Abstract
The adult body harbors powerful reservoirs of stem cells that enable tissue regeneration under homeostatic conditions or in response to disease or injury. The hair follicle is a readily accessible mini organ within the skin and contains stem cells from diverse developmental origins that are shown to have surprisingly broad differentiation potential. In this chapter, we discuss the biology of the hair follicle with particular emphasis on the various stem cell populations residing within the tissue. We summarize the existing knowledge on putative hair follicle stem cell markers, the differentiation potential, and technologies to isolate and expand distinct stem cell populations. We also discuss the potential of hair follicle stem cells for drug and gene delivery, tissue engineering, and regenerative medicine. We propose that the abundance of stem cells with broad differentiation potential and the ease of accessibility make the hair follicle an ideal source of stem cells for gene and cell therapies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Hair follicle
- Stem cells
- Tissue engineering
- Regenerative medicine
- Gene therapy
- Drug delivery
- Reprogramming
5.1 Introduction
The hair follicle (HF) is a dynamic “mini” organ supporting important biological functions of the body. HFs protect against cold and potential injuries; they also have an important sensory and immunologic functions in addition to affecting the social behavior of a person [1, 2].
HFs are easily accessible and contain stem cells from diverse developmental origins that continuously self-renew, differentiate, regulate hair growth, and contribute to skin homeostasis. Hair follicle stem cells have been shown to be highly proliferative in vitro and multipotent [3–5] that allows engineering a variety of different tissues for organ replacement and regenerative medicine. In addition, genetic engineering of the hair follicle stem cells in vivo has shown promising results, suggesting that treatment of genetic diseases of skin and hair via the hair follicle may be feasible. This chapter summarizes the existing literature regarding the differentiation potential of hair follicle stem cells, their putative markers, the common isolation methods, and their application in cell and gene therapies.
5.2 Hair Follicle Biology
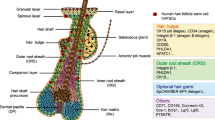
HF is part of the pilosebaceous unit that contains the sebaceous gland, the apocrine gland, and the arrector pili muscle. The HF is composed of two main compartments: the upper part includes the infundibulum and the isthmus, whereas the bulb, matrix, and dermal papilla comprise the lower part. The exact position of the lower part varies during hair cycling. Adjacent to the lower portion of the infundibulum lies the sebaceous gland that waterproofs the skin by secreting sebum. The bulge is a part of the isthmus that is believed to be the stem cell reservoir, which regenerates the HF during hair growth. Cells migrate from the bulge toward the bulb, where they proliferate and differentiate in order to produce the hair shaft and all the epithelial cells that constitute the HF [2]. Finally, the inner and outer root sheaths are composed mainly of keratinocytes surrounding the hair shaft (Fig. 5.1).
HF undergo numerous cycles of growth and retraction throughout life. This dynamic process in adult life has three distinct phases, that is, anagen, catagen, and telogen, each regulated by different signals. Anagen is the growing phase. Stem cells that are located in the bulge region differentiate to all hair lineages, resulting to hair elongation. The duration of anagen in human body varies depending on the anatomic location of the follicle. On the scalp, anagen may last as long as 8 years resulting in long hair, but in other places such as the eyebrow, anagen maybe as short as 3 months. Catagen is the regression phase. At this stage the majority of the HF cells undergo apoptosis, causing reduction of the lower compartment that brings the dermal papilla cells close to the bulge cells. Exchange of signals between the papilla and the bulge regulates the duration of catagen. The cells that escape apoptosis during this phase comprise the reservoir that leads to the next anagen. Telogen is the last phase of the HF cycle, also known as resting phase. In telogen, cells enter a quiescent state waiting for the necessary signals to restart the cycle via the anagen. It is argued that 5–15 % of HF in the scalp remain in telogen [1, 2].
5.3 Location and Differentiation Potential of Hair Follicle Stem Cells
Stem cells can be characterized by three unique properties: self-renewal, capacity to differentiate into one or multiple cell types, and the ability to form tissues in vivo. Based on their differentiation potential, they can be categorized as totipotent, pluripotent, multipotent, and unipotent. Totipotent cells can reproduce all the cells of a living organism including the extraembryonic tissues. Pluripotent cells differ from the totipotent cells in their inability to form the extraembryonic tissues necessary for proper growth of the embryo. Multipotent stem cells have even more restricted differentiation potential, but they can still differentiate into more than one cell type. The lowest in the hierarchy are the unipotent stem cells or progenitor cells, which can generate only one cell type. Stem cells can also be classified into embryonic or adult according to their origin [6]. Although embryonic stem cells have broader differentiation potential, adult stem cells can be isolated from the patient, directly overcoming possible immune rejection after transplantation.
Adult stem cells in vivo reside in multiple tissues usually in a well-protected microenvironment called niche. Examples of stem cell populations that are surrounded by niche include the intestinal stem cells, the neural stem cells, and the HF stem cells. This way, the body holds a powerful reservoir of cells that can readily respond in case of emergency, such as an injury. Some adult stem cells like mesenchymal stem cells (MSC) have multilineage differentiation potential so that a single cell can contribute to the regeneration of multiple tissues such as fat, bone, cartilage, and muscle [7].
Although in vitro stem cells proliferate markedly in response to appropriate signals in the culture media, in vivo they remain quiescent until they are coaxed to proliferate and/or differentiate only when needed, for example, in case of injury. Under homeostatic conditions, the stem cell pool is maintained through asymmetric division, where the parent stem cell divides into two cells with varying differentiation potential: one retaining the stem cell characteristics (self-renewal) and the other assuming a more differentiated phenotype (differentiation). However, expansion of the stem cell pool would require symmetric division, where two stem cells are generated from a parental stem cell [8].
Similar to other organs, the HF contains a rich stem cell pool that resides in different anatomic locations within the HF. As a result, some scientists call the HF as a stem cell “zoo” [9]. In the next chapter, we will present the different stem cell populations, and we will elaborate on their broad differentiation potential.
5.3.1 Bulge and Hair Germ
Due to the complex architecture of the HF, the location of the stem cell reservoir remained elusive for many years. Initial studies reasoned that stem cells resided in the bulb [10], but this hypothesis was abandoned, as removal of the bulb did not inhibit the generation of new hair follicles [11]. In the early 1990s, Cotsarelis and colleagues were the first to propose that stem cells reside in the bulge area of HF. They took advantage of the fact that in vivo stem cells cycle very slowly so that long time after administration of tritiated thymidine, only the cells that retain the label (label-retaining cells) are the slow-cycling stem cells [12, 13]. Several years later, this finding was verified using transgenic mice that were engineered to express the fusion protein histone H2B-GFP under the keratin-5 promoter in a tetracycline-regulatable manner. As a result skin cells expressed GFP except when the mice were fed doxycycline, which suppressed GFP expression. The fast-cycling cells lost the GFP, whereas the slow-cycling stem cells retained it. These label-retaining cells were localized in the bulge region of hair follicles [14]. Furthermore, tracing studies with transgenic mice expressing the LacZ transgene under the control of either keratin-15 or Lgr5 promoter further supported the bulge activation hypothesis, which states that during anagen, stem cells from the bulge migrate in the bulb region where they are induced to proliferate and differentiate to all epithelial cell types of the HF [15, 16].
