Abstract
Lung disease remains a significant health and economic burden to societies throughout the world and is projected to increase in prevalence. Recent medical advances have enhanced clinical care but further research is needed in the areas of inhibition of lung fibrosis, altering airway inflammation and manipulating the pulmonary vascular endothelium. Stem cells have the potential to address these deficiencies by their remarkable ability to differentiate into various tissue lines and regulate internal repair systems. These unique regenerative abilities provide a novel approach to management for those suffering from pulmonary disease.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Introduction
While therapies based on embryonic or induced pluripotent stem cell delivery to treat disease are many years away, adult stem cell treatment is much closer to the clinic. Among adult stem cells, mesenchymal stem cells (MSC) hold particular promise for the treatment of lung diseases. In this chapter we will review the potential clinical applications of MSC for lung disease by highlighting relevant preclinical and early human studies before touching on the implications of the discovery of a lung-resident population of MSC.
A putative pulmonary niche for lung-resident mesenchymal stromal cells. Lung-resident mesenchymal stromal cells (MSC, red fluorescent PKH-26 staining) reside in the alveolar region in close proximity to alveolar type 2 cells (green fluorescent cytokeratin staining) either in the corners of the alveoli (arrow) or attached to the alveolar septa (solid arrow). Blue is nuclear DAPI staining. Like bone marrow-derived MSC, lung-resident cells are multipotent and immunomodulatory [41]. Reprinted with permission from the American Thoracic Society. Copyright of American Thoracic Society
5.1.1 The Burden of Pulmonary Disease
Respiratory disorders are a leading cause of death worldwide with further increases in mortality expected in the future. In Western countries, respiratory disease ranks second only to cardiovascular disease in terms of mortality, incidence, prevalence and socioeconomic cost. The aetiology of lung disease can be generally summarised into environmental, occupational, genetic, lifestyle and other causes. Nonetheless, for a significant number of disorders no aetiology is discernable and these are termed idiopathic. The societal burden is substantial and future management strategies need to focus on prevention and effective therapies introduced early in the disease process. In terms of non-malignant lung disease, the most pressing avenues for research are centred on altering airway inflammation, inhibiting lung fibrosis, manipulating the pulmonary vascular endothelium and developing novel therapeutic options for patients with hereditary and congenital disease processes.
5.1.2 Pulmonary Disease: Where Is the Clinical Need?
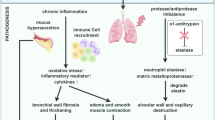
Chronic obstructive pulmonary disease (COPD) is a common respiratory disease characterised by irreversible airways obstruction culminating in progressive decline in lung function. Currently, COPD is the fifth leading cause of death worldwide with estimates of prevalence in the USA of 5% and 6.3% in Asia [1, 2]. Data from the UK General Practice Research Database estimate about 883,200 patients in the UK have a diagnosis of COPD. In England, in 2002/2003, COPD was recorded as the reason for hospital admission in 109,243 cases and accounted for 1,094,922 bed-days, with a median duration of stay of 6 days [3]. Mortality from COPD is expected to continue to increase in the coming decades while deaths from heart and cerebrovascular disease are expected to decrease. Reducing the burden of COPD requires better evaluation and diagnosis but also improved management strategies aimed at preserving lung function. Pathophysiologically COPD is a multicomponent disease with inflammation central to its pathogenesis along with parenchymal destruction and airway remodelling. Emphysema is almost invariably induced by cigarette smoking, and it is widely accepted that the disease is caused by excessive proteolytic enzyme activity by proteases and a chronic inflammatory process characterised by a cellular influx consisting of macrophages, neutrophils and T lymphocytes.
Medical therapy with inhalers and pulmonary rehabilitation has no significant impact on lung function trajectory in patients with COPD. Lung volume reduction either through novel bronchoscopic techniques or the conventional surgical approach targets only those patients with upper lobe dominant emphysema disease—at most just 25% of patients. Lung transplantation is an option for a select group of patients, but lack of donor organ availability, advancing age of potential recipients and the high economic cost of transplantation limit more widespread application. A theoretical protective effect of MSC transplantation on pulmonary emphysema may be partly mediated by modulation of T cell function and inhibiting the apoptosis of lung cells by influencing the vascular endothelial growth factor signalling pathway [4].
