Abstract
Colonoscopic surveillance for risk of colorectal cancer (CRC) associated with inflammatory bowel disease (IBD) is recommended annually beginning at 8 years disease duration in ulcerative pancolitis and Crohn’s colitis. Family history of sporadic CRC raises the risk of CRC by twofold, and association with primary sclerosing cholangitis by fourfold. Optimized conditions for endoscopy, random and targeted biopsy requirements, and patient information and compliance are essential for success of the surveillance program, as well as experienced endoscopic detection and classification of neoplastic lesions as (1) sporadic lesions (in uninvolved colon) and in involved bowel, (2) polypoid dysplastic lesions, and (3) non-polypoid dysplastic lesions. Endoscopic en bloc resection (with free margins) is recommended for all resectable lesions without invisible dysplasia, whereas sphincter-preserving colectomy is recommended for endoscopically unresectable non-polypoid lesions and invisible lesions detected by random biopsy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Ulcerative colitis
- Crohn’s colitis
- Colonoscopic surveillance
- Colorectal cancer (CRC) risk
- Dysplasia, polypoid and non-polypoid
- Invisible dysplasia
- Targeted biopsy vs. random biopsy
- Microvessel pattern
- Endoscopic resection, indications
- Colectomy, indications
The cumulative risk of colorectal cancer (CRC) in patients with ulcerative colitis (UC) amounts to 2% at 10 years, 9% at 20 years, and 18% at 30 years after onset of symptoms [1]. The risk of CRC is increased about 2.5-fold for Crohn’s disease (CD) [2] and 5.6-fold for Crohn’s colitis [3] and is equivalent in long-standing UC and Crohn’s colitis [4].
In spite of the known increased risk of CRC and in spite of endoscopic surveillance, overall 5-year survival for CRC associated with inflammatory bowel disease (IBD) may not be better than for sporadic CRC. In a study of 28 patients with CD-associated CRC and 52 with UC-associated CRC, the overall 5-year survival rates were only 46% for CD- and 50% for UC-associated CRC; the median duration of IBD was 15 years for CD and 18 years for UC. Dysplasia was associated with CRC in 73% of CD and 79% of UC [4]. Hence, the detection rate of dysplastic lesions must rise in surveillance colonoscopy for IBD. We must intensify detection and analysis of neoplasias in IBD surveillance using HD endoscopy with magnification (>50-fold) and chromoendoscopy (indigo carmine) or (to a lesser extent) virtual chromoendoscopy (NBI). (Compare Chap. 1.)
1 Factors Increasing Risk of Colonic Neoplasias in IBD
The relative risk of CRC is significantly elevated after 8–10 years of colitis in IBD, which is the time point when surveillance should start in both UC and Crohn’s colitis, whereas in left-sided UC, surveillance may start at 15-year disease duration [1, 2, 5, 6]. A more recent study stressed that 9–15% of cancers occur earlier and would be missed with this recommendation [7], in spite of evidence gathered from retrospective analyses [2, 5, 6].
Young onset of IBD even raises the risk of CRC [5, 8]. The risk of CRC is increased twofold in UC patients with first- or second-degree relatives with CRC [8], and fourfold with long-standing primary sclerosing cholangitis (PSC) [8, 9] (Table 12.1). Patients with IBD and PSC should undergo annual colonoscopy beginning at the time of diagnosis of PSC and continuing indefinitely, even after liver transplantation [9, 10].
2 Colonoscopic Surveillance for CRC Risk in Long-Standing IBD
2.1 Surveillance Protocol
Surveillance colonoscopy (using white-light imaging, WLI) is best performed in stable remission of colitis (Truelove activity index ≤2) [11] and combined with chromoendoscopy (CE) using indigo carmine (or methylene blue) for targeted biopsy strategy [4]. After 8 years of UC, screening and further surveillance colonoscopy is recommended, with targeted biopsies of suspicious lesions or quadrant biopsies every 10 cm from suspicious sections of the involved colon (e.g., with pseudopolyposis or postinflammatory narrowing). Analogous recommendations pertain to Crohn’s colitis when at least 30% of the colon is involved [4, 12, 13]. The typical result is a minimum of 28–32 biopsy samples. However, a recent randomized multi-center prospective study showed equality of targeted biopsy for detecting neoplastic lesions compared with random biopsy [14]. The procedure report in UC or Crohn’s colitis should number the locations of all biopsies sampled from flat mucosa, from any superficial lesion 0-II (image documented), or any suspicious polypoid lesion sampled or removed.
