Abstract
The male gonadal axis function is strongly affected by physical exercise. Relatively short, intense exercise usually increases while more prolonged exercise usually decreases serum testosterone levels. The exercise-associated increment in circulating testosterone is considered not to be mediated by LH whereas a neuroendocrine dysfunction has been indicated as the cause of testosterone reduction in response to prolonged exercise, along with increased cortisol levels, a primary testicular dysfunction, or even an accumulation of metabolic waste materials. Chronic physical exercise may induce a state of oligospermia, a reduction of the total number of motile sperm and an increase in abnormal or immature spermatozoa. Alterations in the hormonal milieu but also the oxidative stress associated with endurance exercise may be involved.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Androgens exert strong anabolic effects on skeletal muscle protein synthesis (1, 2), satellite cell number (3), and skeletal muscle growth (4, 5). Because these changes are of great importance to muscle strength, androgens have been recognized as important hormones that influence sports performance (6). Exercise-induced changes in testosterone concentrations can moderate or support neuromuscular performance through various short-term mechanisms (e.g. second messengers, lipid/protein pathways, neuronal activity, behaviour, cognition, motor-system function, muscle properties, and energy metabolism) (7).
On the other hand, the gonadal axis function is strongly affected by physical exercise depending on the intensity and duration of the activity, the fitness level, and the nutritional-metabolic status of the individual (8, 9). Moreover, circulating testosterone and its bioavailable fractions are affected by weight and age. They are also changed by different kinds of stress which may appear as physical stress (i.e. endurance training, sleep deprivation in extreme sports, changes of air pressure in altitude training) or mental stress in relation to sport events and training (9).
In this chapter, the effects of physical exercise on testicular steroidogenesis and on spermatogenesis will be revised.
Physiology of the Male Gonadal Axis
The male gonadal axis consists of the testes and the hypothalamus–pituitary unit that controls their function. The testes posses a dual function, i.e. the production of androgens and of sperm.
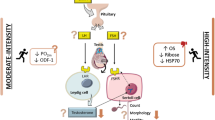
Figure 1 depicts an outline of the male gonadal axis and of the hormonal regulation of the testicular function.
Schematic diagram of the male gonadal axis. CRH corticotropin releasing hormone; DA dopamine; DHT dihydrotestosterone; FSH follicle-stimulating hormone; GABA gamma-aminobutyric acid; GnRH gonadotropin-releasing hormone; IL-1 interleukin-1; LH luteinizing hormone; NE norepinephrine; NPY neuropeptide Y; PRL prolactin; ROS reactive oxygen species.
The pituitary gland is the central structure controlling gonadal function: it releases the gonadotropins luteinizing hormone (LH) and follicle-stimulating hormone (FSH) and is regulated by the hypothalamic gonadotropin-releasing hormone (GnRH), which is secreted in a pulsatile fashion with peaks every 90–120 min. In man, the major hormone controlling GnRH secretion is testosterone, which inhibits gonadotropin secretion via negative feedback both at the hypothalamic and pituitary level. Dihydrotestosterone (DHT) and estradiol also modulate gonadotropin secretion acting at the hypothalamic and/or pituitary level (10, 11). In addition, several neurotransmitters and neuromodulators might influence GnRH secretion: the noradrenergic system and neuropeptide Y (NPY) show stimulatory activity, whereas interleukin-1, opioid peptides, dopamine, serotonine, and gamma-aminobutyric acid (GABA) are inhibitory. Leptin, which is produced by the fat cells, has been shown to stimulate GnRH and gonadotropin secretions (11). Ghrelin, a peptide hormone with growth hormone-releasing action, exerts multiple endocrine and non-endocrine effects including inhibition of the gonadal axis at both the central and peripheral level (12, 13). Furthermore, the adverse effect of stress on reproductive function is well known. Several factors are involved: corticotropin-releasing hormone (CRH) inhibits GnRH secretion, prolactin further reduces the GnRH pulse rate (10), and cortisol inhibits both the hypothalamus–pituitary and gonadal functions.
LH and FSH are produced and secreted by the gonadotropic cells of the anterior pituitary. LH regulates testicular androgenesis whereas FSH, together with locally produced testosterone, is responsible for spermatogenesis. LH binds to specific receptors on the surface of Leydig cells in the testis and regulates the biosynthesis of testosterone. FSH binds to receptors on the Sertoli cells and promotes spermatogenesis: in addition to a number of other proteins, the hormones inhibin B and activins are formed in the Sertoli cells under the influence of FSH. Inhibin B plays an important role in the feedback regulation of FSH secretion, whereas the physiological role of activins has not been conclusively clarified (10).
