Abstract
Stem cells have enormous potential for regenerative medicine to treat fatal diseases and injuries that cannot otherwise be healed. In particular, adult stem cell-based therapies have been studied for several decades. Mesenchymal stem cells/marrow stromal cells (MSCs) have shown safety and therapeutic efficacy in preclinical models of various diseases such as cardiovascular disease, cancer, bone defects, renal failure, and neurodegenerative disorders. In spite of the great potential, several factors including low survival rate, low efficiency of MSC homing to injured sites, and poor levels of engraftment and retention have been major technical challenges to be overcome before MSC-based therapy can be applied to clinical applications in a consistently therapeutic manner. Genetically modified MSCs can be one option to overcome some of these problems and to deliver therapeutic agents. MSCs are powerful delivery vehicles and potent protein synthesis factories, and therefore the use of gene-modified MSCs to provide growth factors and other signals to improve the repair of damaged or diseased tissues holds much promise. Here we review the basic biology of human MSCs and the current status of preclinical and clinical trials using genetically engineered MSCs.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Mesenchymal stem cells
- Gene therapy
- Cellular therapy
- Preclinical models
- Growth factor production
- Revascularization
- Cancer
- Renal disease
- Bone disease
- Neurodegenerative disorders
- Clinical trials
- Regenerative medicine
15.1 Human Diseases and Stem Cells
15.1.1 Degenerative Diseases
The human body is made up of millions of cells. When cells are injured, we may experience disease or disability. Those with type I diabetes have damaged or decreased numbers of islet cells, which are unable to produce sufficient amounts of insulin. Such individuals must substitute what is missing by taking frequent, daily insulin injections. Those with spinal cord injuries have damaged nerve cells which are no longer able to conduct messages from limbs to the brain and back and as a result have lost the ability to move some part of their body. Some organs like the liver and skin are excellent at repairing and regenerating themselves, while other organs or tissues have far less capacity to do so.
Islet or whole organ transplantation is one of the methods to cure these types of diseases but immune rejection is a major problem and the patients must often remain immunosuppressed, increasing risk of infections and cancers. Organ transplantation is also hampered by severe donor shortages. In spinal cord injury, transplantation is not even an option to consider. One promising new way to treat some diseases and disabilities is to regenerate injured or missing cells with stem cells, either by replacing those that have been damaged in the tissue or organ (e.g., pluripotent cell-derived differentiated cell types), or to provide cells that deliver factors that can encourage endogenous recovery (e.g., mesenchymal stem cells).
15.1.2 Pluripotent Stem Cells and Their Therapeutic Potential
Both embryonic stem cells (ESCs) and adult stem cells (e.g., hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs)) are under examination in clinical trials of cell therapy. Bone marrow (BM) transplantation, which has provided great success in transplantation for more than 50 years, has shown a great therapeutic benefit for the blood-forming system and this may extend to promoting healing of other tissues [1].
Recently, researchers’ attention has been focused on human (h) ESCs, which theoretically have the potential to differentiate into all types of adult human tissues (pluripotency) and can grow indefinitely (self-renewal) [2]. Since their initial derivation, hESCs have become a promising tool for developmental biology and regenerative medicine. However, concerns related to ethical objections regarding the use of human embryos for hESC derivation have dramatically restricted research using these cells and therefore have set back the development of hESCs for clinical trials, although recent first-in-human phase I clinical trials from the company Geron were initiated in 2011 [3]. Later, Geron announced that they pulled out their entire program due to the financial reasons, but to date, safety has been demonstrated [4].
Due to their allogeneic nature, immune rejection of transplanted cells or tissues derived from hESCs is another potential drawback to therapeutic applications. The immune system of the patient recognizes transplanted “foreign” cells or tissues and escalates a rapid response, attacking the graft. This attack can result in the loss of the graft, which can lead to the death of patients if it was an organ on which the patient was reliant (e.g., heart). Immunosuppressive drug regimens, similar to those used for current human tissue and organ transplant procedures, might lessen the severity of the anticipated immune rejection, but at the same time may put the tissue recipient at an increased risk for infections. This risk can be lessened by application of human leukocyte antigen (HLA)-matched tissue, as is currently being practiced in organ transplantation, or could be completely eliminated by the use of the patient’s own tissue. The latter possibility might, in the future, be achieved by reprogramming the patient’s own somatic cells to induced pluripotent stem cells (iPSCs) [5].
Takahashi and Yamanaka pioneered methods to generate iPSCs by virally transducing four transcription factors into human somatic cells and showed that the resultant iPSCs have similar characteristics to ESCs [5]. Recent reports, however, have indicated that iPSCs are not exactly the same as ESCs and may be more prone to genetic mutations during the reprogramming and expansion phases [6]. Also, abnormal gene expression in some differentiated cells from iPSCs can induce T-cell-dependent immune responses in autologous transplantations done in mice [7]. Differentiated progenies from iPSCs and hESCs still have an immature status similar to early human fetal tissues (<6 weeks), and it is too early to tell whether it will be appropriate to apply these cells for transplantation therapies in humans [8].
15.1.3 Mesenchymal Stem Cells and Clinical Trials
Over the last half century, adult stem cell therapies in the form of bone marrow, mobilized peripheral blood, and umbilical cord blood transplantations have rescued thousands of patients from induced or genetic disorders [9]. After the first human hematopoietic stem cell transplantation in 1956, the technique gradually evolved to become a standard clinical procedure (reviewed in [10]). Mesenchymal stem cells (MSCs) were first described as adherent “marrow stromal cells” and were studied for their role in supporting hematopoiesis and were then engineered to provide factors for other cells (reviewed in [11]). Later, these cells were found to differentiate into cartilage, bone, fat, tendon, and fibroblasts [9]. Over the past three decades of study, MSCs have become a tool for regenerative applications either through direct differentiation into specific tissues (e.g., bone), or indirectly through protein or cytokine secretion and immune suppression [9, 11–14]. MSCs have become a promising cell-based therapeutic because they are easily accessible from various tissues (e.g., bone marrow, fat and umbilical cord tissue) and are easily grown in culture. MSCs can be expanded in vitro to a clinical scale and can be cryopreserved without the loss of their integrity.
MSCs have demonstrated systemic migration after intravenous injection, in particular to areas of hypoxia or tissue damage [15]. The systemic administration of allogeneic MSCs has not been observed to cause any adverse effects in numerous treated patients, in part due to immunomodulatory effects [16, 17]. Also, MSCs have been considered safe as they do not show tumor formation after transplantation [18] and have been widely tested and shown efficacy in preclinical and clinical studies for cardiovascular, neurodegenerative, graft-versus-host (GvHD), and autoimmune diseases [9, 11]. Due to the MSC’s osteoblast differentiation potential, Caplan and colleagues applied allogeneic MSCs to osteogenesis imperfecta patients [19]. In addition, LeBlanc et al. investigated the immunomodulatory effects of transplanted MSC for steroid-resistant GvHD [16], and similar methods were applied to other diseases [9]. These early studies have established a good clinical record of safety for systemic MSC administration.
15.2 Genetic Modification of Mesenchymal Stem Cells
Even though there have been remarkable advances in demonstrating safety in MSC clinical trials, therapeutic efficacy is still debated [9]. Unsolved problems such as low cell survival and engraftment efficiency after MSC transplantation still remain to be resolved [13, 14]. Genetic engineering of MSCs is a potential means to improve their therapeutic potential. MSCs can be modified to express therapeutic agents, to improve cell survival or to possess an enhanced ability to home to a disease site. In the following section, we will briefly discuss the pros and cons of several gene modification methods.
15.2.1 Choice of Vector Systems
Genetic modification of MSCs can be achieved by permanent integration or episomal expression of target genes via viral vector transduction or by transient expression of specific genes using nonviral delivery [20]. Viral delivery of desired genes to MSCs is one of the most utilized methods in preclinical studies due to the ability to achieve high infectivity with broad tropism. However, the transduction efficiency depends on the target cells (Table 15.1). Clonal analysis of transduced MSCs has shown that these cells often contain several thousand copies of transgene RNA per cell and can maintain transgene expression for 6 months or longer [11, 21–23]. In MSC studies, retroviral, lentiviral, adenoviral, and adeno-associated virus (AAV) vectors are generally used [20]. Adenoviral and AAV vectors do not integrate into the host genome but can express transgenes in an episomal manner. In non-dividing cells, these viral particles can sustain long-term transgene expression but would be diminished as cells proliferate in dividing cells due to the dilution of viruses. However, it is well documented that the capsids of adenoviral vector can be recognized by the patient’s innate immune system and can cause adverse events [24]. Even though success has been shown with the delivery of factor IX with AAV into the hepatocytes of human hemophilia patients, transduced hepatocytes were cleared due to an immune reaction to capsids from the AAV [25]. To achieve short-term transgene expression in MSCs for applications such as angiogenic growth factor expression after myocardial infarction or surface antigen modification for increase cell survival, it may be beneficial to choose AAV vectors.
MoMuLV-based retroviral and HIV-based lentiviral vectors can offer long-term expression of transgenes in target cells due to their permanent integration into the host genome. While lentiviral vectors can transduce both dividing and non-dividing cells, retroviral vectors can only transduce dividing cells (Table 15.1). In terms of chromosomal integration sites, there are differences in the integration hot spots between these two viral vectors, but most integration is into the active regions of chromosomes. Retroviral vectors tend to favor integration into transcriptional start sites, promoter regions, or CpG islands, while lentiviral vectors do not appear to have preferential integration regions [26, 27]. Numerous papers, including work from our own lab, have demonstrated that MSCs can be transduced with retroviral or lentiviral vectors and can retain transgene expression for many passages. In addition, transduced cells retain in vitro lineage-specific differentiation and in vivo engraftment, with no detectable complications caused by viral integration [11, 18, 22, 23, 28–31].
