Abstract
Blood vessels and tumor angiogenesis are generally associated with tumor growth and poor clinical outcome of cancer patients. However, it has been recently discovered that some blood vessels present within the tumor microenvironment can be associated with a good prognosis by contributing to tumor suppression rather than tumor growth. These specialized blood vessels, designated high endothelial venules (HEVs), are normally found in lymph nodes where they mediate high levels of lymphocyte extravasation from the blood. A high density of tumor HEVs in human breast carcinomas and melanomas was associated with high levels of cytotoxic lymphocyte infiltration, indicating that HEVs may participate in the eradication of tumors by facilitating the access of “killer” lymphocytes into tumor tissues. A better understanding of the mechanisms regulating tumor HEVs could thus have an important impact for cancer therapy. Dendritic cells and the lymphotoxin pathway have been shown to be critical for maintenance of HEV differentiation in lymph nodes and may also regulate tumor HEVs. In this chapter, we will first describe the unique properties of lymph node HEVs and their role in lymphocyte trafficking. We will then review the phenotypic characteristics of tumor HEVs and their association with lymphocyte infiltration and favorable clinical outcome of cancer patients. Finally, we will discuss the promising potential of tumor HEVs for cancer therapy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Secondary Lymphoid Organ
- Human Solid Tumor
- High Endothelial Venule
- Postcapillary Venule
- Lymphocyte Homing
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 High Endothelial Venules (HEVs) and Lymphocyte Trafficking to Lymph Nodes
The immune system plays an important role in the homeostatic maintenance of the body. It is responsible for detecting and eliminating foreign pathogens as well as recognizing and targeting tumor cells. The circulatory system is an important aspect of immune surveillance, as it provides the necessary network for immune cells to travel through the body, monitoring for disease and infection. Lymphocytes, specialized immune cells that are designed to interact with specific targets, travel to and from secondary lymphoid organs, such as the lymph nodes (LNs), Peyer’s patches, and spleen, through the blood vessels seeking their specific cognate antigens that signal an infection (Butcher and Picker 1996; von Andrian and Mempel 2003). Dendritic cells (DCs), multifunctional antigen-presenting cells, reside in tissues monitoring their surroundings and then migrate to lymphoid organs to interact with lymphocytes. The trafficking of lymphocytes and DCs to a common location (i.e., LNs) maximizes the probability that lymphocytes will encounter their specific cognate antigens presented by DCs (von Andrian and Mempel 2003).
Lymphocytes circulating in the blood enter LNs at the level of specialized postcapillary venules designated as high endothelial venules (HEVs; Fig. 16.1) (Girard et al. 2012; Girard and Springer 1995). Lymphocyte extravasation through HEVs occurs via a well-defined adhesion cascade (Girard et al. 2012). First, they roll and tether along the surface of the blood vessel wall (von Andrian and Mempel 2003). During rolling, lymphocytes are stimulated by locally expressed chemokines. Lymphocytes then firmly adhere to HEVs before transmigrating between endothelial cells to the lymph node parenchyma underneath. Once entered, lymphocytes will localize to specific areas of the lymph node to interact with DCs before exiting through the efferent lymph vessel and back into the blood (Girard et al. 2012). HEV blood vessels are also found in other secondary lymphoid tissues such as Peyer’s patches and support a similar process. However, they have not been observed in the spleen.
