Abstract
Unexplained fullness after eating, inability to finish a normal-sized meal (early satiety), and/or epigastric pain or burning are common symptoms. In clinical practice the likeliest explanation is functional dyspepsia, where by definition esophagogastroduodenoscopy is normal, with no other structural explanation found. Symptoms may be primarily after eating, as in postprandial distress syndrome, or may have no relationship to meals (epigastric pain syndrome). The diagnosis of functional dyspepsia requires exclusion of organic disease. The pathogenesis is incompletely understood and is likely multifactorial. In a minority, Helicobacter pylori (H. pylori) infection plays a role, but some patients have microscopic duodenal inflammation characterised by eosinophils, and sometimes mast cells. Currently, treatment involves a stepwise approach. If H. pylori infection is present, eradication therapy may be beneficial. Acid suppression is otherwise first-line therapy. An antidepressant (a low-dose tricyclic agent) or prokinetic agent is second-line therapy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Case
A 57-year-old woman with a background history of mild asthma and allergic rhinitis presents with a 30-year history of unexplained gnawing, or sometimes burning, epigastric pain after eating. She also reports feeling bloated and uncomfortable after most meals and an inability to finish a meal as large as her sister or husband (early satiety). When asked about food triggers, ingestion of wheat was identified, but reduced ingestion of gluten gave limited relief. She also described what had been labelled as heartburn a few days per week (lower retrosternal and epigastric burning), but no acid regurgitation or dysphagia. Her bowel habits were normal, and there was no history of weight loss, vomiting, or dysphagia. There was no history of an infection preceding the onset of symptoms. She had a history of anxiety, but not depression. Ten years ago, an esophagogastroduodenoscopy (EGD) was normal, including testing for H. pylori, and she was treated with a standard-dose proton pump inhibitor (PPI) with only slight improvement. The dominant symptoms remained postprandial fullness and early satiety and were at times bad enough to result in time off work. Her sleep was affected. Physical examination revealed a normal weight female, with a soft and non-tender abdomen. The working diagnosis was PPI-resistant non-erosive gastroesophageal reflux disease (GERD), and she was referred for a second opinion and further workup.
A repeat EGD was normal with no evidence of peptic ulceration or esophagitis. Duodenal biopsies from the second portion were obtained, with confirmation that the patient was eating gluten at the time of biopsy. There was no evidence of celiac disease, but the duodenal eosinophil count in 5 high-power fields (5 hpf) was increased to 39/5 hpf (normal <22/5 hpf). Gastric biopsies were normal. An oesophageal impedance-pH study off PPI therapy was normal. A diagnosis of functional dyspepsia (FD) (postprandial distress (PDS) subtype) was made.
Objectives
-
Recognise patients presenting with symptoms of FD in clinical practice.
-
Differentiate FD from other causes of dyspepsia, especially GERD.
-
Understand current treatment options for FD.
Epidemiology
Unexplained fullness after eating, early satiety, and/or epigastric pain or burning are common complaints in the community and in clinical practice. Although there is a broad list of differential diagnoses for these symptoms, including peptic ulcer disease, GERD, medication side effects (e.g. non-steroidal anti-inflammatory drugs (NSAIDs)), and rarely gastroesophageal malignancy or gastroparesis, the majority of those who consult have no explanation identified by EGD or other routine tests and are labelled as having FD [1].
In the USA, about 10% of the population report typical FD symptoms, although many are mislabelled as having GERD. Heartburn and FD symptoms overlap more than expected by chance, suggesting a common underlying pathophysiology, and therefore in some cases, it can be difficult to differentiate the two conditions. One clue is early satiety; this symptom is a good discriminator and points to FD rather than GERD [1, 2]. Frequent dominant heartburn on the other hand points to GERD.