Notably, transplantation of keratin-15+ or Lgr5+ cells along with dermal fibroblasts in the dermis of nude mice generated new HF with high efficiency [15, 16]. Interestingly, damage of the bulge from autoimmune disease lichen planopilaris resulted in permanent hair loss [17], further highlighting the importance of bulge-derived stem cells for hair regeneration. In addition to hair regeneration, bulge stem cells were found to contribute to wound healing following skin injury by migrating and differentiating to epidermal keratinocytes [18, 19]. However, they are not necessary for the maintenance of the epidermis (ablation of the cells does not affect the homeostasis of the epidermis), and in the long run, they fail to stay at the sites of injury [19]. Additional studies have shown robust multipotency of bulge stem cells in vivo, where they were found to participate in angiogenesis, and in vitro where they were coaxed to differentiate into neurons, glial cells, melanocytes, keratinocytes, and mesenchymal cells [20–24].
Although it is widely accepted that the bulge harbors stem cells, the exact stem cell population is still under debate. Jaks and colleagues challenged the notion of label-retaining cells as the true stem cell population in HF, as Lgr5+ cells can regenerate the whole follicle but do not coincide with the label-retaining cells of the bulge. The same study reported that Lgr5+ cells were found in the hair germ, a region between the dermal papilla and the bulge, which remains discrete during telogen but overlaps with the matrix during anagen [16]. Others believe that the hair germ originates from the bulge and contributes to the generation of the new HF in the beginning of anagen [25]. In agreement, Greco and colleagues showed that the transcriptional profile of hair germ cells resembles that of bulge cells. They also found that hair germ cells proliferate faster than bulge cells and respond first to the dermal papilla signals at the late telogen. However, they also lose their proliferative capacity faster than bulge cells during long-term expansion in vitro [26].
5.3.2 Isthmus/Infundibulum
Cells located above the bulge are believed to retain multipotent properties. Studies have reported that they can differentiate not only into the epithelial lineages of the HF but also into the sebaceous gland and the epidermis. However, it is yet not known whether these cells represent a unique stem cell population, or a subset of bulge stem cells, or even progenitors with limited differentiation capacity.
Isolated cells from the area between the bulge and the sebaceous gland were found to be distinct from the bulge-derived stem cells since they did not express bulge-specific markers such as keratin-15 and CD34. Although they maintained their high clonogenic potential in vitro, they were also actively proliferating in vivo – in contrast to the notion that in vivo stem cells are the slow-cycling, label-retaining cells [27]. Similarly Jensen and colleagues reported that cells isolated from the upper bulge region and were not quiescent in vivo could generate new follicles after implantation, suggesting that stem cells need not be slow-cycling cells in vivo in order to be multipotent [28].
Although, during homeostasis, bulge-derived cells do not contribute to the generation of epidermis [15, 16, 19], several studies showed that cells derived from a region above the bulge can give rise to epidermis and persist there for a long time following injury [28–30].
5.3.3 Sebaceous Gland
There are two theories with regard to the origins of the sebaceous gland. The first asserts that stem cells residing in the bulge region migrate and give rise to resident gland cells. This theory is supported by transplantation studies showing that bulge cells generated functional sebaceous gland in vivo [15, 16]. The second theory suggests that stem cells located above the bulge differentiate into sebocytes [29–31]. Horsley and colleagues identified a unique cell population in the region of sebaceous gland that expresses the transcription factor Blimp1 and has unipotent differentiation potential into sebocytes. Loss of Blimp1 in HF resulted in activation of bulge cells, which may suggest a possible connection between bulge and sebaceous gland. The same study also showed that implanted bulge stem cells could give rise into Blimp1+ cells [31].
5.3.4 Dermal Papilla and Dermal Sheath
Dermal papilla (DP) and dermal sheath (DS) are cell populations within the HF that are believed to contain stem cells. Whereas bulge cells originate from ectoderm, DP and DS cells are derived from mesoderm, and they are known to regulate hair cycling by exchanging signals with the bulge [2]. Multiple studies showed that DP and DS cells have broad differentiation potential. In a pioneering study, Lako and colleagues demonstrated that DP and DS cells could reconstitute multiple lineages of the hematopoietic system in lethally irradiated mice [32]. Rat and human HF-derived DP and DS cells could also be induced to differentiate toward the myogenic, osteogenic, chondrogenic, and adipogenic lineage resembling bone marrow mesenchymal stem cells [3–5, 33, 34]. A recent study showed that DP/DS stem cells are the precursors of dermal stem cells and contribute to dermal maintenance and wound healing [35].
5.4 Putative Hair Follicle Stem Cell Markers
The majority of the studies in HF have been conducted in murine models. However, there are several differences that have to be taken into account between human and murine models, and conclusions derived from experiments with mice models do not necessarily apply in human HF cells. Whereas humans have only two types of hair (vellus and heavily pigmented hairs), mice are endowed with several distinct hair types (pelage, vibrissae, cilia, hairs on the tail, ear, genital, perianal area, nipples, and around the feet). In addition, the biological cycles of human and mouse HF are different; while human HF cycle independently after birth, mouse HF cycle in synchrony [2]. Finally, the biological markers characterizing the stem cell populations in human and mouse are strikingly different. Table 5.1 summarizes the most common markers of HF stem cells based on the species they are derived from and the location where they are expressed.
5.4.1 Murine Hair Follicles
5.4.1.1 Bulge
Several markers have been proposed to characterize murine bulge stem cells. In addition to keratin-15 and Lgr5, CD34 is co-expressed with keratin-15 and has also been proposed as a potential stem cell marker of the bulge. CD34+ cells are relatively quiescent and have higher clonogenic potential in vitro as compared to CD34− cells [36]. Several transcription factors have been identified in the bulge region including Tcf3, Sox-9, Lhx2, and NFATc1. Tcf3 was shown to maintain the undifferentiated cell state by repressing numerous genes that induce sebaceous gland and HF differentiation [38]. Another key transcription factor that is expressed in the bulge area is Sox-9. Sox-9+ cells are first detected during the formation of hair placode, the precursor of HF during prenatal life. The cells co-localize with early label-retaining cells, which subsequently give rise to bulge stem cells. Notably, deletion of Sox-9 decreased the proliferation of bulge stem cells, impaired the generation of proliferative matrix cells, and resulted in inhibition of HF morphogenesis [41, 51].
Similar to Sox-9, Lim-homeodomain transcription factor, Lhx2 is also expressed during hair placode formation as was seen by microarray analysis in the P-cadherin+ cells that mark early hair progenitors. In postnatal life, Lhx2 is expressed in the bulge and suppresses differentiation, prompting some investigators to hypothesize that it may be required for stem cell maintenance [40]. However, a recent study challenged this notion and reported that Lhx2 is required for the induction of anagen and not for the maintenance of stem cells [52]. The fourth bulge-specific transcription factor is NFATc1, which is regulated by the intracellular levels of calcium. Under high calcium conditions, NFATc1 is dephosphorylated and translocates to the nucleus, where it downregulates cyclin-dependent kinase 4 and suppresses proliferation in bulge region. As a result, downregulation of NFATc1 leads to activation of bulge-derived stem cells [39]. Interestingly, NFATc1-expressing cells coincide only partially with CD34+, Tcf3+, Lhx2+, and Sox-9+ cells in the bulge region, suggesting there is no unique marker of bulge stem cells but rather a group of transcription factors that regulate stem cell maintenance and activation through a series of complex and dynamic interactions.
