Abstract
Doxorubicin and daunorubicin are notable members of the type II polyketide synthase family and clinically important cancer chemotherapeutic agents and are produced by a mutant strain S. peucetius ATCC 27952. They belong to the anthracycline-type antitumor drugs. Doxorubicin remains one of the most widely used antitumor drugs for the treatment of various cancers because of its broad spectrum of activity. As a result, numerous works have been carried to unravel the biosynthetic pathway and the underlying regulatory mechanisms to gain insight into the mechanisms of the genes involved. Consenquently, there is a need to develop an overproducing strain at the industrial scale, to produce doxorubicin as an anticancer drug. Therefore a significant amount of progress has been made in unraveling the bottlenecks in the pathway, manipulating the biosynthesis, improving production, and generating novel derivatives by engineering S. peucetius strain.
Here we review in depth, various pathway engineering approaches and strategies that have been applied during these courses of time, since the discovery of these compounds, for the efficient production of daunorubicin and doxorubicin. The major pathway engineering approaches discussed in this chapter are divided into three parts: the first part includes the engineering of the thymidine diphosphate-l-daunosamine biosynthesis pathway genes which is important for the enhanced production of the glycone which in turn is used for the glycosylation reaction. Similarly the second part includes the engineering of the polyketide genes responsible for the production of the aglycone moiety that undergoes several modifications to generate the important compounds doxorubicin and daunorubicin. Lastly, we discuss the engineering of the several regulatory genes involved either directly or indirectly in regulation and control of the production of daunorubicin and doxorubicin.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Daunorubicin
- Doxorubicin
- Anthracycline
- Anticancer drug
- Polyketide synthase
- Biosynthetic pathway
- Pathway engineering
- Streptomyces peucetius
7.1 Introduction
Streptomyces belonging to the group of actinomycetes are filamentous gram-positive bacteria that undergo morphological and physiological differences to produce a wide range of secondary natural products. The soil-dwelling Streptomyces are the key producers of numerous molecules including antibiotics , antivirals, anticancer, and other bioactive molecules, owing to their secondary metabolism. These products may include useful therapeutic agents, such as antibiotics, antifungals, and antitumor , and thus a better understanding of the biosynthetic pathway and the regulatory mechanism of genes at the molecular level would provide useful insights into the fundamental issue of secondary metabolism in Streptomyces spp., ultimately helping to engineer strains for overproduction of these useful metabolites (Hao and Hutchinson 2006). Polyketide secondary metabolites in microbes are mainly produced via various polyketide synthases (type I, type II, type III) and non-ribosomal polyketide synthases (NRPKS) enzymes. Among these, type II polyketide synthases consist of a significant and chemically diverse group of bacterial secondary metabolites, such as tetracyclines and actinorhodin produced by S. coelicolor; rhodomycinone, doxorubicin, and daunorubicin produced by S. peucetius; jadomycin A produced by S. venezuelae; pradimicins produced by Actinomadura sp.; charteusin produced by S. lysosuperificus; chromomycin produced by Actinomyces aburaviensis var. verrucosus; and many more (Fig. 7.1).
Doxorubicin and daunorubicin are notable members of the type II polyketide synthase family, and clinically important cancer chemotherapeutic agents. These molecules are produced by Streptomyces peucetius ATCC 27952 which is a mutant strain of S. peucetius 29050 (Arcamone et al. 1969). Daunorubicin was discovered in 1962, when it was first isolated from Streptomyces caeroleorubidus in France; however, it was also isolated from S. peucetius in Italy and the Soviet Union with different names (Aubel-Sadron and Londos-Gagliardi 1984), such as rubidomycin, daunomycin, and rubomycin. Daunorubicin is reported to be produced from a number of organisms; however, doxorubicin was exclusively produced by S. peucetius subsp. caesius (Grein 1987). Doxorubicin is a chemotherapy medication belonging to the anthracycline and antitumor drug family and is also known by its trade name Adriamycin. It is routinely used in the treatment of numerous human cancers, including breast, ovarian, liver, lung, bladder, gastric, and thyroid cancers, multiple myeloma, non-Hodgkin’s and Hodgkin’s lymphoma, Kaposi’s sarcoma, neuroblastoma, soft tissue sarcoma, and pediatric cancers (Cortes-Funes and Coronado 2007; Thorn et al. 2011). Because of its broad spectrum of activity, doxorubicin remains one of the most widely used antitumor drugs for the treatment of various cancers (Allwood et al. 2002).
The biosynthesis of both daunorubicin and doxorubicin is initiated by a type II polyketide synthase starting from one propionyl-CoA starter unit and extended by nine malonyl-CoA units, to produce a decaketide that is converted to aklanonic acid that leads to the formation of an aglycone ε-rhodomycinone (Hutchinson 1997). The aglycone of daunorubicin, ε-rhodomycinone, is a tetracyclic ring consisting of quinone-hydroquinone groups lying adjacent to each other, along with a methoxy group, a short carbonyl side chain. The sugar is attached to aglycone by a glycosidic bond and is known as l-daunosamine, which consists of 3-amino-2, 3, 6-trideoxy-l-fucosyl moiety, and is synthesized from d-glucose 1-phosphate using a variety of genes. Finally, series of post-modifications, like methylation, decarboxylation, and hydroxylation, leads to the formation of daunorubicin and ultimately doxorubicin. Doxorubicin is the C-14 hydroxylated form of its immediate precursor, daunorubicin. The only difference between these two molecules is the side chain of doxorubicin, which terminates with a primary alcohol, whereas daunorubicin terminates with a methyl group (Fig. 7.1) (Minotti et al. 2004).
S. peucetius has a self-resistance system that helps it to overcome the toxicity of the antibiotic daunorubicin and doxorubicin inside the cell. The four genes, namely, drrA, drrB, drrC, and drrD, present in the doxorubicin biosynthetic gene cluster of S. peucetius mediate the self-resistance. The first two genes drrA and drrB belong to ABC transporter type I and together form an ATP-dependent efflux pump to remove daunorubicin out of the cell (Brown et al. 2017; Guilfoile and Hutchinson 1991; Kaur and Russell 1998), whereas the third gene, drrC, imparts resistance through excisional repair by binding to DNA at regions intercalated by daunorubicin and then removing it (Prija and Prasad 2017). DrrD is a flavin-binding protein involved in the self-resistance mechanism, and DrrD devoid mutant exhibits partial loss of self-resistance to daunorubicin (Karuppasamy et al. 2015).