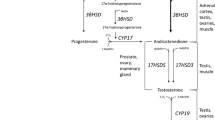
Testosterone is the most important steroid produced by the testis and is responsible for the development and maintenance of male sex characteristics as well as a number of other anabolic and metabolic effects (e.g. muscle and bone metabolism). Normal testosterone concentrations in adult males range between 12 and 30 nmol/L: testosterone concentrations in blood follow a circadian rhythm with higher levels in the morning hours and about 25% lower levels in the evening (11).
Effects of Physical Exercise on Testicular Steroidogenesis
Short, Intense Exercise Increases Circulating Testosterone
The effects of physical activity on the male gonadal axis vary with the intensity and duration of the activity, the fitness level of the individual, and his nutritional-metabolic status. Relatively short, intense exercise usually increases while more prolonged exercise usually decreases serum testosterone levels (8, 9, 14). Increased serum testosterone levels have been reported during relatively strenuous free and treadmill running, weight training, rock climbing, and ergometer cycling (15–17). Short-term sprints can be seen as strength outburst and are comparable to strength training rather than endurance training: sprint exercise increased plasma testosterone concentrations in adolescent boys (18).
The testosterone response increases with increased exercise load (19). Similar workloads produce similar responses, regardless of whether the load is aerobic or anaerobic (20).
Immediate and 5 min post-exercise measurements showed an increase in testosterone levels both in men and women (21). Acute exercise-induced testosterone increments are also seen in older men (22). This acute hormone response was confirmed and described to be markedly stronger in young men compared to old in a study involving ten men with mean age 26.5 years and ten men with mean age 70.0 years (23).
As muscle mass increases with strength training (4) and is correlated with testosterone levels, it could be expected that the testosterone response to acute exercise is higher in persons constantly involved in strength training. Consistently, a 6-month sprint training programme increased plasma testosterone concentrations in response to sprint exercise in adolescent boys (18). Experienced weight lifters compared to beginners showed similar basal levels of testosterone but were able to evoke a stronger testosterone response during exercise (15). Contrary to these findings, a long-term training period of 12 weeks involving younger (mean 23 years) and older men (mean 63 years) showed no significant changes concerning testosterone levels before or immediately after exercise (24).
Ronnestad et al. (25) have recently investigated the effects of testosterone and growth hormone (GH) transient increase during exercise, indicating that performing leg exercises prior to arm exercises, thereby increasing the levels of testosterone and GH, induced superior strength training adaptations compared to arm training without acute elevation of hormones. It has been found that acute elevation in endogenous testosterone (by strength training) potentiates the androgen receptor (AR) response to a strength training session compared to no acute elevation of endogenous testosterone (26). It may thus be speculated that the results by Ronnestad et al. are due to an increased AR expression and, through an improved testosterone-receptor interaction, an increased protein synthesis, leading to superior strength training adaptations. This hypothesis has also been evaluated by Ahtiainen et al. (27), who have described a correlation of individual pre- to post-training changes in resting AR protein concentration with the changes in cross-sectional area of muscle fibres in a combined group of young and elderly subjects who performed heavy resistance exercise bouts before and after a training period. Overall, these findings suggested that the individual changes of AR protein concentration in skeletal muscle following resistance training may have an impact on training-induced muscular adaptations.
Mechanisms Underlying Increases in Circulating Testosterone Following Short, Intense Exercise
No conclusive evidence about gonadotropin response to an acute exercise bout is available. In fact, LH and FSH levels have been reported to be increased, decreased, or unchanged by short-term strenuous exercise (28–31).
The exercise-associated increment in circulating testosterone is considered not to be mediated by LH, due to the inconsistent LH response and to the evidence that testosterone levels increase more quickly than LH in response to exercise. Possible mechanisms such as hemoconcentration, reduced clearance and/or increased testosterone synthesis may be involved (29, 31–33). However, the timing of testosterone response differs from that of other circulating steroids (e.g. androstenedione and dehydroepiandrosterone increase simultaneously with cortisol) thus suggesting that specific testicular mechanisms are involved (31). These mechanisms may include the activation of the sympathetic system, which stimulates testicular testosterone production during exercise via a direct neural pathway in some species (34). Catecholamine levels also increase significantly during exercise. Beta-adrenergic blockade inhibits testosterone responses to exercise, whereas l-dopa, phentolamine, and clonidine had no effect (35). An anticipatory elevation in resting testosterone levels has also been described pre-exercise and seems to be independent of hepatic perfusion or hemoconcentration (28, 31). Ultimately, the exact mechanisms involved in increasing testosterone concentrations in specific exercise protocols are yet to be defined.
Prolonged, Submaximal Exercise and Chronic Exercise Training Decrease Circulating Testosterone
In contrast to the short-term testosterone increment during and immediately after short, intense exercise, a suppression of serum testosterone levels occurs during and subsequent to prolonged exercise, in the hours following intense exercise, as well as during chronic exercise training.