Finally, other studies provided evidence that nestin is expressed in the mouse bulge stem cells. Transgenic mice expressing GFP under the nestin promoter showed that nestin-positive cells are located in the bulge region during telogen but in the upper two thirds of the outer root sheath during anagen. In vivo these cells participated in the formation of new blood vessels, and in vitro they could be coaxed to differentiate into neurons, glial cells, smooth muscle cells, melanocytes, and keratinocytes, demonstrating the multipotency of hair follicle stem cells [20, 21, 53].
5.4.1.2 Upper Bulge
Several markers have been identified over the years that target putative murine stem cells in the upper bulge region. Lgr6, an orphan G protein-coupled receptor, is expressed in the region immediately above the bulge. Lgr6+ cells were shown to play a critical role in the formation of HF, sebaceous gland, and epidermis during development [30]. MTS24, a cell surface glycoprotein, also marked potential stem cells in a region above the bulge. MTS24+ cells exhibited increased colony-forming capacity as compared to MTS24− cells and showed similar gene expression profile with CD34+ bulge cells [27]. However, the differentiation potential of these cells was not examined. In addition, cells residing in the upper isthmus were shown to be multipotent as they could form HF, sebaceous gland, and epidermis after implantation. These cells expressed low levels of integrin α6, were negative for the hematopoietic markers CD34 and Sca-1, and exhibited distinct gene expression profile as compared to bulge cells [28]. Finally, another putative stem cell marker characterizing the region right above the bulge is transmembrane protein leucine-rich repeats and immunoglobulin-like domain protein 1 or Lrig1. Lrig1 was shown to regulate epidermal growth factor signaling by promoting the degradation of epidermal growth factor receptor [54] and to keep cells in this region in a quiescent state [42]. Indeed, in vivo Lrig1+ cells appeared to be quiescent and multipotent, two of the main attributes of stem cells [29].
5.4.1.3 Dermal Papilla and Dermal Sheath
The DP and DS are known to induce HF generation by interacting with epidermal stem cells [55]. In 1999, Kishimoto et al. reported that cells in DP express the proteoglycan versican, which is usually present in the condensed mesenchyme. The same group employed the versican promoter to express either LacZ or GFP and found that when implanted on the back of nude mice along with keratinocytes, the versican+ cells could reconstitute the HF but versican− cells could not [44]. Others observed that nexin-1, a protease inhibitor, was highly expressed in DP during anagen and that the nexin-1 expression level correlated with the rate of hair growth [46]. Similarly, the expression of alkaline phosphatase – an enzyme expressed in bone cells and embryonic stem cells – correlated with hair growth and was also highly expressed in DP during anagen, suggesting a positive correlation between hair induction and alkaline phosphatase activity [45]. Finally, CD133 was expressed in DP cells during HF development, but its expression was greatly diminished after birth. Nevertheless, when co-implanted with embryonic epithelial cells, CD133+ cells enabled generation of HF in vivo [47]. Interestingly, a subpopulation of CD133+ Sox2+ cells within the DP was shown to be essential for the formation of particular types of hair such as awl/auchene follicles [43]. Rendl and colleagues compared the transcriptional profile of five distinct cell populations within the HF, namely, melanocytes, dermal papilla, matrix, outer root sheath, and dermal fibroblasts. This approach successfully identified several genes and signaling pathways that were unique to each population and need to be further explored in the future [56].
5.4.2 Human Hair Follicles
Murine HFs have been largely explored with respect to stem cell markers; however, human HF have remained unexplored. In contrast to murine bulge, the human bulge cannot be identified as a distinct anatomic projection, rendering isolation of bulge cells very challenging. Screening a number of markers in vivo, Kloepper and colleagues identified CD200 and keratin-15 and keratin-19 as putative bulge stem cell markers, although their location is not restricted to the bulge but extends to a wider area of isthmus as well. In contrast to the mouse, human bulge does not express CD34, nestin, or Lhx2 [48]. In a more recent study keratin-15high/CD200+/CD34−/CD271− bulge-derived cells showed increased clonogenic potential as compared to keratin-15low/CD200+/CD34−/CD271− cells [50]. In agreement, CD200-expressing cells that were isolated from a population of label-retaining cells using laser capture microdissection showed increased clonogenic potential in vitro [49]. However, multipotency of CD200+ cells has not been examined. More recently our laboratory reported that DP/DS cells display a cell surface profile characteristic of mesenchymal stem cells being positive for CD90, CD44, CD49b, CD105, and CD73 [4, 5]. In addition, these cells are clonally multipotent as they can differentiate in fat, bone, cartilage, and smooth muscle with high efficiency [5].
5.5 Methods for Isolating Hair Follicle Stem Cells
Three techniques have been routinely used for the isolation of putative stem cells from the HF: microdissection, enzymatic digestion, and fluorescence-activated cell sorting (FACS). In the following, we describe each technique and elaborate on their advantages and disadvantages.
5.5.1 Microdissection
Microdissection is a technique that has been commonly applied for the isolation of cells from DP [11, 32, 33, 57, 58] as well as the bulge [49, 59]. This technique requires the use of fine forceps and blades for the isolation of the area of interest. Subsequently the isolated areas are transferred into tissue culture plates, where the cells migrate out of the tissue and proliferate in the presence of appropriate culture medium.
For DP cell isolation, application of pressure on the suprabulbar region by forceps was shown to compress the bulb and facilitate removal of the connective tissue sheath surrounding the DP, which is subsequently detached from the epithelium using a scalpel blade [60]. Finally, a highly reliable technique that has been used for isolating human bulge cells is laser capture microdissection [49]. A thermolabile membrane is placed on top of the sample, and the area of interest is targeted by laser, which melts the membrane locally marking the cells that are subsequently separated [61]. The major advantage of microdissection is that this approach preserves the whole tissue, thereby increasing the efficiency of cell isolation. However, this technique is quite laborious and requires experienced technicians.
5.5.2 Enzymatic Digestion
Another approach that has been employed for isolation of HF stem cells involves enzymatic digestion of the follicle from the surrounding dermis, usually with dispase or collagenase. The incubation time and concentration of enzymes used vary depending on the amount of extracellular matrix present around the follicle. Generally collagenase treatment requires few hours of incubation at 37 °C, whereas dispase needs overnight treatment [3–5, 23, 49]. Others use a combination of enzymes to isolate DP cells. Specifically, dispase was employed initially to remove the follicle from the cutaneous fat, followed by collagenase D to digest the dermal sheath and isolate the DP. The remaining dermal sheath fibroblasts could be removed by low-speed centrifugation of the DP [62]. Enzyme digestion is a simple method of HF stem cells isolation but with little control over the type of cells that are obtained leading to possible variations between different isolations.