2.2 Chromoendoscopy and Magnifying Narrow-Band Imaging (NBI)
In well-cleaned, mucus-depleted large bowel, image-enhanced endoscopy (IEE) using panchromoendoscopy with indigo carmine (or methylene blue) should be employed to take targeted biopsies. Panchromoendoscopy and targeted biopsies resulted in a higher yield of dysplasia than systematic four-quadrant biopsies in non–dye-sprayed colon [14,15,16]. Chromoendoscopy and virtual chromoendoscopy using NBI do not differ significantly for detection of colitis-associated neoplasia. Given the shorter procedural time and easier applicability, NBI may replace classic chromoendoscopy in the future [17].
Note
Recommendations of surveillance for IBD-associated CRC [12, 18]:
-
Start at 8 years after onset of symptoms in pancolitis.
-
Examine when colonic disease is in remission.
-
IEE-colonoscopy every 1–2 years after onset of surveillance, or every 2–3 years after two negative examinations (no dysplasia/CRC).
-
Use high-definition (HD) endoscopy with >50× magnifying NBI and indigo carmine CE for analysis of lesions and mucosal patterns.
-
Take representative targeted biopsy specimens from each anatomic section (or two protocol biopsies every 10 cm) of the involved colon.
-
With PSC, colonoscopic surveillance starts at diagnosis and requires annual surveillance colonoscopy.
An exemption is ulcerative proctitis / proctosigmoiditis , which does not carry increased risk and may be managed with average-risk recommendations.
3 Diagnosis of Visible Dysplasia on Surveillance Colonoscopy
3.1 Lesions in IBD
To detect areas suspicious for neoplastic lesions in regenerative chronic inflammatory mucosa, you must focus on subtle alterations in microsurface structure and vascular pattern, as well as on visible surface alterations likely to harbor premalignant or malignant tissue [6, 14, 19, 20]. Nevertheless, previous intensity of inflammatory activity correlates positively with an increased risk of high-grade intraepithelial neoplasia (HGIN) or cancer. By analogy, the presence of postinflammatory pseudopolyps approximately doubles the risk of HGIN or cancer in UC [4, 6]. Long-standing UC with stricture or foreshortened colon has a high probability of even advanced cancer [19].
Previously, the term “dysplasia-associated lesion or mass” (DALM) was commonly used for endoscopically unresectable, raised dysplastic lesions (0-IIa or 0-Is) with concomitant dysplasia of the surrounding flat mucosa. Also, “adenoma-like lesion or mass” (ALM) was commonly used for endoscopically resectable protruded lesions with a distinct margin and smooth surface. Endoscopic diagnosis of those lesions was subjective, however, and it sometimes became difficult to distinguish between them [21]. Therefore, new criteria, visible polypoid dysplasia (lesion height ≥ 2.5 mm) and non-polypoid dysplasia (lesion height < 2.5 mm) was proposed by SCENIC (Surveillance for Colorectal Endoscopic Neoplasia Detection and Management in Inflammatory Bowel Disease Patients: International Consensus Recommendations) [12, 20] and the European Crohn’s and Colitis Organisation (ECCO) guideline [13].
Note
Be observant for the principal premalignant/malignant lesions in IBD:
-
Sporadic adenoma/dysplasia (outside IBD-involved colon)
-
Visible Polypoid dysplasia (lesion height ≥ 2.5 mm, 0-Is, Isp, Ip)
-
Visible Non-polypoid dysplasia (lesion height < 2.5 mm, 0-IIa, IIb, IIc)
-
Sites for risk of Invisible Dysplasia: (→ random biopsies) in: Pseudopolyposis, postinflammatory narrowing, surroundings of visible lesions
Sugimoto et al. were the first to apply the Paris classification, as proposed by SCENIC, to a well-documented series of visible high-grade dysplasia (HGD) (n = 39) in a cohort of 62 patients diagnosed with HGD or CRC in chronic UC [22]. HGD lesions typically were reddish (80%) or discolored (20%) versus background mucosa in remission; mostly elevated, flat, or sessile; and mainly (80%) located in the rectosigmoid (Table 12.2). Typically, most sessile/elevated lesions spread out in a flat area (Is+IIb/IIa + IIb) but were not classified as mixed types. The two depressed lesions were next to an ulcer. All flat (IIb) and depressed (IIc) HGD showed up in red, sessile (0-Is) and elevated lesions (0-IIa) in red (66% each) or as discolored (a third each). All were diagnosed with targeted biopsy. Borders were indistinct in 57% of HGD lesions on CE, but were distinct in all (100%) on M-NBI [22]. A recent classification (FACILE) for optical diagnosis of visible dysplasia achieved moderate 76% accuracy by experts based on evaluation of 4 criteria on still images in non-magnifying CE [23]. But prospective clinical data on prevalence of macroscopic types of HGD or early CRC in IBD colitis are not yet available.