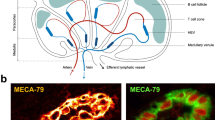
High endothelial venules (HEVs): specialized blood vessel for lymphocyte migration. HEV endothelial cells exhibit a plump, cuboidal morphology and express high levels of sulfated ligands for lymphocytes recognized by the HEV-specific antibody MECA-79 (red staining). The inset illustrates the unique capacity of HEV endothelial cells to capture lymphocytes circulating in the blood
1.1 HEV Morphology
HEV endothelial cells were first identified by Thome in 1898 in the Macacus cynomolgus due to their distinctive morphology that can easily be recognized by light microscopy. He described the cells as being plump, cuboidal endothelial cells that bulged into the vascular lumen (Thome 1898). Von Schumacher confirmed these observations in human LNs in 1899. He noted the close proximity of lymphocytes and postulated the importance of these cells in lymphocyte migration (von Schumacher 1899). Postcapillary venules, in general, consist of an endothelial cell layer, a thin basal lamina, and a thin layer of pericytes. They are the site of fluid and protein exchange and immune cell transmigration during inflammation. HEVs are unique in that contrary to other postcapillary venules, their endothelium is tall and plump and contains many mitochondria, free ribosomes, multivesicular bodies, and a well-developed Golgi apparatus (Girard and Springer 1995). Pericyte-like cells called fibroblastic reticular cells (FRCs) form a perivenular sleeve that surrounds the endothelium and are responsible for a number of homeostatic and maintenance functions including the production of fibronectin, collagen IV, and laminins which form a thick basal lamina layer. Between the basal lamina and the FRC sheath, a perivascular channel is present through which lymphocytes travel during their migration from the blood into the lymphoid organs. HEVs are able to store lymphocytes in this area in “HEV pockets,” up to 4 or 5 at a time between an individual endothelial cell and the FRC layer underneath (Mionnet et al. 2011). These pockets act as a sort of waiting area until the LNs can make room for new lymphocytes by releasing others that are ready to move on, giving them the role of gatekeepers rather than simply passive transfer points for lymphocytes to pass through.
1.2 HEV Ligands for Lymphocyte Homing Receptor L-Selectin
HEVs have a functionally unique protein expression profile that distinguishes them from other blood vessels. The endothelium expresses a specific group of cell surface mucin-like glycoproteins, called peripheral node addressins (PNAds), characterized by their ability to be recognized by the HEV-specific antibody MECA-79. PNAds, which include CD34, podocalyxin, endomucin, endoglycan and nepmucin in human and mouse, and glycosylation-dependent cell adhesion molecule-1 (GlyCAM-1) in mouse only, are defined as L-selectin (CD62L) ligands (Rosen 2004). L-selectin is a cell surface protein expressed on lymphocytes which together with PNAds mediates the initial tethering and rolling of lymphocytes to HEVs. Specifically, it is the 6-sulfo sialyl LewisX carbohydrate motif presented on PNAds that is the L-selectin ligand, and therefore, for L-selectin to be able to bind to PNAds, a series of proper posttranslational glycosylation and sulfation modifications are required (Rosen 2004). HEVs express a number of enzymes necessary for these posttranslational modifications, some exclusive to HEVs such as N-acetylglucosaminyl 6-O-sulphotransferase 2 (GlcNAc6ST2) and fucosyltansferase VII (FUT7) (Girard et al. 2012). L-selectin has a unique, low-affinity bond with its ligand on PNAds, with rapid association and dissociation constants (“catch bond”), resulting in a rolling behavior of the lymphocytes on the HEVs (Rosen 2004). This allows lymphocytes to slow down in the blood stream and navigate their way along the endothelium to find an optimal location to transmigrate as well as interact with locally expressed chemokines.
1.3 HEV-Associated Chemokines
HEVs in LNs produce and/or present a number of different chemokines causing a gradient that attracts lymphocytes to the site of highest concentration. HEV-associated chemokines include CCL21, CCL19, CXCL12, and CXCL13 (Girard et al. 2012; Miyasaka and Tanaka 2004). CCL21 and CCL19 are two chemokines that bind the receptor CCR7 found on lymphocytes and mature DCs (Forster et al. 2008). In the mouse, CCL21 is produced by HEVs and CCL19 is produced by neighboring FRCs and transferred to the luminal surface of HEVs (Miyasaka and Tanaka 2004). In humans, both chemokines are produced by stromal cells and trancytosed across HEVs (Baekkevold et al. 2001). They are held there by sulfated glycans such as heparan sulfate (Bao et al. 2010). CXCL12 is also presented on HEVs but is produced by perivascular cells and transported to the luminal surface of the blood vessels (Okada et al. 2002). CXCL13, produced by follicular dendritic cells and marginal reticular cells, has also been reported to be presented on HEVs in Peyer’s patches and mesenteric LNs (Cyster 1999; Okada et al. 2002).
