Abstract
Cultivated carrot (Daucus carota subsp. sativus) is the most important member in the Apiaceae family in terms of economy and nutrition and is considered the second most popular vegetable in the world after potato. Despite its global importance, the systematics of Daucus remains under active revision at the species, genus, and subtribal levels. The phylogenetic relationships among the species of Daucus and close relatives in the Apioideae have been clarified recently by a series of molecular studies using DNA sequences of the plastid genes rbcL and matK; plastid introns rpl16, rps16, rpoC1; nuclear ribosomal DNA internal transcribed spacer (ITS) sequences; and plastid DNA restriction sites. Of these DNA markers, the ITS region consisting of ITS1, the intervening spacer, and ITS2 has served as the main marker used. Recently, next-generation DNA sequencing methodologies have been used. We review these techniques and how they are impacting the taxonomy of the genus Daucus.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Taxonomy of the Apiaceae (Umbelliferae)
The Apiaceae (Umbelliferae) family contains 466 genera and 3820 species (Plunkett et al. in press) and is one of the largest families of seed plants. It is nearly cosmopolitan in distribution, but most diverse in temperate regions of the northern hemisphere (Downie et al. 2000a, b, c; Heywood 1983). It is well supported as a monophyletic family, closely related to the families Araliaceae, Pittosporaceae, and Myodocarpaceae, and these, along with three smaller families, constitute the order Apiales, containing about 5400 species (Judd et al. 2016; Plunkett et al. 1996b).
The Apiaceae is well defined morphologically by a suite of characters, typically including herbs with compound leaves, stems usually hollow in the internodes and with secretory canals containing ethereal oils, resins, and other compounds; alternate compound leaves or simple and deeply divided or lobed leaves with sheathing petioles; determinate inflorescences containing simple to compound umbels often subtended by involucral bracts; small flowers with 5 sepals, 5 petals, 5 stamens, and 2 connate carpels with an inferior ovary; 2 small stigmas; with the fruit a schizocarp (dry fruits breaking into one-seeded segments) with each of the two mericarps attached to an entire and deeply divided forked central stalk (carpophone) (Judd et al. 2016).
This large suite of distinctive characters makes the Apiaceae and its constituent species easily recognized to family, but divisions within the family have been the subject of long dispute including circumscription and relationships of the genus Daucus (Constance 1971; Plunkett and Downie 1999) Traditionally, the Apiaceae has been divided into three subfamilies, the Saniculoideae, Hydrocotyloideae, and Apioideae, with the Apioideae, containing the genus Daucus, by far the largest of these three traditional subfamilies. Drude (1898) recognized 8 tribes and 10 subtribes within the Apioideae. Molecular phylogenetic studies have confirmed the monophyly of the subfamily Apioideae but not many of its tribes and subtribes (Downie et al. 2001). Downie et al. (2001) recognized nine tribes in the Apiaceae subfamily Apioideae, and placed Daucus, and 12 other genera, in tribe Scandiceae Spreng., subtribe Daucinae Dumort. (the other 12 genera being Agrocharis Hochst., Ammodaucus Coss. and Durieu, Cuminum L., Laser Borkh. ex P. Gaertn., B. Mey. and Schreb., Laserpitium L., Melanoselinum Hoffm., Monizia Lowe, Orlaya Hoffm., Pachyctenium Maire and Maire and Polemannia Eckl. and Zeyh., Polylophium Boiss., Pseudorlaya (Murb.) Murb., and Thapsia L.).
A genus-level treatment of Daucus by Sáenz Laín (1981) used morphological and anatomical data and recognized 20 species. Rubatzky et al. (1999) later estimated 25 species of Daucus. The phylogenetic relationships among the species of genus Daucus and close relatives in the Apioideae have been clarified by a series of molecular studies using DNA sequences of the plastid genes rbcL and matK; plastid introns rpl16, rps16, rpoC1; nuclear ribosomal DNA internal transcribed spacer (ITS) sequences; and plastid DNA restriction sites (e.g., Arbizu et al. 2014b, 2016a, b; Banasiak et al. 2016; Downie and Katz-Downie 1996; Downie et al. 1996, 1998, 2000a, b, c, 2001, 2010; Katz-Downie et al. 1999; Lee 2002; Lee and Downie 1999, 2000, 2006; Plunkett et al. 1996a; Spalik and Downie 2007; Spalik et al. 2001a, b; Weitzel et al. 2014). Of these DNA markers, the ITS region consisting of ITS1, the intervening spacer, and ITS2 has served as the main marker. A recent study of ITS, and other DNA regions proposed as standard barcodes (psbA-trnH, matK, and rbcL) in 1957 species in 385 diverse genera in the Apiaceae have shown ITS to serve to identify species 73.3% of the time, higher than any of the other individual markers tested (Liu et al. 2014).
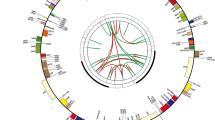
A study by Banasiak et al. (2016) using DNA sequences from nuclear ribosomal ITS and three plastid markers (rps16 intron, rpoC1 intron, and rpoB-trnC intergenic spacer) is the latest of a series of studies to investigate ingroup and outgroup relationships of Daucus (Fig. 2.1). This study redefined and expanded the genus Daucus to include the following genera and species into its synonymy: Agrocharis Hochst. (4 species), Melanoselinum Hoffm. (1 species), Monizia Lowe (1 species), Pachyctenium Maire and Pamp. (1 species), Pseudorlaya (Murb.) Murb. (2 species), Rouya Coincy (1 species), Tornabenea Parl. (6 species), Athamanta dellacellae E. A. Durand and Barratte, and Cryptotaenia elegans Webb ex Bolle (these latter two genera with only some of its members transferred to Daucus).