Bronchial asthma is the most common chronic respiratory illness typified by reversible airflow obstruction and with a varied prevalence in Western countries of 10–12% [5]. Unlike COPD, asthma is an important cause of morbidity and mortality in children. Whilst mortality has fallen dramatically in the last 20 years, asthma rates have increased continuously during recent decades. There are several ways to estimate the burden of disease with one approach being the disability adjusted life year (DALY) score as adopted by the World Health Organization. This disability score describes the number of years of healthy life lost due to disability or premature death. In 2003, asthma was the eleventh-leading contributor to the overall burden of disease in Australia, accounting for 2.4% of the total number of DALYs. In that year, 63,100 years of healthy life were lost due to asthma—59,054 of these due to years lived with disability and 4,045 due to premature death. Morbidity from asthma remains substantial despite medical advances with preventative therapy over the years.
Idiopathic pulmonary fibrosis (IPF) is a relatively common chronic, fibrosing lung disease of unknown cause that is characterised by severe, refractory and progressive breathlessness. The course of disease is relentless with an average survival of 3.6 years from diagnosis and age at onset 61 years. Epidemiological studies suggest an annual incidence of 16.3 cases per 100,000 population and prevalence of 42.7 cases per 100,000 [6]. Although respiratory failure is the most common cause of death, other modes include congestive cardiac failure, lung infection, pulmonary embolism and bronchogenic carcinoma. No recommended or approved therapy exists currently for IPF with the exception of anti-inflammatory and anti-fibrotic agent pirfenidone which is licensed in Europe and Japan for those with mild to moderate disease. However, the Food and Drug Administration in the USA is demanding a further phase 3 trial of pirfenidone given inconsistencies in some previous studies. Central to the pathogenesis of IPF is abnormal epithelial repair and epithelial-mesenchymal transition. Endothelin receptor antagonists (ERA) are another potential treatment line although bosentan, a dual ERA, had no influence on disease progression in a large multicentre randomised controlled trial. MSC have theoretical benefits in the IPF patient in switching injured epithelia down the pathway of repair rather than fibrosis.
Acute lung injury (severe variant known as Adult Respiratory Distress Syndrome or ARDS) is defined as acute onset of severe hypoxia and pulmonary infiltrates within 12–72 h of a precipitating event. Sepsis is the leading cause, followed by pneumonia, aspiration of gastric contents, massive blood transfusion, multiple trauma and other tissue injury. ARDS is a significant issue for intensive care units with an estimated 18 to 34 cases per 100,000 population each year [7]. In published clinical trials, prolonged corticosteroid treatment at an initial dose of 1 mg/kg/day of methylprednisolone [8] significantly improves patient centred outcome variables. Nonetheless, despite recent improvements in critical care, the mortality rate still remains at about 50%. With the pathogenesis of acute lung injury/ARDS involving lung endothelial injury, alveolar epithelial injury and the accumulation of a protein rich cellular debris in the alveolar space, one possible candidate for therapy is the MSC.
Idiopathic pulmonary arterial hypertension (PAH) is a syndrome characterised by a progressive increase in pulmonary vascular resistance leading to right ventricular overload and eventually to right ventricular failure and premature death. The annual incidence of PAH is estimated at 7.1 cases per million population and prevalence at 52 cases per million population [9]. The increase in pulmonary vascular resistance is related to a number of progressive changes in the pulmonary arterioles, including vasoconstriction, obstruction through proliferation of smooth muscle, fibroblasts and endothelial cells, inflammation and in situ thrombosis. The main histological features include medial hypertrophy, intimal thickening and plexiform lesions. The plexifom lesion represents a focal proliferation of endothelial and smooth muscle cells and is pathognomonic of PAH. Medical therapies for PAH centre on selective pulmonary vascular bed vasodilatation, anticoagulation and long-term antifibrotic and remodelling agents. Despite these advances, the condition remains invariably progressive with markedly reduced life expectancy. Future directions of therapy may focus on the delivery of MSC to alter endothelial-mesenchymal transition and directly promote vascular remodelling.