Expert consensus subdivides FD into those with PDS, characterised by postprandial fullness and/or early satiety at least 3 days per week (in fact usually patients have symptoms after most meals), and epigastric pain syndrome (EPS), characterised by intermittent episodes of pain or burning at least 1 day per week. PDS and EPS often overlap, but in the general population, PDS is more prevalent, accounting for about two-thirds of FD cases [2]. FD is important because the symptoms impact on quality of life, including work and relationships. Anxiety and depression, as well as sleep disturbances, are highly prevalent in patients with FD.
Etiology and Pathophysiology
The pathogenesis of FD is not completely understood and is likely multifactorial in nature. Traditionally FD has been considered a disorder arising from the stomach, and most attention has focussed on this organ. Fullness after eating points to a gastric motility problem, and although one in five patients with FD have slower than normal gastric emptying, this abnormality correlates poorly, if at all, with symptoms. Vomiting is not a feature of FD but is frequent in gastroparesis and may help distinguish these two overlapping conditions [3]. Further, there is good evidence that a subset with FD have gastric fundus relaxation failure, and this is associated with the inability to finish a normal-sized meal [4]. Normally, the gastric fundus relaxes after eating creating a pleasant feeling of satiety, but if there is a failure of this normal vagal mechanism, then early satiety often occurs. Certain drugs that relax the gastric fundus can reduce PDS symptoms, including early satiety [5]. In addition to disordered motility, hypersensitivity to mechanical or chemical stimuli is frequently observed in FD, although as with gastric emptying the relationship between this and symptoms of dyspepsia is not completely understood [2].
There is increasing evidence that abnormalities in the duodenum may lead to FD, particularly PDS. Increased duodenal inflammation has been identified in a subset with PDS, most notably increased duodenal eosinophils [6] (defined as >22 eosinophils after counting 5 HPFs) (Fig. 8.1). Further, duodenal eosinophils in FD are associated with increased small intestinal permeability and changes in neuronal structure and function, which may explain intestinal hypersensitivity [7]. In some cases, mast cells can also be seen to be increased along with eosinophils, if special stains are applied [8]. Immune activation has been documented in FD (cytokines and circulating homing small intestinal T cells), and immune activation is associated with (and might explain) slow gastric emptying [1].
Although epigastric pain or burning can be a feature of FD, and some patients respond to acid suppression [9], gastric acid secretion is normal. Eradication therapy for H. pylori leads to complete symptom resolution in only a minority of FD patients. The FD subgroup most likely to respond reports EPS. This suggests epigastric pain may arise from gastric pathology in some FD cases.
Infections other than H. pylori may be associated with FD. As in irritable bowel syndrome (IBS), there is good evidence that FD can arise after an acute intestinal infection, such as Salmonella (post-infectious FD) [10]. It is therefore important to ask for a history of infective symptoms prior to the onset of dyspepsia, although this may be difficult to ascertain in most due to a delay in presentation.
Other putative mechanisms have been suggested and are current areas of investigation. For example, many patients with FD report that certain foods may induce symptoms, such as gluten. It is conceivable that, just as in celiac disease, there is an abnormal immune response to gliadin in FD [11, 12]. The gastroduodenal microbiome has been shown to be perturbed in patients with FD, when compared with non-FD patients. ‘Normalisation’ of gut flora through the use of probiotics may represent a potential therapeutic option, although a Japanese study that investigated this did not report on clinical outcomes [13]. An underlying genetic predisposition is possible but remains to be firmly established. For example, a subgroup with FD have clinical evidence of Ehlers-Danlos type III, but the genetics are unknown [14].
A summary of proposed disease mechanisms based on current understanding is presented in Table 8.1.
Symptoms
The key symptoms are early satiety, postprandial fullness (often described as bloating by patients, unless specific questioning is conducted), epigastric pain, and epigastric burning; these are now considered to constitute dyspepsia [15, 16]. Early satiety is common but is often missed unless specifically asked for whilst eliciting the patient’s history. Patients often describe this as a vague discomfort or excess gas after eating, although what they really mean is that they cannot finish a normal-sized meal because they feel full or uncomfortable. Other symptoms may co-occur, including nausea and heartburn, but these are not considered primary dyspeptic symptoms any longer and may arise through separate mechanisms. Certain symptoms, such as vomiting, require evaluation for alternative or coexistent disease such as gastroparesis. The Rome IV criteria (Table 8.2) provides a symptom-based framework for diagnosing FD [2].