Reproduction of the upper part of the Daucus maximum likelihood phylogeny of Banasiak et al. (2016), using combined nuclear internal transcribed spacer region of ribosomal DNA (ITS) and plastid (rps16 intron, rpoC1 intron, and rpoB-trnC intergenic spacer) data, with numbers above the branches representing bootstrap support and posterior probability values. The arrows show hard incongruence between Banasiak et al. (2016) and the nuclear ortholog phylogenies of Arbizu et al. (2014b, 2016b)
Banasiak et al. (2016) made the relevant nomenclatural transfers into Daucus (Table 2.1) and following this classification, the genus Daucus contains ca. 40 species and now includes winged and completely unadorned (“obsolete”) fruits in addition to its traditionally recognized spiny fruits. As summarized in Banasiak et al. (2016) and presented in graphic form in Fig. 5 of this paper, winged versus spiny versus obsolete fruits presented major traditional taxonomic characters at higher levels in the Apiaceae (e.g., Drude 1897–1898). Winged fruits are considered to be adapted to wind dispersal (Jongejans and Telenius 2001; Theobald 1971), and spiny fruits to animal dispersal (Jury 1982; Spalik et al. 2001a; Williams 1994) and likely under strong selective pressure. The above phylogenetic analyses, however, show these fruit characters to be highly homoplastic and of limited value in delimiting monophyletic groups.
The above classification philosophy followed by Banasiak et al. (2016) in placing all members of a monophyletic clade into a single genus (here Daucus) is not universally accepted, and others may revise the circumscription of these genera. For example, a dissenting classification philosophy of relying solely on molecular data for classification is presented by Stuessy and Hörandl (2014), who recognize a “holophyletic” group as one that includes the immediate ancestor and all its descendants, independent of whatever divergence occurs within each of the derivative lineages (Ashlock 1971). A paraphyletic group, in contrast, is one that derives from a common ancestor but that does not contain all its descendants (Hennig 1966) and is an unacceptable taxon following cladistic conventions. Stuessy and Hörandl (2014) point out that adaptive radiation, common in oceanic islands, produces patterns where new populations continue to accrue reproductive isolation and speciation such that they produce quite distinctive new forms, often recognized as new genera, leaving parental populations intact. As examples in the Daucinae, Stuessy et al. (2014) cite the genus Monizia in the Madeira Islands, but other possibilities could be the genus Tornabenea or the species Cryptotaenia elegans on the Cape Verde Islands or the genus Melanoselinum on the Madeira Islands. Critical data bearing on this classification question rest in the distinctiveness and divergence of these new island forms. Because we have not studied these subsumed genera in detail, we currently take no position on these differences in classification, awaiting additional data and perspectives from others, such as Martínez-Flores (2016) and Plunkett et al. (in press) who maintain more traditional classifications of Daucus.
2.2 Distribution of Daucus
Phylogenetic analysis of ITS sequences supports southern Africa as the ancestral origin of the Apiaceae subfamily Apioideae (Banasiak et al. 2013). Phylogenetic analysis of ITS sequences supports an Old World Northern Hemisphere origin for Daucus, with one or two dispersals to the Southern Hemisphere (Spalik et al. 2010). The center of diversity of Daucus in its traditional sense is in the Mediterranean region (Sáenz Laín 1981). Daucus species also occur elsewhere, with one species (D. glochidiatus) in Australia, four species in the American continent (D. carota, D. montanus, D. montevidensis, D. pusillus Michx.). Following the expanded classification of Daucus by Banasiak et al. (2016), the now included genus Agrocharis extends the range of Daucus into tropical Africa (Townsend 1989).
2.3 New Taxonomic Approaches: Next-Generation Sequencing (NGS)
A major innovation in plant systematics is the development of high-throughput, “next-generation” DNA sequencing (NGS) to infer phylogenetic relationships (Egan et al. 2012; E. M. Lemmon and A. R. Lemmon 2013). NGS typically first involves large-scale sequencing of all components of the genome, with the Illumina platform currently the most commonly used. Some genomes, such as plastid and mitochondria, have much higher coverage than single- to low-copy nuclear DNA and can be factored out of the nuclear genome in NGS data by coverage statistics. The utility of NGS sequencing is markedly improved when a high-quality whole-genome “reference” sequence is available that serves as a heterologous template to guide mapping of sequences of related germplasm. Such whole-genome reference sequences are available in carrot for the plastid genome (Ruhlman et al. 2006) and for the plastid and nuclear genome (Iorizzo et al. 2016). As summarized below, recent phylogenetic studies in Daucus have used high-throughput DNA sequencing to infer phylogenetic relationships at the genus level using orthologous nuclear DNA sequences, also at the genus level using whole plastid DNA sequences, and at the species level using genotyping-by-sequencing (GBS).
2.3.1 Next-Generation DNA Phylogenetic Studies at the Genus Level Using Orthologous Nuclear DNA Sequences
In the past, there has been a paucity of validated nuclear orthologs for phylogenetic studies, and hence, most molecular taxonomic studies have relied heavily on a few plastid and/or ribosomal genes (Small et al. 2004). Phylogenies reconstructed with only one or a few independently inherited loci may result in unresolved or incongruent phylogenies due to data sampling (Graybeal 1998), horizontal gene transfer, or differential selection and lineage sorting at individual loci (Maddison 1995). Following a phylogenetic study by Spooner et al. (2013) where eight nuclear orthologs were used in Daucus but designed without NGS techniques, Arbizu et al. (2014b) identified 94 nuclear orthologs in Daucus, constructed a phylogeny with these, and determined 10 of them to provide essentially the same phylogeny as all 94, paving the way for additional and most cost-effective nuclear ortholog phylogenetic studies in carrot. The 94 (and 10) nuclear ortholog phylogeny was highly resolved, with 100% bootstrap support for most of the external and many of the internal clades. They resolved multiple accessions of many different species as monophyletic with strong support, but failed to support other species. This phylogeny had many points of agreement with Banasiak et al. (2016), including resolving two major clades (Daucus I and II in their study, labeled clade A and B in Arbizu et al. 2014b), with a clade A’ containing all examined 2n = 18 chromosome species (D. carota all subspecies, D. capillifolius, D. syrticus), with the other clade A species being and D. aureus and D. muricatus (as sister taxa), and D. tenuisectus. Two non-Daucus species (Rouya polygama and Pseudorlaya pumila) resolved sister to Daucus clade A’. Clade B (Daucus II in Banasiak et al. 2016) contained six wild Daucus species D. glochidiatus, D. guttatus, D. involucratus, D. littoralis, and D. pusillus, but D. guttatus was not monophyletic within this clade.