Note
The flat II-b component, when the demarcation line is identified on M-NBI, must be classified, since mixed lesions (0-Is+IIb; 0-IIa + IIb) are a good indication for endoscopic resection, whereas invisible dysplasia near such lesions suggests colectomy because of high and non-manageable cancer risk.
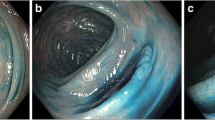
Endoscopic diagnosis of surface (S) and vascular (V) structure of IBD-associated lesions is usually very difficult even for experts. Morphology of the lesions in IBD patients is altered by long-standing inflammation, and regeneration of the tissue comes in a variety of forms. At present, there is no consensus regarding pit pattern diagnosis or NBI magnification findings in IBD patients. It is sometimes difficult to distinguish between sporadic dysplasia and UC-associated dysplasia by gross appearance, surface structure, and vascular pattern of the lesions. A p53 immunohistochemical (IHC) staining is mandatory to distinguish between them, after complete removal of the lesion (Figs. 12.1 and 12.2). However, chromoendoscopy with magnification and NBI with magnification (M-NBI) are very useful to identify abnormal surface structure and vascular pattern of the lesions in IBD patients, although interpretation of those findings has not yet been established (Figs. 12.3 and 12.4).
A case of sporadic mucosal cancer . (a) A laterally spreading tumor (LST)-NG (PD) around 25 mm located at the sigmoid colon was found by WLI in a 63-year-old man with long-lasting ulcerative colitis (UC) , which was in remission. (b) Demarcation line and surface structure become much clear with indigo carmine dye spraying. (c) Irregular and uneven microvessels, as well as irregular surface structure, were observed with NBI magnification. (d) Dense, uneven small pits at the peripheral part of the lesion and loose, unclear small pits at the central part were observed by crystal violet staining and magnification. (f) Margin-free resection was achieved by endoscopic submucosal dissection (ESD). (e, g) Histopathological result was Is+IIc, 28 × 18 mm, tubular adenocarcinoma (tub1) with tubular adenoma, pM, int, INFα, ly0, v0, pHM0, pVM0. (h) p53 immuno-histochemical staining was negative, so this lesion was judged to be a sporadic mucosal cancer
A case of UC-associated mucosal cancer . (a) A slightly reddish IIa lesion about 10 mm in size was found at the upper rectum by WLI in a 65-year-old man with 15 years history of UC, in remission. (b) Demarcation line and surface structure become much clear with indigo carmine dye spraying. (c) Slightly irregular microvessels and irregular surface structure were observed with NBI magnification. (d) Dense, uneven small pits were observed at the central part of the lesion by crystal violet staining and magnification. (f) Margin-free resection was achieved by ESD. (e, g) Histopathological result was IIa, 9 × 9 mm, tubular adenocarcinoma (tub1), pM, int, INFα, ly0, v0, pHM0, pVM0. (h) Overexpression of p53 protein was observed by immuno-histochemical staining, so this lesion was judged to be UC-associated mucosal cancer
0-Is lesions in long-standing ulcerative regenerative pancolitis with pseudopolyps (a, b) as well as raised dysplasia (c–f, M-NBI, 100×). (a, b) Regularly arranged, meshed capillary and surface structure, regenerative pseudopolyp. (c) Dense capillary network and slightly irregular surface structure in rectal LST-GM (0-IIa + Is): Tubulovillous adenoma. (d) Irregular meshed capillary and enlarged irregular surface structure in sigmoid 0-Is lesion. Histology: Tubulovillous low-grade intraepithelial neoplasia (LGIN). (e, f) Highly irregular capillary and surface structure in rectal 0-Is lesion. Histology: HGIN (differentiated mucosal cancer T0 m1)
A case of flat dysplasia (0-IIc like advanced cancer). (a) An irregular depressed lesion about 2 cm in size is seen in the descending colon of a 63-year-old man with 16 years history of ulcerative pancolitis. (b) Rigid colonic wall and tense nodules. (c) Non-structured surface and sparse vascular network was observed with NBI magnification. (d) Stainability of crystal violet was poor within the depressed area. Because the biopsy revealed adenocarcinoma, the patient underwent total colectomy. This lesion had already became advanced cancer although the lesion size was just 2 cm. Final histopathological diagnosis: Tubular adenocarcinoma (tub2 + por2), 0-IIa + IIc, 20 mm, pT2(MP), sci, INFc, ly0,v0,pPM0, pDM0, pRM0
Sometimes, invisible dysplasias or flat dysplasias (similar to lesions type 0-IIb or IIc) are detected by chance during surveillance colonoscopy. In cases of HGD, cancer may already be present in 42–67% of patients [18, 24]. By contrast, low-grade dysplasia (LGD) may carry only a 3% initial risk of concomitant CRC, with a 10% subsequent rate of progression to CRC within 10 years [25].