Diagnostic Evaluation
A careful history and examination should be performed in patients presenting with epigastric symptoms. Epigastric pain can arise from many other causes, including biliary and pancreatic pathology. If the pattern of the pain is suggestive (severe, episodic, lasting for hours, radiating to the back) or if there are risk factors for biliary tract disease, appropriate testing is indicated, but beware of false-positive results (e.g. abdominal ultrasound finding incidental gallstones). Peptic ulcers may cause EPS, or less often PDS, so a history of NSAID use and testing for H. pylori is a routine part of the evaluation as, in the absence of these risk factors, peptic ulcer disease is highly unlikely.
Current expert consensus recommends an EGD for any patient aged ≥60 years with dyspeptic symptoms, primarily to exclude gastroesophageal malignancy [17]. On the other hand, performing an EGD in younger patients with dyspepsia generally has a low yield, but should be considered on a case-by-case basis if certain ‘alarm features’ or ‘red flags’ are present. These include vomiting, dysphagia and/or odynophagia, evidence or suspicion of gastrointestinal bleeding, unexplained weight loss, a palpable mass or lymphadenopathy, or a family history of upper gastrointestinal malignancy [2, 17]. However, even in the presence of a red flag, few patients with typical FD symptoms will have malignancy identified at EGD [17].
Gastroparesis is rare, unlike FD [3]. If the patient is vomiting or losing weight, a gastric emptying study should be considered if the EGD and other tests are normal. However, it should be remembered that a mild degree of delayed gastric emptying is common in FD (20%) and is unlikely to adequately explain the symptoms [2].
GERD is common and overlaps with FD. This can make differentiating the two conditions troublesome. A rule of thumb is that the presence of early satiety most likely indicates true FD, not GERD. Some cases of PPI failure in patients thought to have non-erosive GERD are explained by misdiagnosis of FD as GERD [18]. IBS also overlaps with FD more than expected by chance, but altered bowel habits in association with bloating or pain characterise IBS, not FD. As with IBS, anxiety and less often depression are common in those with FD, and depression should be screened for, as its presence alters treatment. There is some evidence that these central nervous system disturbances may arise primarily from the gut in some cases, rather than the brain, indicating the communication is bidirectional [19].
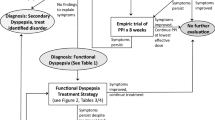
Celiac disease is a great mimic and can present with dyspepsia. It is our practice to ask about wheat intolerance, check a complete blood count, and consider tissue transglutaminase testing for celiac disease. A diagnostic algorithm to workup suspected FD is shown in Fig. 8.2.
Treatment
A firm diagnosis, followed by reassurance, explanation, and a treatment plan work best in clinical practice. The prognosis is excellent, and FD is not linked to any increase in mortality [20]. A treatment algorithm is shown in Fig. 8.3.
Helicobacter Pylori
If H. pylori is detected, either by non-invasive means (e.g. breath test, stool antigen) or on biopsies during EGD, eradication therapy should be offered [21]. Patients should be counselled regarding potential side effects, and it is important to remember that the majority of patients with FD will not respond symptomatically to eradication (suggesting the H. pylori infection is often incidental and asymptomatic).
Acid Suppression
Acid suppression is otherwise first-line therapy [17]. A standard dose of a PPI before breakfast is superior to placebo. A double-dose PPI adds no established benefit. The mechanism by which a PPI works is unknown but may increase the duodenal pH, positively alter the microbiome, or possibly suppress duodenal eosinophils [22]. An alternative is a histamine type-2 receptor antagonist (H2RA) , although current evidence indicates that PPIs are slightly more effective, and the therapeutic effect of H2RAs may wear off over time (tachyphylaxis).