Although a promising method, nonviral gene transfer must overcome low efficiency (discussed in [32]). To test gene delivery methods, McMahon et al. tested GFP expression via adenoviral, AAV, lentiviral, plasmid transfection, and electroporation in rat MSCs [33]. Lentiviral delivery showed the highest GFP expression with minimal cell death. Adenoviral vectors provided effective GFP expression but with a reduced transduction efficiency and an increased cell toxicity compared to lentiviral vectors. In that study, AAV vectors could not effectively deliver transgenes into rat MSCs, although another study showed that AAV vectors could infect human MSCs [34]. To improve the transduction efficiency for AAV vectors, specific serotypes of AAV must be chosen as only serotype 2 AAV vectors were shown to have a high MSC transduction efficiency [35]. Transfection and electroporation of plasmids were not as effective as viral delivery of GFP and were harmful to MSCs due to the associated cytotoxicity. Furthermore, transfected plasmids can randomly integrate as concatamers into host chromosomes at a frequency of 1/3000 to 1/5000 [36].
15.2.2 Safety Considerations for Genetic Modifications
Even though long-term gene expression can be achieved by retroviral and lentiviral vectors, the risk of insertional mutagenesis remains a concern when considering genetically engineered MSC for clinical trials. In the hematopoietic stem cell (HSC) clinical trial that used retroviral vector for X-linked severe combined immunodeficiency disease (X-SCID) in France, four out of eleven children developed leukemia [37, 38]. One out of ten patients treated by hematopoietic stem cell gene therapy for Wiskott–Aldrich syndrome also developed acute lymphocytic leukemia [39]. Later, it was discovered that the leukemia in both cases was due to the long-terminal repeat (LTR) of the retroviral cassette having integrated in the proximity of the LMO2 proto-oncogene promoter. As a result, the integrated LTR acted as a promoter to drive LMO2 expression and lead to leukemia, in cells that were greatly expanded already due to the selection process in both trials, and delivery of a growth factor receptor in the case of XSCID.
Naldini and colleagues compared the in vivo tumor induction capacity by both retroviral and lentiviral vectors [40]. Using the tumor-prone p16 knockout mouse strain, this group found that retroviral vectors triggered a dose-dependent induction of tumor onset, while lentiviral vectors showed low genotoxicity upon integration. Later, the same group also showed that the retroviral LTR is the major component capable of generating unregulated cell growth in this tumor-prone mouse model, by swapping between retroviral vector LTR and self-inactivating (SIN)-LTR in lentiviral plasmids [41]. Indeed, additional gene-modified stem cell clinical trials using SIN-LTR lentiviral vectors have avoided this outcome [42]. The possibility of adverse events can be monitored by serial transplantation experiments in vivo [18, 40].
In contrast to hematopoietic stem cells which are capable of long-term self-renewal and differentiation in vivo, gene-modified MSCs have not been reported to cause tumors, using in vivo assays. We have completed a comprehensive decade-long study of the biosafety of MSCs stably transduced by retro- and lentiviral vectors and did not observe adverse events arising from the human cells, in sensitive xenograft assays [18]. However, it should be noted that we did not perform serial transplantations and the follow-up of the human MSCs in immune-deficient mice was limited to 18 months, due to the natural lifespan of the mice.
A way to potentially avoid risks from random gene insertion would be to use human embryonic stem cell (ESC) or human induced pluripotent stem cell (hiPSC)-derived MSCs [43] which have safe harbor integrations of the transgenes and have been subsequently expanded in vitro. The lifespan of primary MSCs are limited up to 40 doublings during in vitro expansion [44]. Aging significantly reduces the in vitro and in vivo survival and differentiation potential of primary MSCs [45]. This could be potentially overcome in the future by the use of hESC or hiPSC-derived MSCs, since these cell types could theoretically be expanded indefinitely after transduction and prior to differentiation to the MSC lineage (discussed in [46]).
15.3 Gene-Modified MSCs in Pre-clinical Models
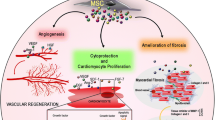
MSCs genetically modified to secrete cytokines and other growth factors have been successfully used in animal models for various diseases, and are therefore poised to be tested in human clinical trials [11]. Accumulating evidence indicates that genetically modified MSCs have therapeutic potential in various disease models and that genetic engineering of the cells can improve cell survival, homing to the disease sites, secretion of therapeutic agents and differentiation into different cell types. Here we will describe the current status of gene-modified MSCs in various preclinical models.
15.3.1 MSC Survival After Transplantation
The majority of intravenously transplanted MSCs appear to die within several hours or lodge nonspecifically in the lung, spleen, liver, or kidney [11, 14, 22, 47]. To improve MSC survival after transplantation, several approaches have been examined including the overexpression of proliferation-related or anti-apoptotic-related genes or preconditioning using hypoxia or other approaches prior to transplantation (Table 15.2).
Several laboratories have focused on the overexpression of Akt, a protein which inhibits apoptosis. MSCs engineered to overexpress Akt survived longer than unmodified MSCs after transplantation in a variety of animal models. Dzau and colleagues documented that Akt-overexpressing MSCs had a higher survival rate after transplantation into an ischemic heart model and showed that the improvement of cardiac function following transplantation was due to the paracrine factors secreted from the surviving MSCs [48]. Another paper from the same group further demonstrated that transplanted Akt-modified MSCs balanced the metabolism and pH of the myocardium [49]. Recently, a swine myocardial infarction model also added to the evidence showing that Akt transduced MSCs survived longer and showed greater efficacy than unmodified MSCs [50]. Our group has shown that, rather than performing gene modification, hypoxic preconditioning of human MSCs at 3% oxygen for 24 h prior to transplantation will upregulate AKT activity, enhance cell homing and survival, and enhance their in vivo capacity to promote revascularization in a xenograft model of hindlimb ischemia [61].
Overexpression of connexin43, a gap junction protein, also showed a higher MSC survival rate and improvement of cardiac function. This result was shown to be due to the fact that these cells expressed more Bcl-2, one of the negative regulators in the apoptotic pathway, along with phosphorylated Akt. These cells also expressed less Bax, a pro-apoptotic protein [51]. In the same fashion, Hsp20 overexpression in MSCs has been shown to increase the cell survival rate along with reduced fibrosis. Hsp20 can protect other proteins against heat-induced cellular stress. The beneficial effects of these gene-modified MSCs were associated with enhanced Akt activation and increased secretion of growth factors such as VEGF, FGF-2, and IGF-1 [52].
MSCs engineered to express Bcl-2, which is one of the key anti-apoptotic proteins, have shown better survival and improved cardiac function due to reduced apoptotic events and increased VEGF secretion [53]. Recent studies using Bcl-xL- (one of the Bcl-2 family members) modified MSCs also supports this concept [54]. Direct intra-articular injection of Bcl-xL overexpressing MSCs into a rabbit articular cartilage defect model improved MSC survival and increased cartilage healing. Several studies mediated Heme oxygenase (HO-1), which is known for protection against apoptosis in rat [55–58], mouse [59], and swine cardiac ischemic models [60]. It is well known that HO-1 itself has therapeutic potential in the treatment of cardiac disease [62].
Although overexpression of pro-proliferative and anti-apoptotic genes in MSCs improved cell survival after transplantation, there must be extreme caution for any consideration of application to human clinical trials as these genes are related to cancer activity and overexpression may lead to cell transformation. Therefore it is most prudent to learn from the overexpression data in these instances and apply this knowledge to developing novel ways to precondition cells prior to transplantation to achieve similar effects. For example, our group others have explored the use of hypoxic prestimulation prior to transplantation [61, 63, 64].
15.3.2 MSC Homing
Efficient MSC homing to the tissue of interest is one of the most important aspects of effective MSC therapy. Extensive studies have shown that MSC migration is mediated by growth factor or chemokine/receptor pairs such as SDF-1/CXCR4, HGF/c-MET, VEGF/VEGFR, MCP-1/CCR2, and others (detailed review in [14]). The well-known homing receptor CXCR4 is a chemotactic receptor for stromal cell-derived factor-1α (SDF-1). CXCR4 is absent in populations of in vitro expanded MSCs, but freshly isolated MSCs have a small positive population [65–67]. It has been shown that hypoxic (tissue normoxic) preculture can induce CXCR4 expression [64]. Surface antigen modification of CD44 by the FUT VI enzyme improved homing efficiency of BM-MSCs into the bone in NOD/SCID mice without the expression of CXCR4 [67]; ex vivo engineered E-selectin, which is not expressed naturally in MSCs, was also shown to be sufficient to home MSCs into bone[67]. Also, our group has also shown that modification of MSCs with bone-homing ligands tethered to bisphosphonate has also resulted can result in homing of MSCs back to the bone [68].
In a rat myocardial infarction model, MSCs engineered to overexpress CXCR4 showed greater numbers of cells that had homed to ischemic sites and improved left ventricular function, as compared to unmodified MSCs [69] (Table 15.3). Huang et al. further analyzed that CXCR4-overexpressing mouse MSCs migrated to the infarction site and released the collagen degrading enzyme, matrix metalloproteinase-9 (MMP-9), which lead to a reduction of the remodeling of infarcted myocardium [70]. Dzau and colleagues turned their focus on a different chemokine receptor, CCR1 [71]. This receptor is one of the G protein-coupled receptors known to bind to CCL7 and is usually expressed by MSCs at low levels.. This group noticed that infarcted hearts have higher expression levels of CCL7. To better guide MSCs to ischemic sites, they overexpressed CCR1 in murine MSCs. These cells had better survival, reduced cardiac remodeling and increased cardiac functions in comparison with non-engineered MSCs.
Since permanent expression of E-selectin is not required to home MSC to bone, transient expression of these homing proteins can be considered [67]. Nonviral methods such as plasmid transfection, cytokine treatment, hypoxia, and others that can increase levels of homing receptors can be an alternative method to improve MSC localization to bone, to the perivascular space, and to damaged tissues in general.