3.2 Prevalent Versus Incident Low-Grade Dysplasia
A review of 10 prospective studies reported that when LGD was detected at initial surveillance colonoscopy (prevalent LGD), HGD or CRC developed in 29% (16 of 55 patients) at some time during further follow-up, and CRC developed in 13% (7 patients) [26]. But when LGD was found during further surveillance (incident LGD), only 16% (33 of 204 patients) progressed to HGD or CRC, with 8% (17 patients) progressing to CRC [5]. When no dysplasia was found on initial colonoscopy, the rate of progression to CRC ranges from 1% to 3% per year [4, 27].
3.3 Focality of Dysplasia
The overall 5-year progression rate of flat prevalent LGD to either HGD or CRC was 53%. The rate was nearly identical among 39 patients with unifocal LGD and 7 patients with multifocal LGD [4, 28].
4 Management of Neoplastic Lesions in IBD Patients
Sporadic adenomas in uninvolved parts of the colon in UC (or Crohn’s colitis) carry a low risk (<5%) of associated dysplasia or CRC, as do protruding lesions in non-dysplastic mucosa of IBD-involved colon [26]. Those are very good indications for endoscopic resection. The clinical course of UC-associated lesions can be followed with a strict surveillance program after complete endoscopic resection of dysplastic lesions [27]. Therefore, it is recommended to conduct endoscopic resection for both polypoid dysplastic lesions and non-polypoid dysplastic lesions without invisible dysplasia around the lesion or distant area, if technically possible [12, 13]. Endoscopic submucosal dissection (ESD) by expert endoscopists is feasible for neoplasia in UC patients and may avoid unnecessary surgery [29]. However, patients with endoscopically unresectable non-polypoid dysplasia should undergo a total colectomy, as a meta-analysis involving 477 patients indicated that even low-grade flat dysplasia (LGD) had a risk of 22% for synchronous cancer and a 5-year progression rate of 36% to advanced neoplasia (HGD or CRC) [4].
Note
According to SCENIC and ECCO guidelines [4, 12, 13]:
-
Endoscopic resection (en bloc) is indicated for:
-
Sporadic lesions
-
Polypoid dysplasia and non-polypoid dysplasia without invisible dysplasia around the lesion or in a distant area
-
-
Total colectomy is indicated for IBD with:
-
Endoscopically unresectable non-polypoid dysplasia
-
Invisible HGD detected by random biopsy
-
5 Cases: Neoplastic Lesions in IBD
Case 1: Flat adenoma in Chronic Ulcerative Colitis
In a patient with long-lasting UC, surveillance colonoscopy showed discolored, slightly irregular mucosa in the sigmoid colon on a background of UC in remission (Fig. 12.5). Irregular surface structure with less vascular network was observed by NBI magnification. Uneven, irregular pits were observed by crystal violet staining magnification. The lesion was resected en bloc by ESD; histology revealed tubular adenoma, resected R0, 46 × 33 mm.