During the last decades, an increasing number of investigative research studies have pointed to how chronic exposure to endurance exercise training can result in the development of a dysfunction within the reproductive components of the neuroendocrine system. The majority of these studies have concentrated upon women. However, the effects of endurance exercise training on the male reproductive neuroendocrine system have been investigated beginning in the 1980s (36). Most studies observed athletes during training and competition, giving the impression of generally lowered androgen levels, but lack the comparison with a control group (9).
A controlled study examining the effects of endurance training on the hypothalamus–pituitary–testis axis in males involved 53 men undergoing endurance training for at least 5 years and a control group of 35 age-matched, sedentary men. Baseline serum testosterone levels of the exercising men were significantly lower than in the control group. Differences in gonadotropins were not seen. Normal regulation would require LH levels to rise with falling testosterone levels, as these have a positive feedback on pituitary gonadotropin release. A suppression in the regulatory axis could explain this finding (37).
Contrary to these observations, basal testosterone levels in trained weight lifters were not altered, nor did an increase in the daily training volume change these levels (38). Similarly, basal testosterone, free testosterone, bioavailable testosterone, and sex hormone-binding globulin concentrations were not significantly different in high top-class athletes (sprinters and jumpers) vs. untrained subjects (17).
Endurance training can be seen as a factor of exposure not only to physical but also to psychological stress. It has been demonstrated in a controlled study that the reactivity patterns of mental/psychological and physical stress response of the hypothalamus–pituitary–adrenal axis are the same in a specific individual. Differential reactivity is rather seen between the so-called high and low responders. Each group has a specific endocrine reactivity pattern concerning the hypothalamus–pituitary–adrenal axis (39). It seems that the decrease of testosterone levels under the stressful situations of endurance sport is not sufficiently answered by the pituitary. There is no adequate rise in LH levels, which seem to be unaltered or even show a tendency to decrease with the growing amount of stress impact. Nevertheless, age-dependent effects seem to exist in this regard, and the ratio of androgen to estradiol is shifted by physical activity to a more favourable pattern (higher androgen and lower estradiol levels) in older men compared to younger men performing regular mild physical activity (40).
The “Exercise-Hypogonadal Male”: Clinical Issues
It has recently been demonstrated that among subjects engaged in chronic exercise training, a selected group of men develop alterations in their reproductive hormonal profile, i.e. persistently low basal resting testosterone concentrations (41). In particular, the majority of these men exhibit clinically “normal” testosterone concentrations, but these concentrations are at the low end of normal range or even reach subclinical status.
The health consequences of such hormonal changes are increased risk of abnormal spermatogenesis, male infertility problems, and compromised bone mineralisation (41–43). The prevalence of such health problems seems low, but investigative studies examining this condition and its consequences are few in number (41, 42). The specific terminology used to refer to this condition has not been universally agreed upon. In 2005, Hackney and associates proposed the use of “the Exercise-Hypogonadal Male” as a label for this condition (44).
The “Exercise-Hypogonadal Male”: Pathophysiological Mechanisms
Exercise-hypogonadal men frequently display a lack of significant elevation in basal LH in correspondence with the reduced testosterone concentration, reflecting hypogonadotropic-hypogonadism characteristics (36, 41, 45). These LH abnormalities may involve disparities in luteinizing pulsatility (i.e. pulse frequency and amplitude), although evidence for altered LH pulsatile release is conflicting (46, 47). Moreover, gonadotropin response to GnRH has been reported both reduced and increased following prolonged, exhaustive exercise (48, 49).
Exercise-hypogonadal men have been shown to have altered basal prolactin (41). At either excessively low or high circulating levels, PRL can result in suppression of testosterone levels in men (50). It has been speculated that the absence of prolactin at the testicle alters the effectiveness of LH to stimulate testosterone production. This theory is based upon the proposed synergistic effects of prolactin upon testicular LH receptors (36). However, not all investigators reporting low resting testosterone in endurance-trained men have reported the concomitant existence of low resting prolactin levels (50). Some investigations have looked at a potential relationship between high prolactin levels and low testosterone, speculating that any “stressful” situation might provoke disproportionate prolactin responses in exercise-hypogonadal men, and this ultimately promotes a reproductive axis disruption (51).
Leptin is an adipocyte-released hormone associated in part with communicating to the hypothalamus satiety and energy reserves status (52). It is also linked to reproductive function both in women and in men. Acute and chronic exercise can impact upon resting leptin concentrations, independent of changes in body adiposity (53). However, to date no research studies have examined whether leptin concentrations are altered in exercise-hypogonadal men.
Ghrelin is another hormone associated with appetite regulation. Newly emerging experimental evidence in animals and in humans suggests that ghrelin may function as a metabolic modulator of the gonadal axis, with predominant inhibitory effects in line with its role as signal of energy deficit (12, 13). Acute and chronic exercise has been shown to influence ghrelin concentration levels (54). However, no research has yet examined whether ghrelin levels in exercise-hypogonadal men are normal.
