Abstract
In this chapter, we will present and discuss the challenges of assessing oncology products from a health economic perspective. We will provide a brief introduction on the need for economic evaluation in health care and focus on cost-effectiveness and comparative aspects of the evaluation of oncology products, which are of paramount interest to HTA decision-making bodies using economic evaluation in their decision-making framework. As the burden of oncology is well-documented, we do not discuss it in detail here. Before we address the specific issue of oncology, we will briefly define the critical aspects of HTA assessment and also define what a cost-effectiveness analysis is and why economic modelling is the most appropriate tool to assess the cost-effectiveness of oncology products. We will touch upon the prices of oncology drugs and the questions that high prices raise regarding funding and availability. We then present an overview of the general structure of an oncology cost-effectiveness model. Usually, this is quite simple, representing response, progression, advanced-stage disease and death. Despite the relative simplicity of these models, some issues may render the evaluation more complex; we will touch upon these in this chapter:
-
Issue with clinical inputs due to the design of randomised clinical trials (e.g. cross-over designs involving a treatment switch)
-
Need for survival extrapolation and limitations of current parametric models
-
Rare conditions with limited economic and comparative evidence available
-
High pace of clinical development
Finally, we will conclude with a discussion of the uncertainty around the evaluation of oncology products and the major evolution expected in health economics in oncology.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
As resources are scarce and economic growth is flattening, it is becoming unavoidable that funding of health care interventions is based on economic considerations as well as medical benefit. Cost-effectiveness analysis (CEA) has become increasingly popular for prioritising interventions for funding purposes, as it aims to ensure that health care is delivered as equitably and efficiently as possible. Almost all countries have installed formal processes to assess the costs of new health care interventions in the light of their expected benefits, before actually committing to funding them. Most of the new and promising interventions have a higher price than currently available alternatives and do not generate savings when total expenditure is considered. A minor change to an intervention strategy can lower the cost without a substantial loss of benefit, or increase the benefit without increasing the cost (Kumar 2013). CEA is the best tool to compare different strategies accurately. It allows quantifying benefits related to effectiveness (e.g. decreases in mortality and/or morbidity) and the economic costs of achieving these benefits.
CEA compares a new intervention with alternative health care interventions (standard of care or no intervention), taking future costs and benefits into account and estimating the cost per life-year gained with the different interventions (Gold et al. 1996). Cost-effectiveness is typically evaluated using an incremental cost-effectiveness ratio (ICER) comparing the new treatment with the reference comparator. Usually, ICER is expressed as an incremental cost per life-year gained. However, there are two significant limitations related to CEA. First, while CEA is useful for comparing different treatments for the same disease, it does not allow to compare treatments for different diseases that vary in outcome measures. Second, CEA cannot combine reductions in morbidity and reductions in mortality into a single index; thus, it does not allow direct comparisons between treatments that differ on these two dimensions. As a result, cost-utility analysis (CUA), which addresses some of these issues, has gained popularity among decision-makers.
Thanks to the development of “utility”-based outcome measures, like quality-adjusted-life-years (QALYs), CUA enables comparison between treatments for different diseases with varying treatment outcomes. Results of CUA are expressed as cost per QALY gained. The National Institute for Health and Care Excellence (NICE) defines a QALY as “A measure of the state of health of a person or group in which the benefits, regarding length of life, are adjusted to reflect the quality of life. One QALY is equal to 1 year of life in perfect health. QALYs are calculated by estimating the years of life remaining for a patient following a particular treatment or intervention and weighting each year with a quality-of-life score (on a 0–1 scale).” It is especially useful when decision-makers operate within a limited budget and need to choose between financing specific treatments and forgoing others. Thus, CUA enables to compare the value of cancer therapy to that of, for instance, an anti-hypertensive drug (Miller et al. 2014).
CUA is, ultimately, a CEA where the outcome is defined as QALY, and HTA agencies often do not distinguish CUA as a separate type of analysis, presenting it as part of CEA in their publications.
In the last decade, we have seen major advances in the management of cancers, and the progress seems to be accelerating. Survival rates have dramatically increased over the last five decades from an average of 24% the early 1970s to about 50% nowadays. The introduction of new drugs is what mainly drives this process. Therefore, the value of oncology drugs must be recognised, and premium prices can be seen as legitimate. In a growing number of countries, the amount of the premium is defined based on the threshold the country is willing to pay for an additional benefit (often the willingness to pay for one QALY). The threshold is estimated taking into account different factors—often the country’s per capita gross domestic product (GDP) (e.g. the ICER threshold could be three times the GDP per capita) (Murray et al. 2000; Sarin 2008).
