Abstract
Neuromelanin, a black pigment present in dopaminergic and noradrenergic neurons in the substantia nigra pars compacta (SNc) and locus ceruleus (LC), has paramagnetic T1-shortening effects. Conventional magnetic resonance imaging (cMRI) techniques fail to depict the contrast generated by neuromelanin, but there are some neuromelanin-sensitive techniques that allow direct visualization of the SNc and LC as evident high-signal areas. In Parkinson disease, neuromelanin-related signals from the SNc and LC are attenuated suggesting neuronal loss in both these nuclei. In other neurodegenerative and psychiatric disorders, signal alterations of SNc and/or LC can also be shown. Neuromelanin-sensitive MRI is a promising technique to assess changes in these nuclei that occur in Parkinson disease and other related disorders.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Parkinson Disease
- Multiple System Atrophy
- Progressive Supranuclear Palsy
- Progressive Supranuclear Palsy
- Conventional Magnetic Resonance Imaging
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Neuromelanin is a black pigment that is mainly present in the substantia nigra pars compacta (SNc) and locus ceruleus (LC) of the human brain. These nuclei can be identified as evident black or blue areas in gross specimens, but neuroimaging techniques used to visualize these nuclei in vivo are not well developed. Recent development of some magnetic resonance imaging (MRI) techniques that can capture contrast as a result of neuromelanin enables direct visualization of the SNc and LC [1–3]. In this chapter, we describe the imaging techniques, normal findings, and clinical applications of neuromelanin-sensitive MRI.
Neuroimaging Techniques for Visualizing Neuromelanin
Neuromelanin as a Paramagnetic Agent
Neuromelanin , a dark polymer pigment, is structurally similar but not identical to peripheral melanins and consists of eumelanin, pheomelanin, cysteinyldopa, and a small amount of lipid/protein components [4, 5]. Neuromelaninis a byproduct of catecholamine metabolism and is mainly present within the dopaminergic neurons of the SNc and noradrenergic neurons of the LC. It has been shown that neuromelanin plays an important role in protecting these neurons against toxic metabolites and oxidative stress and interacts with α-synuclein [6, 7]. Similar to peripheral melanins, neuromelanin acts as a paramagnetic agent when bound to metals such as iron and copper during MRI [8]. Longitudinal and transverse relaxivities (R1 and R2) of neuromelanin have been reported to be approximately 1.0 mM− 1s− 1 and 1.3 mM− 1s− 1, respectively [9], indicating that neuromelanin mainly possesses T1-shortening effects and can be visualized on T1-weighted imaging (T1WI).
T1-Weighted Fast Spin-Echo Imaging at 3 T
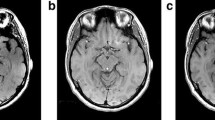
Conventional T1WI with either spin-echo or gradient-echo (GRE) techniques show minimal neuromelanin-related signal changes even in the SNc and LC. A recent study reported that high-resolution two-dimensional (2D) fast spin-echo (FSE) T1WI at 3 T can depict these nuclei as high-signal areas [1]. In this technique, T1-shortening effects of intraneuronal neuromelanin become conspicuous due to the additive effects of high signal-to-noise ratio, high spatial resolution, T1 prolongation of the brain tissue, and the augmentation of inherent magnetization transfer contrast (MTC) effects of multislice FSE; all these are characteristic features of MRI at 3 T. The scanning parameters of neuromelanin-sensitive T1WI are provided in Table 10.1. The distribution of high-signal areas on images obtained using these parameters correlated well with the distribution of neuromelanin-containing neurons of SNc and LC [10], indicating that this technique can be used to directly visualize and detect changes in these nuclei (Fig. 10.1a, b).
Neuromelanin-sensitive magnetic resonance imaging of the substantia nigra and locus ceruleus (LC). a, b Two-dimensional (2D) fast spin-echo (FSE) images obtained at 3 T; c, d three-dimensional (3D) spoiled gradient-echo (GRE) images with magnetization transfer contrast pulse at 3 T; a, c: axial sections at the level of the midbrain; b, d axial sections at the level of the upper pons. Neuromelanin-sensitive images obtained by either 2D-FSE or 3D-GRE techniques clearly depict the high-signal areas at locations that correlate well with the locations of the substantia nigra pars compacta (a, c; arrows) and LC (b, d; arrows). On the 3D-GRE image, a punctate hyperintense area is seen at the midline of the midbrain tegmentum, indicating the ventral tegmental area (c, arrowhead)
T1-Weighted Three-Dimensional Gradient Echo Imaging with Magnetization Transfer Contrast
Neuromelanin-sensitive 2D-FSE T1WI has several limitations such as relatively low spatial resolution, long acquisition time, limited coverage, and signal nonuniformity due to B1 heterogeneity [1]. These technical issues can cause substantial errors in the qualitative and quantitative assessment of the signal alterations in the SNc and LC in Parkinson disease and other related disorders.