Other research investigations have focused on alterations in testicular ability to produce and secrete testosterone and to respond to exogenous stimuli (i.e. LH or hCG). Whereas animal studies have demonstrated that exercise training compromises testicular enzymatic activity (55), data in exercise-hypogonadal men are contradictory. In fact, some investigations suggest testicular steroidogenesis is normal, while some indicate it is marginally impaired when challenged with exogenous stimuli (41).
Another potential disruptive hormone to the gonadal axis is cortisol. Studies in a wide range of sports (e.g. cycling, marathon running, football, handball, rugby, tennis, swimming, and wrestling) have almost all shown increased cortisol concentrations during exercise (56, 57). Cortisol secretion increases in response to exercise intensity and duration, as well as to the training level of subjects (58–61), at least in part to mobilize energy stores. An inhibitory effect of the hypothalamus–pituitary–adrenal axis on the reproductive system has been demonstrated in both sexes (62, 63). In fact, glucocorticoids suppress gonadal axis function at the hypothalamic–pituitary level (62). Moreover, Inder et al. (64) have demonstrated that dexamethasone administration in humans reduces circulating testosterone and downregulates the muscular expression of the AR. Finally, CRH and its receptors have been identified in the Leydig cells of the testis, where CRH exerts inhibitory actions on testosterone biosynthesis (65).
Interestingly, a sport event and also training for such represent both a physical and a mental stress (9). The release of cortisol by activation of the hypothalamic–pituitary–adrenal axis as reaction to mental stress is well documented (39, 66). Stress responses by the hypothalamic–pituitary–gonadal axis are constantly found as well.
Along this line, anticipatory stress was measured in 50 males before a one-day experimental stress event (participation in stressful clinical research protocol). Cortisol levels rose significantly, while both testosterone and LH secretion were decreased (67). Psychological stress markers as measured by scales for anxiety, hostility, and depression were correlated with serum levels of testosterone in a group of males aged 30–55 years. Those classified as highly stressed had significantly lower testosterone levels than their counterparts (68). A cross-sectional study involving 439 males all aged 51 years showed those with low levels of testosterone (adjusted for body mass index) to exhibit a cluster of psychosocial stress indicators (69). Nevertheless, other hormonal profile studies reporting the existence of low testosterone in trained men did not show elevated resting cortisol levels (36, 70, 71). However, resting cortisol levels do not necessarily reflect a hyperactivity of the hypothalamus–pituitary–adrenal axis, which can be better defined either by serial blood or salivary sampling (72) or by assay of urinary free cortisol.
Thus, at this time the role of cortisol to the changes found in the gonadal axis of trained men is in need of further study.
Effects of Physical Exercise on Spermatogenesis
Clinical expression of impaired reproductive function in men engaged in chronic exercise training seems uncommon (42, 47, 73). However, chronic physical exercise may induce a state of oligospermia, a reduction of the total number of motile sperm and an increase in abnormal or immature spermatozoa.
Vaamonde et al. (74) have analysed the semen profiles of three male populations with different types and levels of physical activity (physically active non-professional subjects, water polo players, and triathletes) and found that sperm concentration, velocity, and morphology were significantly different among the practitioners of the three different training modalities. The differences were more marked as intensity and volume of exercise increased, especially for morphology which was the parameter showing the greatest difference (74).
Safarinejad et al. (49) performed a longitudinal study on the effects of intensive, long-term treadmill running on reproductive hormones and semen quality. A total of 286 subjects were randomly assigned to moderate-intensity exercise (∼60% VO2 max) and high-intensity exercise (∼80% VO2 max) groups. The two groups exercised for 60 weeks in five sessions per week. This was followed by a 36-week low-intensity exercise recovery period. After 24 weeks of exercise, the subjects exercising with high intensity demonstrated significantly declined semen parameters (sperm density, motility, and morphology) compared with those exercising with moderate intensity. At 36 and 48 weeks, these differences were more significant. A significant correlation was found between high-intensity exercise, its duration, and sperm count, as well as mean sperm motility and sperm morphology. Serum testosterone and free testosterone began to decrease, and serum SHBG began to increase at the end of 12 weeks with both moderate- and high-intensity exercises. Both semen and hormone parameters improved to their pre-exercise level during the recovery period (49).
In a recent study, Wise et al. (75) have examined the association between regular physical activity and semen quality in a large cohort of 2,261 men attending an infertility clinic. They found that none of the semen parameters (semen volume, sperm concentration, sperm motility, sperm morphology, and total motile sperm) were materially associated with regular exercise. However, in the subgroup of men who reported bicycling as their primary form of exercise, bicycling at levels of >5 h/week was associated with low sperm concentration and total motile sperm. These findings generally agree with earlier studies that have shown deleterious effects of bicycling on semen parameters among competitive cyclists (73, 76). It remains unclear as to whether the changes associated with bicycling are due to mechanical trauma (i.e. caused by compression of scrotum on the bicycle saddle), to a prolonged increase in core scrotal temperature (i.e. related to exercise itself or wearing of constrictive clothing), or some other factors (77).