However, the spiralling increase in cancer drug prices has caused growing concerns. As early as 2013, a group of experts in chronic myeloid leukaemia expressed strong concerns regarding the unsustainable prices of cancer drugs. The expert group identified four critical issues with prices: 1—too high, 2—unsustainable, 3—may compromise access to highly effective therapy and 4—harmful to the sustainability of our national health care systems (Experts in Chronic Myeloid Leukemia 2013). It was indeed estimated that prices of many cancer drugs lead to ICERs far above the thresholds above; for instance, the price of cetuximab was $800,000 per year of increased survival (Fojo and Grady 2009). “Financial distress”, linked to out-of-pocket payments of costly oncology drugs (OD), was the basis for the development of the new concept called “financial toxicity” (Zafar et al. 2013; de Souza et al. 2014).
As innovative, high-cost cancer therapies continue to come to market, economic modelling is needed to enable health care decision-makers to assess their value (Toumi 2017). In the next paragraphs, we describe briefly cost-effectiveness models in oncology and discuss specific issues applicable to the assessment of products in this therapy area.
2 Brief Overview of Cost-Effectiveness Models in Oncology
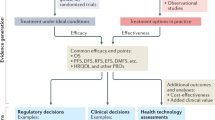
A cost-effectiveness model, or decision analysis model, allows simulating treatment received by patients and permits the assessment of complicated clinical issues that would require years to test through prospective studies. Such a model synthesises evidence on health consequences and costs consequences of introducing a new intervention from many different sources, including data from clinical trials, observational studies, insurance claim databases, case registries, public health statistics, and preference surveys (Weinstein et al. 2003).
Cost-effectiveness models in oncology are usually quite simple, utilising a Markov structure composed of four health states representing no progression/response, progression, advanced-stage disease and death (Fig. 1) (Marsh et al. 2014). Some of the models may not include a state representing advanced disease, being composed only of progression-free, post-progression and death states (Bai et al. 2017).
Markov models are useful when a decision problem involves risk that is continuous over time, when the timing of events is important, and when important events may happen more than once (Sonnenberg and Beck 1993). In a Markov model, the prognosis for long-term diseases is divided into several stages, and patients’ progress through these stages is simulated over a specified period. These characteristics make Markov models suitable for oncology economic modelling.
Although some models include a subsequent treatment line and even extrapolate the results over a lifetime horizon, they remain quite simple from the structural perspective. Because of the relative simplicity of the structure and the clarity of the outcome in cancer (death), it is the quality of the inputs and the extrapolation that mostly drives the quality of the model in oncology. In the subsequent paragraphs, we discuss the specific issues associated with cost-effectiveness modelling in oncology.
3 Issue with Clinical Inputs Due to Crossover (Switch) in Randomised Clinical Trials
Trials of new oncology treatments often involve crossover that allows patients receiving the control treatment to cross over to receive the experimental treatment at disease progression, or when sufficient evidence about the efficacy of the new treatment is achieved. The primary reason for this design is that it would be unethical to maintain patients under the reference therapy that appears less effective than the new therapy. It is also worth noting that some authors use the term “treatment switching” rather than “treatment crossover”, since the latter term may be erroneously associated with cross-over trials, which are a different entity (Latimer and Abrams 2014).
Crossover is often a complex challenge to address in oncology HTA modelling. When an interim trial analysis shows a significant benefit on progression-free survival (PFS), it is common that all patients switch to the new and more effective treatment, as described above. Although this is ethically appropriate, it means the trial cannot reach unbiased estimates of key endpoints, such as overall survival (OS), as following the switch all patients receive the same treatment, regardless of initial randomisation.