15.3.3 Cardiovascular Diseases
Cardiovascular diseases are the leading cause of death in the USA. An estimated 79 million American adults (1 in 3) have one or more types of cardiovascular diseases [72]. Ischemia and hypertensive heart failure cause irreversible loss of cardiomyocytes. Potent pharmacological treatments have significantly improved morbidity and mortality [73]. These methods along with the development of implantable cardioverter-defibrillators [74] and left ventricular assist devices have all significantly increased survival rates [75]. Despite all these improvements in clinical management, the prevalence of heart failure remains. The current best therapy for cardiac failure, heart transplantation, is hampered by the shortage of organ donors. Stem/progenitor cell transplantation for curing cardiac diseases remains an attractive concept that is studied in numerous preclinical and clinical trials.
So far, most gene-modified MSC studies using anti-apoptotic and proliferative genes showed improvement of cardiac function in acute cardiac infarction cases due to better survival of transplanted MSCs and secretion of various growth factors (Table 15.4). Among the growth factors, the most heavily studied is vascular endothelial growth factor (VEGF). VEGF-overexpressing MSCs administered to treat acutely infarcted heart in mouse [76] and rat [77, 78] significantly increased vascular density, reduced the infarcted area and improved cardiac function. Human MSCs genetically modified to secrete VEGF were also found to significantly enhance blood flow recovery in an immune-deficient mouse model of hindlimb ischemia [83]. Hepatocyte growth factor (HGF) is also one of the promising options to improve cardiac ischemia. Ectopic expression of HGF in MSCs improved cardiac function, reduced ventricular remodeling, and enhanced vascular density in rat models [79–81]. Another study confirmed that HGF or VEGF-expressing MSCs also improved cardiac function [82].
In coronary or peripheral artery diseases, bypass surgery or angioplasty is popular solution. Our laboratory examined cell fates, proliferation of growth factor overexpressing MSCs and angiogenesis using VEGF-overexpressing human MSCs in an immune-deficient mouse ischemic hind limb injury model [30]. Other studies have focused on the therapeutic potential of factor releasing MSCs, but cell fate decisions and the proliferation potential of vector containing MSCs are less well illustrated. We showed that bFGF or PDGF-B overexpression in MSCs increased proliferation. When cultured in differentiation conditions, both bFGF and PDGF-B overexpressing MSCs showed enhanced osteogenesis, but strong inhibition was shown for adipogenesis in MSC overexpressing PDGF-B and only mildly affected in the MSCs overexpressing bFGF. Overexpression of TGF-β1 blocked both osteogenic and adipogenic differentiation but VEGF overexpression did not vary in any of these differentiation assays, most probably due to the lack of VEGF receptor expression on MSCs. Therefore, due to the lack of autocrine effects on the MSCs that would produce it, we further examined the role of MSCs engineered to produce VEGF165a in vivo. VEGF overexpressing MSCs were demonstrated to significantly enhance blood flow restoration in a xenograft model of hind limb ischemia, without adverse events [30].
15.3.4 Cancers
Cancer is the second leading cause of death in the USA, accounting for 1 in every 4 deaths over all ages in 2010 [84]. It is estimated that approximately 1 in 2 men (44%) and 1 in 3 women (38%) have a lifetime probability of being diagnosed with an invasive cancer. Since the declaration of “War on Cancer” in 1971, there have been tremendous advancements in cancer biology and successful drug treatments. In consensus, metastatic cancer is the major cause of deaths, not the primary cancer. Metastatic cells spread to the bones, lung, kidney, liver, brain, and other organs, and it is very difficult to locate these metastases by established diagnostics. The short half-life of some drugs limits their delivery to some metastatic tumor sites and side effects on non-tumor cells is one of the major impediments to curing cancers.
MSCs have been proposed as one of the several treatment modalities for cancer therapy due to supposed antitumor effects, but this is still highly controversial. Some papers claimed that MSCs had antitumor properties such as inhibiting the proliferation of glioma, melanoma, lung cancer, hepatoma, and breast cancer [85]. Others showed that MSCs secreted IL-6 and this increased proliferation [86] or production of CCL5 from MSCs and increased metastasis of breast cancer cells [87]. One thing that both sides agreed on is that MSCs migrate into cancer sites with not fully understood mechanisms [85]. With this notion in mind, many groups modified MSCs as delivery vehicles for therapeutic reagents; categorized as immunostimulatory agents, cytotoxic agents, prodrug activators, and viral vector delivery (detailed review in [85]).
15.3.4.1 Immunostimulatory Agents
Cancers have an ability to modulate their environments to hide their identity [88]. Stimulating endogenous immune systems by cytokines is one of the interesting options to treat cancers. Interleukins are known to regulate inflammatory and immune responses [89]. IL-12 and IL-18 are known to kill tumors directly and to recruit T cells and natural killer cells and those cells can eradicate tumors [90]. Administration of MSCs expressing IL-12 compared to adenoviral delivery of IL-12 every 5 days for 4 times showed reduction in the spread of metastatic melanoma, breast cancer, and hepatoma [91] (Table 15.5). IL-12 delivered by adenoviral vector showed toxicity and the levels of IL-12 were only elevated in the serum, but not the intratumoral environment. However, MSC overexpressing IL-12 showed increased apoptosis of tumor cells and higher levels of IL-12 in the intratumoral samples.
The same concept to eradicate renal carcinoma was applied by Gao et al. [92]. They injected MSCs bearing IL-12 once in xenografted nude mouse models and showed reduction of tumor growth and prolonged survival compared to systemic administration of adenoviral delivery of IL-12. Other teams reported that IL-12 expressing MSCs showed therapeutic efficacy on melanoma and cervical cancers [93] as well as intracranial glioma [94]. Similarly, IL-18 modified MSCs also have been investigated to treat glioma in a rat model [95]. IL-18 expressing MSCs were systemically administered and showed inhibition of glioma growth and prolonged survival of rats bearing glioma. IL-2 expressing MSCs also showed similar efficacy in a rat glioma model [96].
Interferons (IFNs) are cytokines released from the host cells and have functions to activate natural killer cells or macrophages and to increase antigen presentation to be recognized by T cells [90]. IFNα and IFNβ were pursued to treat various tumors using MSCs as the vehicle to deliver them, because systemic administrations of IFNs cause toxicity in vivo. As described earlier, MSCs are prone to migrate into tumor sites and IFN expressing MSCs recruit cells of the host immune system. IFNα overexpressing MSCs were evaluated in mouse melanoma lung metastasis models [97] and a mouse plasmacytoma model [98]. Both studies showed that intravenously [97] and subcutaneously [98] injected MSCs producing IFNα increased tumor apoptosis and decreased cancer proliferation along with prolonged survival of mice bearing tumors. Several laboratories utilized IFNβ expressing MSCs to treat various tumors in rodent models [99, 100, 102, 103]. Studeny et al. showed that IFNβ-modified MSCs inhibited melanoma growth in vivo [99]. Interestingly, therapeutic efficacy was only shown when MSCs had migrated to tumor sites but systemically delivered IFNβ or that produced by MSCs at a site distant from the tumors did not. Similar approaches were applied to a breast cancer model [100, 101], prostate cancers [102], and pancreatic cancers [103].
15.3.4.2 Cytotoxic Agents
Tumor necrosis factor-related apoptosis-induced ligand (TRAIL) is a pro-apoptotic protein that will enter cancer cells but normal cells are not affected [104, 105]; TRAIL-induced apoptosis occurs via the caspase pathway. A major drawback of systemic administration of TRAIL is a large amount of TRAIL needed to kill cancers due to the fast clearance of TRAIL by the kidney [106]. Several groups investigated MSCs expressing TRAIL as a vehicle to deliver locally to tumor sites and to sustain the TRAIL expression enough to kill the cancer (Table 15.6). Szegezdi et al. showed that MSCs were not sensitive to TRAIL-induced apoptosis because TRAIL receptors in MSCs were inactive and downstream genes of the TRAIL pathway were rarely expressed [119]. Another report also confirmed that TRAIL did not affect the MSC characteristics such as cell proliferation and differentiation into osteogenic and adipogenic lineages, and that it enhanced the migration of MSCs [120]. With these characteristics, the therapeutic efficacy of TRAIL-secreting MSCs was evaluated in various cancer models. MSC-mediated TRAIL delivery in a human/mouse xenograft model was performed by Mohr et al. [107]. They showed that TRAIL-expressing MSCs could reduce the growth of human lung carcinoma xenografted into immune-deficient mice. TRAIL-expressing MSCs were evaluated in different cancer types including glioma [108, 109, 111, 112, 116, 117], lung cancer [110], breast cancer [110, 113], squamous cancer [110], cervical cancer [110, 113], pancreatic cancer [113, 115], and colon cancer [113, 114, 118].
Several papers have shown that some cancers have a subset that are resistant to TRAIL-mediated apoptosis due to low levels of TRAIL receptors. To address this problem, a variety of methods to sensitize the cancer cells to TRAIL were applied; several laboratories conducted combination studies using TRAIL secreting MSCs in conjunction with drugs [113], RNAi [114], irradiation to the cancers [115], or 5-fluorouracil (5-FU) [118]. Grisendi et al. found that BT549 breast cancer cell lines survived in the high concentration of TRAIL due to the lack of the expression of TRAIL receptor, DR4 and DR5 [113]. They treated BT549 with the proteosome inhibitor PS-341, also known as Bortezomib, which upregulates expression of the DR5 receptor. With the combination of Bortezomib and TRAIL-producing MSCs, tumor apoptosis was increased. Mohr et al. investigated the use of RNAi in combination with TRAIL secreting MSCs to treat metastatic pancreatic carcinoma to treat TRAIL-resistant cells [115].
