breast cancer, and the metabolic activity of osseous breast cancer metastases provided prognostic information [60, 61]. In a retrospective analysis, bone metastases in 102 patients were assessed with FDG PET/CT before and after treatment, and a decrease in FDG uptake was a significant predictor of the response duration in univariate and multivariate analyses [62].
The early detection and accurate restaging of recurrent breast cancer are of significant importance for selecting the best therapeutic option for better prognosis and lower mortality. For breast cancer with suspicious recurrence, however, there is no standard follow-up protocol to date, and further examination of radiologic imaging, such as CT, bone scintigraphy, MRI, and PET/CT, may be needed. FDG PET/CT is a valuable technique that can show functional information for early detection of whole-body multifocal malignant lesions, thus enabling a correct diagnosis of recurrence that might be missed by conventional imaging modalities. Because it allows better discrimination between posttreatment scarring or fibrosis and viable tumor tissue, FDG PET/CT is efficient for detecting locoregional recurrence, especially in the chest wall, axilla, and extra-axillary lymph node basins, with better performance than CT or MRI (Fig. 6.3). A meta-analysis systematically summarized the overall diagnostic value of FDG PET/CT for the diagnosis of recurrence in breast cancer patients. The pooled sensitivity was 0.90 (95% CI, 0.88–0.92), indicating a high capacity for FDG PET/CT analysis in the early detection of recurrent breast cancer [63]. In addition, the pooled specificity was 0.81 (95% CI, 0.78–0.84), which showed a relatively higher ability to exclude recurrence compared with that of the other imaging modalities, such as CT or MRI. In other words, a negative test of FDG PET/CT can indicate the absence of recurrent breast cancer, with 81% probability.
A 58-year-old female patient underwent mastectomy due to breast cancer. During follow-up, her tumor marker levels started to increase. On her control mammography and breast USG, there was no sign of local recurrence, but on her FDG PET/CT images, there were metastatic lymph nodes with increased FDG uptake in the left posterior cervical region (upper row) and in the mediastinum at the right lower paratracheal and para-aortic regions (middle row). In the mastectomy region (lower row), there was no pathologic FDG uptake
F-18 sodium fluoride (NaF) is a positron emitter that is used for bone imaging in PET/CT machines. Its mechanism of uptake is quite similar to that of Tc-99m diphosphonate, which is the SPECT radiopharmaceutical for bone scintigraphy. Studies comparing the utility of NaF PET/CT with Tc-99m diphosphonate whole-body bone scintigraphy have shown that NaF PET/CT generally has higher sensitivity and specificity than bone scanning. The higher uptake of NaF than Tc-99m diphosphonate in the skeleton and the faster blood clearance yield a better target/background ratio in a shorter time period. Factors that contribute to the success of NaF PET/CT include NaF uptake in both lytic and blastic metastases, sectional imaging along with the advantage of whole-body scanning, easy detection of small lesions with improved resolution of PET technology, and better visualization of bone marrow lesions [64]. Recently, the frequent use of SPECT/CT utility along with planar whole-body scintigraphy has augmented the specificity of Tc-99m diphosphonate bone scintigraphy and reduced the demand for NaF PET/CT.
In addition to FDG and NaF, other PET radiopharmaceuticals have been used in breast cancer in both preclinical and clinical settings. Radiolabeled hypoxia-avid compounds, such as F-18-labeled fluoromisonidazole (FMISO), can be used to evaluate oxygenation status in experimental or human tumors. This PET radiotracer has affinity for hypoxic cells with functional nitroreductase enzymes; therefore, it accumulates in hypoxic cells but not in necrotic cells. F-18-labeled fluorothymidine (FLT) has been proposed as an early molecular imaging biomarker to evaluate treatment response with taxanes [65]. Uptake of FLT is correlated with the Ki-67 labeling index, another proliferation parameter, in breast cancer. Some studies have reported a strong correlation of FLT uptake with cell proliferation in untreated patients with breast cancer, enabling detection of response as early as 1 week after chemotherapy. Pio et al. compared FDG and FLT imaging in 14 patients with newly diagnosed primary or metastatic breast cancer to monitor and predict tumor response to chemotherapy [66]. The group concluded that FLT may be more accurate than FDG 2 weeks after the end of the first course of chemotherapy for predicting longer-term efficacy of chemotherapy for women with breast cancer. F-18-labeled fluoroestradiol (FES) is a novel radiopharmaceutical that noninvasively measures ER expression in tumors and has emerged as a valuable method to predict response to hormone therapy in recurrent or metastatic breast cancer patients [67, 68]. Level of FES uptake predicted the likelihood of response to tamoxifen and aromatase inhibitor treatment, and some studies support its use in treatment response assessment in some groups with recurrent or metastatic breast cancer [69].