7.1.1 Objective
Doxorubicin and daunorubicin have been of interest since their discovery in 1962 and their use as a potent anticancer drug in various forms of cancer. There has been significant work involving the enhanced production of these important metabolites from S. peucetius using various approaches. The drive to develop a recombinant strain of industrial importance for mass production of this anticancer drug has been addressed in this chapter. Using the fermentation process combined with pathway engineering strategies and engineering the regulatory genes with modifications at the molecular level have been discussed and explained in this chapter thus providing an overview of the doxorubicin and daunorubicin biosynthesis in S. peucetius.
7.1.2 Mode of Action of Doxorubicin
Doxorubicin is one of the most potent US Food and Drug Administration-approved anthracycline classes of anticancer agents. It exerts its antiproliferative activity on tumor cells via three proposed mechanisms: (1) DNA binding by intercalation between DNA double helix and disrupt DNA replication and transcription process, (2) disruption of topoisomerase-II-dependent DNA repair, and (3) production of free radicals ultimately damaging cell components such as cell membranes, nucleic acids, and proteins (Fig. 7.2). Collectively, these modes of actions result in DNA disruption and loss of DNA mismatch repair function that ultimately leads to cell death (Gewirtz 1999; Thorn et al. 2011). However, cardiotoxicity is the major factor limiting its medicinal use as it alters iron and calcium regulations in mitochondria (Swain et al. 2003; Carvalho et al. 2009).
Mode of action of doxorubicin (DXR) inside the cancer cell. Doxorubicin interacts with the DNA by intercalation, disruption of topoisomerase-II-dependent DNA repair, and inhibition of the replication and transcription process. Doxorubicin is oxidized to doxorubicin semiquinone which is unstable intermediate and converted back to DXR-releasing reactive oxygen species (ROS) that causes oxidative stress and induces damage to cell membrane, lipid peroxidation, and DNA damage leading to cell death
Doxorubicin and most of the anthracycline class of compounds intercalate between deoxyribonucleic acid (DNA) base pairs and bind with DNA associated enzymes (topoisomerase II), inhibiting DNA replication and ribonucleic acid (RNA) transcription. The aglycone molecule intercalates in between DNA strands, whereas the sugar unit binds to the minor groove in the DNA by displacing water molecules and ions. The amino sugar (l-daunosamine) attached at the seventh hydroxyl position of the ring sits in the minor groove and intercalates the flanking base pairs immediately adjacent to the flanking site, which stabilizes the binding complex of doxorubicin and the DNA molecule. The amino group and hydroxyl group of the sugar facing outside the minor groove interact with the polymerase enzymes, thus inhibiting their function in DNA replication (Pigram et al. 1972; Frederick et al. 1990). Alternatively inside the cell, doxorubicin undergoes one electron reduction to form a DOX-semiquinone radical, an unstable metabolite. It is reoxidized back to doxorubicin and leads to the formation of reactive oxygen species and hydrogen peroxide. These reactive radicals cause oxidative stress to the cell that destructs multiple cell components such as cell membrane, nucleic acids, and ultimately trigger apoptotic pathways of cell death (Fig. 7.2) (Doroshow 1986; Thorn et al. 2011).
7.2 Biosynthesis of Daunorubicin and Doxorubicin
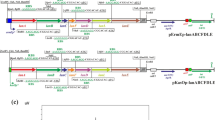
The gene cluster of S. peucetius has been studied extensively, because of its ability to produce doxorubicin and daunorubicin, which have broad clinical applications. S. peucetius 29050 complete genetic map was first published in 1999 by Lomovskaya et al. The S. peucetius ATCC 27952 strain was sequenced, and its genome was analyzed in 2004 (Parajuli et al. 2004). The 8.7-Mb genomic sequence analysis of S. peucetius ATCC 27952 identified a 40 kb daunorubicin and doxorubicin biosynthetic gene cluster, which is 5.3 % of the total genome consisting of 37 open reading frames (Fig. 7.3). Biosynthesis of doxorubicin is completed in three stages: (A) formation of the aglycone, ε-rhodomycinone through condensation of one propionyl-coenzyme A and nine malonyl coenzyme A precursor units; (B) formation of activated amino sugar moiety, thymidine diphosphate (TDP)-l-daunosamine from d-glucose-1-phosphate; and finally (C) glycosylation of ε-rhodomycinone by glycosyltransferase , followed by different post-modifications like decarboxylation, methylation, and hydroxylation to produce the final compound (Hutchinson and Colombo 1999). In recent years, detail study of the daunorubicin and doxorubicin biosynthetic gene cluster that comprises catalytic enzymes, transcription factors, and resistance gene has improved the understanding of the biosynthesis machinery and regulatory mechanisms that control the doxorubicin biosynthesis.
7.2.1 Biosynthesis of ε-Rhodomycinone
Doxorubicin biosynthesis starts with the formation of an important intermediate ε-rhodomycinone (Dickens et al. 1995), whose entire carbon backbone is synthesized by a type II polyketide synthase (PKS) enzyme that is encoded by the dpsABCDGEFY genes (Fig. 7.4). A 21-carbon decaketide is initially formed by serial condensation of 9 malonyl-CoA units to 1 propionyl-CoA starter unit, and this multistep reaction involves enzymes from the polyketide synthase family, like 3-oxoacyl ACP synthase (dpsA) (Meurer and Hutchinson 1995), ketosynthases (dpsB and dpsC) (Grimm et al. 1994; Bao et al. 1999), acyltransferase (dpsD), and an acyl carrier protein (dpsG) (Lomovskaya et al. 1999). The ketoreductase (dpsE) carries out reduction of the decaketide, followed by aldol condensation, and then three steps of ring cyclization catalyzed by DpsF and DpsY, to form 12-deoxyalkanoic acid (Lomovskaya et al. 1998). A keto group is introduced into this intermediate by monooxygenase (dnrG) to form alkalonic acid, which is subsequently converted to aklaviketone by alkalonic acid-S-adenosyl-l-methionine methyl ester transferase, encoded by a homodimeric protein dnrC (Madduri and Hutchinson 1995). Further, alkalonic acid methyl ester cyclase, encoded by dnrD, carries out the cyclation reaction. Finally, the 7-oxo moiety of aklaviketone is reduced to a hydroxyl group, to form ε-rhodomycinone in two sequential steps executed by the enzymes aklaviketone reductase, encoded by dnrH, and a hydroxylase, encoded by dnrF (Filippini et al. 1995).