2.3.2 An Expansion of the Above Study—The Daucus Guttatus Complex
As mentioned above, the nuclear ortholog study of Arbizu et al. (2014b) resolved a monophyletic group (clade B) of six wild Daucus species D. glochidiatus, D. guttatus, D. involucratus, D. littoralis, and D. pusillus. Some of these species are morphologically similar and difficult to distinguish, causing frequent misidentifications. Arbizu et al. (2016b) used the group of ten nuclear orthologs mentioned above in the study of Arbizu et al. (2014b), and morphological data (Arbizu et al. 2014a), and a greatly expanded subset of accessions of these species, to refine phylogenetic structure of the group. The nuclear ortholog data resolved four well-supported clades (Fig. 2.2), that in concert with morphological data, and nomenclatural data from a study of type specimens (Martínez-Flores et al. 2016) served to identify four phenetically most similar species D. bicolor, D. conchitae, D. guttatus, and D. setulosus. Internested among these four similar species were phenetically more distinctive species D. glochidiatus, D. involucratus, D. littoralis, and D. pusillus. They presented a key to better distinguish all of these eight species. In summary, their research clarified species variation in the D. guttatus complex, resolved interspecific relationships, provided the proper names for the species, and discovered morphological characters allowing proper identification and key construction of members of the D. guttatus complex and related species.
Maximum parsimony phylogenetic reconstruction of the Daucus guttatus complex using 10 nuclear orthologs showing resolution of the species in the Daucus guttatus complex. Numbers above branches represent bootstrap values. Clades 1, 2, and 3 were identified in Arbizu et al. (2014b)
2.3.3 Next-Generation DNA Phylogenetic Studies at the Genus Level Using Whole Plastid DNA Sequences
The plastid genome has many features that make it useful for plant phylogenetic studies, including its small size (generally 120–160 kbp), high copy number (as many as 1000 per cell), generally conservative nature (Wolfe et al. 1987), and varying rates of change in different regions of the genome, allowing studies at different phylogenetic levels (Raubeson and Jansen 2005). Hence, earlier sequence-based plant phylogenetic studies used genes or gene regions from the plastid. Relative to the Apioideae, the subfamily of the Apiaceae including Daucus, systematic studies have used plastid restriction site data; DNA sequence data from plastid genes; from plastid introns; from plastid intergenic spacer regions. Using NGS sequencing approaches, Downie and Jansen (2015) sequenced five complete plastid genomes in the Apiales (Apiaceae + Araliaceae): Anthriscus cerefolium (L.) Hoffm., Crithmum maritimum L., Hydrocotyle verticillata Thunb., Petroselinum crispum (Mill.) Fuss, and Tiedemannia filiformis (Walter) Feist and S. R. Downie subsp. greenmanii (Mathias and Constance) Feist and S. R. Downie, and compared the results obtained to previously published plastomes of Daucus carota subsp. sativus and Panax schin-seng T. Nees. They discovered the rpl32-trnL, trnE-trnT, ndhF-rpl32, 5’rps16-trnQ, and trnT-psbD intergenic spacers to be among the most fast-evolving loci, with the trnD-trnY-trnE-trnT combined region presenting the greatest number of potentially informative characters overall that may possess ideal phylogenetic markers in these families.
Spooner et al. (2017) explored the phylogenetic utility of entire plastid DNA sequences in Daucus, using Illumina sequencing, and compared the results with prior phylogenetic results using plastid and nuclear DNA sequences. The phylogenetic tree of the entire data set (Fig. 2.3) was highly resolved, with 100% bootstrap support for most of the external and many of the internal clades. Subsets of the plastid data, such as matK, ndhF, or the putative maximally informative regions of the plastid genome outlined by Downie and Jansen (2015) are only partly successful in Daucus, resulting in polytomies and reduced levels of bootstrap support. Additionally, there are areas of hard incongruence (strongly supported character conflict because of differences in underlying evolutionary histories) with phylogenies using nuclear data (Fig. 2.1).
Maximum likelihood cladogram of the entire plastid DNA sequences of Spooner et al. (2017), with the three main clades indicated, with arrows highlighting hard topological incongruence with the nuclear ortholog phylogenies of Arbizu et al. (2014b, 2016b); the two accessions of Daucus syrticus resolve as a sister group to all accessions of D. carota. a Represents expanded topological detail of the upper portion of the entire tree shown on b. The values above the branches are bootstrap support values
Incongruence between plastid and nuclear genes are not uncommon in phylogenetic studies in the Apiaceae (e.g., Lee and Downie 2006; Yi et al. 2015; Zhou et al. 2009), indeed throughout many angiosperms (Wendel and Doyle 1998). These incongruent results showed the value of resequencing data to produce a well-resolved plastid phylogeny of Daucus, and highlighted caution to combine plastid and nuclear data, if at all. The value of generating phylogenies from both nuclear and plastid sequences is that hard incongruence can be quite informative, suggesting such evolutionary processes as “plastid capture” where incongruence can be caused by a history of hybridization between plants with differing plastid and nuclear genomes (Rieseberg and Soltis 1991), and backcrossing to the paternal parent but retaining the plastid genome that is (typically) maternally inherited. Other possible processes that can lead to such incongruence, however, are gene duplication (Page and Charleston 1997), horizontal gene transfer (Doolittle 1999), and incomplete lineage sorting (Pamilo and Nei 1988).
2.3.4 Next-Generation DNA Phylogenetic Studies at the Species Level—Genotyping-by-Sequencing (GBS) for the Daucus Carota Complex
The genus Daucus contains cultivated carrot (Daucus carota L. subsp. sativus Hoffm.), the most important member of Apiaceae in terms of economic importance and nutrition (Rubatzky et al. 1999; Simon 2000), and is considered the second most popular vegetable worldwide after potato (Heywood 2014). Daucus carota has many formally named subspecies and varieties, and the species is widely naturalized in many countries worldwide. The great morphological variation in D. carota has resulted in more than 60 infraspecific taxa, making D. carota the most problematic species group in the Apiaceae (Heywood 1968a, b; Small 1978; Thellung 1926). Cultivated carrots and closely related wild carrots (other subspecies and varieties of D. carota sensu lato) belong to the Daucus carota complex. Its constituent taxa all possess 2n = 18 chromosomes and have weak biological barriers to interbreeding. D. carota undergoes widespread hybridization experimentally and spontaneously with commercial varieties of carrot and the wild subspecies of D. carota (e.g., Ellis et al. 1993; Hauser 2002; Hauser and Bjørn 2001; Krickl 1961; McCollum 1975, 1977; Nothnagel et al. 2000; Rong et al. 2010; Sáenz de Rivas and Heywood 1974; Steinborn et al. 1995; St. Pierre and Bayer 1991; St. Pierre et al. 1990; Umiel et al. 1975; Vivek and Simon 1999; Wijnheijmer et al. 1989). In addition, there are other closely related wild species with 2n = 18 chromosomes (D. sahariensis, D. syrticus) based on shared karyotypes (Iovene et al. 2008), the genus-level phylogenetic studies summarized above, and they represent gene pool 1 species to cultivated carrot. The haploid chromosome number for the genus Daucus (sensu stricto) ranges from n = 8 to n = 11. In addition to the n = 8 diploid species, diploid chromosome numbers in Daucus range from 2n = 16 to 22, and a tetraploid (D. glochidiatus) and a hexaploid (D. montanus) species have been reported (Table 2.1).