Positron Emission Mammography
To overcome the limited resolution of PET equipment as well as space limitations of current CT acquisition protocols, which cause false-negative evaluations by FDG PET/CT protocols in small breast tumors, a new imaging modality, PEM, has emerged. PEM, which is a high-resolution tomographic molecular imaging device, has a pair of dedicated gamma radiation detectors that are placed above and below the breast. Mild breast compression, similar to conventional mammography, is necessary both to attain higher spatial resolution (1–2 mm for PEM vs 4–6 mm for PET) and to reduce the radiation dose by reducing breast thickness [70, 71]. The crystal detectors, which are constructed to provide improved spatial resolution and count rate efficiency, collect gamma rays emitted from the breast tissue due to previous injection of FDG. The result is a set of 12 slices each in the craniocaudal and mediolateral oblique positions, similar to conventional mammography.
The advantage of PEM is its ability to detect small hypermetabolic lesions. PEM can detect lesions <2 cm due to its higher spatial resolution (up to 2.4 mm) compared to that of whole-body PET [70]. Even small tumors <1 cm can be detected by PEM with a sensitivity of 60–70% [72]. Studies that compared PEM with MRI and whole-body PET/CT showed similar high sensitivities for PEM (93% for known index lesions, 85% for unsuspected additional lesions) and MR but low sensitivity for whole-body PET/CT (67.9%) [73, 74]. As both MRI and PEM have similar sensitivities, the indications for both of the exams are quite similar: in preoperative surgical planning or prechemotherapy evaluation to detect and characterize primary breast lesions [70]. PEM can be an alternative for patients who cannot tolerate MRI or have a contraindication to MRI, but in this context, the radiation exposure in PEM is a disadvantage.
PEM also suffers from the same specificity issues as breast MRI. Nonmalignant lesions, such as fibroadenomas, fibrocystic changes, and fat necrosis, can also accumulate FDG, mimicking a malignant lesion [70]. The specificity for detecting carcinoma ranges from 92% to 97% for PEM and 85% to 92% for MRI [75]. There are commercially available vacuum-assisted biopsy systems that can be used with PEM devices. The positive predictive values of these biopsies are similar to those of MRI-guided biopsies and higher than those of mammography-guided biopsies [70].
Positron Emission Tomography/Magnetic Resonance Imaging
PET/MR imaging is particularly interesting as a possible improvement over PET/CT oncologic whole-body imaging because MRI provides improved lesion detection in the brain, breast, liver, kidneys, and bones compared with lesion detection via CT. For breast malignancies, PET/MR can bring metabolic, anatomic, spectroscopic, and diffusion- and perfusion-based data together in a single examination. In whole-body imaging for breast cancer, PET/MR has been shown to provide improved sensitivity over PET/CT, particularly for breast lesions and liver and bone metastases [76, 77]. In local staging, PET imaging, which provides greater sensitivity for axillary nodes, appears to be complementary with MRI, which provides greater accuracy for satellite lesions. PET/MR has been shown to be more likely to determine the correct maximum diameter of the tumor (T stage) than PET/CT, which may be useful in surgical and oncological planning [78].
When separated out by sequence, dynamic contrast-enhanced (DCE) MRI has been shown to be most useful for breast and brain lesions, diffusion-weighted imaging (DWI) has been shown to be most useful for liver and bone metastases, and PET has been shown to be most useful for lymph node metastases [77]. These variable strengths highlight the advantage of multimodality imaging. In particular, combining PET and DWI may be important because PET has been shown to greatly improve the specificity of DWI in whole-body imaging [79]. In addition, omitting whole-body CT from the PET examination can decrease the radiation dose by half [77]. These data suggest a wider role for PET/MR imaging in breast cancer staging and surveillance, particularly in young patients and in patients undergoing serial examinations.
Radionuclide Therapies in Breast Cancer
Palliative Treatment of Painful Osteoblastic Skeletal Metastases
Postmortem studies indicate that 75% of breast carcinoma patients develop bone metastases [80]. The majority of patients with bone metastases develop severe pain that reduces their quality of life. A multidisciplinary approach to palliating pain is usually necessary. In patients with pain with multifocal, osteoblastic metastatic lesions, low-energy beta-emitting radionuclides, such as samarium-153-ethylenediaminetetramethylenephosphonate (Sm-153 EDTMP) and strontium-89, can be used to deliver high radiation to metastases but only a negligible dose to the hematopoietic marrow. Radionuclide therapy is indicated in patients with failure of conventional analgesics and to palliate recurrent pain in a previously irradiated site. The uptake of radiopharmaceuticals in radionuclide therapy depends on the osteoblastic activity and the calcification of the tumor tissue. The response rate is approximately 75%, and 25% of the patients may even become pain-free [81]. The majority of patients are able to reduce or withdraw opioid analgesics and continue using nonsteroidal anti-inflammatory medication. The therapy can be repeated if the cell counts are appropriate. Patients should have reasonable bone marrow reserve and must be monitored after treatment for probable temporary bone marrow suppression. Concomitant treatment with bisphosphonates does not interfere with the radionuclide treatment [81].