Chemokines presented on HEVs not only are important for attracting lymphocytes but also induce signaling that upregulates or activates other cell surface molecules. After lymphocytes roll and tether along the blood vessel wall through interaction of L-selectin with PNAds, they need to firmly adhere with the aid of integrins (von Andrian and Mempel 2003). Integrins are normally found in a low-affinity conformation on the surface of lymphocytes but can be rapidly activated after chemokine stimulation. For example, CCL21 and CCL19 binding to CCR7 and CXCL12 binding to CXCR4 stimulate a signaling cascade that ultimately results in a conformational change in the integrin αLβ2 (lymphocyte function-associated antigen-1 – LFA-1), increasing its affinity for its two endothelial ligands, intercellular adhesion molecule (ICAM)-1 and ICAM-2, expressed on HEVs (Shamri et al. 2005). This mediates firm adhesion on the endothelium. Mesenteric LNs and Peyer’s patches HEVs express the adhesion molecule MAdCAM-1. It is also an L-selectin ligand; however, its primary binding partner is the integrin α4β7, and together they mediate the entry of lymphocytes through Peyer’s patches and mesenteric LNs HEVs (von Andrian and Mempel 2003). Interestingly, mucosal vascular addressin cell adhesion molecule 1 (MAdCAM-1) can be found on peripheral LNs HEVs around the time of birth, and it is important for the initial colonization of secondary lymphoid organs; however, it disappears soon after birth (Mebius et al. 1996).
1.4 Microenvironmental Control of HEVs
HEV endothelial cells are highly plastic and their specialized phenotype is heavily dependent on their tissue microenvironment (Girard et al. 2012; Girard and Springer 1995). If HEV endothelial cells are isolated from lymphoid organs and placed ex vivo, they rapidly lose their cuboidal appearance, their HEV-associated genes, and their cell surface markers (Lacorre et al. 2004). Mebius et al. demonstrated the importance of lymph-borne factors since mechanical ligation of afferent lymphatics, caused HEVs to lose their morphology in vivo (Mebius et al. 1991). Interestingly, reestablishment of lymphatic flow restored the HEV phenotype, demonstrating the phenotypic reversibility of these cells. More recently, it was determined that DCs, which enter the LNs in part through the afferent lymph, play an essential role in the maintenance of HEVs (Moussion and Girard 2011). If CD11c+ DCs were depleted in vivo, lymphocyte homing to LNs and overall lymph node cellularity were reduced. This can be attributed to the downregulation of HEV-specific markers such as MECA-79+ L-selectin ligands and posttranslational enzymes which resulted in the inability of lymphocytes to adhere efficiently on HEVs and enter LNs (Moussion and Girard 2011). Interestingly, in DC-depleted mice, MAdCAM-1 was upregulated in peripheral LNs HEVs, suggesting a reversion back to an immature neonatal HEV phenotype. It was also shown that when HEVs were placed ex vivo, they were able to keep their HEV phenotype when DCs were present but not if signaling through the lymphotoxin-beta receptor (LTβR) was inhibited. Blocking LTβR signaling on high endothelial cells in vivo caused a similar phenotype as depleting DCs: reduced cellularity, downregulation of HEV-associated genes, and impaired lymphocyte homing to LNs (Browning et al. 2005; Onder et al. 2013). Lymphotoxin (LT) α1β2 is expressed by T and B lymphocytes as well as DCs; however, HEVs form normally in T and B lymphocyte-deficient mice, suggesting that it is only LTα1β2 expressed on DCs that is required for HEV development and maintenance (Moussion and Girard 2011). LTβR signaling triggers the alternative NFκB signaling pathway via IKKα to induce the expression of GlyCAM-1, GlcNAc6ST2, CCL21, CCL19, and CXCL13 (Drayton et al. 2004). Vascular endothelial growth factor (VEGF), an endothelial cell growth factor, is produced by DCs and has been suggested to play a role in HEV formation and homeostasis; however, studies were done under inflammatory conditions (Webster et al. 2006; Wendland et al. 2011).