Oxidative Stress as a Putative Mechanism Underlying Impaired Spermatogenesis in Exercise-Hypogonadal Men
Several mechanisms have been reported to affect the male reproductive function in exercising subjects. Alterations in the hormonal milieu, as discussed in the previous paragraph, may well play a role, since qualitatively and quantitatively normal spermatogenesis is critically dependent on an intact hypothalamus–pituitary–testis axis. On the other hand, it has been reported that endurance exercise is associated with oxidative stress (78). During endurance exercise, there is a 10- to 20-fold increase in whole-body oxygen (O2) consumption, and O2 uptake in the active skeletal muscle increases 100- to 200-fold (79). This increase in O2 utilization may result in the production of reactive oxygen species (ROS) at the rates that exceed the body’s capacity to detoxify them (80). An increase in the formation of ROS decreases fertility, as the ROS will attack the membranes of the spermatozoa, decreasing their viability (81). However, some studies have suggested that exercise training enhances antioxidant capacity (82, 83). Indeed, the machinery eliminating ROS adapts after regular exercise and actually lowers the amount of ROS that is produced, especially in the major organs (muscles) of oxygen consumption and ROS production. Exercise training tends to decrease ROS also in body fluids, although no data concerning seminal fluid seem to be available.
Regardless of the exercise protocol studied, increases in DNA damage in peripheral human white cells have been reported, generating the consensus that exercise does indeed induce DNA damage (84). After an exercise bout, DNA damage persists for up to 7 days (85). The presence of high ROS levels has been reported in the semen of between 25 and 40% of infertile men (86). This is because ROS, at high levels, are potentially toxic to sperm quality and function (87). Therefore, persistent ROS formation during continuous strenuous exercise might be harmful for normal spermatogenesis. However, the participation of other maybe unknown factors affecting sperm quality seems plausible (49).
References
Urban RJ, Bodenburg YH, Gilkison C, et al. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am J Physiol. 1995;269:E820–6.
Ferrando AA, Sheffield-Moore M, Yeckel CW, et al. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab. 2002;282:E601–7.
Sinha-Hikim I, Roth SM, Lee MI, Bhasin S. Testosterone-induced muscle hypertrophy is associated with an increase in satellite cell number in healthy, young men. Am J Physiol Endocrinol Metab. 2003;285:E197–205.
Bhasin S, Storer TW, Berman N, et al. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;335:1–7.
Sinha-Hikim I, Artaza J, Woodhouse L, et al. Testosterone-induced increase in muscle size in healthy young men is associated with muscle fiber hypertrophy. Am J Physiol Endocrinol Metab. 2002;283:E154–64.
Viru A, Viru M. Preconditioning of the performance in power events by endogenous testosterone: in memory of professor Carmelo Bosco. J Strength Cond Res. 2005;19:6–8.
Crewther BT, Cook C, Cardinale M, Weatherby RP, Lowe T. Two emerging concepts for elite athletes: the short-term effects of testosterone and cortisol on the neuromuscular system and the dose-response training role of these endogenous hormones. Sports Med. 2011;41:103–23.
Vingren JL, Kraemer WJ, Ratamess NA, Anderson JM, Volek JS, Maresh CM. Testosterone physiology in resistance exercise and training: the up-stream regulatory elements. Sports Med. 2010;40:1037–53.
Zitzmann M. Exercise, training, and the hypothalamic-pituitary-gonadal axis in men. In: Ghigo E, Lanfranco F, Strasburger CJ, editors. Hormone use and abuse by Athletes, vol. 29. New York, USA: Springer; 2011. p. 25–30. doi:10.1007/978-1-4419-7014-5.
Jockenhoevel F, Schubert M. Anatomy and physiology of the testis. In: Jockenhoevel F, Schubert M, editors. Male hypogonadism. Bremen: UNI-MED Verlag; 2007. p. 12–30.
Weinbauer GF, Luetjens CM, Simoni M, Nieschlag E. Physiology of testicular function. In: Nieschlag E, Behre H, Nieschlag S, editors. Andrology: male reproductive health and dysfunction. 3rd ed. Heidelberg: Springer; 2009. p. 11–60.
Lanfranco F, Bonelli L, Baldi M, et al. Acylated ghrelin inhibits spontaneous LH pulsatility and responsiveness to naloxone, but not that to GnRH in young men: evidence for a central inhibitory action of ghrelin on the gonadal axis. J Clin Endocrinol Metab. 2008;93:3633–9.
Tena-Sempere M. Ghrelin and reproduction: ghrelin as novel regulator of the gonadotropic axis. Vitam Horm. 2008;77:285–300.