Baczyk et al. reported the results of a randomized controlled trial comparing Sm-13 EDTMP and Sr-89 in metastatic prostate cancer (n = 60) and breast cancer (n = 40) patients [82]. Although there was no difference in pain relief between the two radionuclides, patients with purely blastic metastatic lesions experienced more pain relief than patients with a mixed blastic/lytic pattern of metastases.
Radium-223 (Ra-223) is a bone-seeking alpha particle emitter radionuclide that delivers higher absorbed radiation to the bone surface, thus sparing the bone marrow due to its limited range. A double-blind, randomized, placebo-controlled phase III trial (ALSYMPCA) in prostate cancer patients showed a survival advantage (14 vs 11.2 months) in the Ra-223 arm with a low toxicity profile [83]. The median time of new skeletal events was also longer in the Ra-223 arm (13.6 vs 8.4 months). With respect to its tumoricidal effect in skeletal metastases, Ra-223 promises more than pain palliation in metastatic breast cancer patients.
Radioembolization for Liver Metastases
Radioembolization is a liver-directed therapy that involves injection of micron-sized embolic particles loaded with a radionuclide via percutaneous hepatic artery catheterization under fluoroscopic guidance. Because cancer cells are supplied by the hepatic artery and normal hepatocytes by portal venous blood, radioembolization targets tumor cells with a high dose of lethal radiation while sparing healthy hepatocytes. The antitumor effect is mainly from radiation rather than embolization. Because the hepatic artery is not embolized totally during radioembolization, portal vein thrombosis, which is a contraindication for other transarterial techniques, such as chemoembolization, is not a contraindication for radioembolization.
Yttrium-90 (Y-90) is the most commonly used radionuclide in radioembolization. Y-90 is embedded in either glass- or resin-based microspheres. Holmium-166 (Ho-166) microspheres have also been used recently. The procedure is performed on an outpatient basis. The probable complications are less commonly seen than in other locoregional therapies and may include nausea, fatigue, abdominal pain, hepatic dysfunction, biliary injury, and fibrosis. The complications that may be caused by the spread of radioactive microspheres to extrahepatic locations, such as gastrointestinal ulcers, cholecystitis, and radiation pneumonitis, can be avoided by meticulous pretreatment angiographic assessment and dosimetric calculations.
Radioembolization is an effective treatment for both primary and secondary liver tumors. ECOG performance status ≤2, adequate hematological parameters, and pulmonary, renal, and liver function tests are mandatory. Significant extrahepatic tumor burden, which diminishes expected survival, is also an exclusion criterion. When there is a bilobar, multicentric tumor load in the liver, instead of treating the whole liver in one session, sequential treatments are administered 6–8 weeks apart.
Liver metastases in breast cancer patients have been treated by radioembolization, and accumulating experience is encouraging (Fig. 6.4). Bangash et al. investigated Y-90 radioembolization in 27 breast cancer patients with progressing liver metastases on standard polychemotherapy [84]. The response rate was 39.1%, and stable and progressive disease was observed in 52.1% and 8.8%, respectively. Median survival was 6.8 and 2.6 months in patients with ECOG 0 vs 1, 2, and 3. In a multi-institutional study of 44 breast cancer patients with chemorefractory liver metastases, the response to Y-90 radioembolization was 95% when evaluated by PET and 47% when evaluated by CT [85]. Even patients without a PET or CT response had a median survival of 3.6 months. Median survival for the whole patient group was not reached at a follow-up of 14 months. Pieper et al. reported a disease control rate (response+stable disease) of 71.1% and an objective response rate (complete+partial response) of 28.9% in their single-center experience of 44 liver-dominant metastatic breast cancer patients with Y-90 radioembolization [86]. The median time to progression of the treated liver lobe was 101 days, and the median overall survival was 184 days. The authors stated that radioembolization can successfully delay progression of therapy-refractory liver-dominant metastatic breast cancer patients with a low complication rate.
In a 49-year-old female breast cancer patient, multiple metastases were detected in the lungs, liver, and bones 1 year after completion of adjuvant chemotherapy. Although the metastases responded well to second-line chemotherapy, a large metastatic lesion located in the posterior section of the right lobe of the liver did not decrease much in size. Therefore, before continuing with the chemotherapy regimen, radioembolization was planned for this lesion. In pretherapy angiographic evaluation, Tc-99m MAA was given in the posterior branch of the right hepatic artery, and SPECT/CT images (upper row) taken afterward showed homogeneous distribution of the radiopharmaceutical. After dosimetric calculations, 150 Gy of Y-90 microspheres was given via the same vascular route, and images (middle row) taken afterward showed a homogeneous distribution of Y-90 microspheres in the lesion. The control FDG PET/CT imaging (lower row) showed the response to radioembolization as necrosis (shown with an arrow)
There are many ongoing prospective trials examining the role for radioembolization in unresectable liver tumors, one of which includes breast cancer patients (SIRMITOC). The results of these trials will further clarify the efficacy and position of radioembolization.