One of the unique characteristics of HEVs is their ability to maintain blood vessel integrity and selectivity despite the large quantity of lymphocytes that pass between the cells to the parenchyma underneath. HEV endothelial cells are held together by junctional proteins, in particular vascular endothelial (VE)-cadherin. A recent study has shown that podoplanin on FRCs is an activating ligand for C-type lectin-like receptor 2 (CLEC-2) on platelets and together they promote VE-cadherin expression on HEVs (Herzog et al. 2013). If either molecule is not present, it will lead to leaky HEV junctions but only if there is a high rate of lymphocyte extravasation through HEVs, for example, in mesenteric LNs or during immune challenge. CLEC-2 activation by podoplanin on FRCs led to sphingosine-1-phosphate (S1P) release from platelets which in turn caused an upregulation of VE-cadherin on HEVs. Blocking the activity of the S1P receptor S1PR1 on HEV endothelial cells reduced VE-cadherin expression, demonstrating the importance of S1P binding. However, it should be noted that S1P is primarily produced by erythrocytes in the blood and has been shown to regulate vascular integrity after inflammatory challenges (Camerer et al. 2009). In this HEV study, S1P present in blood was not sufficient to rescue the leaky HEV phenotype seen in podoplanin-deficient or CLEC-2-deficient mice. It was specifically CLEC-2 signaling on platelets and S1P release from platelets that increased VE-cadherin expression and overall HEV integrity (Herzog et al. 2013).
1.5 Remodeling of Lymph Node HEVs in Cancer
The immune response against tumor cells is similar to the one against foreign pathogens. However, targeting tumor cells is difficult because they originate from host cells and the immune system has been conditioned to ignore them. Tumors evolved several mechanisms to escape immune control including “hiding” from immune cells, immune cell elimination, and disabling immune cells. One of the strategies used by tumor cells to evade the immune system is to alter LN HEV function. Using a melanoma mouse model, Carriere et al. showed that naïve lymphocyte recruitment to the proximal LN was impaired after tumor development even in the absence of LN metastasis (Carriere et al. 2005). The lymphocyte homing defect was not due to a change in HEV number or HEV morphology but rather to a progressive decrease in the expression of CCL21 within LNs resulting in a diminished capacity of lymphocytes to firmly adhere to HEVs that express low level of CCL21.
Metastasis to the sentinel LN (the first draining LN) is the first step of cancer spreading in many malignancies. Using an animal model of nasopharyngeal carcinoma, Qian et al. showed that prior to the establishment of metastasis in the sentinel LN, the blood vasculature and lymphatic channels were reorganized (Qian et al. 2006). Sentinel LN HEVs were highly dilated and branched out into thin walls. This remodeling of HEV blood vessels favored cancer cell arrival and was associated with poor prognosis. Similar changes in HEVs were observed in axillary LNs of human breast cancer patients and in LNs in the neck region of patients with squamous cell carcinoma of the tongue (Lee et al. 2012; Qian et al. 2006). HEV remodeling preceding metastasis was associated with a poor clinical outcome and it was therefore proposed as an early prognosis marker of sentinel LN metastasis.
2 Tumor HEVs and Lymphocyte Trafficking in Cancer
It has recently been discovered that blood vessels with HEV characteristics are frequently found in the stroma of many human solid tumors (Martinet et al. 2011, 2012). Interestingly, a high density of tumor HEVs significantly correlated with longer survival of breast cancer patients (Martinet et al. 2011). Blood vessels and tumor angiogenesis are generally associated with poor prognosis, and this is the first time that a specific type of blood vessel is associated with good prognosis. Therefore, although blood vessels are generally believed to promote tumor growth, the phenotype of blood vessels is important, and some types of blood vessels present in the tumor microenvironment (i.e., tumor HEVs) may contribute to tumor suppression rather than tumor growth. Tumor HEVs were generally associated with high levels of lymphocyte infiltration into the tumor microenvironment (Martinet et al. 2011, 2012), supporting the possibility that these vessels may limit cancer development by functioning as major gateways for tumor-infiltrating lymphocytes (TILs).
2.1 Phenotype of Tumor HEVs
The frequent presence of MECA-79+ blood vessels within human solid tumors, including melanomas and breast, colon, lung, and ovarian carcinomas, was revealed for the first time by immunohistochemistry on primary tumor sections (Martinet et al. 2011). The MECA-79+ endothelial cells present within human melanomas and breast tumors (Fig. 16.2) were further characterized and shown to express a panel of LN HEV markers: pan-vascular endothelial cell markers CD31 and von Willebrand factor (vWB), postcapillary venule marker Duffy antigen receptor for chemokines (DARC), and HEV-specific markers HECA-452, G72, and G152 (these three HEV-specific monoclonal antibodies recognize the 6-sulfo sialyl Lewisx ligands for lymphocyte L-selectin) (Martinet et al. 2011, 2012). In addition, MECA-79+ endothelial cells present within tumors harbored a plump morphology, similarly to endothelial cells of LN HEVs. Given their phenotypic and morphological similarities to LN HEVs, MECA-79+ blood vessels present within human solid tumors were named tumor HEVs (Martinet et al. 2011).