UC Case 1: (a) Slightly irregular, discolored mucosa was observed in a patient with long-standing UC in remission. (b) Irregular mucosal surface and tumor border became much clearer with indigo carmine dye spraying. (c) The tumor border was clearly seen with NBI. (d) Irregular surface structure with less vascular network was observed by NBI magnification. (e) Crystal violet staining. (f) Uneven, irregular pits were observed by crystal violet staining magnification. The lesion was resected by ESD, and histology revealed tubular adenoma, 46 × 33 mm
Note
-
Endoscopic diagnosis of flat neoplasia and lateral margins is sometimes difficult, but chromoendoscopy and magnification are helpful in recognizing irregular mucosal structure and demarcation line.
Case 2: Flat Carcinoma in Long-Standing Ulcerative Colitis
A small nodule and slight reddish area was recognized during surveillance colonoscopy in a 63-year-old man with 18 years’ history of ulcerative pancolitis in remission (Fig. 12.6). A very flat lesion became obvious after chromoendoscopy with indigo carmine, and a highly dilated neoplastic vessel was observed by NBI magnification. The nodular area showed almost non-structure pit after crystal violet staining, and biopsy revealed well-differentiated adenocarcinoma. The patient underwent total colectomy.
UC Case 2: (a) A small nodule and slight reddish area were recognized during surveillance colonoscopy. (b) An irregular mucosal surface and very flat lesion became obvious after indigo carmine dye spraying. (c, d) A highly dilated neoplastic vessel, suggesting invasive cancer, was observed with NBI magnification. (e, f) An almost non-structure pit pattern, also suggesting invasive cancer, was observed at the nodular area with crystal violet staining magnification. The biopsy specimen revealed well-differentiated adenocarcinoma, and the patient underwent total colectomy. The final histopathological result was a well-differentiated tubular adenocarcinoma (tub1), pSM (200 μm), 11 × 5 mm, int, INFb, ly(+),v(−)
Note
-
Be observant for subtle differences in mucosal color or pattern of flat neoplasia when performing surveillance colonoscopy in patients with ulcerative colitis in remission.
Case 3: Raised Dysplasia
A 51-year-old otherwise healthy man was referred for endoscopic resection of a rectal LST-granular-mixed (LST-GM) of 4 cm estimated diameter, with focal HGD. He had chronic ulcerative pancolitis for 18 years and had been in remission for 3 years. The rectal LST was much larger than expected (diameter 7 cm), and he had a variety of suspicious discolored lesions. Therefore, he was reevaluated with a magnifying (100-fold) endoscope up to the hepatic flexure, looking for additional dysplastic lesions. He presented a variety of raised lesions with irregular surface structure and vascular pattern; challenging endoscopic differential diagnoses of regenerative versus neoplastic lesions were clarified by targeted biopsies (Fig. 12.7).
UC Case 3: (a) Rectal LST-mixed (75 × 35 mm; 0–7.5 cm p.a) in chronic pancolitis in remission. Histology: polypoid dysplasia with focal HGIN (in two areas). Raised lesion with LGIN + focal HGIN. (b) Sessile whitish lesion (0-Is, 15 mm) in descending colon (60 cm p.a.), chronic ulcerative pancolitis. (c) Absent, but very smooth surface, tortuous vascular pattern (NBI 80×; same lesion as in b). Histology: nonneoplastic fibrotic mucosa and submucosa with mucinophages: Fibrotic pseudopolyp. (d) Protruding reddish lesion (0-IIa + Is, 20 mm) in descending colon (60 cm p.a.). Same lesion 0-Is as in Fig. 12.3e, f (NBI 80×). Targeted biopsy: polypoid dysplasia with focal HGIN
Diagnosis: Multiple raised dysplasias (three distantly separated lesions with HGIN) in chronic ulcerative pancolitis. The patient was referred for sphincter-preserving total colectomy with ileoanal pouch.
Note
On such a background of extensive regenerative mucosa and multiple endoscopic lesions in long-standing (mildly active) IBD-associated colitis:
-
Endoscopic diagnosis of neoplasias and malignancy is extremely difficult, even for expert endoscopists.
-
Risk of advanced-stage CRC is very high when endoscopic resection of lesions and follow-up would be attempted.
-
Colectomy with ileal pouch is preferable.
References
Eaden JA, et al. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–35.
Jess T, et al. Increased risk of intestinal cancer in Crohn’s disease: a meta-analysis of population-based cohort studies. Am J Gastroenterol. 2005;100:2724–9.