Conclusion
The general advantage of nuclear medicine imaging is its ability to show deteriorations in a functional level, such as changes in a molecular structure or physiological processes, which makes it very different from radiological techniques that image on the basis of morphological alterations. Scintimammography is indicated for the study of breast lesions in patients in whom mammography or MRI is nondiagnostic or difficult to interpret; it may also be useful for assessing and even predicting the response to chemotherapy. Similar notions are also true for PEM imaging, which is a fairly new technique. Although whole-body FDG PET/CT imaging does not have sufficient utility in the detection of primary disease and is not optimized to replace the SLN procedure for initial axillary staging, FDG PET/CT scanning has efficacy superior to that of conventional imaging for the detection of locoregional and metastatic spread in the appropriate patient population and has a better diagnostic performance for the detection of skeletal metastasis compared with that of routine bone scanning. The major roles for PET/CT in breast cancer are detecting and localizing metastasis, monitoring the response to treatment, and early detection of recurrence. With PET/MR imaging, several drawbacks of PET/CT imaging, such as an inferior image quality in brain and liver lesions, can be improved. On the basis of the abovementioned evidence, the integration of nuclear medicine techniques with radiological techniques offers an interesting opportunity to improve the diagnostic imaging yield in breast cancer, which will eventually lead to better patient management. Another aspect of nuclear medicine, radionuclide treatments, also serves breast cancer patients. Radionuclide treatment for metastatic bone pain palliation is a safe and effective option for patients with multifocal osteoblastic metastases that has been used in breast cancer patients for years. Radioembolization, which is a fairly new radionuclide treatment option, is a novel transarterial locoregional therapy that is gaining recognition as a treatment option for primary and metastatic liver cancers and for which promising experience is also increasing in breast cancer patients.
References
Brem R, Rechtman L. Nuclear medicine imaging of the breast: a novel, physiological approach to breast cancer detection and diagnosis. Radiol Clin N Am. 2010;48:1055–74.
De Cesare A, De Vincentis G, Gervasi S, Crescentini G, Fiori E, Bonomi M, et al. Single photon emission computed tomography (SPECT) with Technetium-99m sestamibi in the diagnosis of small breast cancer and axillary lymph node involvement. World J Surg. 2011;35:2668–72.
Jacobsson H. Single-photon-emission computed tomography (SPECT) with 99mTechnetium sestamibi in the diagnosis of small breast cancer and axillary node involvement. World J Surg. 2011;35:2673–4.
Lee J, Rosen E, Mankoff D. The role of radiotracer imaging in the diagnosis and management of patients with breast cancer: part 1 – overview, detection and staging. J Nucl Med. 2009;50:569–81.
Delmon-Moingeon LI, Piwnica-Worms D, Van den Abbeele AD, Holman BL, Davison A, Jones AG. Uptake of the cation hexakis(2-methoxyisobutylisonitrile)-technetium-99m by human carcinoma cell lines in vitro. Cancer Res. 1990;50:2198–202.
Carvalho PA, Chiu ML, Kronauge JF, Kawamura M, Jones AG, Holman BL, et al. Subcellular distribution and analysis of technetium-99m-MIBI in isolated perfused rat hearts. J Nucl Med. 1992;33:1516–22.
Archer CD, Parton M, Smith IE, Ellis PA, Salter J, Ashley S, et al. Early changes in apoptosis and proliferation following primary chemotherapy for breast cancer. Br J Cancer. 2003;89:1035–41.
Cutrone JA, Yospur LS, Khalkhali I, Tolmos J, Devito A, Diggles L, et al. Immunohistologic assessment of technetium-99m-MIBI uptake in benign and malignant breast lesions. J Nucl Med. 1998;39:449–53.
Zhang A, Li P, Liu Q, Song S. Breast-specific gamma camera imaging with 99mTc-MIBI has better diagnostic performance than magnetic resonance imaging in breast cancer patients: a meta-analysis. Hell J Nucl Med. 2017;20:26–35.
Brem RF, Petrovitch I, Rapelyea JA, Young H, Teal C, Kelly T. Breast-specific gamma imaging with 99m Tc-Sestamibi and magnetic resonance imaging in the diagnosis of breast cancer-a comparative study. Breast J. 2007;13:465–9.
Meissnitzer T, Seymer A, Keinrath P, Holzmannhofer J, Pirich C, Hergan K, et al. Added value of semi-quantitative breast-specific gamma imaging in the work-up of suspicious breast lesions compared to mammography, ultrasound and 3-T MRI. Br J Radiol. 2015;88:20150147.