Tumor HEVs: major gateways for lymphocyte migration into solid tumors. Tumor HEVs were associated with high rates of CD3+ T cell and CD8+ cytotoxic T-cell infiltration in human breast carcinomas. Serial tissue sections were stained with antibodies against HEV-specific marker MECA-79 and antibodies against CD3 (upper panel) or CD8 (lower panel)
Human solid tumors contained a highly heterogeneous number of tumor HEVs that varied among tumor types, tumor anatomic locations, and patients (Martinet et al. 2011, 2012). The mean HEV density (HEV absolute number/mm2) measured on tumor sections was 10-fold higher in melanomas than in breast cancer (Martinet et al. 2012). Furthermore, melanomas located in the trunk of the body contained more HEVs than melanomas of the limbs. Absolute number and density of tumor HEVs were also highly variable among patients without any correlation with patient’s age or sex. Tumor HEVs were observed in ~70 % of all primary solid tumors analyzed; 11 of 18 melanomas, 94 of 127 breast cancers, 4 of 5 colon and lung carcinomas, and 11 of 18 ovarian carcinomas exhibited tumor HEVs (Martinet et al. 2011). Several studies conducted using independent cohorts of patients have also confirmed the presence of tumor HEVs in ~70 % of human solid tumors (Avram et al. 2013; Martinet et al. 2012). Interestingly, the high variability in the density of tumor HEVs was not related to differences in tumor angiogenesis as revealed by the absence of a significant correlation between density of tumor HEVs and density of CD34+ microvessels (Martinet et al. 2011). This strongly suggests that the factors and mechanisms involved in the induction of tumor HEVs are independent of tumor angiogenesis.
In addition to primary tumors, tumor HEVs were also observed in human melanoma metastases (Avram et al. 2013; Cipponi et al. 2012). In contrast to primary melanoma, HEVs of metastatic lesions were associated with ectopic lymphoid structures also called tertiary lymphoid organs (TLOs), which are highly organized lymphoid tissues. Similar to secondary lymphoid organs, TLOs contained T-cell compartments and organized B-cell follicles surrounded by follicular dendritic cells. Some follicles exhibited proliferating germinal centers. Recently, Avram et al. showed that the number of tumor HEVs in melanoma metastases was low compared to primary melanomas (Avram et al. 2013). Furthermore, HEVs associated with TLOs were observed in less than 25 % of the metastatic lesions analyzed (Cipponi et al. 2012). The presence of TLOs containing HEVs has also been reported in non-small cell lung cancer (de Chaisemartin et al. 2011). In this study, HEVs were never observed independently of TLOs.
2.2 Major Gateways for Tumor-Infiltrating Lymphocytes
The density of tumor HEVs within the tumor stroma was a strong predictor of T and B lymphocyte infiltration in both breast cancer and melanomas (Martinet et al. 2011, 2012). Interestingly, similar to HEV density, the mean density of T lymphocytes was 10-fold higher in melanomas than in breast cancer. As revealed by immunostaining, tumor HEVs were specifically located within lymphocyte-rich tumor areas, containing mainly T lymphocytes and, to a lesser extent, B lymphocytes. A large proportion of T lymphocytes were cytotoxic CD8+ T lymphocytes (Fig. 16.2). In addition, TILs were frequently observed extravasated or attached to the luminal surface of tumor HEVs (Martinet et al. 2011, 2012). This strongly suggested that tumor HEVs are actively involved in the recruitment of TILs into human primary tumors. Further analysis of TIL populations associated with tumor HEVs using large-scale flow cytometry revealed that the numbers of naïve (CD45RA+, CD62L+), central memory T cells (CD45RA-, CD62L+) and effector memory T cells (CD45RA-, CD62L+) positively correlated to the density of tumor HEVs. In addition, chemokines regulating lymphocyte homing to LNs (e.g., CCL19, CCL21, CXCL13) and to peripheral tissues (e.g., CCL5, CXCL9, CXCL10, CXCL11) were overexpressed in tumors containing high densities of tumor HEVs (Martinet et al. 2011, 2012).