Ekbom A, et al. Increased risk of large-bowel cancer in Crohn’s disease with colonic involvement. Lancet. 1990;336:357–9.
Farraye FA, et al. AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:746–74.
Bernstein CN, et al. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854–62.
Rutter MD, et al. Thirty-year analysis of a colonoscopic surveillance program for neoplasia in ulcerative colitis. Gastroenterology. 2006;130:1030–8.
Lutgens MW, et al. High frequency of early colorectal cancer in inflammatory bowel disease. Gut. 2008;57:1246–51.
Beaugerie L, et al. Cancers complicating inflammatory bowel disease. N Engl J Med. 2015;372:1441–52.
Soetikno RM, et al. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta-analysis. Gastrointest Endosc. 2002;56:48–54.
Broome U, et al. Primary sclerosing cholangitis and ulcerative colitis: evidence for increased neoplastic potential. Hepatology. 1995;22:1404–8.
Lichtiger S, et al. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med. 1994;330:1841–5.
Laine L, et al. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastrointest Endosc. 2015;81:489–501.
Magro F, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017;11:649–70.
Watanabe T, et al. Comparison of targeted vs random biopsies for surveillance of ulcerative colitis-associated colorectal cancer. Gastroenterology. 2016;151:1122–30.
Kiesslich R, et al. Methylene blue-aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology. 2003;124:880–8.
Marion JF, et al. Chromoendoscopy-targeted biopsies are superior to standard colonoscopic surveillance for detecting dysplasia in inflammatory bowel disease patients: a prospective endoscopic trial. Am J Gastroenterol. 2008;103:2342–9.
Bisschops R, et al. Chromoendoscopy versus narrow band imaging in UC: a prospective randomised controlled trial. Gut. 2018;67:1087–94.
Farraye FA, et al. AGA medical position statement on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:738–45.
Gumaste V, et al. Benign and malignant colorectal strictures in ulcerative colitis. Gut. 1992;33:938–41.
Soetikno R, et al. Paradigm shift in the surveillance and management of dysplasia in inflammatory bowel disease (West). Dig Endosc. 2016;28:266–73.
Chiu K, et al. DALM, rest in peace: a pathologist’s perspective on dysplasia in inflammatory bowel disease in the post-DALM era. Mod Pathol. 2018;31:1180–90.
Sugimoto S, et al. Endoscopic morphologic features of ulcerative colitis-associated dysplasia classified according to the SCENIC consensus statement. Gastrointest Endosc. 2017;85:639–46.
Iacucci M, et al. A multimodal (FACILE) classification for optical diagnosis of inflammatory bowel disease associated neoplasia. Endosc 2019;51:133–41.
Rubio CA, et al. Villous and serrated adenomatous growth bordering carcinomas in inflammatory bowel disease. Anticancer Res. 2000;20:4761–4.
Lim CH, et al. Ten year follow up of ulcerative colitis patients with and without low grade dysplasia. Gut. 2003;52:1127–32.
Odze RD. Adenomas and adenoma-like DALMs in chronic ulcerative colitis: a clinical, pathological, and molecular review. Am J Gastroenterol. 1999;94:1746–50.
Odze RD, et al. Long-term follow-up after polypectomy treatment for adenoma-like dysplastic lesions in ulcerative colitis. Clin Gastroenterol Hepatol. 2004;2:534–41.
Ullman TA, et al. The fate of low grade dysplasia in ulcerative colitis. Am J Gastroenterol. 2002;97:922–7.
Kinoshita S, et al. The role of colorectal endoscopic submucosal dissection in patients with ulcerative colitis. Gastrointest Endosc. 2018;87:1079–84.
Acknowledgments
The contribution of a case by Dr. Gerhard Kleber of Aalen, Germany, and the evaluation of histology by Dr. Daniel Neureiter of Salzburg, Austria, are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer International Publishing
About this chapter
Cite this chapter
Yahagi, N., Maehata, T., Nakayama, A. (2019). Chronic Inflammatory Bowel Disease in Remission: Mucosal Neoplasias. In: Berr, F., Oyama, T., Ponchon, T., Yahagi, N. (eds) Atlas of Early Neoplasias of the Gastrointestinal Tract. Springer, Cham. https://doi.org/10.1007/978-3-030-01114-7_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-01114-7_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-01113-0
Online ISBN: 978-3-030-01114-7
eBook Packages: MedicineMedicine (R0)