Novikov SN, Krzhivitskii PI, Kanaev SV, Krivorotko PV, Ilin ND, Jukova LA, et al. Axillary lymph node staging in breast cancer: clinical value of single photon emission computed tomography-computed tomography (SPECT-CT) with 99mTc methoxyisobutylisonitrile. Ann Nucl Med. 2015;29:177–83.
Hendrick RE. Radiation doses and cancer risks from breast imaging studies. Radiology. 2010;257:246–53.
Guo C, Zhang C, Liu J, Tong L, Huang G. Is Tc-99m sestamibi scintimammography useful in the prediction of neoadjuvant chemotherapy responses in breast cancer? A systematic review and meta-analysis. Nucl Med Commun. 2016;37:675–88.
Lee HS, Ko BS, Ahn SH, Son BH, Lee JW, Kim HJ, et al. Diagnostic performance of breast-specific gamma imaging in the assessment of residual tumor after neoadjuvant chemotherapy in breast cancer patients. Breast Cancer Res Treat. 2014;145:91–100.
Bromham N, Schmidt-Hansen M, Astin M, Hasler E, Reed MW. Axillary treatment for operable primary breast cancer. Cochrane Database Syst Rev. 2017;04:CD004561.
Johnson MT, Guidroz JA, Smith BJ, Graham MM, Scott-Conner CE, Sugg SL, et al. A single institutional experience of factors affecting successful identification of sentinel lymph node in breast cancer patients. Surgery. 2009;146:671–6.
Sugie T, Kassim K, Tsuji W, Takeuchi M, Yamashiro M, Ueno T, et al. Sentinel lymph node navigation surgery with indocyanine green fluorescence in early breast cancer. Cancer Res. 2009;69 Abstract nr:1017.
Tagaya N, Tsumuraya M, Nakagawa A, Iwasaki Y, Kato H, Kubota K. Indocyanine green (ICG) fluorescence imaging versus radioactive colloid for sentinel lymph node identification in patients with breast cancer. J Clin Oncol. https://doi.org/10.1200/jco.2010.28.15_suppl.674.
Liu J, Huang L, Wang N, Chen P. Indocyanine green detects sentinel lymph nodes in early breast cancer. J Int Med Res. 2017;45:514–24.
Liu J, Guo W, Tong M. Intraoperative indocyanine green fluorescence guidance for excision of nonpalpable breast cancer. World J Surg Oncol. 2016;14:266.
Caruso G, Cipolla C, Costa R, Morabito A, Latteri S, Fricano S, et al. Lymphoscintigraphy with peritumoral injection versus lymphoscintigraphy with subdermal periareolar injection of technetium-labeled human albumin to identify sentinel lymph nodes in breast cancer patients. Acta Radiol. 2014;55:39–44.
Somasundaram SK, Chicken DW, Waddington WA, Bomanji J, Ell PJ, Keshtgar MRS. Sentinel node imaging in breast cancer using superficial injections: technical details and observations. Eur J Surg Oncol. 2009;35:1250–6.
Aliakbarian M, Memar B, Jangjoo A, Zakavi SR, Reza Dabbagh Kakhki V, Aryana K, et al. Factors influencing the time of sentinel node visualization in breast cancer patients using intradermal injection of the radiotracer. Am J Surg. 2011;202:199–202.
Mudun A, Sanli Y, Ozmen V, Turkmen C, Ozel S, Eroglu A, et al. Comparison of different injection sites of radionuclide for sentinel lymph node detection in breast cancer: single institution experience. Clin Nucl Med. 2008;33:262–7.
Hindie E, Groheux D, Brenot-Rossi I, Rubello D, Moretti JL, Espié M. The sentinel node procedure in breast cancer: nuclear medicine as the starting point. J Nucl Med. 2001;52:405–14.
Lyman GH, Giuliano AE, Somerfield MR, Benson AB 3rd, Bodurka DC, Burstein HJ, et al. American Society of Clinical Oncology. American Society of Clinical Oncology guideline recommendations for sentinel lymph node biopsy in early-stage breast cancer. J Clin Oncol. 2005;23:7703–20.
Manca G, Rubello D, Tardelli E, Giammarile F, Mazzarri S, Boni G, et al. Sentinel lymph node biopsy in breast cancer: indications, contraindications, and controversies. Clin Nucl Med. 2016;41:126–33.
Giammarile F, Alazraki N, Aarsvold JN, Audisio RA, Glass E, Grant SF, et al. The EANM and SNMMI practice guideline for lymphoscintigraphy and sentinel node localization in breast cancer. Eur J Nucl Med Mol Imaging. 2013;40:1932–47.
Sun X, Liu JJ, Wang YS, Wang L, Yang GR, Zhou ZB, et al. Roles of preoperative lymphoscintigraphy for sentinel lymph node biopsy in breast cancer patients. Jpn J Clin Oncol. 2010;40:722–5.