Biosynthetic pathways of doxorubicin (DXR), daunorubicin (DNR), and ε-rhodomycinone from propionyl-CoA and malonyl-CoA along with biosynthetic pathway for thymidine diphosphate-l-daunosamine starting from d-glucose-1-phosphate. Also shown in the figure is the pathway for the glycosylation and post-modification of the final compound daunorubicin and doxorubicin into 13-dihydrodaunorubicin and baumycin-like higher glycosides
7.2.2 Biosynthesis of Thymidine Diphosphate-l-Daunosamine
The biosynthesis of thymidine diphosphate-l-daunosamine involves a seven gene cluster, namely, dnmL, dnmM, dnmU, dnmT, dnmJ, and dnmV. The biosynthesis begins from d-glucose-1-phosphate. The sequential action of two enzymes glucose-1-phosphate thymidylyl transferase and thymidine diphosphate-d-glucose 4, 6-dehydratase, encoded by dnmL and dnmM, respectively, catalyzes the first two enzymatic reactions to generate the intermediate thymidine diphosphate-4-keto-6-deoxy-d-glucose (TKDG) (Gallo et al. 1996). DnmU, an epimerase, carries out the epimerization of thymidine diphosphate-4-keto-6-deoxy-d-glucose to thymidine diphosphate-4-keto-6-deoxy-l-glucose, to which a keto group and an amino group is added at the C-3 position, followed by the enzyme hydratase and aminotransferase, which are encoded by dnmT and dnmJ, respectively. Finally, dnmV, a ketoreductase, reduces the C-4 ketone to a hydroxyl group, to generate thymidine diphosphate-d-daunosamine (Fig. 7.4) (Otten et al. 1997).
7.2.3 Glycosylation and Post-modifications
After the completion of the polyketide stage, ε-rhodomycinone is converted to rhodomycin D, a daunosamine conjugated derivative by the enzyme DnrS. Rhodomycin D is then converted to 13-deoxycarminomycin (Furuya and Hutchinson 1998) by the DnrP esterase, and this is followed by O-methylation by the methyltransferase encoded by DnrK, to produce 13-deoxydaunorubicin (Dickens et al. 1997). The latter metabolite undergoes C-13 oxidation by the cytochrome P450 enzyme in two stages, first forming an intermediate 13-dihydrodaunorubicin, and then daunorubicin. DoxA is responsible for both steps (Walczak et al. 1999). Daunorubicin is eventually hydroxylated by the same DoxA enzyme at the C-14 position, to generate doxorubicin (Fig. 7.4).
7.2.4 Regulation of Daunorubicin and Doxorubicin Biosynthesis
S. peucetius has various types of regulatory genes that control the production of daunorubicin/doxorubicin, which include transcription factors dnrO, dnrN, and dnrI, transcriptional repressor drrD/dnrW, transcriptional control by a coherent feed forward loop, self-resistance, and feedback regulation (Jiang and Hutchinson 2006). The dnrO is the major transcriptional regulator located adjacent to the dnrN gene. It encodes a protein that has a helix-turn-helix DNA binding domain close to its N-terminal region and belongs to a member of the TetR family of transcriptional regulators. The inactivation of dnrO leads to the complete loss of anthracycline antibiotics biosynthesis in S. peucetius. DnrO is essential for the expression of the pathway-specific dnrN transcriptional activator, and this in turn activates dnrI (Otten et al. 2000). DnrI, being the master regulator, binds to the several regions of polyketide synthases and activate the efflux regulatory genes (Madduri and Hutchinson 1995; Tang et al. 1996). In contrast, DnrO negatively regulates biosynthesis pathway genes due to self-repression phenomena (Lei and Parekh 2005). The self-repression of dnrO is an important event, as it is the key factor for the feedback regulation of daunorubicin biosynthesis, and this activates the transcription of dnrN and dnrI, which in turn leads to the activation of daunorubicin biosynthesis in a sequential manner (Ajithkumar and Prasad 2010). Thus, the existence of tightly regulated antibiotic biosynthesis machinery has been explained and extensively studied by making use of dnrO, dnrN, and dnrI transcription regulator mutants, leading to the better understanding of the doxorubicin biosynthesis in S. peucetius (Vasanthakumar et al. 2013).
7.3 Pathway Engineering and Production of Daunorubicin and Doxorubicin
The production of daunorubicin and doxorubicin from S. peucetius is hindered by several factors such as (1) the low availability of thymidine diphosphate-l-daunosamine sugar, (2) low efficiency of glycosylation reaction, (3) cytotoxicity, and (4) regulatory mechanisms. This could be overcome by generating a robust S. peucetius strain capable of producing practical amount of target molecules using recent biotechnological tools. Till date, several studies have been performed to enhance the production of daunorubicin and doxorubicin from this strain. The basic approaches used to enhance the production of daunorubicin and doxorubicin are summarized in Fig. 7.5.