X-linked inhibitor of apoptosis protein (XIAP), which prevents apoptosis by inhibition of caspase-3 and caspase-9 activation, leading to the resistance to TRAIL treatment, was silenced by the shRNA technique and in combination with TRAIL-expressing MSCs and RNAi, metastatic pancreatic cancer in human to mouse xenograft models went into remission. TRAIL-secreting MSCs alone showed reduced growth of tumor but could not block the tumor growth enough. Sequential treatments of irradiation and TRAIL expressing MSCs showed killing of TRAIL-resistant glioma cells [116]. Mueller et al. found that a subset of colon carcinoma cells were resistant to TRAIL-induced apoptosis [118]. They injected 5-FU, which is an active form of prodrug to kill cancers, with TRAIL-expressing MSCs, and showed improved efficacy.
15.3.4.3 Prodrug Activators
Prodrug systems, which convert nontoxic prodrugs into cytotoxic materials, are also utilized to treat various types of cancer (detailed review in [121]). Currently, several types of prodrug activation systems are available. Cytosine deaminase (CD) converts nontoxic 5-fluorocytosine (5-FC) to 5-fluorouracil (5-FU) and herpes simplex virus (HSV) thymidine kinase (TK) is sensitive to ganciclovir. Activated prodrugs are known to kill cancers through the bystander effect [121]. Several papers have exploited the CD-mediated prodrug activation approach (Table 15.7). MSCs producing CD can convert 5-FC to 5-FU, then 5-FU diffuses out from MSCs to kill rapidly dividing cells. Several cancer models such as colon cancer [122], melanoma [123], gastric cancer [124], prostate cancer [125], glioma [126], and a rat glioblastoma model [127] have been evaluated with intravenously injected CD-producing MSCs and have shown inhibition of tumor growth. In the prostate cancer [125] and rat glioblastoma model [127], cytosine deaminase::uracil phosphoribosyltransferase (CD::UPRT), which is a better converter than CD alone, has been exploited.
For TK-mediated cancer treatment, TK-expressing MSCs were able to deliver cytotoxic effects to human glioblastoma cells, but delivery to HeLa cells and MCF7 breast cancer cells was not achieved with the same efficacy [128]. It turned out that cytotoxic effects were transferred into adjacent cells by gap junctions, and HeLa and MCF7 cells did not form gap junctions with MSCs, making TK-mediated induction of apoptosis less effective. Intravenously injected TK-expressing MSCs were effective in reducing tumor volume in the nude mouse model. The same approach was applied to prostate cancer [129] and glioma [130, 131]. Huang et al. reconstructed the gap junction connection by overexpressing Connexin43 in combination with TK [131]. Using this approach, they showed enhanced inhibition of tumor growth as compared to MSC therapy with TK-alone.
15.3.4.4 Viral Vector Delivery
Oncolytic viruses such as adenovirus are able to replicate and selectively kill cancer cells, sparing normal cells [132] (Table 15.1). Direct injection of oncolytic viruses to intratumoral sites showed efficacy and tumor regression in clinical trials, but intravenous injection did not [132]. However, it is well known that adenoviral vectors cause a host immune reaction. High concentrations of adenovirus can cause a transient elevation in liver enzymes, a sign of an immune reaction, and has been associated with severe adverse events.
Since MSCs are used as a vehicle to deliver to tumor sites and as a reservoir for the adenovirus, it is feasible to apply this approach for cancer treatment (Table 15.8). Komarova et al. first utilized MSCs to deliver oncolytic adenovirus [133]. Several papers also showed efficacy with adenovirus-loaded MSCs using a breast cancer lung metastasis xenograft model [134], intracranial glioma [135–137], ovarian cancer [133], and metastatic neuroblastoma [138]. Instead of adenovirus, Mader et al. utilized oncolytic measles virus-loaded MSCs to treat ovarian cancer in a xenograft model [139]. The measles virus is known to induce cytopathic effects on cancers, but native viruses are neutralized by preexisting antiviral antibodies. MSCs bearing measles viruses formed syncytia in the presence of antiviral antibodies and enhanced the survival of mice bearing tumors.
In Spain, there has been progress towards the clinical application of adenovirus-loaded MSCs to treat neuroblastoma [138]. In this study, 4 children from ages 2 to 5 with metastatic refractory stage IV neuroblastoma were infused at least twice with MSCs bearing oncolytic adenovirus. The clinical team followed the patient’s renal and liver functions, white and red blood cell and platelets counts, and they checked the adenovirus concentration in serum and urine every 2 weeks. One of the four patients showed that metastatic tumors had disappeared and is now in complete remission for 36 months after the first treatment.
15.3.5 Bone-Related Diseases
It is not surprising that one of the first human clinical trials using MSCs was to treat osteogenesis imperfecta, a genetic bone disease, because MSCs can form bone [9]. Several laboratories tried to enhance osteogenesis by overexpressing bone morphogenetic protein 2 (BMP2), insulin-like growth factor 1 (IGF1), VEGF, and human telomerase reverse transcriptase (hTERT) (Table 15.9). Shi et al. examined whether hTERT overexpression can maintain MSC proliferation and osteogenic differentiation potential in ex vivo culture [147]. An in vitro osteogenic differentiation assay in hTERT overexpressing MSCs showed more mineralized bone structure than unmodified MSCs. In their following paper, hTERT overexpressing MSCs were subcutaneously injected into beige mice and showed more osteogenic cells than MSCs alone, along with an increased osteogenic potential due to the upregulation of CBFA1, osterix, and osteocalcin [140].
Chang et al. evaluated the possibility of whether non-canonical Wnt-4 regulates the osteogenic pathway [141]. They tested two different bone defect models, a periodontal bone defect model and a craniofacial defect model with Wnt-4 expressing MSCs embedded in polylactic co-glycolide polymer scaffolds. Wnt-4 overexpressing MSCs increased osteogenesis and showed extensive periodontal bone regeneration and improved the repair of craniofacial defects in vivo. Several papers used BMP2 expressing MSCs to enhance bone formation. Li et al. evaluated BMP2-expressing canine MSCs on ulnar bone defects in the canine model [142]. Sixteen weeks after the transplantation, BMP2 overexpressing MSCs increased the area of newly formed bone and healed or partially healed all of the bone defects.
Ponnazhagan and colleagues looked at bone regeneration in an osteopenic mouse model with BMP2 expressing MSCs [143]. Intravenously injected BMP2 secreting MSCs enhanced bone mineral deposits and more trabecular bone formation than MSCs alone. Chen et al. engineered MSCs to express BMP2 and implanted them into a periodontal defect rabbit model [144]. BMP2 overexpressing MSCs regenerated cementum with Sharpey’s fiber and enhanced bone formation where it attached to periodontal fibers. To enhance bone formation compared to BMP2 expressing MSCs, Runx2, one of the master regulators of osteogenesis, was co-expressed [145]. Runx2/BMP2 co-expressing MSCs were embedded within a PLGA scaffold and implanted subcutaneously into athymic nude mice. BMP2/Runx2 expressing MSCs showed enhanced bone formation compared to MSC only and BMP2 expressing MSCs. Instead of BMP2, efficacy using IGF1 overexpressing MSCs was recently evaluated in a tibia fracture model [146]. IGF1 secreting MSCs were intravenously injected into the tail of insulin-receptor-substrate knock-out (Irs(−/−)) mice, which lack the ability to repair fractures. Authors claimed that IGF1 expressing MSCs improved new bone formation and restored the tibia fracture in Irs(−/−) mice. From the in vitro and in vivo assays, they showed that IGF1 induced osteogenesis via the Irs1-PI3K signaling pathway, with autocrine and paracrine effects.
15.3.6 Renal Failure
Most kidney diseases are related to the characteristics of ischemic, inflammatory and immunologic injury. MSC-mediated treatments were pursued as cellular therapy to improve these problems. It is known that erythropoietin (EPO) is downregulated at the end stage of renal failures. Eliopoulos et al. transduced EPO into murine MSCs and injected them subcutaneously into syngeneic mice with chronic renal failure [148] (Table 15.10). Among various doses, higher doses showed increased hematocrit levels to normal compared to controls and better survival of the mice. In a follow-up study, the same group co-introduced IGF-1 and EPO secreting mouse MSCs subcutaneously to the renal failure mouse model. An enhanced hematocrit level was achieved and cardiac function was improved [149]. Our group has also overexpressed EPO from human MSCs, in the late 1990s, and found a significant increase in hematocrit and differentiation of co-transplanted human hematopoietic stem cells to the red cell lineage [31]. However, toxicity occurred from the very high RBC counts resulting from the high unregulated dosages of EPO that MSCs can produce if the transgene is not under the control of a regulated inducible promoter (reviewed in [11]). These studies confirmed that MSCs can be powerful in vivo delivery vehicles, but suggest that, with growth factor expression, it will be important to regulate the amounts of protein produced.
For the acute renal failure model, Hagiwara et al. examined the production of kallikrein, which makes cells resistance to oxidative stress-induced apoptosis, from gene-modified modified MSCs. Kallikrein engineered rat MSCs showed significant reduction of apoptosis induced by H2O2 and inhibition of neutrophil and monocyte infiltration [150]. A recent paper showed VEGF-mediated protection and improvement of acute renal failure in a nude mouse model [151]. In that report, VEGF-engineered human fetal MSCs also showed better survival of renal epithelium by increased cell proliferation and reduced apoptosis, better renal function, and increased peritubular capillary density [151].
15.3.7 Neurological Diseases
MSC therapies have shown efficacy in preclinical models of various neurological diseases such as Parkinson’s disease, Huntington’s disease, multiple sclerosis, and stroke (detailed review in [152]). Here we review genetically engineered MSCs in preclinical models of Parkinson’s disease, Huntington’s disease, and amyotrophic lateral sclerosis (Table 15.11). Stroke models are reviewed in [157].