Ahmed M, Purushotham AD, Horgan K, Klaase JM, Douek M. Meta-analysis of superficial versus deep injection of radioactive tracer and blue dye for lymphatic mapping and detection of sentinel lymph nodes in breast cancer. Br J Surg. 2015;102:169–81.
Hidar S, Bibi M, Gharbi O, Tebra S, Trabelsi A, Korbi S, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy in inflammatory breast cancer. Int J Surg. 2009;7:272–5.
Lyman GH, Somerfield MR, Bosserman LD, Perkins CL, Weaver DL, Giuliano AE. Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;10(35):561–4.
Veronesi U, Paganelli G, Viale G, Luini A, Zurrida S, Galimberti V, et al. Sentinel-lymph-node biopsy as a staging procedure in breast cancer: update of a randomised controlled study. Lancet Oncol. 2006;7:983–90.
Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Ashikaga T, et al. National Surgical Adjuvant Breast and Bowel Project. Technical outcomes of sentinel lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomized phase III trial. Lancet Oncol. 2007;8:881–8.
Hunt KK, Ballman KV, McCall LM, Boughey JC, Mittendorf EA, Cox CE, et al. Factors associated with local-regional recurrence after a negative sentinel node dissection: results of the ACOSOG Z0010 trial. Ann Surg. 2012;256:428–36.
Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ. Panel members. Strategies for subtypes dealing with the diversity of breast cancer: highlights of the St. Gallen International expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol. 2011;22:1736–47.
Jung SY, Rosenzweig M, Sereika S, Linkov F, Brufsky A, Weissfeld JL. Factors associated with mortality after breast cancer metastasis. Cancer Causes Control. 2012;23:103–12.
Schneider JA, Divgi CR, Scott AM, Macapinlac HA, Seidman AD, Goldsmith SJ, et al. Flare on bone scintigraphy following Taxol chemotherapy for metastatic breast cancer. J Nucl Med. 1994;35:1748–52.
Coombes RC, Dady P, Parsons C, McCready VR, Ford HT, Gazet JC, et al. Assessment of response of bone metastases to systemic treatment in patients with breast cancer. Cancer. 1983;52:610–4.
Choi YJ, Shin YD, Kang YH, Lee MS, Lee MK, Cho BS, et al. The effects of preoperative 18F-FDG PET/CT in breast cancer patients in comparison to the conventional imaging study. J Breast Cancer. 2012;15:441–8.
Groheux D, Giacchetti S, Moretti JL, Porcher R, Espié M, Lehmann-Che J, et al. Correlation of high 18F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur J Nucl Med Mol Imaging. 2011;38:426–35.
Tchou J, Sonnad SS, Bergey MR, Basu S, Tomaszewski J, Alavi A, et al. Degree of tumor FDG uptake correlates with proliferation index in triple negative breast cancer. Mol Imaging Biol. 2010;12:657–62.
Jeong YJ, Kang DY, Yoon HJ, Son HJ. Additional value of F-18 FDG PET/CT for initial staging in breast cancer with clinically negative axillary nodes. Breast Cancer Res Treat. 2014;145:137–42.
Manohar K, Mittal BR, Bhoil A, Bhattacharya A, Singh G. Role of 18F-FDG PET/CT in identifying distant metastatic disease missed by conventional imaging in patients with locally advanced breast cancer. Nucl Med Commun. 2013;34:557–61.
Seo MJ, Lee JJ, Kim HO, Chae SY, Park SH, Ryu JS, et al. Detection of internal mammary lymph node metastasis with (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with stage III breast cancer. Eur J Nucl Med Mol Imaging. 2014;41:438–45.
Fuster D, Duch J, Paredes P, Velasco M, Muñoz M, Santamaría G, et al. Preoperative staging of large primary breast cancer with [18F]fluorodeoxyglucose positron emission tomography/computed tomography compared with conventional imaging procedures. J Clin. 2008;26:4746–51.
Groheux D, Giacchetti S, Espié M, Vercellino L, Hamy AS, Delord M, et al. The yield of 18F-FDG PET/CT in patients with clinical stage IIA, IIB, or IIIA breast cancer: a prospective study. J Nucl Med. 2011;52:1526–34.
Jeong YJ, Kang DY, Yoon HJ, Son HJ. Additional value of F-18 FDG PET/CT for initial staging in breast cancer with clinically negative axillary nodes. Breast Cancer Res Treat. 2014;145:137–42.
Segaert I, Mottaghy F, Ceyssens S, De Wever W, Stroobants S, Van Ongeval C, et al. Additional value of PET-CT in staging of clinical stage IIB and III breast cancer. Breast J. 2010;16:617–24.
Champion L, Lerebours F, Cherel P, Edeline V, Giraudet AL, Wartski M, et al. 18F-FDG PET/CT imaging versus dynamic contrast-enhanced CT for staging and prognosis of inflammatory breast cancer. Eur J Nucl Med Mol Imaging. 2013;40:1206–13.