To put the taxonomic problem of the Daucus carota complex into historical context, several molecular approaches have examined its diversity and genetic relationships. St. Pierre et al. (1990) used isozymes to study 168 accessions of the D. carota complex from 32 countries and could not separate named subspecies into distinct groups. Nakajima et al. (1998) used random amplified polymorphic DNA (RAPD) and amplified fragment length polymorphism (AFLP) data and showed all accessions of D. carota group into a major clade. Vivek and Simon (1998, 1999) used restriction fragment length polymorphisms (RFLPs) of nuclear, plastid, and mitochondrial DNA and interpreted their results to be generally concordant with the classification proposed by Sáenz Laín (1981), but studied just one additional subspecies (subsp. drepanensis). Using AFLPs, Shim and Jørgensen (2000) showed wild and cultivated carrot clustered separately. Bradeen et al. (2002) used AFLPs and intersimple sequence repeats (ISSR) and concluded wild carrots had no substructure. Rong et al. (2014) obtained a Daucus phylogeny using SNPs and found the subspecies of D. carota to be intermixed with each other. Lee and Park (2014) proposed D. sahariensis, D. syrticus, and D. gracilis to be the likely closest relatives to D. carota. In an attempt to characterize the populations of D. carota present in São Miguel Island (Azores, Portugal), Matias Vaz (2014) used one nuclear ortholog, nuclear ribosomal DNA ITS, and morphological descriptors and concluded that the classification of D. carota remained problematic. Other morphological studies (Arbizu et al. 2014a; Mezghani et al. 2014; Small 1978; Spooner et al. 2014; Tavares et al. 2014) likewise not distinguish the subspecies of D. carota. However, Iorizzo et al. (2013) used 3326 single nucleotide polymorphisms (SNPs) to study the genetic structure and domestication of carrot and found a clear separation between wild (subsp. carota) and cultivated (subsp. sativus) accessions of D. carota.
These taxonomic problems have practical considerations for germplasm curators and taxonomists who have relied on local floras for identifying these taxa such as floras from Algeria (Quézel and Santa 1963), the Azores (Schäfer 2005), Europe (Heywood 1968b), the Iberian Peninsula and Balearic Islands (Pujadas Salvà 2003), Libya (Jafri and El-Gadi 1985), Morocco (Jury 2002), Palestine (Zohary 1972), Portugal (Franco 1971), Syria (Mouterde 1966), Tunisia (Le Floc’h et al. 2010; Pottier-Alapetite 1979), and Turkey and the East Aegean Islands (Cullen 1972). Unfortunately, the keys and descriptions in these floras lack consensus about both the number of infraspecific taxa and characters best distinguishing them. For instance, 11 wild subspecies were recognized by Heywood (1968a, b), five by Sáenz Laín (1981: subsp. carota, subsp. gummifer, subsp. hispanicus, subsp. maritimus, and subsp. maximus), five by Arenas and García-Martin (1993), and Pujadas Salvà (2002) proposed nine subspecies for the Iberian Peninsula plus Balearic Islands (subsp. carota, subsp. cantabricus, subsp. commutatus, subsp. gummifer, subsp. halophilus, subsp. hispanicus, subsp. majoricus, subsp. maximus, and subsp. sativus).
Molecular investigations are trying to resolve the natural taxa in D. carota. “Reduced-representation” methods obtain partial DNA polymorphisms throughout the genome and have been shown to be very useful at the species level. Genotyping-by-sequencing (GBS) is one such reduced-representation method that generates sequence variants or single nucleotide polymorphisms (SNPs) (Elshire et al. 2011). GBS provides a powerful and cost-effective molecular approach for phylogeny reconstruction, producing abundant large-scale genomic data to infer phylogenetic relationships among recently diverged species or populations (e.g., Balfourier et al. 2007; Escudero et al. 2014; Good 2011; Wong et al. 2015). It captures both neutral genetic diversity and loci that affect quantitative traits of interest, because of the full-genome coverage of the GBS markers. It shows little to no ascertainment bias because markers are developed directly on the population being genotyped. Genetic relatedness among genotypes calculated using GBS markers is based on patterns of neutral and functional genetic variation across the genome.
Arbizu et al. (2016a) used GBS to examine the subspecies of D. carota. They obtained SNPs covering all nine D. carota chromosomes from 162 accessions of Daucus and related genera. They scored a total of 10,814 or 38,920 SNPs with a maximum of 10 or 30% missing data, respectively. Consistent with prior results, the phylogenetic tree separated species with 2n = 18 chromosome from all other species in a single clade. Most interestingly, there was a strong geographic component to this phylogeny, with the wild members of D. carota from central Asia in a clade with eastern members of subsp. sativus. The other subspecies of D. carota were in four clades associated with geographic groups, suggesting that the subspecies are not natural groups. In summary, the wide range of morphological and molecular studies summarized above documents poor substructure of either morphologically or phylogenetically stable groups in D. carota. These results were concordant with results from recent morphological studies that led Spooner et al. (2014) to question whether many wild subspecies recognized within D. carota are valid taxa.
2.4 Conclusions
In summary, the taxonomy of Daucus at both the genus and species levels has been improved markedly in the last years by a series of morphological and molecular studies. Earlier studies using limited sets of plastid and nuclear markers have shown nuclear ribosomal ITS to be the most useful marker. Next-generation sequencing techniques are corroborating many of these studies, but adding details, especially cautioning combining nuclear and plastid data in combined data approaches. The phylogenetic study of Banasiak et al. (2016) has clarified ingroup and outgroup relationships and has resulted in an expanded concept of the genus. Continuing studies at the species and genus levels with NGS data and with additional collections are helping to refine our understanding of Daucus and should eventually lead to a much needed formal taxonomic revision taking into account phylogeny, keys, descriptions, illustrations, typifications, distributions, and maps.
