Abstract
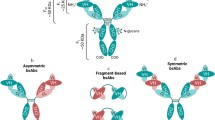
In recent years, both laboratory and clinical studies have demonstrated that bispecific antibodies (BsAbs) may have significant potential application in cancer therapy either by targeting tumor cells with cytotoxic agents including effector cells, radionuclides, drugs, and toxins, or by simultaneously blocking two tumor-associated targets, e.g., tumor growth factors and/or their cell surface receptors. A major obstacle in the development of BsAb has been the difficulty of producing the materials in sufficient quality and quantity by traditional technologies such as the hybrid hybridoma and chemical conjugation methods. The development of recombinant BsAbs as therapeutic agents will depend heavily on the advances made in the design of the constructs (or formats) and production efficiency. Here we describe a recombinant method for the construction and production of a tetravalent IgG-like BsAb molecule, IgG–scFv fusion, in which, a single-chain Fv (scFv) antibody fragment of one antigen specificity is genetically fused to the c-terminal of a conventional IgG of a different antigen specificity.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Fischer N, Leger O (2007) Bispecific antibodies: molecules that enable novel therapeutic strategies. Pathobiology 74:3–14
Jain M, Kamal N, Batra SK (2007) Engineering antibodies for clinical applications. Trends Biotechnol 25:307–316
Demarest SJ, Glaser SM (2008) Antibody therapeutics, anti-body engineering, and the merits of protein stability. Curr Opin Drug Discov Devel 11:675–687
Chames P, Baty D (2009) Bispecific antibodies for cancer therapy. Curr Opin Drug Discov Devel 12:276–283
Thakur A, Lum LG (2010) Cancer therapy with bispecific antibodies: clinical experience. Curr Opin Mol Ther 12:340–349
Kipriyanov SM, Le Gall F (2004) Recent advances in the generation of bispecific antibodies for tumor immunotherapy. Curr Opin Drug Discov Devel 7:233–242
Lum LG, Thakur A (2011) Targeting T cells with bispecific antibodies for cancer therapy. BioDrugs 25:365–379
Shen J, Zhu Z (2008) Catumaxomab, a rat/murine hybrid trifunctional bispecific monoclonal antibody for the treatment of cancer. Curr Opin Mol Ther 10:373–384
Marvin JS, Zhu Z (2006) Bispecific antibodies for dual-modality cancer therapy: killing two signaling cascades with one stone. Curr Opin Drug Discov Devel 9:184–193
Lu D, Zhang H, Koo H et al (2005) A fully human recombinant IgG-like bispecific antibody to both the epidermal growth factor receptor and the insulin-like growth factor receptor for enhanced antitumor activity. J Biol Chem 280:19665–19672
Wu C, Ying H, Grinnell C et al (2007) Simultaneous targeting of multiple disease mediators by a dual-variable-domain immunoglobulin. Nat Biotechnol 25:1290–1297
Schaeter G, Haber L, Crocker L et al (2011) A two-in-one antibody against HER3 and EGFR has superior inhibitory activity compared with monospecific antibodies. Cancer Cell 20:472–486
Michaelson JS, Demarest SJ, Miller B et al (2009) Anti-tumor activity of stability-engineered IgG-like bispecific antibodies targeting TRAIL-R2 and LTbetaR. MAbs 1:128–141
Dong J, Sereno A, Aivazian D et al (2011) A stable IgG-like bispecific antibody targeting the epidermal growth factor receptor and the type I insulin-like growth factor receptor demonstrates superior anti-tumor activity. MAbs 3:273–288
Baeuerle P (2012) Revival of bispecific antibodies, resource of 3rd annual world bispecific antibody summit (Boston, USA). http://bispecific.com/uploads/ffiles/2012/05/904821.pdf
Marvin JS, Zhu Z (2005) Recombinant approaches to IgG-like bispecific antibodies. Acta Pharmacol Sin 26:649–658
Dong J, Sereno A, Snyder WB et al (2011) Stable IgG-like bispecific antibodies directed towards the type I insulin-like growth factor receptor demonstrate enhanced ligand blockade and anti-tumor activity. J Biol Chem 286:4703–4717
de Haard HJ, van Neer N, Reurs A et al (1999) A large non-immunized human Fab fragment phage library that permits rapid isolation and kinetic analysis of high affinity antibodies. J Biol Chem 274:18218–18230
Hoet RM, Cohen EH, Kent RB et al (2005) Generation of high-affinity human antibodies by combing donor-derived and synthetic complementarity-determining-region diversity. Nat Biotechnol 23:344–348
Lu D, Shen J, Vil MD et al (2003) Tailoring in vitro selection for a picomolar affinity human antibody directed against vascular endothelial growth factor receptor 2 for enhanced neutralizing activity. J Biol Chem 278:43496–43507
Reiter Y, Brinkmann U, Lee B et al (1996) Engineering antibody Fv fragments for cancer detection and therapy: disulfide-stabilized Fv fragments. Nat Biotechnol 14:1239–1245
Worn A, Pluckthun A (2001) Stability engineering of scfv fragments. J Mol Biol 305:989–1010
Miller BR, Demarest SJ, Lugovskoy A et al (2010) Stability engineering of scFvs for the development of bispecific and multivalent antibodies. Protein Eng Des Sel 23:549–557
Demarest SJ, Glaser SM (2008) Antibody therapeutics, antibody engineering, and the merits of protein stability. Curr Opin Drug Discov Devel 11:675–687
Mabry R, Lewis KE, Moore M et al (2010) Engineering of stable bispecific antibodies targeting IL-17A and IL-23. Protein Eng Des Sel 23:115–127
Mabry R, Snavely M (2010) Therapeutic bispecific antibodies: the selection of stable single-chain fragments to overcome engineering obstacles. IDrugs 13:543–549
Lu D, Jimenez X, Witte L, Zhu Z (2004) The effect of variable domain orientation and arrangement on the antigen-binding activity of a recombinant human bispecific diabody. Biochem Biophys Res Commun 28:507–513
Orcutt KD, Ackerman ME, Cieslewicz M et al (2009) A modular IgG-scFv bispecific antibody topology. Protein Eng Des Sel 23:221–228
Dimasi N, Gao C, Fleming R et al (2009) The design and characterization of oligospecific antibodies for simultaneous targeting of multiple disease mediators. J Mol Biol 393:672–692
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media, LLC
About this protocol
Cite this protocol
Lu, D., Zhu, Z. (2014). Construction and Production of an IgG-Like Tetravalent Bispecific Antibody, IgG–Single-Chain Fv Fusion. In: Steinitz, M. (eds) Human Monoclonal Antibodies. Methods in Molecular Biology, vol 1060. Humana, Totowa, NJ. https://doi.org/10.1007/978-1-62703-586-6_11
Download citation
DOI: https://doi.org/10.1007/978-1-62703-586-6_11
Published:
Publisher Name: Humana, Totowa, NJ
Print ISBN: 978-1-62703-585-9
Online ISBN: 978-1-62703-586-6
eBook Packages: Springer Protocols