5.5.3 Fluorescence-Activated Cell Sorting
FACS is a common method for isolating stem cells, especially from murine HF [15, 16, 20, 28, 30, 43, 44, 47]. Fluorescently labeled antibodies are used to tag the cell surface, and cells are sorted based on fluorescence intensity, which is proportional to the expression level of the particular target receptor. FACS can also be applied for isolating cells based on markers that are not expressed on the cell surface. Our group made use of the smooth muscle alpha-actin (αSMA) promoter-driven GFP to isolate a homogeneous population of smooth muscle cells (SMC) from ovine and human HF-MSC [33, 34]. FACS yields highly purified cell populations that can be further expanded or directly analyzed for mRNA or protein expression. Regrettably, lack of reliable stem cell markers hampers use of this method in sorting human HF stem cells.
5.6 Hair Follicle Stem Cells for Tissue Engineering and Cell Therapy
5.6.1 Tissue-Engineered Vascular Grafts
Cardiovascular disease is the leading cause of death in USA as being reported by American Heart Association. In 2006 heart diseases accounted for more than 600,000 deaths. Almost half of the deaths were caused by coronary heart diseases, and 400,000 surgical bypass operations were performed highlighting the importance of an artificial arterial substitute (www.americanheart.org). A functional arterial graft should contain both endothelial cells (ECs) and SMCs. ECs line the lumen of a vessel, endow it with thromboresistant properties, and are selectively permeable to substances circulating in the blood. SMCs form the medial layer of an artery and are mainly responsible for the dilatation and constriction of the vascular wall in response to vasoactive agonists.
Our laboratory showed that DS cells of ovine and human HF stained positive for αSMA, a marker of SMC (Fig. 5.2). This finding prompted us to hypothesize that functional SMC can be derived from HF. To this end, HF were transduced with a lentivirus encoding for GFP under the control of the αSMA promoter, and GFP+ cells were sorted out using flow cytometry. We found that both ovine and human HF-derived SMC exhibited significantly higher proliferation and clonogenic potential compared to vascular SMC. In addition, tissue-engineered vascular grafts prepared from HF-derived SMCs displayed high reactivity in response to vasoactive agonists and generated significant mechanical force as shown by compaction of fibrin hydrogels [3–5, 63]. More recent studies in our laboratory showed that these vascular grafts could be implanted into the arterial circulation of an ovine animal model where they remained patent for at least 3 months [64] (Row S. et al., 2013, manuscript in preparation), suggesting that the HF may be a readily accessible source of stem cells for cardiovascular tissue regeneration and cell therapies.
Cells comprising the dermal sheath of hair follicle are positive for αSMA. (a) H&E staining from neonatal ovine dermis. (b) Immunohistochemistry showing αSMA+ cells in the dermal sheath of hair follicles (Image taken from Peng et al. [63])
5.6.2 Tissue Engineering of Cartilage, Bone, and Fat
In addition to myogenic differentiation, rodent DP/DS cells have the capacity to differentiate into the osteogenic, chondrogenic, and adipogenic lineage, similar to bone marrow-derived MSCs [33, 34]. Extending these studies, we demonstrated that human HF cells also possess multilineage differentiation potential [4]. We also showed that single clones give rise to all four lineages, strongly indicating that human HF-MSC represent a true stem cell population and not a mixed population of progenitors with uni-lineage differentiation potential [5]. These results suggest that human HF can be an easily accessible source of true MSC that could be employed for regeneration of bone and cartilage for the replacement of joints or for meniscus repair.
5.6.3 Skin Regeneration
Several studies suggested that HF cells migrate to the epidermis during homeostasis and to a larger extent following skin injury [18, 19, 30, 35, 65], suggesting that HF cells could be used to generate the epidermis and enhance wound healing. Indeed, Hoeller and colleagues reported generation of bioengineered skin by introducing fibroblasts and HF tissue into the dermis. Interestingly, epidermal keratinocytes migrated out of the hair follicle and developed multiple layers of epidermis and stratum corneum [66]. In addition, HF-derived melanocytes have been used to develop a pigmented skin equivalent [67]. Most importantly, transplantation of tissue-engineered skin from HF-derived stem cells was shown to enhance healing of ulcers and burns significantly [68–70]. Notably, when hair buds were introduced into bioengineered skin before implantation, they sped up and guided nerve regeneration, suggesting that HF may recover the lost sense of touch [71].
5.6.4 Nerve Regeneration
Mouse HF-derived nestin+/K15− stem cells have the capacity to differentiate into neurons in vitro, suggesting a possible application to nerve regeneration in a variety of central and peripheral nervous system diseases [22]. Indeed, Amoh and colleagues transplanted mouse HF nestin + stem cells into a severed sciatic nerve or spinal cord, where they differentiated into Schwann cells and promoted nerve regeneration [53, 72]. The same group also reported that human HF stem cells have the capacity to restore the function of injured nerves [73, 74]. HF-derived neuronal and Schwann cells have also been introduced into acellular sciatic nerve conduit, where they exhibited long-term survival and significant electrophysiological properties in vitro but failed to induce repeated potentials [75].
5.6.5 Engineering Functional Hair Follicle
An important application of HF stem cells is bioengineering of HF to restore abnormal hair loss (alopecia). Common forms of alopecias include (a) the androgenetic alopecia which results from the miniaturization of the hair; (b) the alopecia areata, which results from an autoimmune response that damages the hair follicle; and (c) permanent alopecia which can be caused, for example, from severe trauma [1].
Bioengineering a HF has been a topic of intense scientific research over many years. To date two strategies have been developed to achieve this goal. The first approach includes the transplantation of intact HF from a HF-rich area into the bald area. This technique requires initially the surgical excision of a thin strip of scalp that contains dense HF and subsequently the isolation of the individual follicles and implantation back to the bald scalp [76]. Although transplantation of whole follicles is considered as the gold standard for hair restoration, studies demonstrated that segments of the HF can also induce hair growth after transplantation [57, 77–82]. Transplantation of a truncated human HF after amputating the bulb has shown hair renewal suggesting bulb reformation possibly from the DS compartment [77, 78, 82]. Interestingly, transplantation of intact DP and/or DS into murine models demonstrated mesenchymal interaction with the host epithelium and subsequent hair induction as shown with the transplantation of both murine [57, 79] and human dermal compartments [81]. However, in contrast to human DS when human DP was transplanted into human skin, it failed to induce hair regeneration [80].
In severe cases of alopecias, the number of available HF is not sufficient to restore the bald site. On the other hand, HF stem cells can be expanded in culture into large numbers that may be sufficient to cover the whole area and result in hair restoration. Jahoda and colleagues were the first to report that implantation of DP cells resulted in the hair growth in mice [55]. Although the hair-inductive properties of DP cells were lost after long-term expansion in vitro, coculture with keratinocytes or in keratinocyte-conditioned medium could maintain the inductive properties of DP cells for almost 70 passages [83]. Similar to DP, DS cells were also found to induce HF growth [84]. Finally, HF restoration was enhanced by the mixture of bulge/hair germ stem cells from adult HF with neonatal dermal cells [15, 16, 29, 30, 85]. Notably, when mixed with embryonic mouse dermal and epidermal cells, mouse bone marrow-derived cells differentiated into HF cells, suggesting hair-inductive properties of bone marrow cells [86]. Although the results with mouse models are very encouraging, the significance of these findings in large animal models or humans has yet to be demonstrated.