Three-dimensional (3D) spoiled GRE technique with MTC pulse, also used for source imaging in magnetic resonance angiography, is sensitive to neuromelanin-related contrast [3]. This technique can obtain high-resolution volume images with minimal signal inhomogeneity during a short acquisition (< 10 min) and can overcome the limitations of the 2D-FSE technique (Fig. 10.1c, d). In addition, this method can be used for visualizing the SNc and LC at both 3 and 1.5 T [3].
Normal Image Findings of Neuromelanin-Containing Nuclei
Substantia Nigra
Axial neuromelanin-sensitive images show distinct band-like high-signal areas in the posteromedial part of the crus cerebri, mainly at the level of the lower midbrain (see Fig. 10.1a) [1, 11]. Horizontal and vertical distributions of the hyperintense areas correspond well with that of neuromelanin-containing neurons in gross specimens, but are substantially different from the distribution of low-signal-intensity areas on T2-weighted images and gray matter signal areas on proton-density-weighted or short inversion–time inversion recovery images [11]. Thus, compared with other structural imaging techniques, neuromelanin-sensitive MRI is more appropriate for direct assessment of the SNc. Sometimes, 3D-GRE images show a punctate hyperintense area at the midline of the midbrain tegmentum, indicating dopaminergic neurons of the ventral tegmental area (see Fig. 10.1c).
Locus Ceruleus (LC)
The LC is one of the gray matter structures that are “invisible” on conventional MRI but can be visualized as a pair of rod-shaped hyperintense areas along the lateral edge of the fourth ventricle floor in the upper pontine tegmentum by using neuromelanin-sensitive imaging techniques (see Fig. 10.1b, d) [1, 11]. Distribution of the hyperintense areas is identical to that of the LC in gross specimens [10]. In addition, rostrocaudal variations in the signal intensity have been reported to be closely related to the variations in the number of the neuromelanin-containing neurons [12], suggesting that the degree of signal intensity can reflect neuronal number and/or intraneuronal neuromelanin content.
Neuromelanin-Sensitive Imaging for Evaluating the Changes that Occur in Neurodegenerative Diseases
Idiopathic Parkinson Disease (iPD)
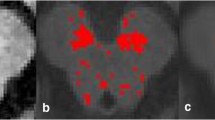
Neuromelanin-sensitive imaging techniques have been mainly utilized for evaluating the pathological changes in the SNc or LC of patients with iPD, because conventional MRI techniques fail to detect significant changes related to the principal pathological changes in iPD, i.e., neuronal depletion of the SNc and LC [11, 13]. Several studies have shown significant signal attenuation and/or volume reduction of hyperintense SNc areas on neuromelanin-sensitive images of iPD patients, indicating degeneration of dopaminergic neurons (Fig. 10.2b) [1, 2, 14]. In addition, such changes were more pronounced in the later stages than in the early stages of the disease, reflecting pathological progression of iPD [2]. Significant signal decrease in the LC areas was also seen in iPD (see Fig. 10.2d) [1]. Thus, neuromelanin-sensitive MRI can be used to visualize degenerative changes in the neuromelanin-containing neurons occurring in iPD. However, the performance of neuromelanin-sensitive MRI in terms of early and differential diagnoses is not known and should be evaluated in further investigations.
Neuromelanin-sensitive imaging of patients with idiopathic Parkinson disease. a, b Images of a 60-year-old healthy woman; c, d images of a 57-year-old woman with early-stage Parkinson disease (Hoehn-Yahr II); a, c axial images at the level of the lower midbrain; b, d axial images at the level of the upper pons; upper row: gray-scale images; lower row: color-coded images. Window level and width were set as a signal intensity of the midbrain tegmentum and 70 % of that (a, c), or were set as that of the pontine tegmentum and 35 % of that (b, d). Signal intensities of the substantia nigra pars compacta and locus ceruleus are evidently lower for the patient with Parkinson disease (c, d; arrows) than for the healthy subject (a, b)
Other Neurological and Psychiatric Disorders
A few investigations have used neuromelanin imaging to assess other parkinsonian and psychiatric disorders. A significant decrease in the volume of hyperintense SNc areas was noted in patients with multiple system atrophy (MSA) , progressive supranuclear palsy (PSP), and corticobasal syndrome (CBS) , but this finding could not be used to discriminate these disorders from iPD [15]. Signal attenuation for the LC , particularly in its rostral two-thirds, was observed for depressive patients suggesting dysfunction of the ascending noradrenergic system [12]. On the other hand, signal augmentation for the SNc was observed in patients with schizophrenia , which may reflect an increase in the dopamine activity [16]. Further, a recent report showed that color-coded MR images could be visually assessed to discriminate depressive patients from healthy individuals with relatively high sensitivity and specificity [17].