JH O, Choi WH, Han EJ, Choi EK, Chae BJ, Park YG, et al. The prognostic value of (18)F-FDG PET/CT for early recurrence in operable breast cancer: comparison with TNM stage. Nucl Med Mol Imaging. 2013;47:263–7.
Aogi K, Kadoya T, Sugawara Y, Kiyoto S, Shigematsu H, Masumoto N, et al. Utility of (18)F FDG-PET/CT for predicting prognosis of luminal-type breast cancer. Breast Cancer Res Treat. 2015;150:209–17.
Kadoya T, Aogi K, Kiyoto S, Masumoto N, Sugawara Y, Okada M. Role of maximum standardized uptake value in fluorodeoxyglucose positron emission tomography/computed tomography predicts malignancy grade and prognosis of operable breast cancer: a multi-institute study. Breast Cancer Res Treat. 2013;141:269–75.
García Vicente AM, Soriano Castrejón A, López-Fidalgo JF, Amo-Salas M, Muñoz Sanchez Mdel M, Álvarez Cabellos R, et al. Basal 18F-FDG PET/CT as a prognostic biomarker in patients with locally advanced breast cancer. Eur J Nucl Med Mol Imaging. 2015;42:1804–13.
Song BI, Lee SW, Jeong SY, Chae YS, Lee WK, Ahn BC, et al. 18F-FDG uptake by metastatic axillary lymph nodes on pretreatment PET/CT as a prognostic factor for recurrence in patients with invasive ductal breast cancer. J Nucl Med. 2012;53:1337–44.
Rousseau C, Devillers A, Sagan C, Ferrer L, Bridji B, Campion L, et al. Monitoring of early response to neoadjuvant chemotherapy in stage II and III breast cancer by [18F]fluorodeoxyglucose positron emission tomography. J Clin Oncol. 2006;24:5366–72.
Schwarz-Dose J, Untch M, Tiling R, Sassen S, Mahner S, Kahlert S, et al. Monitoring primary systemic therapy of large and locally advanced breast cancer by using sequential positron emission tomography imaging with [18F]fluorodeoxyglucose. J Clin Oncol. 2009;27:535–41.
Wang Y, Zhang C, Liu J, Huang G. Is 18F-FDG PET accurate to predict neoadjuvant therapy response in breast cancer? A meta-analysis. Breast Cancer Res Treat. 2012;131:357–69.
Morris PG, Lynch C, Feeney JN, Patil S, Howard J, Larson SM, et al. Integrated positron emission tomography/ computed tomography may render bone scintigraphy unnecessary to investigate suspected metastatic breast cancer. J Clin Oncol. 2010;28:3154–9.
Morris PG, Ulaner GA, Eaton A, Fazio M, Jhaveri K, Patil S, et al. Standardized uptake value by positron emission tomography/computed tomography as a prognostic variable in metastatic breast cancer. Cancer. 2012;118:5454–62.
Tateishi U, Gamez C, Dawood S, Yeung HW, Cristofanilli M, Macapinlac HA. Bone metastases in patients with metastatic breast cancer: morphologic and metabolic monitoring of response to systemic therapy with integrated PET/CT. Radiology. 2008;247:189–96.
Xiao Y, Wang L, Jiang X, She W, He L, Hu G. Diagnostic efficacy of 18F-FDG-PET or PET/CT in breast cancer with suspected recurrence: a systematic review and meta-analysis. Nucl Med Commun. 2016;37:1180–8.
Schirrmeister H, Guhlmann A, Kotzerke J, Santjohanser C, Kühn T, Kreienberg R. Early detection and accurate description of extent of metastatic bone disease in breast cancer with fluoride ion and positron emission tomography. J Clin Oncol. 1999;17:2381–9.
Dittmann H, Jusufoska A, Dohmen BM, Smyczek-Gargya B, Fersis N, Pritzkow M, et al. 3′-deoxy-3′-[18F]fluorothymidine (FLT) uptake in breast cancer cells as a measure of proliferation after doxorubicin and docetaxel treatment. Nucl Med Biol. 2009;36:163–9.
Pio BS, Park CK, Pietras R, Hsueh WA, Satyamurthy N, Pegram MD, et al. Usefulness of 3′-[F-18]fluoro-3′-deoxythymidine with positron emission tomography in predicting breast cancer response to therapy. Mol Imaging Biol. 2006;8:36–42.
Peterson LM, Mankoff DA, Lawton T, Yagle K, Schubert EK, Stekhova S, et al. Quantitative imaging of estrogen receptor expression in breast cancer with pet and 18F-fluoroestradiol. J Nucl Med. 2008;49:367–74.
Kenny LM, Al-Nahhas A, Aboagye EO. Novel PET biomarkers for breast cancer imaging. Nucl Med Commun. 2011;32:333–5.
Linden HM, Stekhova SA, Link JM, Gralow JR, Livingston RB, Ellis GK, et al. Quantitative fluoroestradiol positron emission tomography imaging predicts response to endocrine treatment in breast cancer. J Clin Oncol. 2006;24:2793–9.