5.6.6 Drug Delivery Through the Hair Follicle
Skin is an easily accessible organ that has been widely considered as a unique target for drug delivery. In contrast to the conventional delivery methods (oral, injections), the transdermal route allows drug administration to the circulation through the dermal vasculature and may increase drug bioavailability while avoiding painful injections. However, the presence of stratum corneum, the outermost layer of the skin, severely limits the penetration of hydrophilic and high molecular weight substances [87]. To bypass this drawback, microscale devices have been developed to enable transdermal delivery including liquid jet injectors, microneedles, and thermal ablation devices [87].
Alternatively scientists have focused on drug administration via the follicular route. The HF disrupts the stratum corneum and provides an opening to the epidermis. In certain areas such as the scalp or the face, the total area of openings can reach up to 10 % of the skin area, contributing significantly to solute permeation [88–90]. In addition the dense network of blood vessels that are associated with the HF suggests that drug release to the circulation may be feasible [91]. The heterogeneity of the harboring cell population in the HF (stem cells, gland cells, immune cells, etc.) may enable cell-specific drug targeting for treatment of skin diseases or vaccination [92–94]. Last but not least, the relatively large volume of infundibulum renders the HF a reservoir for sustained drug release to the circulation, further highlighting the importance of follicular delivery [91].
Several studies highlighted the contribution of follicular penetration during drug delivery through the skin. Mitragori and colleagues modeled the permeability of hydrophilic and hydrophobic compounds in skin, assuming that the solutes can transport through one or more of the following mechanisms: free-volume diffusion, lateral diffusion of the lipids, diffusion through pores, or diffusion through shunts (hair follicles and glands). The model predicted that high molecular weight and highly hydrophilic molecules penetrate the skin preferentially through the shunts [95]. Others suggested that there is a critical value of octanol/water partition coefficient beyond which the flux through the follicle is greatly diminished [96]. However, most studies omit the significance of sebum (a lipophilic product of sebaceous gland) during drug delivery due to lack of representative experimental models. The presence of sebum in the HF and its upward flow may hinder the delivery of hydrophilic compounds and may favor the delivery of hydrophobic compounds. Indeed, apart from molecular weight and molecular orientation, diffusion through the sebum was found to be affected by compound lipophilicity [97].
To further improve tissue targeting and drug delivery via the HF, studies have incorporated particle-based formulations. Lademann et al. demonstrated that nanoparticle-containing dye could penetrate up to 1,400 μm into the follicle of porcine skin whereas the non-particle formulation reached only 500 μm. Interestingly, the nanoparticles prolonged the storage of the dye into the follicle [98]. Nanoparticle size was shown to play critical role in follicular penetration, which was optimal for particles between 750 and 1,500 nm and decreased for larger particles [99]. In addition to this, Vogt et al. demonstrated that the size of the particles affects its uptake by the cells. They reported that only the 40 nm size nanoparticles could enter Langerhans cells that are localized around the HF. This suggests that size-specific particle formulation can be engineered to target antigen-presenting cells via the follicular route and deliver vaccines [93].
Finally, systemic delivery of a chemical through the HF has also been examined in vivo [91, 100]. Caffeine was introduced into a shampoo formulation, and its delivery into the circulation via the skin was examined in human subjects. Interestingly, the follicular route not only accelerated the delivery, but it also prolonged detection of caffeine in the blood indicating that HF may act as reservoir of chemical compounds.
5.6.7 Cell and Gene Therapy Using Hair Follicle Stem Cells
The goal of gene therapy is to restore the lost tissue function by introducing the correct gene copy at the sites where the gene is missing or is mutated [101]. Application of gene therapy for hair restoration has been attempted and showed promising results. Transduction of rat bulge-derived hair follicle stem cells with LacZ-encoding retrovirus showed stable expression of the transgene in the HF epithelial compartments for at least 6 months after implantation of transduced cells in an immunodeficient mouse model [102]. Retroviral gene transfer of the streptomyces tyrosinase gene was used to treat albinism. Specifically, transduction of ex vivo cultured skin from albino mice restored melanin production from the skin HF [103]. Direct gene transfer into the skin in vivo has also been reported to restore hair growth. Intradermal administration of the Sonic Hedgehog gene into C57BL/6 mice using an adenovirus resulted into anagen induction and subsequently enhanced hair growth [104]. More recently, in vivo transfection of the human telomerase reverse transcriptase DNA complexed with polyethylenimine induced telogen to anagen transition in the rat dorsal skin [105]. In addition to the treatment of hair- or skin-related disorders, gene transfer to HF could be used for delivery of proteins into the systemic circulation through the vascular plexus surrounding the follicles. To this end, it may be feasible to engineer HF that produce insulin and reverse diabetes as we have previously shown with epidermal cells using a diabetic mouse model [106].
5.6.8 Reprogramming of Hair Follicle Stem Cells
In a breakthrough study in 2006, Yamanaka and colleagues demonstrated that introduction of four transcription factors (OCT4, SOX2, KLF4, and c-Myc) into mouse embryonic fibroblasts or adult fibroblasts endowed them with enhanced proliferation capacity and potential for differentiation into all three germ layers, similar to embryonic stem cells (ESC) [107–110]. The Thomson group demonstrated that two of the transcription factors (KLF4 and c-Myc) could be replaced by NANOG and LIN28 with similar outcome [111]. The resulting cells were designated as induced pluripotent cells (iPSCs). An explosion of studies that followed demonstrated that iPSC could be generated from many human cells including blood cells [112, 113], MSC [114], fetal [114] and neonatal fibroblasts [111, 114], adipose-derived stem cells [115], adult testis [116], β-pancreatic cells [117], and T lymphocytes [118]. Interestingly, HF-derived primary keratinocytes could be reprogrammed with 100-fold higher efficiency than fibroblasts [119]. HF-derived MSC were also reprogrammed and used to understand the feedback loops that sustain self-renewal using global genomic and proteomic strategies [120]. DP cells were shown to reprogram using only two factors (Oct4, Klf4) [121], possibly suggesting the presence of endogenous factors that facilitated reprogramming. Reprogramming with fewer transcription factors or higher efficiency suggests that HF cell-derived iPSC may be useful for regenerative medicine applications as well as for development of models to study the genetics and pathophysiology of human disease.
5.7 Conclusions: Future Directions
In summary, HF stem cells have great potential for tissue engineering and regenerative medicine applications. The ease of accessibility along with the broad differentiation capacity of HF stem cells makes the HF an ideal stem cell source. However, human HF stem cells remain relatively unexplored as compared to their mouse counterparts or other human adult stem cells. As a result more studies are required to address a number of challenges that hinder application of these cells in regenerative medicine. To this end, identification of reliable HF stem cell markers is urgently needed to facilitate HF stem cell isolation. More studies are also needed to evaluate the differentiation potential of human HF stem cells and establish culture conditions for efficient differentiation. The ease of reprogramming should be further explored to identify potential small molecules that may induce reprogramming even in the absence of genetic modification [122]. Finally, more studies are necessary to establish the HF as a site for drug and gene/protein delivery, for treatment of skin diseases and wound healing, or to the blood circulation for treatment of systemic disorders.