15.3.7.1 Huntington’s Disease
Huntington’s disease (HD) is an autosomal dominant neurodegenerative disease, which is caused by the excessive expression of cytotoxic polyglutamine (poly-Q) in the mutant huntingtin protein HTT and death of medium spiny neurons due to HTT toxicity and the lower expression of brain-derived neurotrophic factor (BDNF) [158]. Unfortunately, there is currently no cure for HD [159]. Canals et al. showed that disease onset is dependent on BDNF expression levels in the R6/2 HD mouse model and BDNF can improve symptoms and extend life span [160]. With this notion in mind, efficacy of MSCs expressing BDNF and nerve growth factor (NGF) transplantation in the YAC128 HD mouse model were evaluated [153]. For the YAC128 HD model, hyperkinesis starts at 3 months of age with progressive motor neuron impairment at 6 months of age and neurodegenerative features showing at 9 months of age. In the report by Dey et al., they transplanted BDNF secreting MSCs in a preventive manner at 4 months of age, which is ahead of the onset of motor neuron impairment. As expected, BDNF expressing MSCs transplanted into the striatum of HD mice showed significant improvement in motor function, as measured by performance on the rotarod, and significant reduction in levels of hindlimb clasping, a hallmark phenotype of affected HD mice. The least amount of neuronal loss within the striatum of the YAC128 mice at 13 months of age was observed in those transplanted with the growth factor-producing MSCs.
The underlying mechanisms for the beneficial effects from BDNF overexpressing MSCs are not completely known. These effects may represent a combination of the anti-apoptotic and axon-extending properties of MSCs, combined with the effects of the naturally produced neurotrophins in conjunction with the additional BDNF expression. BDNF therapies for HD have been extensively reviewed by Zuccato and Cattaneo [161–163]. It will be interesting to see if the effects of BDNF expressing MSCs can help to prevent the worsening of the symptoms or reverse the course of disease progression in this currently untreatable severe neurodegenerative disorder.
15.3.7.2 Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig’s disease, is a neurodegenerative disorder caused by the loss of motor neurons connected to muscle, and failure of this neuromuscular junction leads to paralysis of patients [152]. Most ALS cases are sporadic events and only 10% of them are familial cases. Currently, the underlying disease etiology of sporadic ALS is unknown but in familial cases, it is linked to point mutations of cytosolic Cu2+/Zn2+ superoxide dismutase 1 (SOD1). Currently, there are no treatments available that prevent neuromuscular decline to significantly delay the progression of ALS.
Glial cell-derived growth factor (GDNF) has been shown to have neuroprotective function in motor neurons of the SOD1 ALS mouse [164]. Following intramuscular transplantation of MSCs engineered to express GDNF in a rat model of familial ALS, Svendsen and colleagues showed an increased number of neuromuscular connections and motor neuron cell bodies in the spinal cord at mid-stages of the disease [154]. Furthermore, they showed that GDNF secreting MSCs could delay the disease progression and significantly extended lifespan in the SOD1 rat model [154].
15.3.7.3 Parkinson’s Disease
Parkinson’s disease (PD) is a neurodegenerative disease caused by the progressive degeneration of dopaminergic (DA) neurons in the midbrain [165]. Currently, there is no cure for PD, although implantation of deep brain stimulation devices and pharmacological agents can ameliorate clinical symptoms [166]. Different growth factors have been evaluated in an attempt to recover the damaged DA neurons, or to delay the rate of decline. GDNF secreting MSCs promoted rejuvenation of host striatal DA fibers and improvement in DA-dependent behavioral function in a rat model of PD [155]. Similar results were confirmed using BDNF secreting MSCs, where intrastriatally injected BDNF overexpressing MSCs showed improved clinical symptoms and rejuvenated striatal DA fibers in a rat model of Parkinson’s disease [156].
15.4 Beyond the Preclinical Models: Future Directions for Genetically Engineered MSC Therapy in Working Toward Human Clinical Trials
In human clinical trials, safety is one of the major concerns and it is critical to identify and minimize risks associated with treatment [46]. In current MSC culture protocols, MSCs are cultured in fetal bovine serum-containing media. To generate safe and clinically acceptable MSC expansion protocols, xeno-free cell culture media should be better developed to allow optimized growth and subsequent in vivo function, while minimizing the risk of transmitting pathogens or causing human immune reactions [167]. In the case of hESC culture, Martin et al. found that xenogeneic serum replacement is the source of nonhuman sialic acid Neu5Gc, which causes immunological reactions involving human antibodies [168]. Therefore, human clinical applications of MSCs would best employ chemically defined media.
Karyotypic stability is a highly important criterion for any cell type expanded in culture, prior to consideration of clinical trials. In contrast to murine MSCs which can easily undergo transformation in culture, there has been no documented evidence that human MSCs can be transformed during short-term in vitro expansion due to the development of chromosomal abnormalities. There has been one report that was retracted because it was found that the MSC cultures were contaminated with HeLa cells [169–171]. This type of contamination can occur readily in laboratories that use aspiration flasks to remove media from culture flasks, since all cell cultures are eventually mixed by regurgitation from the hose line, in spite of new sterile pipettes for each culture. Aspiration flaks must never be used in Good Laboratory Practices or in Good Manufacturing Practice Facilities, to ensure the identity of the culture. A second group, de la Fuente et al. retracted their paper because they could not reproduce the transformation data [172]. Nevertheless, well characterized in vitro MSC culture protocols and carefully adhered to standard operating procedures must be followed, along with the establishment of sensitive techniques to investigate chromosomal abnormalities.
Even though genetically engineered MSCs showed enhanced efficacy in the various preclinical disease models discussed here (including cardiovascular, cancer, bone formation defects, renal damage, neurological diseases, and others), there are so far no studies approved to move forward with human clinical trials. Currently, there are 123 human clinical trials registered using MSCs worldwide and all of the studies are utilizing unmodified MSCs [173].
There are barriers toward human clinical trials using genetically modified MSCs as there is no safe standard protocol to engineer MSCs to express transgenes. Each vector system has its own advantages and limitations with regard to efficacy and safety for the planned human clinical trials. As discussed earlier, most preclinical studies have utilized permanently integrating viral vectors as a delivery method for the gene of interest in order to continually express the transgene. Transient expression of genes of interest are not effective in most cases, but can be used to modify surface antigens of MSCs in order to increase MSC homing capacity and survival after transplantation. The risk of insertional mutagenesis caused by viral cassette integration into the host genome must be considered prior to the planned clinical trial. The potential risk to benefit ratio for that disorder or disease must be carefully evaluated, as is currently done for hematopoietic stem cell gene therapy applications [20].
Site-specific integration can be one of the options to eliminate insertional mutagenesis. It is well known that AAV vectors including rep protein integrate into chromosome 19 [174]. With rep, however, the cloning capacity of AAV vectors is reduced significantly. Annealing two inverted repeats (ITRs) can extend its cloning capacity to double the size of the insert [175]. These modified AAV vectors can be one of the options to avoid insertional mutagenesis with sustained expression of the genes of interest. Zinc-finger nuclease (ZFN)-mediated homologous recombination (HR) could be another option to modify MSCs safely, if success rates can be improved [176–179]. Zinc fingers have specific binding sites to DNA and engineered zinc-fingers with nucleases can cut the specific genomic regions of DNA. After the cleavage of specific DNA by ZFNs, the therapeutic cassette can be inserted by Homologous Recombination to create safe site-specific integration. Even though there are tremendous efforts to optimize ZFNs, nonspecific cleavages by ZFNs are still problematic [180].
Benabdallah et al. investigated whether ZFN-mediated targeted gene addition to safe harbor sites are possible [181]. They inserted Epo into the C–C chemokine receptor 5 (CCR5) gene loci, a putative safe harbor site, in MSCs by ZFN-mediated HR. Up to 40% of MSCs were successfully modified with EPO in CCR5 loci. Then, they injected these modified MSCs into NOD/SCID interleukin-2Rγ null (NSG) mice and these mice showed higher hemocrit levels in comparison with unmodified MSCs.
In the practical setting, identification of genetically engineered MSCs, which have safe harbor integrations of the transgenes, is restricted due to the limited lifespan of primary MSCs during in vitro expansion. Aging, moreover, significantly reduces the survival and differentiation potential of BM-MSCs [45]. hESC or hiPSC-derived MSCs can be considered in this case. With human pluripotent stem cells (hESC or hiPSC), a vector integration site could be mapped and cells with safe harbor integrations could potentially be expanded nearly indefinitely to generate differentiated MSCs with safe harbor integrations. Our group and others are working toward this future goal [46]. We have shown that pluripotent stem cell-derived MSCs can perform in vivo in a manner analogous to adult MSCs, by homing into areas of hypoxic injury [43].
With current techniques, it is difficult to track where the transplanted MSCs go in humans and to evaluate their long-term survival and function [14, 46]. Gene marking studies, using non-therapeutic genes such as eGFP or luciferase to track transplanted cells, are prohibited in clinical trials. Therefore, mysteries remain and can only be deciphered from large animal models. To ensure integrity and safety of the transplanted MSCs, suicide genes can be utilized to eliminate gene-modified MSCs if they are found to cause problems in patients [182, 183]. Schuldiner et al. showed that HSV-TK expressing hESCs had self-renewal and pluripotency and were sensitive to ganciclovir to kill cells [182]. They, moreover, could ablate teratoma that had arisen from the subcutaneous injection of undifferentiated TK-hESCs by intraperitoneal injection of ganciclovir. However, caution must be used when considering this strategy for MSCs, since TK has bystander effects on nearby cells and MSCs are known to effectively transfer the protein.
15.5 Conclusions
MSCs have been shown to be safe and have early evidence of efficacy in various clinical trials for heart attack, stroke, graft-vs.-host disease, and multiple sclerosis, among others [184–188], but some problems still need to be solved and efficiency and reproducibility need to be improved. Genetically modified MSCs can potentially overcome these barriers to increase the efficiency of therapy for many disorders. Given the possibility of immune reaction or insertional mutagenesis for vector transduced MSCs, long-term observation of modified MSCs must be followed carefully to meet safety regulations. The field looks to future applications of gene delivery to safe harbor sites to improve biosafety. Since they are powerful delivery vehicles and potent protein synthesis factories, the use of gene-modified MSCs to provide missing enzymes or growth factors and other signals to improve the repair of damaged or diseased tissues holds almost unlimited potential.