Overall scheme used for enhanced production of daunorubicin and doxorubicin (DNR/DXR) from S. peucetius . The scheme shows the pathway engineering strategy and modification of regulatory genes; structural genes; sugar synthesis genes, overexpressing the positive regulators; and the resistance genes followed by inactivation of the post-modification genes thereby improving the yield of both daunorubicin and doxorubicin
7.3.1 Engineering of Thymidine Diphosphate-l-Daunosamine Biosynthesis Pathway Genes
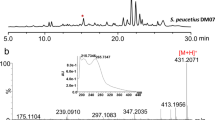
The deoxysugar moieties constitute a very important role in the production of daunorubicin and doxorubicin. Thymidine diphosphate-l-daunosamine sugar formation and its glycosylation by the enzyme DnrS along with DnrQ are considered the rate-limiting step in the biosynthesis of doxorubicin (Dekleva et al. 1985). During daunorubicin and doxorubicin biosynthesis, there is a limited production of glycosylated intermediates because of the low glycosylation efficiency of DnrS/DnrQ glycosyltransferases . Therefore, Malla et al. (2009) explored the overall effects of glycosyltransferase expression for the efficient glycosylation of ε-rhodomycinone and expression of sugar genes to increase the thymidine diphosphate-l-daunosamine pool. Homologous TDP-sugar biosynthesis genes from S. venezuelae ATCC 15439, desIII (glucose-1-phosphate thymidylyltransferase), and desIV (thymidine diphosphate-d-glucose 4, 6-dehydratase) were cloned and overexpressed. Additionally, dnrS, along with dnrQ, which codes for the activator protein DnrQ, were also overexpressed, and their effects were analyzed in S. peucetius ATCC 27952. Introduction of multicopies of dnrS/dnrQ produced noticeable 2.8-fold enhancement over the parental strain. Furthermore, co-overexpression of dnrS/dnrQ along with desIII/desIV increased the doxorubicin production by a 5.6-fold more than the S. peucetius parental strain.
DnrH encodes a glycosyl transferase involved in the post-modification stages of the daunorubicin and doxorubicin biosynthesis. Studies involving the dnrH mutant produced by inactivation of this gene led to an eightfold increase in daunorubicin production and twofold decrease in ε-rhodomycinone accumulation. Introduction of dnmT mutant into the dnrH mutant, daunorubicin production increased ninefold compared to the wild-type S. peucetius. Doxorubicin production was also improved approximately threefold in the dnrH mutant in comparison to the wild-type strain (Scotti and Hutchinson 1996). This is due to the fact that daunorubicin and doxorubicin are further modified into baumycin, like higher glycoside, by these post-modification enzymes, and thus their deletions lead to high production titers of both daunorubicin and doxorubicin. It has also been reported that the DnmT enzyme is present in limiting amounts in the S. peucetius (Dickens et al. 1996).
7.3.2 Engineering of the Polyketide Synthase Genes
Earlier studies carried out by Ye et al. (1994), Gerlitz et al. (1997), Bao et al. (1999), Lomovskaya et al. (1999), and Strohl et al. (1998) have shown the importance of the early polyketide synthase genes, like dpsABCDFGY, to the doxorubicin pathway, as their inactivation leads to complete or partial loss in daunorubicin and doxorubicin production. Thus there is a strong possibility that the overexpression of these genes may lead to a significant increase in the production of these anthracyclines in S. peucetius. Studies carried out by the disruption of the late modifying genes of the doxorubicin biosynthetic pathway , such as dnrU, dnrV, and dnrX, found that individual dnrX or dnrU mutants produced more doxorubicin than their parental strains, whereas the production of daunorubicin and ε-rhodomycinone decreased (Lomovskaya et al. 1998; Lomovskaya et al. 1999). Doxorubicin production increased approximately twofold in the double dnrX and dnrU mutant when compared with only dnrX mutant, which was an approximate sevenfold increase, when compared with the wild-type strain.
This increase in production is accredited to daunorubicin not being able to be converted to 13-dihydrodaunorubicin, and neither daunorubicin and doxorubicin being able to be further modified to acid-sensitive metabolites, due to the deletion of these modifying enzymes (Walczak et al. 1999). Additionally, when the dnrV and doxA genes were introduced and overexpressed in the above dnrX, dnrU, and dnrH mutants, a smaller increase in doxorubicin production was observed (Lomovskaya et al. 1999), possibly due to the fact that the oxidation rate of daunorubicin to doxorubicin is 170-fold less efficient than the conversion rate of 13-dihydrodaunorubicin to daunorubicin. Hence, the increased levels of DoxA are less likely to change the extent of doxorubicin production in S. peucetius.
As mentioned earlier, S. peucetius ATCC 27952 self-resistance system imparts resistance against the toxicity of the antibiotic daunorubicin and doxorubicin inside the cell, and helping in this endeavor are the four resistance genes drrA, drrB, drrC, and drrD. Owing to this fact, when three of these resistance genes dnrABC were cloned under strong ermE* promoter into the pIBR25 expression vector, the recombinant expression strains, pDrrAB25, pDrrC25, and pDrrABC25, produced more doxorubicin than the parental strain, with a 2.2-fold increase in pDrrAB25, a 5.1-fold increase in pDrrC25, and a 2.4-fold increase in pDrrABC25. Thus, doxorubicin production is positively affected when the resistance genes are introduced in multiple copies (Malla et al. 2010a).
7.3.3 Engineering of the Regulatory Genes
Secondary metabolite production in Streptomyces spp. is regulated by two different classes of regulatory genes: cluster-situated regulators and global regulators or pleiotropic regulatory genes. Most of these cluster-situated regulators control the biosynthesis of a particular antibiotic and are also known as pathway-specific regulators. On the other hand, the global regulatory genes may not always be present in biosynthetic gene cluster but regulate morphological and physiological differentiation and secondary metabolite biosynthesis in Streptomyces (Umeyama et al. 2002). The study of these regulatory genes provides a theoretical basis for antibiotic biosynthesis in Streptomyces and also helps to increase the yield of antibiotics by the use of pathway engineering and manipulation of these regulatory genes at molecular level.
Similarly in S. peucetius, as discussed earlier, DnrI, DnrN, and DnrO act as transcriptional regulator and control production of daunorubicin and doxorubicin (Fig. 7.6). The DnrO is the major transcription regulator, and its inactivation leads to complete loss of antibiotic production. In S. peucetius, DnrI is required for the transcription of biosynthetic and resistance genes of the daunorubicin and doxorubicin gene cluster and thus controls the expression of almost all of the biosynthetic and resistance genes (Madduri and Hutchinson 1995), while DnrN controls the expression of DnrI (Otten et al. 1995). Consequently, the introduction of positively acting regulatory genes like DnrI and DnrN has profound effects on the production of antibiotics like daunorubicin. In S. peucetius, the production of daunorubicin was increased 2.5-fold, whereas the ε-rhodomycinone yield was raised to nearly 10-fold (Otten et al. 1995; Stutzman-Engwall et al. 1992).