Two noteworthy congenital and genetic respiratory conditions respectively are neonatal bronchopulmonary dysplasia (BPD) and cystic fibrosis. BPD is a chronic lung disease that develops in infants born prematurely, particularly if they require treatment with oxygen and positive pressure ventilation. It has a complex pathogenesis incorporating contributions of hyperoxia, hypoxia, shear stress from mechanical ventilation, vascular maldevelopment, inflammation, malnutrition and genetics. The clinical picture of BPD has evolved with advances in medical care including surfactant replacement, antibiotic management and protective modes of mechanical ventilation. A significant number of infants with BPD are now surviving to adulthood, manifesting with a range of chronic lung diseases including emphysema [10]. Prevention of alveolar growth arrest with cell-based therapies remains an attractive and durable long-term therapeutic goal. Finally, cystic fibrosis is the most common autosomal recessive inherited condition with an incidence of approximately 1 in 2,400 births. The condition is typically caused by mutations in the gene coding for a protein called the cystic fibrosis transmembrane conductance regulator (CFTR). The defect in CFTR results in poor sodium-chloride ion flow regulation across cell membranes and the accumulation of thick tenacious mucus in the lung and digestive tract. Cystic fibrosis is potentially a model disease for stem cell treatment as the continued lung inflammation and infection may promote engraftment of circulating progenitor cells, corrected for the chloride channel defect [11].
5.2 The Lung: An Attractive Target for MSC Therapy
Due to their immunosuppressive properties, capacity to remodel extracellular matrix and perhaps also their ability to differentiate into lung epithelia, MSC have been proposed as a potential cellular therapeutic agent for lung diseases. One of the difficulties with MSC therapy for other organs may also be an inherent advantage when pulmonary biotherapy is considered. One of the major barriers to non-pulmonary cellular therapy is the pulmonary first pass effect. Following intravenous infusion, due to the filtering function of the pulmonary vasculature, only a small proportion of cells pass through into the systemic circulation. This effect is particularly pertinent to MSC-based therapy due to the large physical size of the mesenchymal stromal cells. The first pass effect has impeded the development of regenerative therapy approaches such as MSC therapy for heart disease [12] and has led to the development of strategies to deliver MSC directly to the affected organ. For instance, in the case of the heart this has involved using direct intracoronary and myocardial stem cell injection, but local stem cell delivery strategies increase the potential risks and side effects of therapy (e.g., bleeding and tissue injury following direct tissue injection or occlusion and embolisation following direct arterial administration). Therefore, the ability to deliver cellular therapy to the lung via a simple intravenous approach is a major advantage and gives the potential for large-scale engraftment. Even more attractively, engrafting cells appear to target areas of injured lung [13, 14]. Direct intra-tracheal, intrapulmonary [15] and intrapleural [16] inoculation represent additional relatively non-invasive routes of administration which are specific to the lung.
Another advantage of MSC therapy is the ability to potentially transplant cells across the human leukocyte antigen (HLA) barrier. While it is clear that MSC have reduced immunogenicity when compared with many cell types [17] since they express only low levels of class I HLA, and no class II HLA or co-stimulatory molecules [18, 19], there is a substantial body of data which questions the degree of immunologic privilege awarded MSC [20]. For instance, MSC are immunogenic in that they can induce memory T-cell responses [21] and, as MSC express the activating NK cell-receptor ligands NKG2D and UL16 [22], they are susceptible to lysis by NK cells [23]. The practical implication is that preclinical work in HLA-matched and/or immunosuppressed animals needs to be cautiously interpreted in the planning of human phase I studies which are likely to involve HLA mismatching.
Despite this caveat, MSC represent an attractive and novel therapeutic agent for inflammatory and fibrotic lung diseases where the clinical need for treatment advances is strong. Although MSC are multipotent and are able to differentiate down lung epithelial lineages [24, 25], it is unlikely that the degree of parenchymal cell engraftment required to achieve a therapeutic effect will ever be achieved. It is much more likely that a therapeutic role for MSC will be created by exploiting their ability to remodel extracellular matrix [26, 27], or their ability to suppress the immune response through contact-dependent and soluble mediators [28–30].
The demonstrated immunosuppressive ability of MSC has translated to clinical trials currently being undertaken in graft-versus-host disease (GVHD) following allogeneic haematopoietic stem cell (HSC) transplantation, Crohn’s disease, multiple sclerosis, lupus, COPD, insulin-dependent diabetes mellitus and in the renal transplant setting. The tissue repair capability of MSC is being investigated in clinical trials for cardiac repair, bone disorders (osteogenesis imperfecta), bone fracture, meniscectomy and liver repair (cirrhosis), as well as for enhancing engraftment after HSC transplantation. Studies have also been carried out using MSC to treat various metabolic disorders, ischaemic stoke and neurological disorders. We will review the preclinical studies which have identified a potential niche for MSC therapy in the treatment of human lung disease, the current early phase human trials and finally the possible role of lung-resident MSC in the pathogenesis of lung disease.