2.3 Tumor HEVs and Clinical Outcome
The presence of a high proportion of TILs within the tumor microenvironment has been associated with a favorable clinical outcome in several human solid tumors (Galon et al. 2006; Pages et al. 2005; Zhang et al. 2003). The strong correlation between the density of tumor HEVs and the density of TILs suggests that tumor HEVs are key players in the recruitment of lymphocytes into tumors and consequently in antitumor immunity and clinical outcome (Fig. 16.3). The highest density of tumor HEVs was observed in melanomas and breast cancers showing signs of tumor regression (Martinet et al. 2011, 2012) supporting tumor HEV density as a prognosis marker in solid tumors. One of the most powerful prognostic markers for primary melanoma staging is the Breslow tumor thickness. It has been shown that the thickness of the tumor lesions was inversely correlated with the density of tumor HEVs (Martinet et al. 2012). Moreover, in a retrospective cohort of 146 primary invasive breast tumors, it was shown that high densities of tumor HEVs were associated with a lower risk of relapse and significantly correlated with longer metastasis-free, disease-free, and overall survival rates (Martinet et al. 2011). Remarkably, in breast carcinoma sections containing both in situ and invasive carcinoma components, the highest density of tumor HEVs was observed within the in situ carcinoma component (Martinet et al. 2013). This suggests that loss of tumor HEVs may represent a critical step during breast cancer progression.
Tumor HEVs, lymphocyte infiltration, and clinical outcome of cancer patients. Unlike classical tumor blood vessels, which are generally associated with tumor growth and poor clinical outcome, tumor HEVs have been shown to be associated with tumor regression and favorable clinical outcome. They may contribute to the eradication of cancer cells by allowing the entry of various T and B lymphocyte populations into tumors, including “killer” T lymphocytes
2.4 Mechanisms Regulating Tumor HEVs
Given the association of tumor HEVs with good prognosis, it is important to understand the cellular and molecular mechanisms which govern the formation of tumor HEVs. As described above, CD11c+ DCs are required for the maintenance of HEVs in LNs, in an LTα1β2-dependent manner (Moussion and Girard 2011). Consistent with a potential role of DCs in the maintenance/induction of tumor HEVs, mature DCs (DC-LAMP+) were frequently observed around human melanoma HEVs and breast cancer HEVs (Martinet et al. 2012, 2013). A positive correlation between density of mature DCs and density of tumor HEVs was found in both types of solid tumors (Martinet et al. 2012, 2013). In addition, a decreased proportion of DCs was observed in invasive breast carcinomas in comparison to in situ carcinomas, similarly to tumor HEVs. Finally, high expression levels of LTβ were associated with high densities of tumor HEVs, and DCs were shown to be the major producers of LTα1β2 within breast tumor microenvironment (Martinet et al. 2013).
In contrast to DCs, Tregs appear to be potential negative players in tumor HEV development. In a methylcholanthrene-induced fibrosarcoma mouse model, the presence of tumor HEVs was only observed in the absence of Foxp3+ Tregs (Hindley et al. 2012). In human breast tumors, whereas a higher density of Tregs was observed in tumors containing high densities of tumor HEVs, the ratio of Tregs to CD3+ cells was diminished (Martinet et al. 2013). This suggests that Tregs may not completely prevent HEV development but may limit it, possibly by controlling DC production of LTα1β2.
3 The Future: Tumor HEVs and Cancer Therapy
As discussed in this chapter, blood vessels in human tumors are not all the same, and some types of blood vessels found in the tumor microenvironment (i.e., high endothelial venules, HEVs) can contribute to the fight against cancer by mediating tumor suppression rather than tumor growth. Increasing the density of the “good” HEV blood vessels within solid tumors thus represents a promising new strategy for cancer therapy. This concept is very novel because the current dogma in the field is that blood vessels and tumor angiogenesis contribute to tumor growth and are generally associated with poor prognosis.
Tumor HEVs were observed in many different types of human solid tumors including melanomas and breast, ovarian, lung, and colon carcinomas. The concept of “tumor HEVs as the good blood vessels against cancer” is thus not limited to a specific type of tumor, and it could have broad applications for diagnosis and therapy of many types of human solid tumors.