References
Arbizu C, Ellison SL, Senalik D, Simon PW, Spooner DM (2016a) Genotyping-by-sequencing provides the discriminating power to investigate the subspecies of Daucus carota (Apiaceae). BMC Evol Biol 16:234
Arbizu C, Reitsma KR, Simon PW, Spooner DM (2014a) Morphometrics of Daucus (Apiaceae): a counterpart to a phylogenomic study. Amer J Bot 101:2005–2016
Arbizu C, Ruess H, Senalik D, Simon PW, Spooner DM (2014b) Phylogenomics of the carrot genus (Daucus, Apiaceae). Amer J Bot 101:1666–1685
Arbizu C, Simon PW, Martínez-Flores F, Ruess H, Crespo MB, Spooner DM (2016b) Integrated molecular and morphological studies of the Daucus guttatus complex (Apiaceae). Syst Bot 41:479–492
Arenas JA, García-Martin F (1993) Atlas carpológico y corológico de la subfamilia Apioideae Drude (Umbelliferae) en España peninsular y Baleares. Ruizia 12:222–234
Ashlock PD (1971) Monophyly and associated terms. Syst Zool 20:63–69
Balfourier F, Roussel V, Strelchenko P, Exbrayat-Vinson F, Sourdille P, Boutet G, Koenig J, Ravel C, Mitrofanova O, Beckert M, Charmet G (2007) A worldwide bread wheat core collection arrayed in a 384-well plate. Theor Appl Genet 114:1265–1275
Banasiak Ł, Piwczyński M, Uliński T, Downie SR, Watson MF, Shakya B, Spalik K (2013) Dispersal patterns in space and time: a case study of Apiaceae subfamily Apioideae. J Biogeor 40:1324–1335
Banasiak Ł, Wojewódzka A, Baczyński J, Reduron J-P, Piwczyński M, Kurzyna-Młynik R, Gutaker R, Czarnocka-Cieciura A, Kosmala-Grzechnik S, Spalik K (2016) Phylogeny of Apiaceae subtribe Daucinae and the taxonomic delineation of its genera. Taxon 65:563–585
Bradeen JM, Bach IC, Briard M, le Clerc V, Grzebelus D, Senalik DA, Simon PW (2002) Molecular diversity analysis of cultivated carrot (Daucus carota L.) and wild Daucus populations reveals a genetically nonstructured composition. J Amer Soc Hort Sci 127:383–391
Constance L (1971) History and classification of the Umbelliferae (Apiaceae). In: Heywood VH (ed) The biology and chemistry of the Umbelliferae. Academic Press, London, pp 1–11
Cullen J (1972) Daucus. In: Davis PH (ed) Flora of Turkey and the East Aegean Islands. Edinburgh University Press, Edinburgh, pp 531–536
Doolittle WF (1999) Lateral genomics. Trends Cell Biol 9:M5–M8
Downie SR, Jansen RK (2015) A comparative analysis of whole plastid genomes from the Apiales: expansion and contraction of the inverted repeat, mitochondrial to plastid transfer of DNA, and identification of highly divergent noncoding regions. Syst Bot 40:336–351
Downie SR, Katz-Downie DS (1996) A molecular phylogeny of Apiaceae subfamily Apioideae: evidence from nuclear ribosomal DNA internal transcribed spacer sequences. Amer J Bot 83:234–251
Downie SR, Katz-Downie DS, Cho KJ (1996) Phylogenetic analysis of Apiaceae subfamily Apioideae using nucleotide sequences from the chloroplast rpoC1 intron. Mol Phylo Evol 6:1–18
Downie SR, Katz-Downie DS, Spalik K (2000a) A phylogeny of Apiaceae tribe Scandiceae: evidence from nuclear ribosomal DNA internal transcribed spacer sequences. Amer J Bot 87:76–95
Downie SR, Katz-Downie DS, Watson MF (2000b) A phylogeny of the flowering plant family Apiaceae based on chloroplast DNA rpl16 and rpoC1 intron sequences: towards a suprageneric classification of subfamily Apioideae. Amer J Bot 87:273–292
Downie SR, Plunket GM, Watson MF, Spalik K, Katz- Downie DS, Valiejo-Roman CM, Terentieva EI, Troitsky AV, Lee BY, Lahham J, El-Oqlah A (2001) Tribes and clades within Apiaceae subfamily Apioideae: the contribution of molecular data. Edinb J Bot 58:301–330
Downie SR, Ramanath S, Katz-Downie DS, Llanas E (1998) Molecular systematics of Apiaceae subfamily Apioideae: phylogenetic analyses of nuclear ribosomal DNA internal transcribed spacer and plastid rpoC1 intron sequences. Amer J Bot 85:563–591
Downie SR, Spalik K, Katz-Downie DS, Reduron JP (2010) Major clades within Apiaceae subfamily Apioideae as inferred by phylogenetic analysis of nrDNA ITS sequences. Plant Diver Evol 128:111–136
Downie SR, Watson MF, Spalik K, Katz-Downie DS (2000c) Molecular systematics of Old World Apioideae (Apiaceae): relationships among some members of tribe Peucedaneae sensu lato, the placement of several island endemic species, and resolution within the apioid superclade. Canad J Bot 78:506–528
Drude CGO (1897–1898) Umbelliferae. In: Engler A, Prantl K (eds) Die natürlichen Pflanzenfamilien, vol. 3, no. 8. Engelmann, Leipzig, p 63–250
Egan A, Schlueter J, Spooner DM (2012) Applications of next-generation sequencing in plant biology. Amer J Bot 99:175–185
Ellis P, Hardman J, Crowther T, Saw P (1993) Exploitation of the resistance to carrot fly in the wild carrot species Daucus capillifolius. Ann Appl Biol 122:79–91
Elshire RJ, Glaubitz JC, Sun Q, Poland JA, Kawamoto K, Buckler ES, Mitchell SE (2011) A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS ONE 6:e19379
Escudero M, Eaton DAR, Hahn M, Hipp AL (2014) Genotyping-by-sequencing as a tool to infer phylogeny and ancestral hybridization: a case study in Carex (Cyperaceae). Mol Phylo Evol 79:359–367
Franco JA (1971) Nova flora de Portugal (Continente e Açores). Sociedade Astória, Lisbon