5.2.1 Preclinical Studies: Acute Lung Injury
As outlined above, acute lung injury is a common complication in patients admitted to the intensive care unit, and still carries a substantial risk of mortality and residual morbidity despite decades of research. Unfortunately, care remains largely supportive. Due to their immunomodulatory effects, effects on epithelial repair and potential to reduce alveolar oedema, MSC have been considered as a potential treatment option [31]. Preclinical studies largely employing the endotoxin-induced model of acute lung injury have been encouraging [15, 32]. The therapeutic effect of MSC in this setting appears to be mediated largely by paracrine rather than contact-dependent effects, perhaps through the secretion of the keratinocyte growth factor (KGF) [32].
5.2.2 Preclinical Studies: Idiopathic Pulmonary Fibrosis
As highlighted above, IPF is a relatively common chronic, fibrosing lung disease of unknown cause. IPF affects older individuals (typically older than 50) and is characterised by severe, refractory and progressive breathlessness. On clinical examination, patients usually have fine bibasal crackles on auscultation of the chest and digital clubbing. Open lung biopsy, if performed, reveals geographic and temporally heterogeneous fibrosis with areas of active fibrosis (fibroblastic foci) and areas of normal lung. The course is usually relentless, with an average survival from diagnosis of only 3.6 years. To date, there is no approved or recommended therapy for the treatment of IPF, other than lung transplantation in highly selected individuals.
The therapeutic potential for MSC in IPF was first recognised when it was noted that lung fibrosis was diminished in a study designed to assess the effect of bleomycin-induced pulmonary fibrosis on pulmonary engraftment of MSC [27]. Since that study, multiple preclinical studies, summarised in Table 5.1, have demonstrated the therapeutic efficacy of MSC in the bleomycin model with MSC leading to reduced lung connective tissue (hydroxyproline and collagen) content and fibrosis scores.
Although there appears to be a consistent effect of MSC if delivered soon after the administration of bleomycin, the therapeutic effect diminishes considerably if treatment is delayed until 7 days after administration [27, 33]. This effect highlights an inherent deficiency of the bleomycin model. Bleomycin induces an initial inflammatory response which is followed by a fibrotic response, whereas IPF is now known to be a fibrotic disease from the outset with minimal or no preceding fibrosis. Agents which have a predominantly anti-inflammatory, rather than anti-fibrotic effect may therefore appear effective in preclinical studies but be ineffective in humans. Successful later delivery of potential therapeutics is more likely, therefore, to reliably predict efficacy in human IPF. This is particularly important to recognise since the timing of MSC delivery appears to determine the fate of the engrafting cell, with later delivery favouring the differentiation of MSC into cells which are pro-fibrotic [14]. Taken together, however, and in the absence of suitable large animal models of IPF, the small animal studies performed to date have provided sufficient evidence for potential efficacy in human IPF for phase I trials to be planned.
5.2.3 Preclinical Studies: Asthma
While most patients with asthma enjoy excellent disease control due to the efficacy of currently available inhaled corticosteroid +/− long-acting β2-agonist therapy, a minority of patients are less responsive and have persistent asthmatic symptoms (cough, wheeze and breathlessness) and airflow obstruction. This group typically has largely irreversible airway remodelling with persistent airflow obstruction despite maximal use of bronchodilator therapy. MSC have been trialled in preclinical studies to determine their ability to reverse the airway remodelling characteristic of chronic asthma, with early reports of success [34]. Our group is currently exploring the use of murine MSC in a murine model of allergic asthma due to house dust mite (K. Atkinson, personal communication), and if successful preclinical data are obtained we will take it into a phase I clinical trial in people with severe treatment-refractory asthma.
5.2.4 Preclinical Studies: Other Applications
MSC have also been studied in the preclinical setting in other lung diseases where a strong clinical need for improved therapeutics exists. Post-transplant obliterative bronchiolitis is the major cause of long-term mortality and morbidity after lung transplantation and is refractory to treatment. In a heterotopic tracheal transplant model, Grove and colleagues have recently demonstrated the therapeutic potential of intravenously delivered MSC to attenuate airway obliteration through the production of IL-10 and modulation of TGFβ expression [35]. MSC have also been shown to be of benefit in preclinical studies of PAH [36, 37], particularly when they are transgenically treated to induce hyper-expression of heme oxygenase-1 [36].