References
Paus R, Cotsarelis G (1999) The biology of hair follicles. N Engl J Med 341:491–497
Schneider MR, Schmidt-Ullrich R, Paus R (2009) The hair follicle as a dynamic miniorgan. Curr Biol 19:R132–R142
Liu JY, Peng HF, Andreadis ST (2008) Contractile smooth muscle cells derived from hair-follicle stem cells. Cardiovasc Res 79:24–33
Liu JY, Peng HF, Gopinath S, Tian J, Andreadis ST (2010) Derivation of functional smooth muscle cells from multipotent human hair follicle mesenchymal stem cells. Tissue Eng Part A 16:2553–2564
Bajpai VK, Mistriotis P, Andreadis ST (2012) Clonal multipotency and effect of long-term in vitro expansion on differentiation potential of human hair follicle derived mesenchymal stem cells. Stem Cell Res 8:74–84
Goessler UR, Riedel K, Hormann K, Riedel F (2006) Perspectives of gene therapy in stem cell tissue engineering. Cells Tissues Organs 183:169–179
Pittenger MF, Mackay AM, Beck SC et al (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Morrison SJ, Kimble J (2006) Asymmetric and symmetric stem-cell divisions in development and cancer. Nature 441:1068–1074
Jaks V, Kasper M, Toftgard R (2010) The hair follicle-a stem cell zoo. Exp Cell Res 316:1422–1428
Kligman AM (1959) The human hair cycle. J Invest Dermatol 33:307–316
Oliver RF (1966) Whisker growth after removal of the dermal papilla and lengths of follicle in the hooded rat. J Embryol Exp Morphol 15:331–347
Cotsarelis G, Sun TT, Lavker RM (1990) Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 61:1329–1337
Morris RJ, Potten CS (1999) Highly persistent label-retaining cells in the hair follicles of mice and their fate following induction of anagen. J Invest Dermatol 112:470–475
Tumbar T, Guasch G, Greco V et al (2004) Defining the epithelial stem cell niche in skin. Science 303:359–363
Morris RJ, Liu Y, Marles L et al (2004) Capturing and profiling adult hair follicle stem cells. Nat Biotechnol 22:411–417
Jaks V, Barker N, Kasper M et al (2008) Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet 40:1291–1299
Mobini N, Tam S, Kamino H (2005) Possible role of the bulge region in the pathogenesis of inflammatory scarring alopecia: lichen planopilaris as the prototype. J Cutan Pathol 32:675–679
Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM (2000) Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell 102:451–461
Ito M, Liu Y, Yang Z et al (2005) Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med 11:1351–1354
Li L, Mignone J, Yang M et al (2003) Nestin expression in hair follicle sheath progenitor cells. Proc Natl Acad Sci USA 100:9958–9961
Amoh Y, Li L, Yang M et al (2004) Nascent blood vessels in the skin arise from nestin-expressing hair-follicle cells. Proc Natl Acad Sci USA 101:13291–13295
Amoh Y, Li L, Katsuoka K, Penman S, Hoffman RM (2005) Multipotent nestin-positive, keratin-negative hair-follicle bulge stem cells can form neurons. Proc Natl Acad Sci USA 102:5530–5534
Yu H, Fang D, Kumar SM et al (2006) Isolation of a novel population of multipotent adult stem cells from human hair follicles. Am J Pathol 168:1879–1888
Yu H, Kumar SM, Kossenkov AV, Showe L, Xu X (2010) Stem cells with neural crest characteristics derived from the bulge region of cultured human hair follicles. J Invest Dermatol 130:1227–1236
Ito M, Kizawa K, Hamada K, Cotsarelis G (2004) Hair follicle stem cells in the lower bulge form the secondary germ, a biochemically distinct but functionally equivalent progenitor cell population, at the termination of catagen. Differentiation 72:548–557
Greco V, Chen T, Rendl M et al (2009) A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 4:155–169
Nijhof JG, Braun KM, Giangreco A et al (2006) The cell-surface marker MTS24 identifies a novel population of follicular keratinocytes with characteristics of progenitor cells. Development 133:3027–3037
Jensen UB, Yan X, Triel C, Woo SH, Christensen R, Owens DM (2008) A distinct population of clonogenic and multipotent murine follicular keratinocytes residing in the upper isthmus. J Cell Sci 121:609–617
Jensen KB, Collins CA, Nascimento E et al (2009) Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell 4:427–439
Snippert HJ, Haegebarth A, Kasper M et al (2010) Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science 327:1385–1389
Horsley V, O’Carroll D, Tooze R et al (2006) Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell 126:597–609
Lako M, Armstrong L, Cairns PM, Harris S, Hole N, Jahoda CA (2002) Hair follicle dermal cells repopulate the mouse haematopoietic system. J Cell Sci 115:3967–3974
Jahoda CA, Whitehouse J, Reynolds AJ, Hole N (2003) Hair follicle dermal cells differentiate into adipogenic and osteogenic lineages. Exp Dermatol 12:849–859
Hoogduijn MJ, Gorjup E, Genever PG (2006) Comparative characterization of hair follicle dermal stem cells and bone marrow mesenchymal stem cells. Stem Cells Dev 15:49–60
Biernaskie J, Paris M, Morozova O et al (2009) SKPs derive from hair follicle precursors and exhibit properties of adult dermal stem cells. Cell Stem Cell 5:610–623
Trempus CS, Morris RJ, Bortner CD et al (2003) Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J Invest Dermatol 120:501–511
Merrill BJ, Gat U, DasGupta R, Fuchs E (2001) Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev 15:1688–1705
Nguyen H, Rendl M, Fuchs E (2006) Tcf3 governs stem cell features and represses cell fate determination in skin. Cell 127:171–183
Horsley V, Aliprantis AO, Polak L, Glimcher LH, Fuchs E (2008) NFATc1 balances quiescence and proliferation of skin stem cells. Cell 132:299–310
Rhee H, Polak L, Fuchs E (2006) Lhx2 maintains stem cell character in hair follicles. Science 312:1946–1949
Vidal VP, Chaboissier MC, Lutzkendorf S et al (2005) Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr Biol 15:1340–1351
Jensen KB, Watt FM (2006) Single-cell expression profiling of human epidermal stem and transit-amplifying cells: Lrig1 is a regulator of stem cell quiescence. Proc Natl Acad Sci USA 103:11958–11963
Driskell RR, Giangreco A, Jensen KB, Mulder KW, Watt FM (2009) Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development 136:2815–2823
Kishimoto J, Ehama R, Wu L, Jiang S, Jiang N, Burgeson RE (1999) Selective activation of the versican promoter by epithelial-mesenchymal interactions during hair follicle development. Proc Natl Acad Sci USA 96:7336–7341
Iida M, Ihara S, Matsuzaki T (2007) Hair cycle-dependent changes of alkaline phosphatase activity in the mesenchyme and epithelium in mouse vibrissal follicles. Dev Growth Differ 49:185–195