Schematic representation of genes involved in regulation of daunorubicin and doxorubicin (DNR/DXR) production in S. peucetius . Global regulatory genes like asfR and Metk1-sp and pathway-specific regulatory genes like dnrO, dnrI, and dnrN act as positive regulatory genes and thus have positive effect in daunorubicin and doxorubicin production, whereas the pleiotropic downregulatory genes such as iclR, wblA, ndgR, and doxR decrease the daunorubicin and doxorubicin production and hence the negative regulatory genes. Overexpression or inhibition of the regulatory genes has a profound effect in the production of the final compound doxorubicin
Introduction of regulatory genes such as dnrN, dnrI, afsR, and metK1-sp under strong ermE* promoter increased doxorubicin production by 1.2-fold in recombinant strains NI (with dnrN-dnrI), 1.4-fold in NIS (with dnrN-dnrI-metK1-sp), and 4.3-fold in NIR (with dnrN-dnrI-afsR) (Malla et al. 2010b). AfsR is a global regulator which constitutes the AfsK-AfsR system. The expression of afsR from both S. peucetius ATCC 27952 and S. venezuelae in S. peucetius enhanced production of doxorubicin by fourfold and eightfold, respectively (Parajuli et al. 2005). Furthermore the overexpression of this pleiotropic activator afsR enhanced other antibiotics such as actinorhodin in S. lividans, clavulanic acid in S. clavuligerus, and streptomycin in S. griseus (Maharjan et al. 2009).
S. peucetius does not contain a functional copy of bldA-tRNA. Although bldA is non-essential for the survival of the Streptomyces species, it plays an important role in secondary metabolism. When the regulatory gene dnrO codon was thoroughly examined, a TTA codon was found which is hardly encoded by bldA-tRNA. Multiple engineered strains of S. peucetius were generated by heterologously expressing bldA and dnrO individually and a combination of both bldA and dnrO. Overexpression of these pathway-specific negative regulators enhanced the production of daunorubicin 1.25-fold, as compared to the parental strain (Pokhrel et al. 2016). Likewise these genes, engineering of genes, like dephosphocoenzyme A (coaE), which catalyzes the last step in the biosynthesis of the cofactor coenzyme A, has been shown to have positive increase in doxorubicin production. When these two genes coaA and coaE were overexpressed independently in the doxorubicin-producing wild-type strain, there was 1.4- and 1.5-fold increase in doxorubicin production, respectively. Both genes in combination exhibited 2.1-fold enhancement in doxorubicin production (Lee et al. 2014).
Besides overexpressing positive regulators to enhance production titer, as mentioned above, there are a few negative regulators present in S. peucetius, whose overexpression or inactivation may have a negative or positive effect on daunorubicin and doxorubicin production (Fig. 7.6). One such negative regulator is wblA, which controls antibiotic production and morphological differentiation in actinomyces, and when this wblA regulator from S. coelicolor was introduced into the doxorubicin-overproducing strain, it led to significant decrease in the production of doxorubicin (Kang et al. 2007).
The dox R regulator belonging to the IclR type family of transcription regulator was found in the genome of S. peucetius, and when overexpressed in S. peucetius strain, it strongly repressed the production of antibiotics . Furthermore, it exerted an adverse consequence on the regulatory system of doxorubicin, wherein the binding of DoxR inhibited the dnrI expression, leading to the blockade of doxorubicin production (Chaudhary et al. 2014). Another regulatory gene ndgR which is a regulator for nitrogen source-dependent growth and antibiotic production, similar to an IclR-like regulator from S. coelicolor, can bind to the promoters in the doxorubicin biosynthetic gene cluster in S. peucetius (Yang et al. 2009), and its inactivation in S. coelicolor leads to increased actinorhodin production. Thus, the deletion of the doxR and the ndgR regulatory genes may also have a positive effect on daunorubicin and doxorubicin production.
7.4 Conclusion
Because of low production yield of doxorubicin and high market demand, engineering of S. peucetius strain is a beneficial goal. Until the late 1990s, the annual production of doxorubicin was over 225 kg, and it was the most widely used anticancer drug. Moreover, doxorubicin is also considered as lead molecule to generate other value-added derivatives by enzymatic and chemical modifications with improved pharmacological properties for clinical cancer treatment (Arcamone et al. 1997; Allwood et al. 2002). Although doxorubicin can be produced semi-synthetically from its precursor daunorubicin, the process is tiresome, and the yield is quite low. Thus, sustainable fermentation technology combined with pathway engineering approaches is currently needed to enhance the production of these drugs (Hutchinson and Colombo 1999; Malla et al. 2010c).
In summary, we conclude that by identification of the key steps in S. peucetius that hinder daunorubicin and doxorubicin production, like the low availability of thymidine diphosphate-l-daunosamine sugar and the low efficiency of glycosylation, cytotoxicity, and the regulatory mechanisms, daunorubicin and doxorubicin production can be raised significantly in the wild-type strain of S. peucetius by genetic engineering . This would involve overexpression of the genes regulating doxorubicin production and also the genes in the biosynthetic pathway , along with the deletion of negative regulators and inhibiting the post-modification steps of daunorubicin and doxorubicin into other metabolites (Table 7.1). Additionally, high-daunorubicin-and doxorubicin-producing strains can be generated by overexpression of the genes in the sugar pathway of thymidine diphosphate-l-daunosamine, a very important step in the production of daunorubicin and doxorubicin, along with the engineering of the polyketide synthase genes. Engineering of wild-type strain using combined effect of regulatory genes and other biosynthesis genes along with self-resistance and cofactors limiting genes using state-of-the-art systems/synthetic biology and metabolic engineering tools could certainly generate a high-doxorubicin-producing strain for commercial production of these valuable anticancer drugs.