5.2.5 Human Studies of MSC Therapy in Lung Disease
The largest study of MSC therapy in human lung disease began recruitment in 2008 and is listed as closed to recruitment but ongoing (http://www.clinicaltrial.gov/ct2/show/NCT00683722, accessed 1 June 2011). The primary aim of this phase II clinical trial was to establish the safety and efficacy of multiple administrations of allogeneic MSC (Prochymal™, Osiris Therapeutics Inc., osiristx.com) in patients with moderate and severe COPD. Human adult MSC were derived from the bone marrow of normal healthy adult volunteer donors. A total of 62 patients, between the ages of 47 and 80 years, with a diagnosis of moderate (n = 23) or severe (n = 39) COPD have been enrolled and are being followed for 2 years in this placebo-controlled study. The primary outcome measure is safety, with secondary outcome measures listed as pulmonary function tests, exercise capability and quality of life. Interim 6-month results were made available on 23 June 2009 (http://investor.osiristx.com/releasedetail.cfm?releaseid=391580, accessed 17 March 2010) but have not been formally published. All patients in the trial completed the planned course of four infusions without any evidence of infusional toxicity. Adverse event rates were comparable for patients receiving Prochymal™ and placebo, but the pulmonary function efficacy endpoint was not met [38].
Our group has initiated two human phase I trials of MSC therapy for lung disease. In the first study (http://clinicaltrials.gov/ct2/show/NCT01175655), the primary objective is to establish the safety of infusions of bone marrow-derived MSC from related or unrelated HLA-identical or HLA-mismatched donors in the management of bronchiolitis obliterans syndrome (BOS) after lung transplantation. The secondary objectives are to document changes in lung function, 6 min walk distance (6MWD) and survival following MSC infusion. Patients (n = 10) with single, bilateral or heart-lung allografts and deteriorating chronic allograft dysfunction manifesting as either BOS grades 2 and 3, or grade 1 [39] with an additional risk factor for subsequent death, will receive open label treatment with 2 × 106 MSC/kg bodyweight twice weekly for 2 weeks.
In the second study, a phase I, open-label, investigator-driven, non-randomised dose-escalation evaluation of the safety and feasibility of MSC treatment for subjects diagnosed with IPF, we will be assessing the feasibility and safety of delivering allogeneic placenta-derived MSC to patients (n = 8) with IPF. A total of eight subjects will be studied, four will receive 1 × 106 cells/kg and the next four will receive 2 × 106 cells/kg. The primary endpoint is to provide evidence of safe delivery of MSC in doses as per protocol. The secondary endpoints are the effectiveness at 1, 3 and 6 months post MSC infusion, compared to baseline, as assessed by lung function, exercise capacity (6MWD) and gas exchange as assessed by resting PaO2 and pulse oximetry during exercise testing. Enrolled patients will have moderate disease as assessed by honeycombing > 5% in 0–3 of 6 lung zones; forced vital capacity (FVC) > 50% of predicted and a diffusing capacity for carbon monoxide (DLCO) > 25% of predicted capacity.
The only other human study listed on www.clinicaltrial.gov as currently recruiting involves the intra-tracheal administration of umbilical cord blood-derived MSC to infants with BPD (http://clinicaltrials.gov/ct2/show/NCT01297205, accessed 1 June 2011).
5.2.6 Lung-Resident MSC: Their Role in Lung Physiology and Disease
In 2007 a population of lung-resident MSC were identified in the bronchoalveolar lavage (BAL) fluid of lung transplant recipients. Astonishingly, this cellular population was donor-derived, as discerned from their ability to reflect the sex of the lung donor, even many years after sex mismatched lung transplantation [40]. This startling discovery was consistent with an emerging body of literature suggesting that MSC occupy niches in many non-haematopoietic organs, not simply bone marrow. It is likely that these so-called “tissue resident” MSC have a different function to that of bone marrow-derived MSC, but this area of human biology is in its infancy. The apparently long-lived nature of lung-resident MSC confirms their ability to self-renew or their “stemness”.