Good JM (2011) Reduced representation methods for subgenomic enrichment and next-generation sequencing. In: Orgogozo V, Rockman MV (eds) Molecular methods for evolutionary genetics, methods in molecular biology. Springer, New York, pp 85–103
Graybeal A (1998) Is it better to add taxa or characters to a difficult phylogenetic problem? Syst Biol 47:9–17
Hauser TP (2002) Frost sensitivity of hybrids between wild and cultivated carrots. Conserv Genet 3:73–76
Hauser TP, Bjørn GK (2001) Hybrids between wild and cultivated carrots in Danish carrot fields. Genet Res Crop Evol 48:499–506
Hennig W (1966) Phylogenetic systematics (trans: Davis DD & Zangerl R). University of Illinois Press, Urbana
Heywood VH (1968a) The Daucus carota-gingidium complex. Feddes Rep 79:66–68
Heywood VH (1968b) Daucus. In: Tutin TG, Heywood VH, Burges NA, Valentine DH, Walters SM, Webb DA (eds) Flora Europaea. Cambridge University Press, Cambridge, pp 373–375
Heywood VH (1978) Multivariate synthesis of the tribe Caucalideae. In: Cauwet-Marc A-M, Carbonnier J (eds) Actes du 2ème Symposium International sur les Ombellifères, CNRS, Perpignan, p 727–736
Heywood VH (1983) Relationships and evolution in the Daucus carota complex. Israel J Bot 32:51–65
Heywood VH (2014) The socio-economic importance of the Apiales. J Fac Pharm Istanbul 44:113–130
Iorizzo M, Ellison S, Senalik D, Zeng P, Satapoomin P, Bowman M, Iovene M, Sanseverino W, Cavagnaro P, Yildiz M, Macko-Podgórni A, Moranska E, Grzebelus E, Grzebelus D, Ashrafi H, Zheng Z, Cheng S, Spooner D, Van Deynze A, Simon P (2016) A high-quality carrot genome assembly reveals new insights into carotenoid accumulation and asterid genome evolution. Nat Genet 48:657–666
Iorizzo M, Senalik DA, Ellison SL, Grzebelus D, Cavagnaro PF, Allender C, Brunet J, Van Deynze A (2013) Genetic structure and domestication of carrot (Daucus carota subsp. sativus) (Apiaceae). Amer J Bot 100:930–938
Iovene M, Grzebelus E, Carputo D, Jiang J, Simon PW (2008) Major cytogenetic landmarks and karyotype analysis in Daucus carota and other Apiaceae. Amer J Bot 95:793–804
Jafri SMH, El-Gadi A (1985) Daucus. In: Jafri SMH, El-Gadi A (eds) Flora of Libya. Al-Faateh University, Faculty of Science, Department of Botany Tripoli, p 130–144
Jongejans E, Telenius A (2001) Field experiments on seed dispersal by wind in ten umbelliferous species (Apiaceae). Pl Ecol 152:67–78
Judd WS, Campbell CS, Kellogg EA, Stevens PF, Donoghue MJ (2016) Plant systematics: a phylogenetic approach. Sinauer Associates Inc, Sunderland, Massachusetts
Jury SL (1982) Tuberculate fruits in the Umbelliferae. In: Cauwet-Marc AM, Carbonnier J (eds) Actes du 2ème symposium international sur les ombellifères “contributions pluridisciplinaires à la systématique”. Braun- Brumfield, Ann Arbor, pp 149–159
Jury SL (2002) Daucus. In: Valdés B, Rejdali M, Achhal El, Kadmiri A, Jury JL, Montserrat JM (eds) Catalogue des plantes vasculaires du Nord de Maroc, incluant des clés d’identification. Consejo Superior de Investigaciones Científicas, Madrid, p 467–469
Katz-Downie DS, Valiejo-Roman CM, Terentieva EI, Troitsky AV, Pimenov MG, Lee B, Downie SR (1999) Towards a molecular phylogeny of Apiaceae subfamily Apioideae: additional information from nuclear ribosomal DNA ITS sequences. Plant Syst Evol 216:167–195
Krickl M (1961) Karotten: Zur Frage der Verkreuzung mit der wilden Karotte. Saatgut-Wirtschaft 13:135–136
Le Floc’h É, Boulos L, Véla E (2010) Catalogue synonymique commenté de la flore de Tunisie. République Tunisienne, Ministère de l’Environnement Et Du Développment Durable Banque Nationale de Gènes, Tunis
Lee BY (2002) Taxonomic review on the African Umbelliferous genus Agrocharis: inferences based on molecular data. Israel J Plant Sci 50:211–216
Lee BY, Downie SR (1999) A molecular phylogeny of Apiaceae tribe Caucalideae and related taxa: inferences based on ITS sequence data. Syst Bot 24:461–479
Lee BY, Downie SR (2000) Phylogenetic analysis of cpDNA restriction sites and rps16 intron sequences reveals relationships among Apiaceae tribes Caucalideae, Scandiceae and related taxa. Plant Syst Evol 221:35–60
Lee C-S, Downie SR (2006) Phylogenetic relationships within Cicuta (Apiaceae tribe Oenantheae) inferred from nuclear rDNA ITS and cpDNA sequence data. Canad J Bot 84:453–468
Lee BY, Park C-W (2014) Molecular phylogeny of Daucus (Apiaceae): evidence from nuclear ribosomal DNA ITS sequences. J Spec Res 3:39–52
Lemmon EM, Lemmon AR (2013) High-throughput genomic data in systematics and phylogenetics. Annu Rev Ecol Evol Syst 44:99–121
Liu J, Shi L, Han J, Li G, Lu H, Hou J, Zhou X, Meng F, Downie SR (2014) Identification of species in the angiosperm family Apiaceae using DNA barcodes. Mol Ecol Resour 14:1231–1238
Maddison WP (1995) Phylogenetic histories within and among species. In: Hoch PC, Stevenson AG (eds) Experimental and molecular approaches to plant biosystematics Monographs in systematics. Botanical Garden, St. Louis, Missouri, pp 273–287
Martínez-Flores F (2016) Sistemática del género Daucus L. (Apiaceae): implicaciones taxonómicas y filogenéticas. Doctoral thesis, Universidad de Alicante, Spain