References
Van Laake LW, Van Hoof D, Mummery CL (2005) Cardiomyocytes derived from stem cells. Ann Med 37(7):499–512
Thomson JA et al (1998) Embryonic stem cell lines derived from human blastocysts. Science 282(5391):1145–7
Strauss S (2010) Geron trial resumes, but standards for stem cell trials remain elusive. Nat Biotechnol 28(10):989–90
Frantz S (2012) Embryonic stem cell pioneer Geron exits field, cuts losses. Nat Biotechnol 30(1):12–3
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4):663–76
Barrilleaux B, Knoepfler PS (2011) Inducing iPSCs to escape the dish. Cell Stem Cell 9(2):103–11
Zhao T et al (2011) Immunogenicity of induced pluripotent stem cells. Nature 474(7350):212–5
Patterson M et al (2011) Defining the nature of human pluripotent stem cell progeny. Cell Res. doi:10.1038/cr.2011.133
Parekkadan B, Milwid JM (2010) Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng 12:87–117
Copelan EA (2006) Hematopoietic stem-cell transplantation. N Engl J Med 354(17):1813–26
Meyerrose T et al (2010) Mesenchymal stem cells for the sustained in vivo delivery of bioactive factors. Adv Drug Deliv Rev 62(12):1167–74
Friedenstein AJ et al (1974) Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation 17(4):331–40
Ankrum J, Karp JM (2010) Mesenchymal stem cell therapy: two steps forward, one step back. Trends Mol Med 16(5):203–9
Karp JM, Leng Teo GS (2009) Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell 4(3):206–16
Capoccia BJ et al (2009) Revascularization of ischemic limbs after transplantation of human bone marrow cells with high aldehyde dehydrogenase activity. Blood 113(21):5340–51
Le Blanc K et al (2008) Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 371(9624):1579–86
Aggarwal S, Pittenger MF (2005) Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105(4):1815–22
Bauer G et al (2008) In vivo biosafety model to assess the risk of adverse events from retroviral and lentiviral vectors. Mol Ther 16(7):1308–15
Horwitz EM et al (1999) Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med 5(3):309–13
Waehler R, Russell SJ, Curiel DT (2007) Engineering targeted viral vectors for gene therapy. Nat Rev Genet 8(8):573–87
Mosca JD et al (2000) Mesenchymal stem cells as vehicles for gene delivery. Clin Orthop Relat Res 2000(379 Suppl): S71–S90
Meyerrose T et al (2006) Establishment and transduction of primary human stromal/mesenchymal stem cell monolayers. In: Nolta JA (ed) Genetic engineering of mesenchymal stem cells, Chap 2. Kluwer Academic, Dordrecht, the Netherlands
Meyerrose TE et al (2008) Lentiviral-transduced human mesenchymal stem cells persistently express therapeutic levels of enzyme in a xenotransplantation model of human disease. Stem Cells 26(7):1713–22
Stephenson J (2001) Studies illuminate cause of fatal reaction in gene-therapy trial. JAMA 285(20):2570
Manno CS et al (2006) Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med 12(3):342–7
Bushman F et al (2005) Genome-wide analysis of retroviral DNA integration. Nat Rev Microbiol 3(11):848–58
Cattoglio C et al (2007) Hot spots of retroviral integration in human CD34+ hematopoietic cells. Blood 110(6):1770–8
Brouard N et al (2000) Transplantation of gene-modified human bone marrow stromal cells into mouse-human bone chimeras. J Hematother Stem Cell Res 9(2):175–81
Ding L et al (1999) Bone marrow stromal cells as a vehicle for gene transfer. Gene Ther 6(9):1611–6
Fierro FA et al (2011) Effects on proliferation and differentiation of multipotent bone marrow stromal cells engineered to express growth factors for combined cell and gene therapy. Stem Cells 29(11):1727–37
Dao MA, Pepper KA, Nolta JA (1997) Long-term cytokine production from engineered primary human stromal cells influences human hematopoiesis in an in vivo xenograft model. Stem Cells 15(6):443–54
Papapetrou EP, Zoumbos NC, Athanassiadou A (2005) Genetic modification of hematopoietic stem cells with nonviral systems: past progress and future prospects. Gene Ther 12(Suppl 1):S118–30
McMahon JM et al (2006) Gene transfer into rat mesenchymal stem cells: a comparative study of viral and nonviral vectors. Stem Cells Dev 15(1):87–96
Stender S et al (2007) Adeno-associated viral vector transduction of human mesenchymal stem cells. Eur Cell Mater 13:93–99 (discussion 99)
Chng K et al (2007) Specific adeno-associated virus serotypes facilitate efficient gene transfer into human and non-human primate mesenchymal stromal cells. J Gene Med 9(1):22–32
Wang Z et al (2004) Detection of integration of plasmid DNA into host genomic DNA following intramuscular injection and electroporation. Gene Ther 11(8):711–21
Hacein-Bey-Abina S et al (2008) Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest 118(9):3132–42
Hacein-Bey-Abina S et al (2003) LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science 302(5644):415–9
Boztug K et al (2010) Stem-cell gene therapy for the Wiskott-Aldrich syndrome. N Engl J Med 363(20):1918–27
Montini E et al (2006) Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol 24(6):687–96
Montini E et al (2009) The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of HSC gene therapy. J Clin Invest 119(4):964–75
Aiuti A et al (2009) Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med 360(5):447–58
Gruenloh W et al (2011) Characterization and in vivo testing of mesenchymal stem cells derived from human embryonic stem cells. Tissue Eng A 17(11–12):1517–25
Bruder SP, Jaiswal N, Haynesworth SE (1997) Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem 64(2):278–94
Roobrouck VD, Ulloa-Montoya F, Verfaillie CM (2008) Self-renewal and differentiation capacity of young and aged stem cells. Exp Cell Res 314(9):1937–44
Jung Y, Bauer G, Nolta JA (2012) Induced pluripotent stem cell-derived mesenchymal stem cells: progress toward safe clinical products. Stem Cells 30(1):42–7
Meyerrose TE et al (2007) In vivo distribution of human adipose-derived mesenchymal stem cells in novel xenotransplantation models. Stem Cells 25(1):220–7
Mangi AA et al (2003) Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med 9(9):1195–201
Gnecchi M et al (2009) Early beneficial effects of bone marrow-derived mesenchymal stem cells overexpressing Akt on cardiac metabolism after myocardial infarction. Stem Cells 27(4):971–9
Yu YS et al (2010) AKT-modified autologous intracoronary mesenchymal stem cells prevent remodeling and repair in swine infarcted myocardium. Chin Med J (Engl) 123(13):1702–8
Wang D et al (2010) Connexin43 promotes survival of mesenchymal stem cells in ischaemic heart. Cell Biol Int 34(4):415–23
Wang X et al (2009) Hsp20-engineered mesenchymal stem cells are resistant to oxidative stress via enhanced activation of Akt and increased secretion of growth factors. Stem Cells 27(12):3021–31
Li W et al (2007) Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells 25(8):2118–27
Hu B et al (2010) Enhanced treatment of articular cartilage defect of the knee by intra-articular injection of Bcl-xL-engineered mesenchymal stem cells in rabbit model. J Tissue Eng Regen Med 4(2):105–14
Zeng B et al (2008) Effects of combined mesenchymal stem cells and heme oxygenase-1 therapy on cardiac performance. Eur J Cardiothorac Surg 34(4):850–6
Tsubokawa T et al (2010) Impact of anti-apoptotic and anti-oxidative effects of bone marrow mesenchymal stem cells with transient overexpression of heme oxygenase-1 on myocardial ischemia. Am J Physiol Heart Circ Physiol 298(5):H1320–9
Shu T et al (2010) HO-1 modified mesenchymal stem cells modulate MMPs/TIMPs system and adverse remodeling in infarcted myocardium. Tissue Cell 42(4):217–22
Zeng B et al (2010) Over-expression of HO-1 on mesenchymal stem cells promotes angiogenesis and improves myocardial function in infarcted myocardium. J Biomed Sci 17:80
Liang OD et al (2011) Mesenchymal stromal cells expressing heme oxygenase-1 reverse pulmonary hypertension. Stem Cells 29(1):99–107
Jiang YB et al (2011) Effects of heme oxygenase-1 gene modulated mesenchymal stem cells on vasculogenesis in ischemic swine hearts. Chin Med J (Engl) 124(3):401–7
Rosova I et al (2008) Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells 26(8):2173–82
Liu X et al (2006) Heme oxygenase-1 (HO-1) inhibits postmyocardial infarct remodeling and restores ventricular function. FASEB J 20(2):207–16
Hu X et al (2008) Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg 135(4):799–808
Hung SC et al (2007) Short-term exposure of multipotent stromal cells to low oxygen increases their expression of CX3CR1 and CXCR4 and their engraftment in vivo. PLoS ONE 2(5):e416
Rombouts WJ, Ploemacher RE (2003) Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia 17(1):160–70
Wynn RF et al (2004) A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood 104(9):2643–5
Sackstein R et al (2008) Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med 14(2):181–7
Guan M et al (2012) Directing mesenchymal stem cells to bone to augment bone formation and increase bone mass. Nat Med 18(3):456–62
Cheng Z et al (2008) Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol Ther 16(3):571–9
Huang W et al (2011) Mesenchymal stem cells overexpressing CXCR4 attenuate remodeling of postmyocardial infarction by releasing matrix metalloproteinase-9. Stem Cells Dev 21(5):778–89
Huang J et al (2010) Genetic modification of mesenchymal stem cells overexpressing CCR1 increases cell viability, migration, engraftment, and capillary density in the injured myocardium. Circ Res 106(11):1753–62