Acid suppression appears to be more effective in PDS than in EPS, but given the significant overlap of these in real-world settings, and the potential for coexistent GERD, a trial of acid suppression is generally warranted in all patients with FD, regardless of subtype [9, 17].
Antidepressants
Tricyclic antidepressants (TCAs) are considered second-line therapy [17]. A systematic review found that the beneficial effect of antidepressants for treating FD was limited to TCAs, and highlighted the need to monitor for side effects [23].
Prokinetics
Trials that have compared prokinetic agents with PPI therapy in FD showed a trend towards PPIs being more effective. As such, prokinetic therapy is a potential second-line option [17]. In the USA, prokinetic options are limited, as most of the drugs that were evaluated in randomised trials (e.g. cisapride and mosapride) are not currently available. Prokinetic agents used to treat gastroparesis (e.g. metoclopramide, domperidone) have limited data regarding their efficacy in FD. Acotiamide, which acts by enhancing the release and duration of enteric acetylcholine, has been shown to be superior to placebo in reducing PDS symptoms and is currently available in Japan and India [5, 24].
Further Options
The non-absorbable antibiotic rifaximin provided relief of FD symptoms in one clinical trial, but the duration of benefit is unknown [25]. The benefit of probiotics has not been established. Herbal products such as peppermint oil and STW-5 (Iberogast) may also be of benefit in some patients.
Dietary therapy may be helpful. Just as in IBS, a diet low in fermentable oligo-, di-, and monosaccharides and polyols appears to help some cases, but randomised controlled trial evidence in FD is lacking. Food triggers should be considered, as some patients may respond to gluten restriction (those with early satiety in particular) [12]. Psychological therapies can help some patients, particularly if there is comorbid psychological distress, and we refer for cognitive behavioural therapy if patients are responding poorly. Depression should be treated if present.
Case Study: Follow-Up
As the patient was concerned about the potential side effects of PPI long term, she was switched to a H2RA and reported substantial benefit initially, although this waned over months. A low dose of amitriptyline (starting at 10 mg at night for 1 month, then 25 mg for 1 month, then increasing to 50 mg at night for 6 months) was well-tolerated, improved sleep, and reduced all dyspeptic symptoms.
Clinical Pearls
-
If a patient reports early satiety (remembering you need to ask specifically about this complaint), think about underlying FD high up on your differential diagnosis list.
-
Increased duodenal eosinophils are linked to FD, particularly PDS – but you must ask your pathologist to count 5 high-power fields in order to detect the abnormality, otherwise this will be missed.
-
A young patient (<60 years) with dyspeptic symptoms and no alarm features, no relevant drug history (e.g. NSAIDs), and no evidence of H. pylori on non-invasive testing has FD until proven otherwise; EGD then has a low yield. EGD should be performed in patients ≥60 years of age, and on a case-by-case basis in younger patients with alarm features.
-
First-line therapeutic options for FD are acid suppression or, if H. pylori infected, eradication therapy.
-
Low-dose tricyclic antidepressants and prokinetics are second-line options.
References
Talley NJ, Ford AC. Functional dyspepsia. N Engl J Med. 2015;373(19):1853–63.
Stanghellini V, Chan FKL, Hasler WL, Malagelada JR, Suzuki H, Tack J, et al. Gastroduodenal disorders. Gastroenterology. 2016;150(6):1380–92.
Tack J, Carbone F. Functional dyspepsia and gastroparesis. Curr Opin Gastroenterol. 2017;33(6):446–54.
Carbone F, Tack J. Gastroduodenal mechanisms underlying functional gastric disorders. Dig Dis. 2014;32(3):222–9.
Ueda M, Iwasaki E, Suzuki H. Profile of acotiamide in the treatment of functional dyspepsia. Clin Exp Gastroenterol. 2016;9:83–8.