Summary and Future Directions
Neuromelanin-sensitive imaging by using specific MRI techniques shows promise for use in direct assessment of pathological and functional changes in the neuromelanin-containing neurons of the SNc and LC. However, further technical improvement and clinical investigations are needed to establish the clinical significance of this imaging in terms of preclinical, early, and differential diagnoses as well as evaluation of surrogate markers for clinical severity and drug response.
References
Sasaki M, Shibata E, Tohyama K, et al. Neuromelanin magnetic resonance imaging of locus ceruleus and substantia nigra in Parkinson’s disease. Neuroreport. 2006;31:1215–8.
Schwarz ST, Rittman T, Gontu V, et al. T1-weighted MRI shows stage-dependent substantia nigra signal loss in Parkinson’s disease. Mov Disord. 2011;26:1633–8.
Nakane T, Nihashi T, Kawai H, et al. Visualization of neuromelanin in the substantia nigra and locus ceruleus at 1.5 T using a 3D-gradient echo sequence with magnetization transfer contrast. Magn Reson Med Sci. 2008;7:205–21.
Fedorow H, Tribl F, Halliday G, et al. Neuromelanin in human dopamine neurons: comparison with peripheral melanins and relevance to Parkinson’s disease. Prog Neurobiol. 2005;75:109–24.
Zecca L, Tampellini D, Gerlach M, et al. Substantia nigra neuromelanin: structure, synthesis, and molecular behaviour. Mol Path. 2001;54:414–8.
Zecca L, Zucca FA, Wilms H, et al. Neuromelanin of the substantia nigra: a neuronal black hole with protective and toxic characteristics. Trends Neurosci. 2003;26:578–80.
Fasano M, Bergamasco B, Lopiano L. Modifications of the iron-neuromelanin system in Parkinson’s disease. J Neurochem. 2006;96:909–16.
Tosk JM, Holshouser BA, Aloia RC, et al. Effects of the interaction between ferric iron and L-dopa melanin on T1 and T2 relaxation times determined by magnetic resonance imaging. Magn Reson Med. 1992;26:40–5.
Enochs WS, Petherick P, Boqdanova A, et al. Paramagnetic metal scavenging by melanin: MR imaging. Radiology. 1997;204:417–23.
Shibata E, Sasaki M. Imaging of neuromelanin. In: Naidich TP, Duvernoy HM, Delman BN, et al. editors. Duvernoy’s atlas of the human brain stem and cerebellum. Wien, Springer; 2009. p. 475–482.
Sasaki M, Shibata E, Tohyama K, et al. Monoamine neurons in the human brain stem: anatomy, magnetic resonance imaging findings, and clinical implications. Neuroreport. 2008;19:1649–54.
Shibata E, Sasaki M, Tohyama K, et al. Reduced signal of locus ceruleus in depression in quantitative neuromelanin magnetic resonance imaging. Neuroreport. 2007;18:415–8.
Lehericy S, Sharman MA, Santos CL, et al. Magnetic resonance imaging of the substantia nigra in Parkinson’s disease. Mov Disord. 2012;27:822–30.
Kashihara K, Shinya T, Higaki F. Reduction of neuromelanin-positive nigral volume in patients with MSA, PSP and CBD. Intern Med. 2011;50:1683–7.
Kashihara K, Shinya T, Higaki F. Neuromelanin magnetic resonance imaging of nigral volume loss in patients with Parkinson’s disease. J Clin Neurosci. 2011;18:1093–6.
Shibata E, Sasaki M, Tohyama K, et al. Use of neuromelanin-sensitive MRI to distinguish schizophrenic and depressive patients and healthy individuals based on signal alterations in the substantia nigra and locus ceruleus. Biol Psychiatry. 2008;64:401–6.
Sasaki M, Shibata E, Ohtsuka K, et al. Visual discrimination among patients with depression and schizophrenia and healthy individuals using semiquantitative color-coded fast spin-echo T1-weighted magnetic resonance imaging. Neuroradiology. 2010;52:83–9.
Acknowledgments
This work was partly supported by a grant-in-aid for Advanced Medical Science Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Sasaki, M., Yamashita, F., Kudo, K. (2013). Neuromelanin Imaging in Parkinson Disease. In: Nahab, F., Hattori, N. (eds) Neuroimaging of Movement Disorders. Current Clinical Neurology, vol 44. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-62703-471-5_10
Download citation
DOI: https://doi.org/10.1007/978-1-62703-471-5_10
Published:
Publisher Name: Humana Press, Totowa, NJ
Print ISBN: 978-1-62703-470-8
Online ISBN: 978-1-62703-471-5
eBook Packages: MedicineMedicine (R0)