References
Ajithkumar V, Prasad R (2010) Modulation of dnrN expression by intracellular levels of DnrO and daunorubicin in Streptomyces peucetius. FEMS Microbiol Lett 306(2):160–167. https://doi.org/10.1111/j.1574-6968.2010.01948.x
Allwood J, Stanley A, Wright P (2002) Doxorubicin. In: The cytotoxics handbook. Radcliffe Publishing, Oxford, pp 322–329
Arcamone F, Cassinelli G, Fantini G, Grein A, Orezzi P, Pol C, Spalla C (1969) Adriamycin, 14-hydroxydaimomycin, a new antitumor antibiotic from S. Peucetius var. caesius. Biotechnol Bioeng 11(6):1101–1110. https://doi.org/10.1002/bit.260110607
Arcamone F, Animati F, Capranico G, Lombardi P, Pratesi G, Manzini S, Supino R, Zunino F (1997) New developments in antitumor anthracyclines. Pharmacol Ther 76(1-3):117–124. https://doi.org/10.1016/S0163-7258(97)00096-X
Aubel-Sadron G, Londos-Gagliardi D (1984) Daunorubicin and doxorubicin, anthracycline antibiotics, a physiochemical and biological review. Biochimie 66(5):333–352. https://doi.org/10.1016/0300-9084(84)90018-X
Bao W, Sheldon PJ, Wendt-Pienkowski E, Hutchinson CR (1999) The Streptomyces peucetius dpsC gene determines the choice of starter unit in biosynthesis of the daunorubicin polyketide. J Bacteriol 181(15):4690–4695
Brown K, Li W, Kaur P (2017) Role of aromatic and negatively-charged residues of DrrB in multi-substrate specificity conferred by the DrrAB system of Streptomyces peucetius. Biochemistry 56(13):1921–1931. https://doi.org/10.1021/acs.biochem.6b01155
Carvalho C, Santos RX, Cardoso S, Correia S, Oliveira PJ, Santos MS, Moreira PI (2009) Doxorubicin: the good, the bad and the ugly effect. Curr Med Chem 16(25):3267–3285. https://doi.org/10.2174/092986709788803312
Chaudhary AK, Singh B, Maharjan S, Jha AK, Kim BG, Sohng JK (2014) Switching antibiotics production on and off in actinomycetes by an IclR family transcriptional regulator from Streptomyces peucetius ATCC 27952. J Microbiol Biotechnol 24(8):1065–1072. https://doi.org/10.4014/jmb.1403.03026
Cortes-Funes H, Coronado C (2007) Role of anthracyclines in the era of targeted therapy. Cardiovasc Toxicol 7(2):56–60. https://doi.org/10.1007/s12012-007-0015-3
Dekleva ML, Titus JA, Strohl WR (1985) Nutrient effects on anthracycline production by Streptomyces peucetius in a defined medium. Can J Microbiol 31(3):287–294. https://doi.org/10.1139/m85-053
Dickens ML, Ye J, Strohl WR (1995) Analysis of clustered genes encoding both early and late steps in daunomycin biosynthesis by Streptomyces sp. strain C5. J Bacteriol 177(3):536–543. https://doi.org/10.1128/jb.177.3.536-543.1995
Dickens ML, Ye J, Strohl WR (1996) Cloning, sequencing and analysis of aklaviketone reductase from Streptomyces sp. strain C5. J Bacteriol 178(11):3384–3388. https://doi.org/10.1128/jb.178.11.3384-3388.1996
Dickens ML, Priestley ND, Strohl WR (1997) In vivo and in vitro bioconversion of e-rhodomycinone glycoside to doxorubicin: functions of DauP, DauK and DoxA. J Bacteriol 179(8):2641–2650. https://doi.org/10.1128/jb.179.8.2641-2650.1997
Doroshow JH (1986) Role of hydrogen peroxide and hydroxyl radical formation in the killing of Ehrlich tumor cells by anticancer quinones. Proc Natl Acad Sci U S A 83(12):4514–4518. https://doi.org/10.1073/pnas.83.12.4514
Filippini S, Solinas MM, Breme U, Schluter MB, Gabellini D, Biamonti G, Colombo AL, Garofano L (1995) Streptomyces peucetius daunorubicin biosynthesis gene, dnrF: sequence and heterologous expression. Microbiology 141(4):1007–1016. https://doi.org/10.1099/13500872-141-4-1007
Frederick CA, Williams LD, Ughetto G, Van der Marel GA, Van Boom JH, Rich A, Wang AH (1990) Structural comparison of anticancer drug-DNA complexes: adriamycin and daunomycin. Biochemistry 29(10):2538–2549. https://doi.org/10.1021/bi00462a016
Furuya K, Hutchinson CR (1998) The DrrC protein of Streptomyces peucetius, a UvrA-like protein, is a DNA-binding protein whose gene is induced by daunorubicin. FEMS Microbiol Lett 168(2):243–249. https://doi.org/10.1111/j.1574-6968.1998.tb13280.x
Gallo MA, Ward JM, Hutchinson CR (1996) The dnrM gene in Streptomyces peucetius contains a naturally-occurring frameshift mutation that is suppressed by another locus outside of the daunorubicin-production gene cluster. Microbiology 142(2):269–275. https://doi.org/10.1099/13500872-142-2-269
Gerlitz M, Meurer G, Wendt-Pienkowski E, Madduri K, Hutchinson CR (1997) Effect of the daunorubicin dpsH gene on the choice of starter unit and cyclization pattern reveals that type II polyketide synthase can be unfaithful yet intriguing. J Am Chem Soc 119(31):7392–7393. https://doi.org/10.1021/ja970946h
Gewirtz DA (1999) A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol 57(7):727–741. https://doi.org/10.1016/S0006-2952(98)00307-4
Grein A (1987) Antitumor anthracyclines produced by Streptomyces peucetius. Adv Appl Microbiol 32:203–214. https://doi.org/10.1016/S0065-2164(08)70081-9
Grimm A, Madduri K, Ali A, Hutchinson CR (1994) Characterization of the Streptomyces peucetius ATCC 29050 genes encoding doxorubicin polyketide synthase. Gene 151(1):1–10. https://doi.org/10.1016/S0065-2164(08)70081-9