Walker MM, Aggarwal KR, Shim LSE, Bassan M, Kalantar JS, Weltman MD, et al. Duodenal eosinophilia and early satiety in functional dyspepsia: confirmation of a positive association in an Australian cohort. J Gastroenterol Hepatol. 2014;29(3):474–9.
Cirillo C, Bessissow T, Desmet A-S, Vanheel H, Tack J, Vanden Berghe P. Evidence for neuronal and structural changes in submucous ganglia of patients with functional dyspepsia. Am J Gastroenterol. 2015;110:1205.
Walker MM, Talley NJ, Prabhakar M, Pennaneac’H CJ, Aro P, Ronkainen J, et al. Duodenal mastocytosis, eosinophilia and intraepithelial lymphocytosis as possible disease markers in the irritable bowel syndrome and functional dyspepsia. Aliment Pharmacol Ther. 2009;29(7):765–73.
Pinto-Sanchez MI, Yuan Y, Hassan A, Bercik P, Moayyedi P. Proton pump inhibitors for functional dyspepsia. Cochrane Database Syst Rev. 2017;11:CD011194.
Mearin F, Pérez-Oliveras M, Perelló A, Vinyet J, Ibañez A, Coderch J, Perona M. Dyspepsia and irritable bowel syndrome after a Salmonella gastroenteritis outbreak: one-year follow-up cohort study. Gastroenterology. 2005;129(1):98–104.
Potter M, Walker MM, Talley NJ. Non-coeliac gluten or wheat sensitivity: emerging disease or misdiagnosis? Med J Aust. 2017;207(5):211–5.
Du L, Shen J, Kim JJ, He H, Chen B, Dai N. Impact of gluten consumption in patients with functional dyspepsia: a case–control study. J Gastroenterol Hepatol. 2018;33(1):128–33.
Igarashi M, Nakae H, Matsuoka T, Takahashi S, Hisada T, Tomita J, et al. Alteration in the gastric microbiota and its restoration by probiotics in patients with functional dyspepsia. BMJ Open Gastroenterol. 2017;4(1):e000144.
Fikree A, Chelimsky G, Collins H, Kovacic K, Aziz Q. Gastrointestinal involvement in the Ehlers–Danlos syndromes. Am J Med Genet C: Semin Med Genet. 2017;175(1):181–7.
Vakil NB, Howden CW, Moayyedi P, Tack J. White paper AGA: functional dyspepsia. Clinical Gastroenterol Hepatol. 2017;15(8):1191–4.
Enck P, Azpiroz F, Boeckxstaens G, Elsenbruch S, Feinle-Bisset C, Holtmann G, et al. Functional dyspepsia. Nat Rev Dis Primers. 2017;3:17081.
Moayyedi PM, Lacy BE, Andrews CN, Enns RA, Howden CW, Vakil N. ACG and CAG clinical guideline: management of dyspepsia. Am J Gastroenterol. 2017;112:988.
D’Alessandro A, Zito F, Pesce M, Andreozzi P, Efficie E, Cargiolli M, et al. Specific dyspeptic symptoms are associated with poor response to therapy in patients with gastroesophageal reflux disease. United European Gastroenterol J. 2017;5(1):54–9.
Koloski NA, Jones M, Talley NJ. Evidence that independent gut-to-brain and brain-to-gut pathways operate in the irritable bowel syndrome and functional dyspepsia: a 1-year population-based prospective study. Aliment Pharmacol Ther. 2016;44(6):592–600.
Ford AC, Forman D, Bailey AG, Axon ATR, Moayyedi P. Effect of dyspepsia on survival: a longitudinal 10-year follow-up study. Am J Gastroenterol. 2012;107:912.
Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG clinical guideline: treatment of helicobacter pylori infection. Am J Gastroenterol. 2017;112:212.
Jackson MA, Goodrich JK, Maxan M-E, Freedberg DE, Abrams JA, Poole AC, et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65(5):749–56.