Providing accurate estimates of an OS advantage may not be critical for obtaining approval, as long as the trials give evidence of a favourable benefit-risk ratio. However, HTA agencies almost always require precise estimates of the treatment effect on OS (Weinstein et al. 2003). To decide on price, premium payers need to accurately weigh the benefit of the new drug over current therapies against its additional cost. HTA agencies usually recommend that the model assesses the cost-effectiveness of treating an entire disease population with a novel treatment over a lifetime horizon, especially for interventions that increase survival (National Institute for Health and Care Excellence 2013). An economic model applying biased estimates of treatment effect on OS is likely to generate inaccurate cost-effectiveness results. If control group patients benefit from the experimental treatment, the increase in survival with the new therapy would be underestimated and, consequently, the ICER would likely be overestimated. This may, in turn, influence the HTA decision and lead to inefficient resource allocation. Therefore, it is necessary to provide adjusted estimates of the treatment benefit associated with the new treatment and to assess the sensitivity of cost-effectiveness results to these adjustments, as they are performed post hoc and not part of the statistical analysis plan of the original RCT. Several groups of researchers have reviewed the methods used to analyse trials with crossover (Weinstein et al. 2003; Jönsson et al. 2014; Watkins et al. 2013). Common methods are discussed in the following paragraphs.
Simple methods, which do not adjust for crossover, are commonly applied in RCTs with crossover. Intention-to-treat (ITT) analysis and per protocol (PP) analysis are two such simple methods. In ITT analysis, all randomised subjects are included in the analysis, and treatment groups for the analysis are based only on the initial, randomly assigned treatment, without taking subsequent treatments into account. In PP analysis, patients that switch to the new and more effective treatment arm are excluded from the analysis or censored at the point of switch.
These simple methods are subject to substantial bias. ITT analysis may underestimate the treatment effect on OS, which is composed of PFS and post-progression survival (PPS). It can capture the difference between the two groups in PFS but not in PPS since control group patients are switched at progression to the experimental treatment. PP analysis excluding patients that switch is likely to be subject to selection bias and disrupt randomisation. PP analysis for OS by censoring patients that switch relies on an unlikely assumption that censoring is independent of the outcome, which is often biased. In this case, OS is likely to be artificially inflated in the control arm since patients who progress (and so may not live long) come off the curve, while those who respond well to control treatment remain on study.
Several complex methods are proposed to eliminate or reduce the bias due to crossover. Inverse Probability of Censoring Weights (IPCW) and Rank Preserving Structured Failure Time Model (RPSFTM) are two commonly used complex methods. The IPCW method censors patients at the time of switch and records remaining observations weighted by the inverse of the probability of being censored. The probability of being censored is predicted with each patient’s baseline and time-dependent prognostic factors. The RPSFTM method compares the treatment arm survival time with the counterfactual survival time for control groups as if they did not switch. The counterfactual survival time is calculated with a treatment effect parameter which shrinks the survival time after crossover to remove the treatment effect on the survival time.
However, these methods are also associated with difficulties in adjusting for crossover. Besides being complicated to implement, they rely on certain assumptions which may not always be true. IPCW assumes no unmeasured confounders for estimating the probability of crossover, which is implausible. RPSFTM assumes a constant treatment effect for all patients at any treatment time. In this case, survival time in the treatment group is always assumed proportional to the counterfactual survival time in no treatment group regardless when the patient has a crossover to the treatment group. This restricts its use to cases where this kind of treatment effect is biologically plausible.
Although these methodologies have been developed to address the limitations of oncology trials, they are not widely accepted and remain somewhat controversial. Results obtained using the aforementioned complex adjustment methods are accepted by HTA organisations in the UK (NICE) (National Institute of Health and Care Excellence 2011) and Sweden (Dental and Pharmaceutical Benefits Agency) (Dentaland Pharmaceutical Benefits Agency 2012), but not in France (HAS) and Germany (GBA). A recent article has evaluated the impact of crossover on GBA assessment. The authors concluded that in GBA appraisals, oncology medicines with a crossover in their trials received better additional benefit ratings than that without crossover, but the evidence supporting them was considered of lower level. The authors also stressed that the way in which crossover is implemented might influence the assignment of evidence level by the GBA (Isbary et al. 2017).
4 Need for Survival Extrapolation and Limitations with Current Parametric Model
Health economics evaluation for pharmaceuticals has developed in response to most HTA agencies considering it mandatory for product assessment. The aim of economic evaluation within HTA is to assess all differences between considered treatments regarding costs and outcomes to inform rational decisions on resource allocation. The primary expected outcome of interventions used in oncology is the impact on patients’ survival. Hence, from the perspective of economic evaluation, it is crucial to estimate the absolute gain in survival obtained due to the application of new treatment instead of comparative intervention.