Martínez-Flores F, Arbizu CI, Reitsma K, Juan A, Simon PW, Spooner DM, Crespo MB (2016) Lectotype designation for seven species names in the Daucus guttatus complex (Apiaceae) from the central and eastern Mediterranean basin. Syst Bot 41:464–478
Matias Vaz AM (2014) Estudo morfológico e filogenético das subespécies Daucus carota ssp. azoricus e Daucus carota ssp. maritimus na ilha de S. Miguel. M.Sc. thesis, Universidade dos Açores, Ponta delgada
McCollum GD (1975) Interspecific hybrid Daucus carota × D. capillifolius. Bot Gaz 136:201–206
McCollum GD (1977) Hybrids of Daucus gingidium with cultivated carrots (D. carota subsp. sativus) and D. capillifolius. Bot Gaz 138:56–63
Mezghani N, Zaouali I, Amri WB, Rouz S, Simon PW, Hannachi C, Ghrab Z, Neffati M, Bouzbida B, Spooner DM (2014) Fruit morphological descriptors as a tool for discrimination of Daucus L. germplasm. Genet Res Crop Evol 61:499–510
Mouterde P (1966) Nouvelle Flore de Liban et de la Syrie. Dar El-Machreq, Beirut
Nakajima Y, Oeda K, Yamamoto T (1998) Characterization of genetic diversity of nuclear and mitochondrial genomes in Daucus varieties by RAPD and AFLP. Plant Cell Rep 17:848–853
Nothnagel T, Straka P, Linke B (2000) Male sterility in populations of Daucus and the development of alloplasmic male-sterile lines of carrot. Plant Breed 119:145–152
Page RD, Charleston MA (1997) From gene to organismal phylogeny: reconciled trees and the gene tree/species tree problem. Mol Phylo Evol 7:231–240
Pamilo P, Nei M (1988) Relationships between gene trees and species trees. Mol Biol Evol 5:568–583
Plunkett GM, Downie SR (1999) Major lineages within Apiaceae subfamily Apioideae: a comparison of chloroplast restriction site and DNA sequence data. Amer J Bot 86:1014–1026
Plunkett GM, Pimenov MG, Reduron J-P, Kljuykov EV, van Wyk B-E, Ostroumova TA, Henwood MJ, Tilney PM, Spalik K, Watson MF, Lee B-Y, Pu F-D, Webb CJ, Hart JM, Mitchell AD, Muckensturm B (in press) Apiaceae. In: Kadereit JW, Bittrich V (eds) The families and genera of vascular plants, vol. 15. Springer, Berlin, Germany
Plunkett GM, Soltis DE, Soltis PS (1996a) Evolutionary patterns in Apiaceae: inferences based on matK sequence data. Syst Bot 21:477–495
Plunkett GM, Soltis DE, Soltis PS (1996b) Higher level relationships of Apiales (Apiaceae and Araliaceae) based on phylogenetic analysis of rbcL sequences. Amer J Bot 83:499–515
Pottier-Alapetite G (1979) Daucus. In: Pottier-Alapetite G (ed) Flore de la Tunisie: angiospermes-Dicotylédones (Apetales—Dialypetales). Ministère de l’Enseignement Supérieur et de la Recherche Scientifique et le Ministère de l’Agriculture, Tunis, pp 615–621
Pujadas Salvà AJ (2002) El complejo de Daucus carota L. (Apiaceae) en la flora Ibérica. Anales Jard Bot Madrid 59:368–375
Pujadas Salvà AJ (2003) Daucus. In: Nieto-Feliner G, Jury SL, Herrero A (eds) Flora Iberica: plantas vasculares de la Península Ibérica e islas Baleares. Anales Jard Bot Madrid CSIC 97–125
Quézel P, Santa S (1963) Daucus. In: Quézel P, Santa S (eds) Nouvelle Flore de L’Algérie et des Régions Désertiques Méridionales. Éditions du Centre National de la Recherche Scientifique, Paris, p 659–663
Raubeson LA, Jansen RK (2005) Chloroplast genomes of plants. In: Henry RJ (ed) Plant diversity and evolution: phenotypic variation in higher plants. CABI Publishing, Wallingford, UK, p 45–68
Rieseberg LH, Soltis DE (1991) Phylogenetic consequences of cytoplasmic gene flow in plants. Evol Trends Plant 5:65–84
Rong J, Janson S, Umehara M, Ono M, Vrieling K (2010) Historical and contemporary gene dispersal in wild carrot (Daucus carota ssp. carota) populations. Ann Bot 106:285–296
Rong J, Lammers Y, Strasburg JL, Schidlo NS, Ariyurek Y, de Jong TJ, Klinkhame PGL, Smulders MJM, Vrieling K (2014) New insights into domestication of carrot from root transcriptome analyses. BMC Genom 15:895
Rubatzky VE, Quiros CF, Simon PW (1999) Carrots and related vegetable Umbelliferae. CABI, New York, NY, USA
Ruhlman T, Lee S-B, Jansen RK, Hostetler JB, Tallon LJ, Town CD, Daniell H (2006) Complete plastid genome sequence of Daucus carota: implications for biotechnology and phylogeny of angiosperms. BMC Genom 7:222
Sáenz de Rivas C, Heywood VH (1974) Preliminary study on the Daucus species of peninsular Spain. An Jard Bot AJ Canavilles 31:97–118
Sáenz Laín C (1981) Research on Daucus L. (Umbelliferae). An Jard Bot Madrid 37:481–534
Schäfer H (2005) Flora of the Azores. Margraf Publishers, Weikersheim
Shim SI, Jørgensen RB (2000) Genetic structure in cultivated and wild carrots (Daucus carota L.) revealed by AFLP analysis. Theor Appl Genet 101:227–233
Simon PW (2000) Domestication, historical development, and modern breeding of carrot. Plant Breed Rev 19:157–190
Small E (1978) A numerical taxonomic analysis of the Daucus carota complex. Canad J Bot 56:248–276
Small RL, Cronn RC, Wendel JF (2004) Use of nuclear genes for phylogeny reconstruction in plants. Aust Syst Bot 17:145–170
Spalik K, Downie SR (2007) Intercontinental disjunctions in Cryptotaenia (Apiaceae, Oenantheae): an appraisal using molecular data. J Biogeogr 34:2039–2054
Spalik K, Piwczyński M, Danderson CA, Kurzyna-Młynik R, Bone TS, Downie SR (2010) Amphitropic amphiantarctic disjunctions in Apiaceae subfamily Apioideae. J Biogeogr 37:1977–1994