Yu DW, Yang T, Sonoda T et al (1995) Message of nexin 1, a serine protease inhibitor, is accumulated in the follicular papilla during anagen of the hair cycle. J Cell Sci 108(Pt 12):3867–3874
Ito Y, Hamazaki TS, Ohnuma K, Tamaki K, Asashima M, Okochi H (2007) Isolation of murine hair-inducing cells using the cell surface marker prominin-1/CD133. J Invest Dermatol 127:1052–1060
Kloepper JE, Tiede S, Brinckmann J et al (2008) Immunophenotyping of the human bulge region: the quest to define useful in situ markers for human epithelial hair follicle stem cells and their niche. Exp Dermatol 17:592–609
Ohyama M, Terunuma A, Tock CL et al (2006) Characterization and isolation of stem cell-enriched human hair follicle bulge cells. J Clin Invest 116:249–260
Inoue K, Aoi N, Sato T et al (2009) Differential expression of stem-cell-associated markers in human hair follicle epithelial cells. Lab Invest 89:844–856
Nowak JA, Polak L, Pasolli HA, Fuchs E (2008) Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell 3:33–43
Tornqvist G, Sandberg A, Hagglund AC, Carlsson L (2010) Cyclic expression of lhx2 regulates hair formation. PLoS Genet 6:e1000904
Amoh Y, Li L, Campillo R et al (2005) Implanted hair follicle stem cells form Schwann cells that support repair of severed peripheral nerves. Proc Natl Acad Sci USA 102:17734–17738
Gur G, Rubin C, Katz M et al (2004) LRIG1 restricts growth factor signaling by enhancing receptor ubiquitylation and degradation. EMBO J 23:3270–3281
Jahoda CA, Horne KA, Oliver RF (1984) Induction of hair growth by implantation of cultured dermal papilla cells. Nature 311:560–562
Rendl M, Lewis L, Fuchs E (2005) Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol 3:e331
Cohen J (1961) The transplantation of individual rat and guineapig whisker papillae. J Embryol Exp Morphol 9:117–127
Messenger AG (1984) The culture of dermal papilla cells from human hair follicles. Br J Dermatol 110:685–689
Kobayashi K, Rochat A, Barrandon Y (1993) Segregation of keratinocyte colony-forming cells in the bulge of the rat vibrissa. Proc Natl Acad Sci USA 90:7391–7395
Magerl M, Kauser S, Paus R, Tobin DJ (2002) Simple and rapid method to isolate and culture follicular papillae from human scalp hair follicles. Exp Dermatol 11:381–385
Edwards RA (2007) Laser capture microdissection of mammalian tissue. J Vis Exp (8):309
Wu JJ, Liu RQ, Lu YG, Zhu TY, Cheng B, Men X (2005) Enzyme digestion to isolate and culture human scalp dermal papilla cells: a more efficient method. Arch Dermatol Res 297:60–67
Peng HF, Liu JY, Andreadis ST, Swartz DD (2011) Hair follicle-derived smooth muscle cells and small intestinal submucosa for engineering mechanically robust and vasoreactive vascular media. Tissue Eng Part A 17:981–990
Peng H, Schlaich EM, Row S, Andreadis ST, Swartz DD (2012) A novel ovine ex vivo arteriovenous shunt model to test vascular implantability. Cells Tissues Organs 195:108–121
Levy V, Lindon C, Zheng Y, Harfe BD, Morgan BA (2007) Epidermal stem cells arise from the hair follicle after wounding. FASEB J 21:1358–1366
Hoeller D, Huppertz B, Roos TC et al (2001) An improved and rapid method to construct skin equivalents from human hair follicles and fibroblasts. Exp Dermatol 10:264–271
Liu F, Luo XS, Shen HY, Dong JS, Yang J (2011) Using human hair follicle-derived keratinocytes and melanocytes for constructing pigmented tissue-engineered skin. Skin Res Technol. [Epub ahead of print]; doi: 10.1111/j.1600-0846.2011.00510.x
Limat A, Mauri D, Hunziker T (1996) Successful treatment of chronic leg ulcers with epidermal equivalents generated from cultured autologous outer root sheath cells. J Invest Dermatol 107:128–135
Limat A, Hunziker T (2002) Use of epidermal equivalents generated from follicular outer root sheath cells in vitro and for autologous grafting of chronic wounds. Cells Tissues Organs 172:79–85
Navsaria HA, Ojeh NO, Moiemen N, Griffiths MA, Frame JD (2004) Reepithelialization of a full-thickness burn from stem cells of hair follicles micrografted into a tissue-engineered dermal template (Integra). Plast Reconstr Surg 113:978–981
Gagnon V, Larouche D, Parenteau-Bareil R, Gingras M, Germain L, Berthod F (2011) Hair follicles guide nerve migration in vitro and in vivo in tissue-engineered skin. J Invest Dermatol 131:1375–1378
Amoh Y, Li L, Katsuoka K, Hoffman RM (2008) Multipotent hair follicle stem cells promote repair of spinal cord injury and recovery of walking function. Cell Cycle 7:1865–1869
Amoh Y, Kanoh M, Niiyama S et al (2009) Human hair follicle pluripotent stem (hfPS) cells promote regeneration of peripheral-nerve injury: an advantageous alternative to ES and iPS cells. J Cell Biochem 107:1016–1020
Amoh Y, Aki R, Hamada Y et al (2012) Nestin-positive hair follicle pluripotent stem cells can promote regeneration of impinged peripheral nerve injury. J Dermatol 39:33–38
Lin H, Liu F, Zhang C et al (2011) Characterization of nerve conduits seeded with neurons and Schwann cells derived from hair follicle neural crest stem cells. Tissue Eng Part A 17:1691–1698
Shiell RC (2001) Modern hair restoration surgery. Clin Dermatol 19:179–187
Kim JC, Choi YC (1995) Regrowth of grafted human scalp hair after removal of the bulb. Dermatol Surg 21:312–313
Jahoda CA, Oliver RF, Reynolds AJ, Forrester JC, Horne KA (1996) Human hair follicle regeneration following amputation and grafting into the nude mouse. J Invest Dermatol 107:804–807
Matsuzaki T, Inamatsu M, Yoshizato K (1996) The upper dermal sheath has a potential to regenerate the hair in the rat follicular epidermis. Differentiation 60:287–297
Reynolds AJ, Lawrence C, Cserhalmi-Friedman PB, Christiano AM, Jahoda CA (1999) Trans-gender induction of hair follicles. Nature 402:33–34
Jahoda CA, Oliver RF, Reynolds AJ et al (2001) Trans-species hair growth induction by human hair follicle dermal papillae. Exp Dermatol 10:229–237
Tang L, Madani S, Lui H, Shapiro J (2002) Regeneration of a new hair follicle from the upper half of a human hair follicle in a nude mouse. J Invest Dermatol 119:983–984
Inamatsu M, Matsuzaki T, Iwanari H, Yoshizato K (1998) Establishment of rat dermal papilla cell lines that sustain the potency to induce hair follicles from afollicular skin. J Invest Dermatol 111:767–775
McElwee KJ, Kissling S, Wenzel E, Huth A, Hoffmann R (2003) Cultured peribulbar dermal sheath cells can induce hair follicle development and contribute to the dermal sheath and dermal papilla. J Invest Dermatol 121:1267–1275
Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E (2004) Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 118:635–648
Kataoka K, Medina RJ, Kageyama T et al (2003) Participation of adult mouse bone marrow cells in reconstitution of skin. Am J Pathol 163:1227–1231
Arora A, Prausnitz MR, Mitragotri S (2008) Micro-scale devices for transdermal drug delivery. Int J Pharm 364:227–236
Otberg N, Richter H, Schaefer H, Blume-Peytavi U, Sterry W, Lademann J (2004) Variations of hair follicle size and distribution in different body sites. J Invest Dermatol 122:14–19
Knorr F, Lademann J, Patzelt A, Sterry W, Blume-Peytavi U, Vogt A (2009) Follicular transport route – research progress and future perspectives. Eur J Pharm Biopharm 71:173–180
Wosicka H, Cal K (2010) Targeting to the hair follicles: current status and potential. J Dermatol Sci 57:83–89
Otberg N, Teichmann A, Rasuljev U, Sinkgraven R, Sterry W, Lademann J (2007) Follicular penetration of topically applied caffeine via a shampoo formulation. Skin Pharmacol Physiol 20:195–198
Rolland A, Wagner N, Chatelus A, Shroot B, Schaefer H (1993) Site-specific drug delivery to pilosebaceous structures using polymeric microspheres. Pharm Res 10:1738–1744
Vogt A, Combadiere B, Hadam S et al (2006) 40 nm, but not 750 or 1,500 nm, nanoparticles enter epidermal CD1a+ cells after transcutaneous application on human skin. J Invest Dermatol 126:1316–1322
Vogt A, Mahe B, Costagliola D et al (2008) Transcutaneous anti-influenza vaccination promotes both CD4 and CD8 T cell immune responses in humans. J Immunol 180:1482–1489
Mitragotri S (2003) Modeling skin permeability to hydrophilic and hydrophobic solutes based on four permeation pathways. J Control Release 86:69–92
Frum Y, Bonner MC, Eccleston GM, Meidan VM (2007) The influence of drug partition coefficient on follicular penetration: in vitro human skin studies. Eur J Pharm Sci 30:280–287
Valiveti S, Lu GW (2007) Diffusion properties of model compounds in artificial sebum. Int J Pharm 345:88–94
Lademann J, Richter H, Teichmann A et al (2007) Nanoparticles – an efficient carrier for drug delivery into the hair follicles. Eur J Pharm Biopharm 66:159–164
Toll R, Jacobi U, Richter H, Lademann J, Schaefer H, Blume-Peytavi U (2004) Penetration profile of microspheres in follicular targeting of terminal hair follicles. J Invest Dermatol 123:168–176
Liu X, Grice JE, Lademann J et al (2011) Hair follicles contribute significantly to penetration through human skin only at times soon after application as a solvent deposited solid in man. Br J Clin Pharmacol 72:768–774
Cotsarelis G, Millar SE (2001) Towards a molecular understanding of hair loss and its treatment. Trends Mol Med 7:293–301
Sugiyama-Nakagiri Y, Akiyama M, Shimizu H (2006) Hair follicle stem cell-targeted gene transfer and reconstitution system. Gene Ther 13:732–737
Zhao M, Saito N, Li L et al (2000) A novel approach to gene therapy of albino hair in histoculture with a retroviral streptomyces tyrosinase gene. Pigment Cell Res 13:345–351
Sato N, Leopold PL, Crystal RG (1999) Induction of the hair growth phase in postnatal mice by localized transient expression of Sonic hedgehog. J Clin Invest 104:855–864
Jan HM, Wei MF, Peng CL, Lin SJ, Lai PS, Shieh MJ (2012) The use of polyethylenimine-DNA to topically deliver hTERT to promote hair growth. Gene Ther 19:86–93
Tian J, Lei P, Laychock SG, Andreadis ST (2008) Regulated insulin delivery from human epidermal cells reverses hyperglycemia. Mol Ther 16:1146–1153
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676
Takahashi K, Tanabe K, Ohnuki M et al (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872
Okita K, Ichisaka T, Yamanaka S (2007) Generation of germline-competent induced pluripotent stem cells. Nature 448:313–317
Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S (2008) Generation of mouse induced pluripotent stem cells without viral vectors. Science 322:949–953
Yu J, Vodyanik MA, Smuga-Otto K et al (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318:1917–1920
Ye Z, Zhan H, Mali P et al (2009) Human-induced pluripotent stem cells from blood cells of healthy donors and patients with acquired blood disorders. Blood 114:5473–5480
Loh YH, Agarwal S, Park IH et al (2009) Generation of induced pluripotent stem cells from human blood. Blood 113:5476–5479
Park IH, Zhao R, West JA et al (2008) Reprogramming of human somatic cells to pluripotency with defined factors. Nature 451:141–146
Sun N, Panetta NJ, Gupta DM et al (2009) Feeder-free derivation of induced pluripotent stem cells from adult human adipose stem cells. Proc Natl Acad Sci USA 106:15720–15725
Conrad S, Renninger M, Hennenlotter J et al (2008) Generation of pluripotent stem cells from adult human testis. Nature 456:344–349
Stadtfeld M, Brennand K, Hochedlinger K (2008) Reprogramming of pancreatic beta cells into induced pluripotent stem cells. Curr Biol 18:890–894
Brown ME, Rondon E, Rajesh D et al (2010) Derivation of induced pluripotent stem cells from human peripheral blood T lymphocytes. PLoS One 5:e11373
Aasen T, Raya A, Barrero MJ et al (2008) Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol 26:1276–1284
Lu Y, Loh YH, Li H et al (2012) A self-sustaining feedback loop that regulates proteome diversity and supports self-renewal in pluripotent stem cells. In Revision
Tsai SY, Clavel C, Kim S et al (2010) Oct4 and klf4 reprogram dermal papilla cells into induced pluripotent stem cells. Stem Cells 28:221–228
Zhu S, Wei W, Ding S (2011) Chemical strategies for stem cell biology and regenerative medicine. Annu Rev Biomed Eng 13:73–90
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01 HL086582) and the New York State Stem Cell Science (NYSTEM Contract #C024316) to S.T. Andreadis. The authors would also like to thank Anna Mistriotis for her help with the schematic representation of the hair follicle.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Mistriotis, P., Andreadis, S.T. (2013). Hair Follicle: A Novel Source of Stem Cells for Cell and Gene Therapy. In: Danquah, M., Mahato, R. (eds) Emerging Trends in Cell and Gene Therapy. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-62703-417-3_5
Download citation
DOI: https://doi.org/10.1007/978-1-62703-417-3_5
Published:
Publisher Name: Humana Press, Totowa, NJ
Print ISBN: 978-1-62703-416-6
Online ISBN: 978-1-62703-417-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)