Guilfoile PG, Hutchinson CR (1991) A bacterial analog of the mdr gene of mammalian tumor cells is present in Streptomyces peucetius, the producer of daunorubicin and doxorubicin. Proc Natl Acad Sci U S A 88(19):8553–8557. https://doi.org/10.1073/pnas.88.19.8553
Hao J, Hutchinson CR (2006) Feedback regulation of doxorubicin biosynthesis in Streptomyces peucetius. Res Microbiol 157(7):666–674. https://doi.org/10.1016/j.resmic.2006.02.004
Hutchinson CR (1997) Biosynthetic studies of daunorubicin and tetracenomycin C. Chem Rev 97:2525–2535. https://doi.org/10.1021/cr960022x
Hutchinson CR, Colombo AL (1999) Genetic engineering of doxorubicin production in Streptomyces peucetius: a review. J Ind Microbiol Biotechnol 23(1):647–652. https://doi.org/10.1038/sj.jim.2900673
Jiang H, Hutchinson CR (2006) Feedback regulation of doxorubicin biosynthesis in Streptomyces peucetius. Res Microbiol 157(7):666–674. https://doi.org/10.1016/j.resmic.2006.02.004
Kang SH, Huang J, Lee HN, Hur YA, Cohen SN, Kim ES (2007) Interspecies DNA microarray analysis identifies WblA as a pleiotropic down-regulator of antibiotic biosynthesis in Streptomyces. J Bacteriol 189(11):4315–4319. https://doi.org/10.1128/JB.01789-06
Karuppasamy K, Srinivasan P, Ashokkumar B, Tiwari R, Kanagarajadurai K, Prasad R (2015) Partial loss of self-resistance to daunorubicin in drrD mutant of Streptomyces peucetius. Biochem Eng J 102:98–107. https://doi.org/10.1016/j.bej.2015.02.017
Kaur P, Russell J (1998) Biochemical coupling between the DrrA and DrrB proteins of the doxorubicin efflux pump of Streptomyces peucetius. J Biol Chem 273(28):17933–17939. https://doi.org/10.1128/JB.01789-06
Lee NR, Rimal H, Lee JH, Oh TJ (2014) Characterization of dephosphocoenzyme A kinase from Streptomyces peucetius ATCC27952, and its application for doxorubicin overproduction. J Microbiol Biotechnol 24(9):1238–1244. https://doi.org/10.4014/jmb.1404.04053
Lei H, Parekh SR (2005) Development of improved strains and optimization of fermentation process. In: Barredo JL (ed) Microbial process and products. Humana Press, New York, pp 1–24. https://doi.org/10.1385/1592598471
Lomovskaya N, Doi-Katayama Y, Filippini S, Nastro C, Fonstein L, Gallo M, Colombo AL, Hutchinson CR (1998) The Streptomyces peucetius dpsY anddnrX Genes govern early and late steps of daunorubicin and doxorubicin biosynthesis. J Bacteriol 180(9):2379–2386
Lomovskaya N, Otten SL, Doi-Katayama Y, Fonstein L, Liu XC, Takatsu T, Inventi-Solari A, Filippini S, Torti F, Colombo AL, Hutchinson CR (1999) Doxorubicin overproduction in Streptomyces peucetius: cloning and characterization of the dnrU ketoreductase and dnrV genes and the doxA cytochrome P-450 hydroxylase gene. J Bacteriol 181(1):305–318
Madduri K, Hutchinson CR (1995) Functional and transcriptional analysis of the dnrR1 locus, which controls daunorubicin biosynthesis in Streptomyces peucetius. J Bacteriol 177(5):1208–1215. https://doi.org/10.1128/jb.177.5.1208-1215.1995
Maharjan S, Oh TJ, Lee HC, Sohng JK (2009) Identification and functional characterization of an afsR homolog regulatory gene from Streptomyces venezuelae ATCC 15439. J Microbiol Biotechnol 19(2):121–127. https://doi.org/10.4014/jmb.0803.223
Malla S, Niraula NP, Liou K, Sohng JK (2009) Enhancement of doxorubicin production by expression of structural sugar biosynthesis and glycosyltransferase genes in Streptomyces peucetius. J Biosci Bioeng 108(2):92–98. https://doi.org/10.1016/j.jbiosc.2009.03.002
Malla S, Niraula NP, Liou K, Sohng JK (2010a) Self-resistance mechanism in Streptomyces peucetius: overexpression of drrA, drrB and drrC for doxorubicin enhancement. Microbiol Res 165(4):259–267. https://doi.org/10.1016/j.micres.2009.04.002
Malla S, Niraula NP, Liou K, Sohng JK (2010b) Improvement in doxorubicin productivity by overexpression of regulatory genes in Streptomyces peucetius. Res Microbiol 161(2):109–117. https://doi.org/10.1016/j.resmic.2009.12.003
Malla S, Niraula NP, Singh B, Liou K, Sohng JK (2010c) Limitations in doxorubicin production from Streptomyces peucetius. Microbiol Res 165(5):427–435. https://doi.org/10.1016/j.micres.2009.11.006
Meurer G, Hutchinson CR (1995) Functional analysis of putative beta-ketoacyl: acyl carrier protein synthase and acyltransferase active site motifs in a type II polyketide synthase of Streptomyces glaucescens. J Bacteriol 177(2):477–481. https://doi.org/10.1128/jb.177.2.477-481.1995
Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L (2004) Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev 56(2):185–229. https://doi.org/10.1124/pr.56.2.6
Otten SL, Ferguson J, Hutchinson CR (1995) Regulation of daunorubicin production in Streptomyces peucetius by the dnrR2 locus. J Bacteriol 177(5):1216–1224. https://doi.org/10.1128/jb.177.5.1216-1224.1995
Otten SL, Gallo MA, Madduri K, Liu X, Hutchinson CR (1997) Cloning and characterization of the Streptomyces peucetius dnmZUV genes encoding three enzymes required for biosynthesis of the daunorubicin precursor thymidine diphospho-l-daunosamine. J Bacteriol 179(13):4446–4450. https://doi.org/10.1128/jb.179.13.4446-4450.1997