Cumming DC, Wheeler GD, McColl EM. The effects of exercise on reproductive function in men. Sports Med. 1989;7:1–17.
Kraemer RR, Kilgore JL, Kraemer GR, Castracane VD. Growth hormone, IGF-I, and testosterone responses to resistive exercise. Med Sci Sports Exerc. 1992;24:1346–52.
Sherk VD, Sherk KA, Kim S, Young KC, Bemben DA. Hormone responses to a continuous bout of rock climbing in men. Eur J Appl Physiol. 2011;111:687–93.
Grandys M, Majerczak J, Zapart-Bukowska J, Kulpa J, Zoladz JA. Gonadal hormone status in highly trained sprinters and in untrained men. J Strength Cond Res. 2011;25:1079–84.
Derbré F, Vincent S, Maitel B, et al. Androgen responses to sprint exercise in young men. Int J Sports Med. 2010;31:291–7.
Gotshalk LA, Loebel CC, Nindl BC, et al. Hormonal responses of multiset versus single-set heavy-resistance exercise protocols. Can J Appl Physiol. 1997;22:244–55.
Hackney AC, Premo MC, McMurray RG. Influence of aerobic versus anaerobic exercise on the relationship between reproductive hormones in men. J Sports Sci. 1995;13:305–11.
Kraemer WJ, Staron RS, Hagerman FC, et al. The effects of short-term resistance training on endocrine function in men and women. Eur J Appl Physiol. 1998;78:69–76.
Häkkinen K, Pakarinen A. Acute hormonal responses to heavy resistance exercise in men and women at different ages. Int J Sports Med. 1995;16:507–13.
Häkkinen K, Pakarinen A, Newton RU, Kraemer WJ. Acute hormone responses to heavy resistance lower and upper extremity exercise in young versus old men. Eur J Appl Physiol. 1998;77:312–9.
Craig BW, Brown R, Everhart J. Effects of progressive resistance training on growth hormone and testosterone levels in young and elderly subjects. Mech Ageing Dev. 1989;49:159–69.
Ronnestad BR, Nygaard H, Raastad T. Physiological elevation of endogenous hormones results in superior strength training adaptation. Eur J Appl Physiol. 2011;111:2249–59.
Spiering BA, Kraemer WJ, Vingren JL, et al. Elevated endogenous testosterone concentrations potentiate muscle androgen receptor responses to resistance exercise. J Steroid Biochem Mol Biol. 2009;114:195–9.
Ahtiainen JP, Hulmi JJ, Kraemer WJ, et al. Heavy resistance exercise training and skeletal muscle androgen receptor expression in younger and older men. Steroids. 2011;76:183–92.
Wilkerson JE, Horvath SM, Gutin B. Plasma testosterone during treadmill exercise. J Appl Physiol. 1980;49:249–53.
Metivier G, Gauthier R, de la Cevrotriere J, Grymala D. The effect of acute exercise on the serum levels of testosterone and luteinizing (LH) hormone in human male athletes. J Sports Med Phys Fit. 1980;20:235–7.
Schmid P, Pusch PP, Wolf WW, et al. Serum FSH, LH and testosterone in humans after physical exercise. Int J Sports Med. 1982;3:84–9.
Cumming DC, Brunsting III LA, Strich G, et al. Reproductive hormone increases in response to acute exercise in men. Med Sci Sports Exerc. 1986;18:369–73.
Sutton JR, Coleman MJ, Casey J, Lazarus L. Androgen responses during physical exercise. Br Med J. 1973;1:520–2.
Cadoux-Hudson TA, Few JD, Imms FJ. The effect of exercise on the production and clearance of testosterone in well trained young men. Eur J Appl Physiol Occup Physiol. 1985;54:321–5.
Levin J, Lloyd CW, Lobotsky J, Friedrich EH. The effect of epinephrine on testosterone production. Acta Endocrinol. 1967;55:184–92.
Jezová D, Vigas M. Testosterone response to exercise during blockade and stimulation of adrenergic receptors in man. Horm Res. 1981;15:141–7.
Wheeler GD, Wall SR, Belcastro AN, Cumming DC. Reduced serum testosterone and prolactin levels in male distance runners. JAMA. 1984;27:514–6.
Hackney AC, Fahrner CL, Gulledge TP. Basal reproductive hormonal profiles are altered in endurance trained men. J Sports Med Phys Fitness. 1998;38:138–41.
Fry AC, Kraemer WJ, Ramsey LT. Pituitary-adrenal-gonadal responses to high-intensity resistance exercise overtraining. J Appl Physiol. 1998;85:2352–9.
Singh A, Petrides JS, Gold PW, Chrousos GP, Deuster PA. Differential hypothalamic-pituitary-adrenal axis reactivity to psychological and physical stress. J Clin Endocrinol Metab. 1999;84:1944–8.