Rosamond W et al (2007) Heart disease and stroke statistics–2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 115(5):e69–171
Siu CW, Moore JC, Li RA (2007) Human embryonic stem cell-derived cardiomyocytes for heart therapies. Cardiovasc Hematol Disord Drug Targets 7(2):145–52
Bardy GH et al (2005) Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 352(3):225–37
Rose EA et al (2001) Long-term mechanical left ventricular assistance for end-stage heart failure. N Engl J Med 345(20):1435–43
Wang Y et al (2006) Combining pharmacological mobilization with intramyocardial delivery of bone marrow cells over-expressing VEGF is more effective for cardiac repair. J Mol Cell Cardiol 40(5):736–45
Gao F et al (2007) A promising strategy for the treatment of ischemic heart disease: mesenchymal stem cell-mediated vascular endothelial growth factor gene transfer in rats. Can J Cardiol 23(11):891–8
Matsumoto R et al (2005) Vascular endothelial growth factor-expressing mesenchymal stem cell transplantation for the treatment of acute myocardial infarction. Arterioscler Thromb Vasc Biol 25(6):1168–73
Duan HF et al (2003) Treatment of myocardial ischemia with bone marrow-derived mesenchymal stem cells overexpressing hepatocyte growth factor. Mol Ther 8(3):467–74
Guo Y et al (2008) Locally overexpressing hepatocyte growth factor prevents post-ischemic heart failure by inhibition of apoptosis via calcineurin-mediated pathway and angiogenesis. Arch Med Res 39(2):179–88
Guo YH et al (2008) Hepatocyte growth factor and granulocyte colony-stimulating factor form a combined neovasculogenic therapy for ischemic cardiomyopathy. Cytotherapy 10(8):857–67
Deuse T et al (2009) Hepatocyte growth factor or vascular endothelial growth factor gene transfer maximizes mesenchymal stem cell-based myocardial salvage after acute myocardial infarction. Circulation 120(11 Suppl):S247–54
Fierro F et al (2012) Effects on proliferation and differentiation of multipotent bone marrow stromal cells engineered to express growth factors for combined cell and gene therapy. Stem Cells 29(11):1727–37
Jemal A et al (2010) Cancer statistics, 2010. CA Cancer J Clin 60(5):277–300
Shah K (2012) Mesenchymal stem cells engineered for cancer therapy. Adv Drug Deliv Rev 64(8):739–48
Sasser AK et al (2007) Interleukin-6 is a potent growth factor for ER-alpha-positive human breast cancer. FASEB J 21(13):3763–70
Karnoub AE et al (2007) Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449(7162):557–63
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–74
Rosenberg SA (2001) Progress in human tumour immunology and immunotherapy. Nature 411(6835):380–4
Young HA (2006) Unraveling the pros and cons of interferon-gamma gene regulation. Immunity 24(5):506–7
Chen X et al (2008) A tumor-selective biotherapy with prolonged impact on established metastases based on cytokine gene-engineered MSCs. Mol Ther 16(4):749–56
Gao P et al (2010) Therapeutic potential of human mesenchymal stem cells producing IL-12 in a mouse xenograft model of renal cell carcinoma. Cancer Lett 290(2):157–66
Seo SH et al (2011) The effects of mesenchymal stem cells injected via different routes on modified IL-12-mediated antitumor activity. Gene Ther 18(5):488–95
Ryu CH et al (2011) Gene therapy of intracranial glioma using interleukin 12-secreting human umbilical cord blood-derived mesenchymal stem cells. Hum Gene Ther 22(6):733–43
Xu G et al (2009) Adenoviral-mediated interleukin-18 expression in mesenchymal stem cells effectively suppresses the growth of glioma in rats. Cell Biol Int 33(4):466–74
Nakamura K et al (2004) Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther 11(14):1155–64
Ren C et al (2008) Therapeutic potential of mesenchymal stem cells producing interferon-alpha in a mouse melanoma lung metastasis model. Stem Cells 26(9):2332–8
Sartoris S et al (2011) Efficacy assessment of interferon-alpha-engineered mesenchymal stromal cells in a mouse plasmacytoma model. Stem Cells Dev 20(4):709–19
Studeny M et al (2002) Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res 62(13):3603–8
Studeny M et al (2004) Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst 96(21):1593–603
Ling X et al (2010) Mesenchymal stem cells overexpressing IFN-beta inhibit breast cancer growth and metastases through Stat3 signaling in a syngeneic tumor model. Cancer Microenviron 3(1):83–95
Ren C et al (2008) Cancer gene therapy using mesenchymal stem cells expressing interferon-beta in a mouse prostate cancer lung metastasis model. Gene Ther 15(21):1446–53
Kidd S et al (2010) Mesenchymal stromal cells alone or expressing interferon-beta suppress pancreatic tumors in vivo, an effect countered by anti-inflammatory treatment. Cytotherapy 12(5):615–25
Reed JC (2003) Apoptosis-targeted therapies for cancer. Cancer Cell 3(1):17–22
Wiley SR et al (1995) Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 3(6):673–82
Walczak H et al (1999) Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med 5(2):157–63
Mohr A et al (2008) Mesenchymal stem cells expressing TRAIL lead to tumour growth inhibition in an experimental lung cancer model. J Cell Mol Med 12(6B):2628–2643
Kim SM et al (2008) Gene therapy using TRAIL-secreting human umbilical cord blood-derived mesenchymal stem cells against intracranial glioma. Cancer Res 68(23):9614–23
Sasportas LS et al (2009) Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proc Natl Acad Sci USA 106(12):4822–7
Loebinger MR et al (2009) Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer Res 69(10):4134–42
Yang B et al (2009) Dual-targeted antitumor effects against brainstem glioma by intravenous delivery of tumor necrosis factor-related, apoptosis-inducing, ligand-engineered human mesenchymal stem cells. Neurosurgery 65(3):610–624 (discussion 624)
Menon LG et al (2009) Human bone marrow-derived mesenchymal stromal cells expressing S-TRAIL as a cellular delivery vehicle for human glioma therapy. Stem Cells 27(9):2320–30
Grisendi G et al (2010) Adipose-derived mesenchymal stem cells as stable source of tumor necrosis factor-related apoptosis-inducing ligand delivery for cancer therapy. Cancer Res 70(9):3718–29
Luetzkendorf J et al (2010) Growth inhibition of colorectal carcinoma by lentiviral TRAIL-transgenic human mesenchymal stem cells requires their substantial intratumoral presence. J Cell Mol Med 14(9):2292–304
Mohr A et al (2010) Targeting of XIAP combined with systemic mesenchymal stem cell-mediated delivery of sTRAIL ligand inhibits metastatic growth of pancreatic carcinoma cells. Stem Cells 28(11):2109–20
Kim SM et al (2010) Irradiation enhances the tumor tropism and therapeutic potential of tumor necrosis factor-related apoptosis-inducing ligand-secreting human umbilical cord blood-derived mesenchymal stem cells in glioma therapy. Stem Cells 28(12):2217–28
Choi SA et al (2011) Therapeutic efficacy and safety of TRAIL-producing human adipose tissue-derived mesenchymal stem cells against experimental brainstem glioma. Neuro Oncol 13(1):61–9
Mueller LP et al (2011) TRAIL-transduced multipotent mesenchymal stromal cells (TRAIL-MSC) overcome TRAIL resistance in selected CRC cell lines in vitro and in vivo. Cancer Gene Ther 18(4):229–39
Szegezdi E et al (2009) Stem cells are resistant to TRAIL receptor-mediated apoptosis. J Cell Mol Med 13(11–12):4409–14
Secchiero P et al (2008) Tumor necrosis factor-related apoptosis-inducing ligand promotes migration of human bone marrow multipotent stromal cells. Stem Cells 26(11):2955–63
Cihova M, Altanerova V, Altaner C (2011) Stem cell based cancer gene therapy. Mol Pharm 8(5):1480–7
Kucerova L et al (2007) Adipose tissue-derived human mesenchymal stem cells mediated prodrug cancer gene therapy. Cancer Res 67(13):6304–13
Kucerova L et al (2008) Cytosine deaminase expressing human mesenchymal stem cells mediated tumour regression in melanoma bearing mice. J Gene Med 10(10):1071–82
You MH et al (2009) Cytosine deaminase-producing human mesenchymal stem cells mediate an antitumor effect in a mouse xenograft model. J Gastroenterol Hepatol 24(8):1393–400
Cavarretta IT et al (2010) Adipose tissue-derived mesenchymal stem cells expressing prodrug-converting enzyme inhibit human prostate tumor growth. Mol Ther 18(1):223–31
Chang DY et al (2010) The growth of brain tumors can be suppressed by multiple transplantation of mesenchymal stem cells expressing cytosine deaminase. Int J Cancer 127(8):1975–83
Altanerova V et al (2011) Human adipose tissue-derived mesenchymal stem cells expressing yeast cytosinedeaminase::uracil phosphoribosyltransferase inhibit intracerebral rat glioblastoma. Int J Cancer 130(10):2455–63
Matuskova M et al (2010) HSV-tk expressing mesenchymal stem cells exert bystander effect on human glioblastoma cells. Cancer Lett 290(1):58–67
Song C et al (2011) Thymidine kinase gene modified bone marrow mesenchymal stem cells as vehicles for antitumor therapy. Hum Gene Ther 22(4):439–49
Bak XY, Yang J, Wang S (2010) Baculovirus-transduced bone marrow mesenchymal stem cells for systemic cancer therapy. Cancer Gene Ther 17(10):721–9
Huang Q et al (2010) The anti-glioma effect of suicide gene therapy using BMSC expressing HSV/TK combined with overexpression of Cx43 in glioma cells. Cancer Gene Ther 17(3):192–202
Eager RM, Nemunaitis J (2011) Clinical development directions in oncolytic viral therapy. Cancer Gene Ther 18(5):305–17