It is a common belief that economic evaluations should not be limited to using only the comparative data available directly in clinical trials, especially for assessment of interventions affecting survival. To estimate an absolute gain in survival, consideration of a lifetime horizon in economic evaluations is usually deemed appropriate (Latimer 2011). However, most often survival data available in clinical trials are censored, and the presence of censoring makes in necessary to use data extrapolation for estimating total survival gain. Without extrapolating the outcomes, survival benefit evaluated within the economic analysis would be limited to the outcomes obtained during the follow-up of a particular clinical trial; therefore, it is likely to be underestimated. This underestimation can significantly influence the final results of the economic evaluation, including the conclusion on the cost-effectiveness of the assessed intervention.
The most straightforward and most frequently applied survival models in economic evaluation are so-called parametric models. The use of parametric models is associated with an assumption that survival data follow a particular probabilistic distribution, which can be described with a formula dependent on one to several parameters. Miscellaneous parametric models are available, e.g. exponential, Weibull, Gompertz or lognormal (Fig. 2). Each of these models is characterised by different features, in particular regarding the hazard pattern that can be described using them. Briefly speaking, the hazard is a conditional risk of death (or other events of interest) for patients alive at a certain point in time. The use of parametric models is adequate under the condition that hazard is constant over time or changes monotonically, i.e. only decrease or increases over time. Additionally, some parametric models apply to a situation where hazard initially increases up to a specific point in time and decreases afterwards (National Institute of Health and Care Excellence 2011). The main limitation of the standard parametric models is their lack of applicability to more complex hazards, which are often observed in oncology clinical trials.
Extrapolation of survival data with different models seems to be an unavoidable step of economic evaluation of oncology treatments. However, as with any forecast, there is some level of uncertainty connected with survival extrapolation. The uncertainty of survival extrapolation increases with the length of the extrapolated time horizon and reduced maturity of the data used for extrapolation. Furthermore, many survival models can be considered for use in economic evaluation, taking into account the individual situation, different statistical measures as well as clinical plausibility. Economic evaluations applying different models for survival extrapolation can result in significantly different outcomes and even lead to contradictory conclusions on cost-effectiveness. Consequently, the choice of extrapolation methods may influence recommendations from health authorities, which adds another level of uncertainty connected with survival extrapolation, beyond the uncertain forecast itself.
In the face of the necessity for survival extrapolations within economic evaluation, the variety of possible methods and the impact that the choice of the method may have on the results, one would expect comprehensive guidelines to specify which model should be used in given circumstances. Nevertheless, to the best of our knowledge, such guidelines do not exist, and most probably will not be developed in the short to medium term, as it was deemed too complicated and even inappropriate to indicate specific methods (Watkins et al. 2013). Instead, a systematic process for survival model selection has been proposed. The proposed algorithm aims to improve transparency and consistency of economic evaluations so that they can be readily comprehended not only by HTA agencies but also by an oncologist and the public (Watkins et al. 2013). However, a systematic approach to choosing the survival model has rarely been used in historical economic evaluations (National Institute of Health and Care Excellence 2011).
Furthermore, parametric models are often insufficient to address all the methodological issues appearing within the economic evaluations of new oncology treatments, namely the immaturity of the clinical data, frequent lack of a control group and the availability of intermediate endpoints only. Therefore, there is a need to explore the validity of already available methods, as well as to develop new survival models with better flexibility, which would be more suitable for use in changing circumstances. Many innovative approaches have been proposed in recent years (Latimer 2013; Annemans n.d.), and, for some of them, it has been shown that their use in past economic evaluations would have resulted in more adequate estimates (Gibson et al. 2017).
Moreover, in a particular situation, it may be necessary to develop a novel survival model. This concerns mainly innovative treatments with novel mechanisms of action against cancer, for which features distinguishing them from current treatments can be noticed when analysing survival data. Recent examples of such innovative treatments with unique patterns of survival are immuno-oncology drugs. These agents stimulate the immune system of a patient against cancer. The response to the drug may be delayed, as obtaining effective immune response may take more time than the response to treatments acting directly on cancer cells. However, such a response can be durable in the subset of patients in whom it has been achieved. Delayed response is evident when looking at survival data. Initially, survival of patients using immunotherapy is similar to that with conventional treatments. At a certain point, a separation of the survival curves between these two kinds of therapies can be noticed, together with perceptible plateau achieved with immuno-oncology drugs. The plateau suggests a long-term survival benefit, which should be reflected in the extrapolation. Standard methods for survival modelling are not appropriate in such situations, as parametric models underestimate the long-term response to immunotherapy. Hence, established extrapolation methods prove inadequate for economic evaluation of immune-oncology drugs and new methods had to be developed (Dentaland Pharmaceutical Benefits Agency 2012; Isbary et al. 2017). However, some uncertainty regarding these methods remains from the perspective of the decision-makers (Ziomek et al. 2017). Nevertheless, it can be expected that more and more survival extrapolation methods will be developed in the future to meet new needs arising together with the emergence of new drugs.