Slowinska-Lisowska M, Jozkow P, Medras M. Associations between physical activity and the androgenic/estrogenic status of men. Physiol Res. 2010;59:757–63.
Hackney AC. Effects of endurance exercise on the reproductive system of men: the “exercise-hypogonadal male condition”. J Endocrinol Invest. 2008;31:932–8.
Arce JC, DeSouza MJ. Exercise and male factor infertility. Sports Med. 1993;15:146–69.
Bennell KL, Brukner PD, Malcolm SA. Effect of altered reproductive function and lowered testosterone levels on bone density in male endurance athletes. Br J Sports Med. 1996;30:205–8.
Hackney AC, Moore AW, Brownlee KK. Testosterone and endurance exercise: development of the “exercise-hypogonadal male condition”. Acta Physiol Hung. 2005;92:121–37.
MacConnie S, Barkan A, Lampman RM, et al. Decreased hypothalamic gonadotropin-releasing hormone secretion in male marathon runners. N Engl J Med. 1986;315:411–7.
McColl EM, Wheeler GD, Gomes P, et al. The effects of acute exercise on pulsatile LH release in high-mileage male runners. Clin Endocrinol. 1989;31:617–21.
Di Luigi L, Guidetti L, Baldari C, Fabbri A, Moretti C, Romanelli F. Physical stress and qualitative gonadotropin secretion: LH biological activity at rest and after exercise in trained and untrained men. Int J Sports Med. 2002;23:307–12.
Kujala UM, Alen M, Huhtaniemi IT. Gonadotrophin-releasing hormone and human chorionic gonadotrophin tests reveal that both hypothalamic and testicular endocrine functions are suppressed during acute prolonged physical exercise. Clin Endocrinol. 1990;33:219–25.
Safarinejad MR, Azma K, Kolahi AA. The effects of intensive, long-term treadmill running on reproductive hormones, hypothalamus–pituitary–testis axis, and semen quality: a randomized controlled study. J Endocrinol. 2009;200:259–71.
Hackney AC. The male reproductive system and endurance exercise. Med Sci Sports Exerc. 1996;28:180–9.
Hackney AC, Sharp RL, Runyan WS, Ness RJ. Relationship of resting prolactin and testosterone in males during intensive training. Br J Sports Med. 1989;23:194.
Blueher S, Mantzoros CS. Leptin in reproduction. Curr Opin Endocrinol Diabetes Obes. 2007;14:458–64.
Baylor LS, Hackney AC. Resting thyroid and leptin hormone changes in women following intense, prolonged exercise training. Eur J Appl Physiol. 2003;88:480–4.
Jürimäe J, Cicchella A, Jürimäe T, et al. Regular physical activity influences plasma ghrelin concentration in adolescent girls. Med Sci Sports Exerc. 2007;39:1736–41.
Hu Y, Asano K, Kim S, et al. Relationship between serum testosterone and activities of testicular enzymes after continuous and intermittent training in male rats. Int J Sports Med. 2004;25:99–102.
Deuster PA, Chrousos GP, Luger A, et al. Hormonal and metabolic responses of untrained, moderately trained, and highly trained men to three exercise intensities. Metabolism. 1989;38:141–8.
Le Panse B, Vibarel-Rebot N, Parage G, et al. Cortisol, DHEA, and testosterone concentrations in saliva in response to an international powerlifting competition. Stress. 2010;13:528–32.
Snegovskaya V, Viru A. Elevation of cortisol and growth hormone levels in the course of further improvement of performance capacity in trained rowers. Int J Sports Med. 1993;14:202–6.
Snegovskaya V, Viru A. Steroid and pituitary hormone responses to rowing: relative significance of exercise intensity and duration and performance level. Eur J Appl Physiol Occup Physiol. 1993;64:59–65.
Passelergue P, Robert A, Lac G. Salivary cortisol and testosterone variations during an official and a simulated weightlifting competition. Int J Sports Med. 1995;16:298–303.
Minetto MA, Lanfranco F, Baldi M, et al. Corticotroph axis sensitivity after exercise: comparison between elite athletes and sedentary subjects. J Endocrinol Invest. 2007;30:215–23.
Sakakura N, Takebe K, Nakagawa S. Inhibition of luteinizing hormone secretion induced by synthetic LRH by long-term treatment with glucocorticoids in human subjects. J Clin Endocrinol Metab. 1975;40:774–9.
Chrousos GP, Torpy DJ, Gold PW. Interactions between the hypothalamic–pituitary–adrenal axis and the female reproductive system: clinical implications. Ann Intern Med. 1998;129:229–40.
Inder WJ, Jang C, Obeyesekere VR, Alford FP. Dexamethasone administration inhibits skeletal muscle expression of the androgen receptor and IGF-1—implications for steroid-induced myopathy. Clin Endocrinol. 2010;73:126–332.