Komarova S et al (2006) Mesenchymal progenitor cells as cellular vehicles for delivery of oncolytic adenoviruses. Mol Cancer Ther 5(3):755–66
Stoff-Khalili MA et al (2007) Mesenchymal stem cells as a vehicle for targeted delivery of CRAds to lung metastases of breast carcinoma. Breast Cancer Res Treat 105(2):157–67
Sonabend AM et al (2008) Mesenchymal stem cells effectively deliver an oncolytic adenovirus to intracranial glioma. Stem Cells 26(3):831–41
Yong RL et al (2009) Human bone marrow-derived mesenchymal stem cells for intravascular delivery of oncolytic adenovirus Delta24-RGD to human gliomas. Cancer Res 69(23):8932–40
Ahmed AU et al (2011) A comparative study of neural and mesenchymal stem cell-based carriers for oncolytic adenovirus in a model of malignant glioma. Mol Pharm 8(5):1559–72
Garcia-Castro J et al (2010) Treatment of metastatic neuroblastoma with systemic oncolytic virotherapy delivered by autologous mesenchymal stem cells: an exploratory study. Cancer Gene Ther 17(7):476–83
Mader EK et al (2009) Mesenchymal stem cell carriers protect oncolytic measles viruses from antibody neutralization in an orthotopic ovarian cancer therapy model. Clin Cancer Res 15(23):7246–55
Gronthos S et al (2003) Telomerase accelerates osteogenesis of bone marrow stromal stem cells by upregulation of CBFA1, osterix, and osteocalcin. J Bone Miner Res 18(4):716–22
Chang J et al (2007) Noncanonical Wnt-4 signaling enhances bone regeneration of mesenchymal stem cells in craniofacial defects through activation of p38 MAPK. J Biol Chem 282(42):30938–48
Li H et al (2007) Bone regeneration by implantation of adipose-derived stromal cells expressing BMP-2. Biochem Biophys Res Commun 356(4):836–42
Kumar S, Nagy TR, Ponnazhagan S (2010) Therapeutic potential of genetically modified adult stem cells for osteopenia. Gene Ther 17(1):105–16
Chen YL et al (2008) Periodontal regeneration using ex vivo autologous stem cells engineered to express the BMP-2 gene: an alternative to alveolaplasty. Gene Ther 15(22):1469–77
Lee SJ et al (2010) Enhancement of bone regeneration by gene delivery of BMP2/Runx2 bicistronic vector into adipose-derived stromal cells. Biomaterials 31(21):5652–9
Granero-Molto F et al (2011) Mesenchymal stem cells expressing insulin-like growth factor-I (MSC(IGF)) promote fracture healing and restore new bone formation in Irs1 knock-out mice: analyses of MSC(IGF) autocrine and paracrine regenerative effects. Stem Cells 29(10):1537–48
Shi S et al (2002) Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression. Nat Biotechnol 20(6):587–91
Eliopoulos N et al (2006) Erythropoietin delivery by genetically engineered bone marrow stromal cells for correction of anemia in mice with chronic renal failure. J Am Soc Nephrol 17(6):1576–84
Kucic T et al (2008) Mesenchymal stromal cells genetically engineered to overexpress IGF-I enhance cell-based gene therapy of renal failure-induced anemia. Am J Physiol Renal Physiol 295(2):F488–96
Hagiwara M et al (2008) Kallikrein-modified mesenchymal stem cell implantation provides enhanced protection against acute ischemic kidney injury by inhibiting apoptosis and inflammation. Hum Gene Ther 19(8):807–19
Yuan L et al (2011) VEGF-modified human embryonic mesenchymal stem cell implantation enhances protection against cisplatin-induced acute kidney injury. Am J Physiol Renal Physiol 300(1):F207–18
Joyce N et al (2010) Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med 5(6):933–46
Dey ND et al (2010) Genetically engineered mesenchymal stem cells reduce behavioral deficits in the YAC 128 mouse model of Huntington’s disease. Behav Brain Res 214(2):193–200
Suzuki M et al (2008) Direct muscle delivery of GDNF with human mesenchymal stem cells improves motor neuron survival and function in a rat model of familial ALS. Mol Ther 16(12):2002–10
Glavaski-Joksimovic A et al (2010) Glial cell line-derived neurotrophic factor-secreting genetically modified human bone marrow-derived mesenchymal stem cells promote recovery in a rat model of Parkinson’s disease. J Neurosci Res 88(12):2669–81
Somoza R et al (2010) Intranigral transplantation of epigenetically induced BDNF-secreting human mesenchymal stem cells: implications for cell-based therapies in Parkinson’s disease. Biol Blood Marrow Transplant 16(11):1530–40
van Velthoven CT et al (2009) Regeneration of the ischemic brain by engineered stem cells: fuelling endogenous repair processes. Brain Res Rev 61(1):1–13
Zuccato C et al (2001) Loss of Huntingtin-mediated BDNF gene transcription in Huntington’s disease. Science 293(5529):493–8
Nagahara AH, Tuszynski MH (2011) Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov 10(3):209–19
Canals JM et al (2004) Brain-derived neurotrophic factor regulates the onset and severity of motor dysfunction associated with enkephalinergic neuronal degeneration in Huntington’s disease. J Neurosci 24(35):7727–39
Zuccato C, Cattaneo E (2007) Role of brain-derived neurotrophic factor in Huntington’s disease. Prog Neurobiol 81(5–6):294–330
Zuccato C, Cattaneo E (2009) Brain-derived neurotrophic factor in neurodegenerative diseases. Nat Rev Neurol 5(6):311–22
Zuccato C, Valenza M, Cattaneo E (2010) Molecular mechanisms and potential therapeutical targets in Huntington’s disease. Physiol Rev 90(3):905–81
Acsadi G et al (2002) Increased survival and function of SOD1 mice after glial cell-derived neurotrophic factor gene therapy. Hum Gene Ther 13(9):1047–59
Schapira AH, Jenner P (2011) Etiology and pathogenesis of Parkinson’s disease. Mov Disord 26(6):1049–55
Morley JF, Hurtig HI (2010) Current understanding and management of Parkinson disease: five new things. Neurology 75(18 Suppl 1):S9–15
Halme DG, Kessler DA (2006) FDA regulation of stem-cell-based therapies. N Engl J Med 355(16):1730–5
Martin MJ et al (2005) Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med 11(2):228–32
Rubio D et al (2005) Spontaneous human adult stem cell transformation. Cancer Res 65(8):3035–9
Rosland GV et al (2009) Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res 69(13):5331–9
Armesilla-Diaz A, Elvira G, Silva A (2009) p53 regulates the proliferation, differentiation and spontaneous transformation of mesenchymal stem cells. Exp Cell Res 315(20):3598–610
de la Fuente R et al (2010) Retraction: spontaneous human adult stem cell transformation. Cancer Res 70(16):6682
Trounson A et al (2011) Clinical trials for stem cell therapies. BMC Med 9:52
Smith RH (2008) Adeno-associated virus integration: virus versus vector. Gene Ther 15(11):817–22
Lai CM, Lai YK, Rakoczy PE (2002) Adenovirus and adeno-associated virus vectors. DNA Cell Biol 21(12):895–913
Urnov FD et al (2005) Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature 435(7042):646–51
Lombardo A et al (2007) Gene editing in human stem cells using zinc finger nucleases and integrase-defective lentiviral vector delivery. Nat Biotechnol 25(11):1298–306
Moehle EA et al (2007) Targeted gene addition into a specified location in the human genome using designed zinc finger nucleases. Proc Natl Acad Sci USA 104(9):3055–60
Szczepek M et al (2007) Structure-based redesign of the dimerization interface reduces the toxicity of zinc-finger nucleases. Nat Biotechnol 25(7):786–93
Segal DJ (2011) Zinc-finger nucleases transition to the CoDA. Nat Methods 8(1):53–5
Benabdallah BF et al (2010) Targeted gene addition to human mesenchymal stromal cells as a cell-based plasma-soluble protein delivery platform. Cytotherapy 12(3):394–9
Schuldiner M, Itskovitz-Eldor J, Benvenisty N (2003) Selective ablation of human embryonic stem cells expressing a “suicide” gene. Stem Cells 21(3):257–65
Naujok O et al (2010) Selective removal of undifferentiated embryonic stem cells from differentiation cultures through HSV1 thymidine kinase and ganciclovir treatment. Stem Cell Rev 6(3):450–61
Choi YH, Kurtz A, Stamm C (2011) Mesenchymal stem cells for cardiac cell therapy. Hum Gene Ther 22(1):3–17
Miettinen JA et al (2012) Left ventricular functional recovery after intracoronary injection of autologous bone marrow-derived stem cells in patients with acute myocardial infarction: a dose-response pilot study. Int J Cardiol 154(3):354–6
Hare JM et al (2009) A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol 54(24):2277–86
Kebriaei P et al (2009) Adult human mesenchymal stem cells added to corticosteroid therapy for the treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant 15(7):804–11
Connick P et al (2012) Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. Lancet Neurol 11(2):150–6
Acknowledgement
We thank the California Institute for Regenerative Medicine (CIRM), National Institute of Health (NIH), and philanthropic donors for supporting our research. We apologize to our colleagues whose work could not be cited due to the space limitation.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Jung, Y., Nolta, J.A. (2013). Genetically Engineered Mesenchymal Stem Cells for Cell and Gene Therapy. In: Chase, L., Vemuri, M. (eds) Mesenchymal Stem Cell Therapy. Stem Cell Biology and Regenerative Medicine. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-62703-200-1_15
Download citation
DOI: https://doi.org/10.1007/978-1-62703-200-1_15
Published:
Publisher Name: Humana Press, Totowa, NJ
Print ISBN: 978-1-62703-199-8
Online ISBN: 978-1-62703-200-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)