5 Rare Indications with Limited Economic and Comparative Evidence Available
Economic models in oncology often need to be adapted to cope with data paucity. This is because clinical trial safety and efficacy outcomes for cancer treatments are collected over short periods and may omit information about resource utilisation, costs or patient preferences (Miller et al. 2014). Indeed, 75% of cancer trials have been shown to have 100 or fewer participants, with a median number of 43 patients (Califf et al. 2012). Small, early phase, non-randomised trials with only one study arm are the most challenging from the economic evaluation perspective and lead to high uncertainty about the modelled clinical-economic outcomes. Better economic evidence from oncology drug trials is always in demand, as it can improve the validity of the models (Cressman et al. 2015).
Major challenges in oncology-related health economic modelling arise from the rarity of many indications, or from new potentially innovative therapies targeting the last line of treatment and, thus, lacking an effective comparator. However, the information on the cost of managing such rare or terminal conditions, and on associated utilities, is even scarcer, making the modelling exercise much more uncertain.
6 High Pace of Clinical Development
As someone has put it “patients are dying, and they are dying now”, so regulators have set some new paths to achieve faster access to new therapies, such as breakthrough designation, conditional approval, adaptive approval, approval under exceptional circumstances and early entry (before marketing authorisation is granted). Therefore, innovative drugs—of which oncology products are the most numerous—enjoy faster regulatory approvals than other products (The Economist 2018). As regulatory approval times for cancer treatments have decreased, surrogate endpoints assessed over a short time frame are common in clinical trials of these drugs. Moreover, many products are approved with immature data, leading to substantial uncertainty when modelling the long-term benefit of such therapies.
Furthermore, as new products are being developed, clinical practice may change dramatically, making ongoing clinical trials invalid or useless. For example, while a product is being tested in a clinical trial in the second line, a new first-line drug emerges, making this trial uninformative.
Finally, HTA evaluation frameworks may change as new product classes emerge. NICE has developed a report on immunotherapy called chimeric antigen receptor (CAR) T-cell therapy, where they reviewed the applicability of NICE guidelines for (CAR) T and acknowledged the need to adapt their decision-making framework to such new emerging therapies (Crabb and Stevens 2016) This is even more true for gene therapies.
7 Further Issues with Health Economic Models in Oncology
7.1 Health-Related Quality of Life
Many cancer therapies extend survival only marginally or delay disease progression without extending survival. Therefore, given the high cost of these treatments, a QALY-based CUA is unlikely to reach value-for-money levels that would enable recommendation for financing. Also, the practice of eliciting QALY weights based on valuations from the general population can be challenging, because cancer is dreaded by many people more than other life-threatening conditions (Neumann et al. 2012) Further, utilities are one of the most influential parameters in CUA for advanced tumours submitted to NICE (Oncology Health Economic Modeling Post-Progression Working Group 2017). Therefore, the utility and validity of QALY-based HTA remain an open debate, and some countries have created exceptional HTA pathways for oncology interventions or have eased access to cancer treatments. This was achieved either through modifiers allowing a higher ICER in specific situations (such as end-of-life or disease-severity creating inequity) or through exceptions within HTA policies. Such exceptional circumstances are often applied without being explicitly acknowledged. Indeed, the ICER under which oncology products were recommended by NICE was significantly higher than for non-oncology products (Collins and Latimer 2013). Some countries operate a specific fund for oncology products that are not cost-effective (i.e. Cancer Drugs Fund in the UK) (Prasad and Mailankody 2016), which allows non-cost-effective products to be used within the NHS. However, the Cancer Drugs Fund was heavily criticised for wasting resources without bringing any additional value (Cohen 2017). Following criticism, CDF underwent a substantial reform in 2016 and drugs are now re-assessed after two years.