Lung-resident MSC, like bone marrow-derived MSC, are multipotent in that they are able to differentiate into adipocytes, chondrocytes and osteocytes. Their phenotype is similarly CD73+CD90+CD105+ and CD14−CD34−CD45−, and they are able to inhibit T cell proliferation via a contact-independent mechanism, potentially by the secretion of PGE2 [29]. Although there are multiple similarities to the better characterised bone marrow-derived MSC, lung-resident MSC have a subtly but distinctly different gene expression profile. This is consistent with the concept that tissue resident populations of MSC have organ-specific functions. It is currently not clear what the function of this curious population of lung cells is nor in which pulmonary niche they usually reside.
Since lung-resident MSC were first identified in BAL fluid [40], and as this procedure involves sampling of the intra-alveolar pulmonary compartment, it must be that these MSC either reside within, or are able to migrate into, the alveolar space. In either case their niche must be either intra- or peri-alveolar. Recent evidence from the same group at Ann Arbor, Michigan, using a chimeric pulmonary model suggests that MSC reside in the alveolar region either attached to the alveolar septa or in the corners of the alveoli in close relationship to type 2 alveolar cells [41]. As far as their function is concerned, one can only speculate; however, it seems likely that lung-resident MSC will provide regenerative support to the surrounding epithelium, analogous to the support Sca-1 positive cells provide in the mouse [42] and much like the support their bone marrow cousins provide to adjacent lineages. Further clarification of the role of lung-resident MSC in human lung biology will depend heavily on the identification of suitable and specific markers. One such marker may be forkhead box F1 (FOXF1) [13, 43].
5.3 Conclusions
Lung disease is a major and growing cause of morbidity and mortality. While a number of lung diseases, most notably asthma, are now able to be relatively safely and effectively treated due to huge improvements in the available pharmacologics, substantial therapeutic gaps remain. It is likely that adult stem cells such as MSC may fill some of these gaps; however, in order for this promise to be achieved safely, and in order to avoid a repeat of the problematic headlong introduction of gene therapies to large scale clinical trials [44], a much deeper understanding of basic MSC biology will be required. Of particular interest in the future will be the role of tissue resident MSC in lung physiology and disease. In this way, while initially stem cell technology was seen as potentially therapeutic because of engraftment potential, it is more likely that therapeutic aims will be achieved through the potent paracrine and contact-dependent effects of adult stem cell populations on adjacent somatic and inflammatory cells.
References
COPD (2003) COPD prevalence in 12 Asia-Pacific countries and regions: projections based on the COPD prevalence estimation model. Respirology 8(2):192–198
Stang P, Lydick E, Silberman C, Kempel A, Keating ET (2000) The prevalence of COPD: using smoking rates to estimate disease frequency in the general population. Chest 117(5 Suppl 2):354S–359S
Halpin DM, Miravitlles M (2006) Chronic obstructive pulmonary disease: the disease and its burden to society. Proc Am Thorac Soc 3(7):619–623
Ohnishi S, Nagaya N (2008) Tissue regeneration as next-generation therapy for COPD – potential applications. Int J Chron Obstruct Pulmon Dis 3(4):509–514
Braman SS (2006) The global burden of asthma. Chest 130(1 Suppl):4S–12S
Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G (2006) Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 174(7):810–816
Liu KD, Matthay MA (2008) Advances in critical care for the nephrologist: acute lung injury/ARDS. Clin J Am Soc Nephrol 3(2):578–586
Meduri GU, Golden E, Freire AX, Taylor E, Zaman M, Carson SJ, Gibson M, Umberger R (2007) Methylprednisolone infusion in early severe ARDS: results of a randomized controlled trial. Chest 131(4):954–963