The frequent presence of tumor HEVs in human solid tumors was discovered only recently (Martinet et al. 2011), and very little is known yet about the characteristics of tumor HEVs and the cellular and molecular mechanisms governing their development. Therefore, a better understanding of the phenotype and regulation of tumor HEVs is urgently needed. Similarly, the potential influence of tumor HEVs on the response to cancer therapeutics currently used to treat patients (chemotherapy, radiotherapy, and anti-angiogenic therapy) remains to be determined.
In the future, it may be possible to induce the HEV endothelial cell differentiation program in tumor blood vessels in order to transform regular tumor blood vessels into tumor HEVs. This would increase the density of tumor HEVs without increasing tumor angiogenesis and would suppress tumor growth through enhanced recruitment of cytotoxic T lymphocytes. Concerns have been raised about the idea of “opening up a gateway” between the tumor site and the blood circulation and the possibility that tumor HEVs may provide a potential route for cancer cell metastasis. However, to our knowledge, cellular migration through HEVs is unidirectional and no evidence has yet been provided for the exit of cells from tissues through HEVs. Tumor HEVs may rather limit metastasis of tumor cells to distant sites, since a high density of tumor HEVs was associated with longer metastasis-free survival of breast cancer patients. Novel therapeutic strategies based on the modulation of tumor HEVs could thus have a major impact on tumor growth, tumor cell metastasis, and clinical outcome of cancer patients.
In conclusion, tumor HEVs constitute a new and potentially invaluable discovery in the fight against cancer. However, much work remains to be done before development of novel therapies for cancer patients.
References
Avram G, Sanchez-Sendra B, Martin JM et al (2013) The density and type of MECA-79-positive high endothelial venules correlate with lymphocytic infiltration and tumour regression in primary cutaneous melanoma. Histopathology 63:852–861
Baekkevold ES, Yamanaka T, Palframan RT et al (2001) The CCR7 ligand elc (CCL19) is transcytosed in high endothelial venules and mediates T cell recruitment. J Exp Med 193:1105–1112
Bao X, Moseman EA, Saito H et al (2010) Endothelial heparan sulfate controls chemokine presentation in recruitment of lymphocytes and dendritic cells to lymph nodes. Immunity 33:817–829
Browning JL, Allaire N, Ngam-Ek A et al (2005) Lymphotoxin-beta receptor signaling is required for the homeostatic control of HEV differentiation and function. Immunity 23:539–550
Butcher EC, Picker LJ (1996) Lymphocyte homing and homeostasis. Science 272:60–66
Camerer E, Regard JB, Cornelissen I et al (2009) Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J Clin Invest 119:1871–1879
Carriere V, Colisson R, Jiguet-Jiglaire C et al (2005) Cancer cells regulate lymphocyte recruitment and leukocyte-endothelium interactions in the tumor-draining lymph node. Cancer Res 65:11639–11648
Cipponi A, Mercier M, Seremet T et al (2012) Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases. Cancer Res 72:3997–4007
Cyster JG (1999) Chemokines and cell migration in secondary lymphoid organs. Science 286:2098–2102
de Chaisemartin L, Goc J, Damotte D et al (2011) Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res 71:6391–6399
Drayton DL, Bonizzi G, Ying X et al (2004) I kappa B kinase complex alpha kinase activity controls chemokine and high endothelial venule gene expression in lymph nodes and nasal-associated lymphoid tissue. J Immunol 173:6161–6168
Forster R, Davalos-Misslitz AC, Rot A (2008) CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol 8:362–371
Galon J, Costes A, Sanchez-Cabo F et al (2006) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313:1960–1964
Girard JP, Springer TA (1995) High endothelial venules (HEVs): specialized endothelium for lymphocyte migration. Immunol Today 16:449–457
Girard JP, Moussion C, Forster R (2012) HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol 12:762–773
Herzog BH, Fu J, Wilson SJ et al (2013) Podoplanin maintains high endothelial venule integrity by interacting with platelet CLEC-2. Nature 502:105–109