8 Comparator and Off-Label
When comparing oncology products in health economic models, efficacy data for both treatments should come from head-to-head clinical trials comparing the two drugs. However, new drugs are often approved based on trials versus placebo, so that modelling has to rely on indirect comparisons. Further, 50–75% of cancer care is provided off-label (“Off-Label” Indications for Oncology Drug Use and Drug Compendia 2005) and, to reflect the real-life situation, models may need to compare treatments used in non-approved indications, for which clinical trial data is absent. The missing data can be obtained from other sources, e.g. patient registries, but this can lead to further uncertainty around modelling outcomes.
9 Cost-Effectiveness Vs Affordability
We believe it is essential to raise two points before we conclude this chapter. Firstly, cost-effectiveness does not address budget constraints of payers and may mislead decision-makers by considering that a cost-effectiveness treatment option is also affordable. Secondly, theoretical limitations of CUA are widely described in the literature, so that when assessing a CUA, the reviewer must exert extreme caution in evaluating the methods and assumptions employed in the model, as subtle biases in the specification of the model can dramatically alter the results (Ryder et al. 2009). However, despite the limitations of CUA, no alternative methods have been proposed. Moving forward, integrating additional information into CUA may help to facilitate informed policymaking.
10 Conclusion
High uncertainty is the main challenge in CEA of newly approved oncology products. This uncertainty has increased dramatically over the recent years, with the emergence of new therapies that display efficacy, response features and survival shape distinct from all previously known products. These novel therapies are often associated with very high efficacy that cannot be easily quantified at time of launch due to immature data and come with a very high price tag. The development of gene and cell therapies targeting, often rare, cancers further increases the uncertainty in oncology health economics assessment. New methods for addressing this uncertainty have been developed but remain controversial, and have not yet been adopted by all experts or HTA agencies. In the coming years, we can expect health economics in oncology to evolve substantially, to address the challenges ahead.
References
Annemans L (2018) Extrapolation in oncology modelling: novel methods for novel compounds. Available from: https://www.ispor.org/news/articles/Aug12/Extrapolation-in-Oncology-Modelling.asp. Accessed on 09 Feb 2018
Bai Y, Xu Y, Wu B (2017) Cost-effectiveness and budget impact analysis of apatinib for advanced metastatic gastric cancer from the perspective of health insurance system. Gastroenterol Res Pract 2017:2816737
Califf RM, Zarin DA, Kramer JM et al (2012) Characteristics of clinical trials registered in ClinicalTrials.gov, 2007–2010. Jama 307:1838–1847
Cohen D (2017) Most drugs paid for by £1.27bn cancer drugs fund had no “meaningful benefit”. BMJ 357:j2097. https://doi.org/10.1136/bmj.j2097
Collins M, Latimer N (2013) NICE’s end of life criteria: who gains, who loses? BMJ 346:f1363
Crabb N, Stevens A (2016) Exploring the assessment and appraisal of regenerative medicines and cell therapy products. NICE
Cressman S, Browman GP, Hoch JS et al (2015) A time-trend economic analysis of cancer drug trials. Oncologist 20:729–736
de Souza JA, Yap BJ, Hlubocky FJ et al (2014) The development of a financial toxicity patient-reported outcome in cancer: the COST measure. Cancer 120(20):3245–3253
Dentaland Pharmaceutical Benefits Agency (TLV) (2012) Reimbursement decision for afinitor. TLV, Stockholm, Sweden
Experts in Chronic Myeloid Leukemia (2013) The price of drugs for chronic myeloid leukaemia (CML) is a reflection of the unsustainable prices of cancer drugs: from the perspective of a large group of CML experts. Blood 121(22):4439–4442
Fojo T, Grady C (2009) How much is life worth: cetuximab, non-small cell lung cancer, and the $440 billion question. J Natl Cancer Inst 101(15):1044–1048
Gibson E, Koblbauer I, Begum N et al (2017) Modelling the survival outcomes of immuno-oncology drugs in economic evaluations: a systematic approach to data analysis and extrapolation. PharmacoEconomics 35:1257–1270
Gold MR, Siegel JE, Russell LB, Weinstein MC (1996) Cost-effectiveness in health and medicine. Oxford University Press, New York