Peacock AJ, Murphy NF, McMurray JJ, Caballero L, Stewart S (2007) An epidemiological study of pulmonary arterial hypertension. Eur Respir J 30(1):104–109
Wong PM, Lees AN, Louw J, Lee FY, French N, Gain K, Murray CP, Wilson A, Chambers DC (2008) Emphysema in young adult survivors of moderate-to-severe bronchopulmonary dysplasia. Eur Respir J 32(2):321–328
Spencer H, Jaffe A (2004) The potential for stem cell therapy in cystic fibrosis. J R Soc Med 97(Suppl 44):52–56
Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP (2007) Stem cell transplantation: the lung barrier. Transplant Proc 39(2):573–576
Moodley Y, Atienza D, Manuelpillai U, Samuel CS, Tchongue J, Ilancheran S, Boyd R, Trounson A (2009) Human umbilical cord mesenchymal stem cells reduce fibrosis of bleomycin-induced lung injury. Am J Pathol 175(1):303–313
Yan X, Liu Y, Han Q, Jia M, Liao L, Qi M, Zhao RC (2007) Injured microenvironment directly guides the differentiation of engrafted Flk-1(+) mesenchymal stem cell in lung. Exp Hematol 35(9):1466–1475
Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA (2007) Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol 179(3):1855–1863
Qin ZH, Qu JM, Xu JF, Zhang J, Summah H, Sai-Yin HX, Chen CM, Yu L (2011) Intrapleural delivery of mesenchymal stem cells: a novel potential treatment for pleural diseases. Acta Pharmacol Sin 32(5):581–590
Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O (2003) HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol 31(10):890–896
Beggs KJ, Lyubimov A, Borneman JN, Bartholomew A, Moseley A, Dodds R, Archambault MP, Smith AK, McIntosh KR (2006) Immunologic consequences of multiple, high-dose administration of allogeneic mesenchymal stem cells to baboons. Cell Transplant 15(8–9):711–721
Rasmusson I, Uhlin M, Le Blanc K, Levitsky V (2007) Mesenchymal stem cells fail to trigger effector functions of cytotoxic T lymphocytes. J Leukoc Biol 82(4):887–893
Badillo AT, Beggs KJ, Javazon EH, Tebbets JC, Flake AW (2007) Murine bone marrow stromal progenitor cells elicit an in vivo cellular and humoral alloimmune response. Biol Blood Marrow Transplant 13(4):412–422
Nauta AJ, Westerhuis G, Kruisselbrink AB, Lurvink EG, Willemze R, Fibbe WE (2006) Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood 108(6):2114–2120
Poggi A, Prevosto C, Zancolli M, Canevali P, Musso A, Zocchi MR (2007) NKG2D and natural cytotoxicity receptors are involved in natural killer cell interaction with self-antigen presenting cells and stromal cells. Ann N Y Acad Sci 1109:47–57
Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M (2006) Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells (Dayton, Ohio) 24(1):74–85
Wang G, Bunnell BA, Painter RG, Quiniones BC, Tom S, Lanson NA Jr, Spees JL, Bertucci D, Peister A, Weiss DJ, Valentine VG, Prockop DJ, Kolls JK (2005) Adult stem cells from bone marrow stroma differentiate into airway epithelial cells: potential therapy for cystic fibrosis. Proc Natl Acad Sci USA 102(1):186–191
Sueblinvong V, Loi R, Eisenhauer PL, Bernstein IM, Suratt BT, Spees JL, Weiss DJ (2008) Derivation of lung epithelium from human cord blood-derived mesenchymal stem cells. Am J Respir Critic Care Med 177(7):701–711
Mias C, Lairez O, Trouche E, Roncalli J, Calise D, Seguelas MH, Ordener C, Piercecchi-Marti MD, Auge N, Salvayre AN, Bourin P, Parini A, Cussac D (20009) Mesenchymal stem cells promote matrix metalloproteinase secretion by cardiac fibroblasts and reduce cardiac ventricular fibrosis after myocardial infarction. Stem Cells (Dayton, Ohio)
Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG (2003) Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA 100(14):8407–8411
Gotherstrom C (2007) Immunomodulation by multipotent mesenchymal stromal cells. Transplantation 84(1 Suppl):S35–S37
Jarvinen L, Badri L, Wettlaufer S, Ohtsuka T, Standiford TJ, Toews GB, Pinsky DJ, Peters-Golden M, Lama VN (2008) Lung resident mesenchymal stem cells isolated from human lung allografts inhibit T cell proliferation via a soluble mediator. J Immunol 181(6):4389–4396
Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM (2002) Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99(10):3838–3843
Lee JW, Gupta N, Serikov V, Matthay MA (2009) Potential application of mesenchymal stem cells in acute lung injury. Expert Opin Biol Ther 9(10):1259–1270
Matthay MA, Thompson BT, Read EJ, McKenna DH Jr, Liu KD, Calfee CS, Lee JW (2010) Therapeutic potential of mesenchymal stem cells for severe acute lung injury. Chest 138(4):965–972
Cui A, Dai HP, Dai JW, Pang BS, Niu SJ, Lu YP, Wang C (2007) Effects of bone marrow mesenchymal stem cells on bleomycin induced pulmonary fibrosis in rats. Zhonghua Jie He He Hu Xi Za Zhi 30(9):677–682
Firinci F, Karaman M, Baran Y, Bagriyanik A, Ayyildiz ZA, Kiray M, Kozanoglu I, Yilmaz O, Uzuner N, Karaman O (2011) Mesenchymal stem cells ameliorate the histopathological changes in a murine model of chronic asthma. Int Immunopharmacol 11(8):1120–1126
Grove DA, Xu J, Joodi R, Torres-Gonzales E, Neujahr D, Mora AL, Rojas M (2011) Attenuation of early airway obstruction by mesenchymal stem cells in a murine model of heterotopic tracheal transplantation. J Heart Lung Transplant 30(3):341–350
Liang OD, Mitsialis SA, Chang MS, Vergadi E, Lee C, Aslam M, Fernandez-Gonzalez A, Liu X, Baveja R, Kourembanas S (2011) Mesenchymal stromal cells expressing heme oxygenase-1 reverse pulmonary hypertension. Stem Cells (Dayton, Ohio) 29(1):99–107
Umar S, de Visser YP, Steendijk P, Schutte CI, Laghmani el H, Wagenaar GT, Bax WH, Mantikou E, Pijnappels DA, Atsma DE, Schalij MJ, van der Wall EE, van der Laarse A (2009) Allogenic stem cell therapy improves right ventricular function by improving lung pathology in rats with pulmonary hypertension. Am J Physiol Heart Circ Physiol 297(5):H1606–H1616
Allison M (2009) Genzyme backs Osiris, despite prochymal flop. Nat Biotechnol 27(11):966–967
Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, Mallory GB, Snell GI, Yousem S (2002) Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant 21(3):297–310
Lama VN, Smith L, Badri L, Flint A, Andrei AC, Murray S, Wang Z, Liao H, Toews GB, Krebsbach PH, Peters-Golden M, Pinsky DJ, Martinez FJ, Thannickal VJ (2007) Evidence for tissue-resident mesenchymal stem cells in human adult lung from studies of transplanted allografts. J Clin Invest 117(4):989–996
Badri L, Walker NM, Ohtsuka T, Wang Z, Delmar M, Flint A, Peters-Golden M, Toews GB, Pinsky DJ, Krebsbach PH, Lama VN (2011) Epithelial interactions and local engraftment of lung-resident mesenchymal stem cells. Am J Respir Cell Mol Biol
McQualter JL, Yuen K, Williams B, Bertoncello I (2010) Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc Natl Acad Sci USA 107(4):1414–1419
Walker N, Badri L, Wettlaufer S, Flint A, Sajjan U, Krebsbach PH, Keshamouni VG, Peters-Golden M, Lama VN (2011) Resident tissue-specific mesenchymal progenitor cells contribute to fibrogenesis in human lung allografts. Am J Pathol 178(6):2461–2469
Couzin J, Kaiser J (2005) Gene therapy: as Gelsinger case ends, gene therapy suffers another blow. Science (New York, NY) 307(5712):1028
Zhao F, Zhang YF, Liu YG, Zhou JJ, Li ZK, Wu CG, Qi HW (2008) Therapeutic effects of bone marrow-derived mesenchymal stem cells engraftment on bleomycin-induced lung injury in rats. Transplant Proc 40(5):1700–1705
Bitencourt CS, Pereira PA, Ramos SG, Sampaio SV, Arantes EC, Aronoff DM, Faccioli LH (2011) Hyaluronidase recruits mesenchymal-like cells to the lung and ameliorates fibrosis. Fibrogenesis Tissue Repair 4(1):3
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Chambers, D., Hopkins, P. (2013). Pulmonary Clinical Applications for Mesenchymal Stem Cells. In: Chase, L., Vemuri, M. (eds) Mesenchymal Stem Cell Therapy. Stem Cell Biology and Regenerative Medicine. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-62703-200-1_5
Download citation
DOI: https://doi.org/10.1007/978-1-62703-200-1_5
Published:
Publisher Name: Humana Press, Totowa, NJ
Print ISBN: 978-1-62703-199-8
Online ISBN: 978-1-62703-200-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)