Hindley JP, Jones E, Smart K et al (2012) T-cell trafficking facilitated by high endothelial venules is required for tumor control after regulatory T-cell depletion. Cancer Res 72:5473–5482
Lacorre DA, Baekkevold ES, Garrido I et al (2004) Plasticity of endothelial cells: rapid dedifferentiation of freshly isolated high endothelial venule endothelial cells outside the lymphoid tissue microenvironment. Blood 103:4164–4172
Lee SY, Chao-Nan Q, Seng OA et al (2012) Changes in specialized blood vessels in lymph nodes and their role in cancer metastasis. J Transl Med 10:206
Martinet L, Garrido I, Filleron T et al (2011) Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res 71:5678–5687
Martinet L, Le Guellec S, Filleron T et al (2012) High endothelial venules (HEVs) in human melanoma lesions: major gateways for tumor-infiltrating lymphocytes. OncoImmunology 1:829–839
Martinet L, Filleron T, Le Guellec S et al (2013) High endothelial venule blood vessels for tumor-infiltrating lymphocytes are associated with lymphotoxin beta-producing dendritic cells in human breast cancer. J Immunol 191:2001–2008
Mebius RE, Streeter PR, Breve J et al (1991) The influence of afferent lymphatic vessel interruption on vascular addressin expression. J Cell Biol 115:85–95
Mebius RE, Streeter PR, Michie S et al (1996) A developmental switch in lymphocyte homing receptor and endothelial vascular addressin expression regulates lymphocyte homing and permits CD4+ CD3- cells to colonize lymph nodes. Proc Natl Acad Sci USA 93:11019–11024
Mionnet C, Sanos SL, Mondor I et al (2011) High endothelial venules as traffic control points maintaining lymphocyte population homeostasis in lymph nodes. Blood 118:6115–6122
Miyasaka M, Tanaka T (2004) Lymphocyte trafficking across high endothelial venules: dogmas and enigmas. Nat Rev Immunol 4:360–370
Moussion C, Girard JP (2011) Dendritic cells control lymphocyte entry to lymph nodes through high endothelial venules. Nature 479:542–546
Okada T, Ngo VN, Ekland EH et al (2002) Chemokine requirements for B cell entry to lymph nodes and Peyer’s patches. J Exp Med 196:65–75
Onder L, Danuser R, Scandella E et al (2013) Endothelial cell-specific lymphotoxin-beta receptor signaling is critical for lymph node and high endothelial venule formation. J Exp Med 210:465–473
Pages F, Berger A, Camus M et al (2005) Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 353:2654–2666
Qian CN, Berghuis B, Tsarfaty G et al (2006) Preparing the “soil”: the primary tumor induces vasculature reorganization in the sentinel lymph node before the arrival of metastatic cancer cells. Cancer Res 66:10365–10376
Rosen SD (2004) Ligands for L-selectin: homing, inflammation, and beyond. Annu Rev Immunol 22:129–156
Shamri R, Grabovsky V, Gauguet JM et al (2005) Lymphocyte arrest requires instantaneous induction of an extended LFA-1 conformation mediated by endothelium-bound chemokines. Nat Immunol 6:497–506
Thome R (1898) Endothelien als Phagocyten. Arch Mikrosk Anat 52:820–842
von Andrian UH, Mempel TR (2003) Homing and cellular traffic in lymph nodes. Nat Rev Immunol 3:867–878
von Schumacher S (1899) Ueber Phagocytose und die Abfuhrwege de Leucocyten in den Lymphdrusen. Arch Mikrosk Anat 54:311–328
Webster B, Ekland EH, Agle LM et al (2006) Regulation of lymph node vascular growth by dendritic cells. J Exp Med 203:1903–1913
Wendland M, Willenzon S, Kocks J et al (2011) Lymph node T cell homeostasis relies on steady state homing of dendritic cells. Immunity 35:945–957
Zhang L, Conejo-Garcia JR, Katsaros D et al (2003) Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 348:203–213
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag France
About this chapter
Cite this chapter
Veerman, K.M., Lafouresse, F., Girard, JP. (2014). Tumor High Endothelial Venules and Lymphocyte Trafficking. In: Feige, JJ., Pagès, G., Soncin, F. (eds) Molecular Mechanisms of Angiogenesis. Springer, Paris. https://doi.org/10.1007/978-2-8178-0466-8_16
Download citation
DOI: https://doi.org/10.1007/978-2-8178-0466-8_16
Published:
Publisher Name: Springer, Paris
Print ISBN: 978-2-8178-0465-1
Online ISBN: 978-2-8178-0466-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)