Dufau ML, Tinajero JC, Fabbri A. Corticotropin-releasing factor: an antireproductive hormone of the testis. FASEB J. 1993;7:299–307.
Osterberg K, Karlson B, Hansen AM. Cognitive performance in patients with burnout, in relation to diurnal salivary cortisol. Stress. 2009;12:70–81.
Schulz P, Walker JP, Peyrin L, Soulier V, Curtin F, Steimer T. Lower sex hormones in men during anticipatory stress. Neuroreport. 1996;25:3101–4.
Francis KT. The relationship between high and low trait psychological stress, serum testosterone, and serum cortisol. Experientia. 1981;37:1296–7.
Nilsson PM, Moller L, Solstad K. Adverse effects of psychosocial stress on gonadal function and insulin levels in middle-aged males. J Intern Med. 1995;237:479–86.
Hackney AC, Sinning WE, Bruot BC. Hypothalamic-pituitary-testicular axis function in endurance-trained males. Int J Sports Med. 1990;11:298–303.
Wheeler GD, Singh M, Pierce WD, et al. Endurance training decreases serum testosterone levels in men without change in luteinizing hormone pulsation release. J Clin Endocrinol Metab. 1991;72:422–5.
Minetto MA, Lanfranco F, Tibaudi A, Baldi M, Termine A, Ghigo E. Changes in awakening cortisol response and midnight salivary cortisol are sensitive markers of strenuous training-induced fatigue. J Endocrinol Invest. 2008;31:16–24.
Lucía A, Chicharro JL, Pérez M, Serratosa L, Bandrés F, Legido JC. Reproductive function in male endurance athletes: sperm analysis and hormonal profile. J Appl Physiol. 1996;81:2627–36.
Vaamonde D, Da Silva-Grigoletto ME, Garcia-Manso JM, Vaamonde-Lemos R, Swanson RJ, Oehninger SC. Response of semen parameters to three training modalities. Fertil Steril. 2009;92:1941–6.
Wise LA, Cramer DW, Hornstein MD, Ashby RK, Missmer SA. Physical activity and semen quality among men attending an infertility clinic. Fertil Steril. 2011;95:1025–30.
Gebreegziabher Y, Marcos E, McKinon W, Rogers G. Sperm characteristics of endurance trained cyclists. Int J Sports Med. 2004;25:247–51.
Leibovitch I, Mor Y. The vicious cycling: bicycling related urogenital disorders. Eur Urol. 2005;47:277–86.
Mastaloudis A, Leonard SW, Traber MG. Oxidative stress in athletes during extreme endurance exercise. Free Radic Biol Med. 2001;31:911–22.
Astrand PO, Rodahl K. Circulation. In: van Dalen DB, editor. Textbook of work physiology: physiological basis of exercise. New York: McGraw Hill Book Company; 1986. p. 170–5.
Alessio HM. Exercise-induced oxidative stress. Med Sci Sports Exerc. 1993;25:218–24.
Irvine DS. Glutathione as a treatment for male infertility. Rev Reprod. 1996;1:6–12.
Child RB, Wilkinson DM, Fallowfield JL, Donnelly AE. Elevated serum antioxidant capacity and plasma malondialdehyde concentration in response to a simulated half marathon run. Med Sci Sports Exerc. 1998;30:1603–7.
Clarkson PM, Thompson HS. Antioxidants: what role do they play in physical activity and health? Am J Clin Nutr. 2000;72:637S–46.
Hartmann A, Niess A. Oxidative DNA damage in exercise. In: Sen C, Packer L, Hanninen O, editors. Handbook of oxidants and antioxidants in exercise. Amsterdam: Elsevier; 2000. p. 195–217.
Tsai K, Hsu TG, Hsu KM, et al. Oxidative DNA damage in human peripheral leukocytes induced by massive aerobic exercise. Free Radic Biol Med. 2001;31:1465–72.
Padron OF, Brackett NL, Sharma RK, Lynne CM, Thomas Jr AJ, Agarwal A. Seminal reactive oxygen species, sperm motility and morphology in men with spinal cord injury. Fertil Steril. 1997;67:1115–20.
Saleh R, Agarwal A. Oxidative stress and male infertility: from research bench to clinical practice. J Androl. 2002;23:737–52.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Lanfranco, F., Minetto, M.A. (2013). The Male Reproductive System, Exercise, and Training: Endocrine Adaptations. In: Constantini, N., Hackney, A. (eds) Endocrinology of Physical Activity and Sport. Contemporary Endocrinology. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-62703-314-5_7
Download citation
DOI: https://doi.org/10.1007/978-1-62703-314-5_7
Published:
Publisher Name: Humana Press, Totowa, NJ
Print ISBN: 978-1-62703-313-8
Online ISBN: 978-1-62703-314-5
eBook Packages: MedicineMedicine (R0)