Otten SL, Olano C, Hutchinson CR (2000) The dnrO gene encodes a DNA-binding protein that regulates daunorubicin production in Streptomyces peucetius by controlling expression of the dnrN pseudo response regulator gene. Microbiology 146(6):1457–1468. https://doi.org/10.1099/00221287-146-6-1457
Parajuli N, Basnet DB, Lee HC, Sohng JK, Liou K (2004) Genome analyses of Streptomyces peucetius ATCC 27952 for the identification and comparison of cytochrome P450 complement with other Streptomyces. Arch Biochem Biophys 425(2):233–241. https://doi.org/10.1016/j.abb.2004.03.011
Parajuli N, Viet HT, Ishida K, Tong HT, Lee HC, Liou K, Sohng JK (2005) Identification and characterization of the afsR homologue regulatory gene from Streptomyces peucetius ATCC 27952. Res Microbiol 156(5-6):707–712. https://doi.org/10.1016/j.resmic.2005.03.005
Pigram WJ, Fuller W, Hamilton LD (1972) Stereochemistry of intercalation: interaction of daunomycin with DNA. Nature 235(53):17–19. https://doi.org/10.1038/newbio235017a0
Pokhrel AR, Chaudhary AK, Nguyen HT, Dhakal D, Le TT, Shrestha A, Liou K, Sohng JK (2016) Overexpression of a pathway specific negative regulator enhances production of daunorubicin in bldA deficient Streptomyces peucetius ATCC 27952. Microbiol Res 192:96–102. https://doi.org/10.1016/j.micres.2016.06.009
Prija F, Prasad P (2017) DrrC protein of Streptomyces peucetius removes daunorubicin from intercalated dnrI promoter. Microbiol Res 202:30–35. https://doi.org/10.1016/j.micres.2017.05.002
Scotti C, Hutchinson CR (1996) Enhanced antibiotic production by manipulation of the Streptomyces peucetius dnrH and dnmT genes involved in doxorubicin (adriamycin) biosynthesis. J Bacteriol 178(24):7316–7321. https://doi.org/10.1128/jb.178.24.7316-7321.1996
Strohl WR, Rajgarhia VB, Priesley ND (1998) Streptomyces sp. strain C5 mutant disrupted in daunorubicin-specific polyketide synthase genes, dpsC and dpsD, produce novel anthracyclines suggesting promiscuous starter unit selection. In: Proceedings of the international interdisciplinary conference on polyketides. II-Chemistry, biochemistry and molecular genetics. University of Bristol, Bristol, United Kingdom
Stutzman-Engwall KJ, Otten SL, Hutchinson CR (1992) Regulation of secondary metabolism in Streptomyces spp. and overproduction of daunorubicin in Streptomyces peucetius. J Bacteriol 74(1):144–154. https://doi.org/10.1128/jb.174.1.144-154.1992
Swain SM, Whaley FS, Ewer MS (2003) Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer 97(11):2869–2879. https://doi.org/10.1002/cncr.11407
Tang L, Grimm A, Zhang YX, Hutchinson CR (1996) Purification and characterization of the DnrI DNA-binding protein, a transcriptional activator for daunorubicin biosynthesis in Streptomyces peucetius. Mol Microbiol 22(5):801–813. https://doi.org/10.1046/j.1365-2958.1996.01528.x
Thorn CF, Oshiro C, Marsh S, Hernandez-Boussard T, McLeod H, Klein TE, Altman RB (2011) Doxorubicin pathways: pharmacodynamics and adverse effects. Pharmacogenet Genomics 21(7):440–446. https://doi.org/10.1097/FPC.0b013e32833ffb56
Umeyama T, Lee PC, Horinouchi S (2002) Protein serine/threonine kinases in signal transduction for secondary metabolism and morphogenesis in Streptomyces. Appl Microbiol Biotechnol 59(4-5):419–425. https://doi.org/10.1007/s00253-002-1045-1
Vasanthakumar A, Kattusamy K, Prasad R (2013) Regulation of daunorubicin biosynthesis in Streptomyces peucetius-feed forward and feedback transcriptional control. J Basic Microbiol 53(8):636–644. https://doi.org/10.1002/jobm.201200302
Walczak RJ, Dickens ML, Priestley ND, Strohl WR (1999) Purification, properties, and characterization of recombinant Streptomyces sp. strain C5 DoxA, a cytochrome P-450 catalyzing multiple steps in doxorubicin biosynthesis. J Bacteriol 181(1):298–304
Yang YH, Song E, Kim EJ, Lee K, Kim WS, Park SS, Hahn JS, Kim BG (2009) NdgR, an IclR-like regulator involved in amino-acid-dependent growth, quorum sensing, and antibiotic production in Streptomyces coelicolor. Appl Microbiol Biotechnol 82(3):501–511. https://doi.org/10.1007/s00253-008-1802-x
Ye J, Dickens ML, Plater R, Li Y, Lawrence J, Strohl WR (1994) Isolation and sequence analysis of polyketide synthase genes from the daunomycin-producing Streptomyces sp. strain C5. J Bacteriol 176(20):6270–6280. https://doi.org/10.1128/jb.176.20.6270-6280.1994
Acknowledgments
This research was supported by a grant from the National Research Foundation of Korea to Ramesh Prasad Pandey (Grant No: 2017R1C1B5018056).
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Shrestha, B., Pokhrel, A.R., Darsandhari, S., Parajuli, P., Sohng, J.K., Pandey, R.P. (2019). Engineering Streptomyces peucetius for Doxorubicin and Daunorubicin Biosynthesis. In: Arora, D., Sharma, C., Jaglan, S., Lichtfouse, E. (eds) Pharmaceuticals from Microbes. Environmental Chemistry for a Sustainable World, vol 26. Springer, Cham. https://doi.org/10.1007/978-3-030-01881-8_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-01881-8_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-01880-1
Online ISBN: 978-3-030-01881-8
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)