Glass SB, Shah ZA. Clinical utility of positron emission mammography. Proc (Baylor Univ Med Cent). 2013;26:314–9.
Niklason LT, Kopans D, Hamerg LM. Digital breast imaging: tomosynthesis and digital subtraction mammography. Breast Dis. 1998;10:151–64.
Tejerina Bernal A, Tejerina Bernal A, Rabadan Doreste F, De Lara Gonzalez A, Rosello Llerena JA, Tejerina Gomez A. Breast imaging: how we manage diagnostic technology at a multidisciplinary breast center. J Oncol. 2012;2012:213–421.
Eo JS, Chun IK, Paeng C, Kang KW, Lee SM, Han W, et al. Imaging sensitivity of dedicated positron emission mammography in relation to tumor size. Breast. 2012;21:66–71.
Schilling K, Narayanan D, Kalinyak JE, The J, Velasquez MV, Kahn S, et al. Positron emission mammography in breast cancer presurgical planning: comparisons with magnetic resonance imaging. Eur J Nucl Med Mol Imaging. 2011;38:23–36.
Berg WA, Madsen KS, Schilling K, Tartar M, Pisano ED, Larsen LH, et al. Comparative effectiveness of positron emission mammography and MRI in the contralateral breast of women with newly diagnosed breast cancer. AJR Am J Roentgenol. 2012;198:219–32.
Melsaether AN, Raad RA, Pujara AC, Ponzo FD, Pysarenko KM, Jhaveri K, et al. Comparison of whole-body (18)F FDG PET/MR imaging and whole-body (18)F FDG PET/CT in terms of lesion detection and radiation dose in patients with breast cancer. Radiology. 2016;281:193–202.
Catalano OA, Nicolai E, Rosen BR, Luongo A, Catalano M, Iannace C, et al. Comparison of CE-FDG-PET/CT with CE-FDG-PET/MR in the evaluation of osseous metastases in breast cancer patients. Br J Cancer. 2015;112:1452–60.
Grueneisen J, Nagarajah J, Buchbender C, Hoffmann O, Schaarschmidt BM, Poeppel T, et al. Positron emission tomography/magnetic resonance imaging for local tumor staging in patients with primary breast cancer: a comparison with positron emission tomography/computed tomography and magnetic resonance imaging. Investig Radiol. 2015;50:505–13.
Heusner TA, Kuemmel S, Koeninger A, Hamami ME, Hahn S, Quinsten A, et al. Diagnostic value of diffusion-weighted magnetic resonance imaging (DWI) compared to FDG PET/CT for whole-body breast cancer staging. Eur J Nucl Med Mol Imaging. 2010;37:1077–86.
Roodman GD. Mechanisms of bone lesions in multiple myeloma and lymphoma. Cancer. 1997;80:1557–63.
Fischer M, Kampen WU. Radionuclide therapy of bone metastases. Breast Care (Basel). 2012;7:100–7.
Baczyk M, Czepczynski R, Milecki P, Pisarek M, Oleksa R, Sowinski J. 89Sr versus 153Sm-EDTMP: comparison of treatment efficacy of painful bone metastases in prostate and breast carcinoma. Nucl Med Commun. 2007;28:245–50.
Parker C, Heinrich D, O’Sullivan JM, Fossa S, Chodacki A, Demkow T, et al. Overall survival benefit of radium-223 chloride (Alpharadin) in the treatment of patients with symptomatic bone metastases in castration-resistant prostate cancer (CRPC): a phase III randomised trial (ALSYMPCA). Eur J Cancer. 2011;47(Supplement 2):3.
Bangash AK, Atassi B, Kaklamani V, Rhee TK, Yu M, Lewandowski RJ, et al. 90Y radioembolization of metastatic breast cancer to the liver: toxicity, imaging response, survival. J Vasc Interv Radiol. 2007;18:621–8.
Coldwell DM, Kennedy AS, Nutting CW. Use of yttrium-90 microspheres in the treatment of unresectable hepatic metastases from breast cancer. Int J Radiat Oncol Biol Phys. 2007;69:800–4.
Pieper CC, Meyer C, Wilhelm KE, Block W, Nadal J, Ahmadzadehfar H, et al. Yttrium-90 radioembolization of advanced, unresectable breast cancer liver metastases- a single-center experience. J Vasc Interv Radiol. 2016;27:1305–15.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Turkmen, C., Ozkan, Z.G. (2019). Nuclear Medicine in the Diagnosis and Treatment of Breast Cancer. In: Aydiner, A., Igci, A., Soran, A. (eds) Breast Disease. Springer, Cham. https://doi.org/10.1007/978-3-030-04606-4_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-04606-4_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-04605-7
Online ISBN: 978-3-030-04606-4
eBook Packages: MedicineMedicine (R0)