Spalik K, Wojewódzka A, Downie SR (2001a) The evolution of fruit in Scandiceae subtribe Scandicinae (Apiaceae). Canad J Bot 79:1358–1374
Spalik K, Wojewódzka A, Downie SR (2001b) Delimitation of genera in Apiaceae with examples from Scandiceae subtribe Scandicinae. Edinb J Bot 58:331–346
Spooner DM, Rojas P, Bonierbale M, Mueller LA, Srivastav M, Senalik D, Simon P (2013) Molecular phylogeny of Daucus (Apiaceae). Syst Bot 38:850–857
Spooner DM, Ruess H, Iorizzo M, Senalik D, Simon P (2017) Entire plastid phylogeny of the carrot genus (Daucus, Apiaceae): concordance with nuclear data and mitochondrial and nuclear DNA insertions to the plastid. Amer J Bot 104:296–312
Spooner DM, Widrlechner MP, Reitsma KR, Palmquist DE, Rouz S, Ghrabi-Gammar Z, Neffati M, Bouzbida B, Ouabbou H, El Koudrim M, Simon PW (2014) Reassessment of practical subspecies identifications of the USDA Daucus carota germplasm collection: morphological data. Crop Sci 54:706–718
Steinborn R, Linke B, Nothnagel T, Börner T (1995) Inheritance of chloroplast and mitochondrial DNA in alloplasmic forms of the genus Daucus. Theor Appl Genet 91:632–638
St. Pierre MD, Bayer RJ (1991) The impact of domestication on the genetic variability in the orange carrot, cultivated Daucus carota ssp. sativus and the genetic homogeneity of various cultivars. Theor Appl Genet 82:249–253
St. Pierre MD, Bayer RJ, Weiss IM (1990) An isozyme-based assessment of the genetic variability within the Daucus carota complex (Apiaceae: Caucalideae). Canad J Bot 68:2449–2457
Stuessy TF, Hörandl E (2014) Evolutionary systematics and paraphyly: introduction. Ann Missouri Bot Gard 100:2–5
Stuessy TF, König C, López Sepúlveda P (2014) Paraphyly and endemic genera of oceanic islands: implications for conservation. Ann Missouri Bot Gard 100:50–78
Tavares AC, Loureiro J, Castro S, Coutinho AP, Paiva J, Cavaleiro C, Salgueiro L, Canhoto JM (2014) Assessment of Daucus carota L. (Apiaceae) subspecies by chemotaxonomic and DNA content analyses. Biochem Syst Ecol 55:222–230
Thellung A (1926) Daucus. In: Hegi G (ed) Illustrierte Flora von Mitteleuropa. J. F. Lehmanns Verlag, München, pp 1501–1526
Theobald WL (1971) Comparative anatomical and developmental studies in the Umbelliferae. In: Heywood VH (ed) The biology and chemistry of the Umbelliferae. Academic Press, London, pp 177–197
Townsend CC (1989) Flora of tropical East Africa: Umbelliferae. Balkema, Rotterdam
Umiel N, Jacobsen R, Globerson D (1975) Pollination of the cultivated carrot (Daucus carota L.) by the wild carrot (D. carota var. maximus), and its implications on commercial seed production. Hassadeh 56:478–480. (In Hebrew, English summary)
Vivek BS, Simon PW (1998) Genetic relationships and diversity in carrot and other Daucus taxa based on nuclear restriction fragment length polymorphisms. J Amer Soc Hort Sci 123:1053–1057
Vivek BS, Simon PW (1999) Phylogeny and relationships in Daucus based on restriction fragment length polymorphisms (RFLPs) of the chloroplast and mitochondrial genomes. Euphytica 105:183–189
Weitzel C, Rønsted N, Spalik K, Simonsen HT (2014) Resurrecting deadly carrots: towards a revision of Thapsia (Apiaceae) based on phylogenetic analysis of nrITS sequences and chemical profiles. Bot J Linn Soc 174:620–636
Wendel JF, Doyle JJ (1998) Phylogenetic incongruence: window into genome history and molecular evolution. In: Soltis DE, Soltis PS, Doyle JJ (eds) Molecular systematics of plants II: DNA sequencing. Chapman and Hall, Kluwer, Boston, pp 265–296
Wijnheijmer EHM, Brandenburg WA, Ter Borg SJ (1989) Interactions between wild and cultivated carrots (Daucus carota L.). Euphytica 40:147–154
Williams CF (1994) Genetic consequences of seed dispersal in three sympatric forest herbs. II. Microspatial genetic structure within populations. Evolution 48:1959–1972
Wolfe KH, Li WH, Sharp PM (1987) Rates of nucleotide substitution vary greatly among plant mitochondrial, chloroplast, and nuclear DNAs. Proc Natl Acad Sci USA 84:9054–9058
Wong MML, Gujaria-Verma N, Ramsay L, Yuan HY, Caron C, Diapari M, Vandenberg A, Bett KE (2015) Classification and characterization of species within the genus Lens using genotyping-by-sequencing (GBS). PLoS ONE 10:e122025
Yi T-S, Jin G-H, Wen J (2015) Chloroplast capture and intra- and inter-continental biogeographic diversification in the Asian—New World disjunct plant genus Osmorhiza (Apiaceae). Mol Phylo Evol 85:10–21
Zhou J, Gong X, Downie SR, Peng H (2009) Towards a more robust molecular phylogeny of Chinese Apiaceae subfamily Apioideae: additional evidence from nrDNA ITS and cpDNA intron (rpl16 and rps16) sequences. Mol Phylo Evol 53:56–68
Zohary M (1972) Flora Palaestina. Israel Academy of Sciences and Humanities, Jerusalem
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Spooner, D.M. (2019). Daucus: Taxonomy, Phylogeny, Distribution. In: Simon, P., Iorizzo, M., Grzebelus, D., Baranski, R. (eds) The Carrot Genome. Compendium of Plant Genomes. Springer, Cham. https://doi.org/10.1007/978-3-030-03389-7_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-03389-7_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-03388-0
Online ISBN: 978-3-030-03389-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)