Ford AC, Luthra P, Tack J, Boeckxstaens GE, Moayyedi P, Talley NJ. Efficacy of psychotropic drugs in functional dyspepsia: systematic review and meta-analysis. Gut. 2017;66(3):411–20.
Kusunoki H, Haruma K, Manabe N, Imamura H, Kamada T, Shiotani A, et al. Therapeutic efficacy of acotiamide in patients with functional dyspepsia based on enhanced postprandial gastric accommodation and emptying: randomized controlled study evaluation by real-time ultrasonography. Neurogastroenterol Motil. 2012;24(6):540–e251.
Tan VPY, Liu KSH, Lam FYF, Hung IFN, Yuen MF, Leung WK. Randomised clinical trial: rifaximin versus placebo for the treatment of functional dyspepsia. Aliment Pharmacol Ther. 2017;45(6):767–76.
Essential Reading
Moayyedi PM, Lacy BE, Andrews CN, Enns RA, Howden CW, Vakil N. ACG and CAG clinical guideline: management of dyspepsia. Am J Gastroenterol. 2017;112:988. Evidence-based guidelines for the diagnosis and management of patients with dyspepsia.
Stanghellini V, Chan FKL, Hasler WL, Malagelada JR, Suzuki H, Tack J, et al. Gastroduodenal disorders. Gastroenterol. 2016;150(6):1380–92. Provides a detailed overview of FD and other functional disorders, with reference to the Rome IV Criteria.
Talley NJ, Ford AC. Functional dyspepsia. N Engl J Med. 2015;373(19):1853–63. Expert overview of FD with explanation of disease mechanisms and discussion of relevant clinical trials.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Self-Test
Self-Test
-
Question 1. A 45-year-old man consults regarding a 5-year history of epigastric discomfort, described as burning in nature. Early in its course, the pain was intermittent and tended to occur after meals, although he feels that it is occurring more frequently in recent times. He had a poor response to a PPI. In addition, he has noticed his stools have become increasingly loose and offensive and at times are difficult to flush. EGD with gastric and duodenal biopsies 2 years ago on a normal diet were unremarkable.
Which of the following investigations is most likely to help establish a diagnosis?
-
A.
Repeat EGD and duodenal biopsies.
-
B.
Urease breath test for H. pylori
-
C.
Abdominal CT scan
-
D.
Glucose hydrogen breath test
-
E.
Celiac serology
-
A.
-
Question 2. You are reviewing a 35-year-old female who has returned for follow-up after a normal EGD for symptoms of moderately severe persistent dyspepsia, with normal bowel habits. Gastric biopsies were normal, without evidence of H. pylori. Duodenal biopsies demonstrated an eosinophil count of 30/hpf, on at least 5 high-power fields. There were no typical changes of coeliac disease.
You make a diagnosis of FD, likely PDS, and discuss management options. Which of the following would you recommend as first-line based on randomised controlled trials?
-
A.
Trial of PPI
-
B.
Low FODMAP diet and psychotherapy
-
C.
Trial of a TCA
-
D.
No treatment indicated – reassurance and discharge to primary care physician
-
E.
H2RA
-
A.
-
Question 3. The Rome IV criteria provide a symptom-based framework for the diagnosis of functional dyspepsia (FD) and its subtypes. Which of the following symptoms is least likely to occur in functional dyspepsia, and should alert clinicians to an alternative diagnosis?
-
A.
Excessive belching
-
B.
Heartburn less than once a week
-
C.
Frequent postprandial vomiting
-
D.
Nausea
-
E.
Early satiety
-
A.
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Talley, N.J., Cook, D.R. (2019). Functional Dyspepsia. In: Lacy, B., DiBaise, J., Pimentel, M., Ford, A. (eds) Essential Medical Disorders of the Stomach and Small Intestine. Springer, Cham. https://doi.org/10.1007/978-3-030-01117-8_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-01117-8_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-01116-1
Online ISBN: 978-3-030-01117-8
eBook Packages: MedicineMedicine (R0)