Isbary G, Staab TR, Amelung VE, Dintsios C-M, Iking-Konert C, Mariotti Nesurini S, Walter M, Ruof J (2017) The effect of crossover in oncology clinical trials on evidence levels in early benefit assessment in Germany. Value Health
Jönsson L, Sandin R, Ekman M, Ransberg J et al (2014) Analyzing overall survival in randomized controlled trials with crossover and implications for economic evaluation. Value Health 17:707–713
Kumar R (2013) Health economics and cost-effectiveness research with special reference to hemato-oncology. Med J Armed Forces India 69(3):273–277
Latimer N (2011) NICE DSU technical support document 14: undertaking survival analysis for economic evaluations alongside clinical trials—extrapolation with patient-level data. Available from http://www.nicedsu.org.uk. Accessed on 09 Feb 2018
Latimer NR (2013) Survival analysis for economic evaluations alongside clinical trials—extrapolation with patient-level data: inconsistencies, limitations, and a practical guide. Med Decis Making 33:743–754
Latimer NR, Abrams KR (2014) NICE DSU technical support document 16: adjusting survival time estimates in the presence of treatment switching. Available from http://www.nicedsu.org.uk. Accessed on 09 Feb 2018
Marsh K, Xu P, Orfanos P, Gordon J, Griebsch I (2014) Model-based cost-effectiveness analyses for the treatment of chronic lymphocytic leukaemia: a review of methods to model disease outcomes and estimate utility. Pharmacoeconomics 32(10):981–993
Miller JD, Foley KA, Russell MW (2014) Current challenges in health economic modelling of cancer therapies: a research inquiry. Am Health Drug Benefits 7:153–162
Murray CJ, Evans DB, Acharya A, Baltussen RM (2000) Development of WHO guidelines on generalized cost-effectiveness analysis. Health Econ 9:235–251
National Institute for Health and Care Excellence (2013) Guide to the methods of technology appraisal. NICE, London
National Institute of Health and Care Excellence (2011) Everolimus for the second-line treatment of advanced renal cell carcinoma (NICE technology appraisal guidance 219). NICE, London, UK
Neumann PJ, Bliss SK, Chambers JD (2012) Therapies for advanced cancers pose a special challenge for health technology assessment organizations in many countries. Health Aff (Millwood) 31:700–708
“Off-Label” indications for oncology drug use and drug compendia: history and current status. J Oncol Pract 1:102–105 (2005)
Oncology Health Economic Modeling Post Progression Working Group of the ISPOR Oncology SIG. ISPOR, Boston (2017)
Prasad V, Mailankody S (2016) The UK cancer drugs fund experiment and the US cancer drug cost problem: bearing the cost of cancer drugs until it is unbearable. Mayo Clin Proc 91(6):707–712
Ryder HF, McDonough CF, Tosteson A, Lurie JD (2009) Decision analysis and cost-effectiveness analysis. Semin Spine Surg 21(4):216–222
Sarin R (2008) Criteria for deciding cost-effectiveness for expensive new anti-cancer agents. J Cancer Res Ther 4:1–2
Sonnenberg FA, Beck JR (1993) Markov models in medical decision making: a practical guide. Med Decis Making 13(4):322–338
The Economist. https://www.economist.com/news/finance-and-economics/21736176-progress-developing-treatments-makes-oncology-research-favourite
Toumi M (2017) Introduction to market access for pharmaceuticals. CRC Press
Watkins C, Huang X, Latimer N, Tang Y, Wright EJ (2013) Adjusting overall survival for treatment switches: commonly used methods and practical application. Pharm Stat 12:348–357
Weinstein MC, O’Brien B, Hornberger J, Jackson J, Johannesson M, McCabe C, Luce BR (2003) Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR task force on good research practices—modeling studies. Value Health 6(1):9–17
Zafar SY, Peppercorn JM, Schrag D et al (2013) The financial toxicity of cancer treatment: a pilot study assessing out-of-pocket expenses and the insured cancer patient’s experience. Oncologist 13(4):381–390
Ziomek J, El Mouaddin N, Ng T et al (2017) Analysis of recent approvals of immuno-oncology drugs across England, Scotland, Germany and France. Value Health A399–A811
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Francois, C., Zhou, J., Pochopien, M., Achour, L., Toumi, M. (2019). Oncology from an HTA and Health Economic Perspective. In: Walter, E. (eds) Regulatory and Economic Aspects in Oncology. Recent Results in Cancer Research, vol 213. Springer, Cham. https://doi.org/10.1007/978-3-030-01207-6_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-01207-6_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-01206-9
Online ISBN: 978-3-030-01207-6
eBook Packages: MedicineMedicine (R0)