Abstract
This chapter addresses the relationship between the gut microflora and the neurogastrointestinal system. It is divided into several sections that further dissect the contribution of the bacterial content of the gut towards the development of motility disorders, with a main focus on the most common functional and visceral hypersensitivity disorder—irritable bowel syndrome (IBS).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Irritable Bowel Syndrome
- Bacterial Overgrowth
- Irritable Bowel Syndrome Symptom
- Visceral Hypersensitivity
- Migrate Motor Complex
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
This chapter addresses the relationship between the gut microflora and the neurogastrointestinal system. It is divided into several sections that further dissect the contribution of the bacterial content of the gut towards the development of motility disorders, with a main focus on the most common functional and visceral hypersensitivity disorder—irritable bowel syndrome (IBS).
The Effect of the Brain on Gut Environment
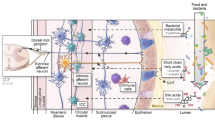
Communication between the brain and the gut (see Fig. 5.1) is modulated by the autonomic nervous system, both sympathetic and parasympathetic. This gut–brain axis controls gut functions ranging from gastrointestinal secretions to motility and immune response. In turn, the vitality of the gut microbiome, at least in part, is determined by the gastrointestinal transit and motility which, when impaired, can affect the delivery of nutrients to the microbiota. For example, impaired intestinal transit caused by disarray of the migrating motor complexes (MMC) can result in the development of small bowel bacterial overgrowth [1]. Disordered MMC contractions are common in IBS where decreased MMC contractions in the small bowel are seen in constipation-predominant IBS, and accelerated intestinal transit in diarrhea-predominant IBS [2]. In addition, the autonomic nervous system modulates gastrointestinal mucus secretion which forms the biofilm, home to the many of the enteric microbiota [3]. It also influences immune activation of the gut, directly, through modulation of the response of the gut immune cells to luminal bacteria, or indirectly, through modification of the ability of luminal bacteria to reach the gut immunocytes. Interestingly, stress and stressful stimuli can enhance the permeability of the intestinal epithelium, which allows bacterial antigens to cross the intestinal epithelium triggering an immune response in the intestinal mucosa [4–9] and causing a significant reduction in the tight junction proteins which leads to a compromise in the epithelial barrier function and the development of leaky gut [10]. Therefore, the brain and its axis can greatly influence the gut milieu.
Mucosal-Gut Microbial Interaction
Stress can also play an important role in mucosal-microbiome interaction and host protection. For example, secretion of mucosal defensins, which are antimicrobial peptides, can play an important role in host defense mechanisms against inflammatory and infectious diseases of the gastrointestinal tract [11]. The secretion of such defensins by Paneth cells is enhanced by stress [12]. Stress can also impact the secretion of neuroendocrine signaling molecules such as catecholamines, serotonin, and cytokines, which are secreted by neurons, immune cells, and enterochromaffin cells, into the gut lumen in response to different stress stimuli [13–15]. Furthermore, norepinephrine release in the intestine during stress and trauma induces expression of virulence factors in Pseudomonas aeruginosa which contributes to gut-derived sepsis [16], stimulates the growth of several other strains of enteric pathogens, and intensify the virulence of Campylobacter jejuni [17]. These reports can help us appreciate the association of stressful events with the development of gastrointestinal disease such gastroenteritis and subsequent development of post infectious IBS [18].
Bidirectional Signaling
The gut microbiome, much like the enteric nervous system, can affect the intestinal motility. For example, Lactobacillus acidophilus and Bifidobacterium bifidum are capable of promoting motility, while other members of the gut microbiome such as Escherichia species can inhibit or slow down the intestinal transit [19]. Gut bacteria can also modulate gut transit indirectly through the production of microbial metabolites such as short-chain fatty acids or peptides such as N-formylmethionyl–leucine–phenylalanine [20–22]. Disturbance in the intricate balance between different enteric microbial populations might, therefore, predispose the host to altered gut motility and secretion, which results in diarrhea or constipation. These changes are, in turn, likely to influence the balance of enteric microbiota. Therefore, the gut microbiome can directly influence gut homeostasis by the regulation of bowel motility and modulation of visceral pain and immune responses [23–26] (Fig. 5.1).
Irritable Bowel Syndrome
IBS is a common disorder afflicting millions of adults and children around the world. From the pediatric perspective, it is estimated that IBS affects up to 25% of school-age children and adolescents, accounts for a significant number (2–4%) of office visits to primary care doctors, and represents about 25–50% of all patients who visit a gastroenterologist’s clinic [27]. The past several years have witnessed an emergence of new concepts related to the pathophysiology of IBS, which include alterations in gut motility, small-bowel bacterial overgrowth, microscopic inflammation, visceral hypersensitivity, and changes related to the brain–gut microbial axis.
Altered Gut Motility
In IBS, disordered gut motility has been observed along the length of the gastrointestinal tract [28–31]. The major migrating complex (MMC), which consists of periodic, contractions that sweep the luminal contents from the stomach to the colon, becomes disorganized. Several studies have shown that patients with IBS tend to have abnormalities in these contractions. For example, Vassallo et al. measured colonic electronic barostat and perfusion manometry in 16 subjects and demonstrated a greater frequency of prolonged, high amplitude and greater preprandial colonic motility, which may explain the increased perception of pain in these patients. Other studies assessing colonic transit times in IBS, showed a shorter colonic transit in patients with diarrhea-predominant IBS [32, 33] consistent with the presenting symptomatololgy. More importantly, the dysmotility seen in these patients can predispose them to develop small-bowel bacterial overgrowth, which is another proposed etiology for the development of IBS symptoms.
The Contribution of the Gut Microbiome Towards IBS
Small-Bowel Bacterial Overgrowth
Small-bowel bacterial overgrowth has emerged as a possible cause of IBS since Vantrappen and colleagues’ work which suggested that it may occur in specific motility disorder such as a reduction in the major migrating complex [1]. Further studies have confirmed this finding in this patient population [34–36]. A large study of 202 patients with IBS found that 78% of these patients had evidence of bacterial overgrowth demonstrated by abnormal lactulose-methane-hydrogen breath testing. In the study, 25 of 47 patients experienced eradication of bacterial overgrowth on follow-up after treatment with antibiotics. Analysis of this subset of patients revealed that those who had successful eradication of bacterial overgrowth reported improvement in their IBS symptoms [37]. Another study by Pimentel showed that 84% of 111 patients with IBS had abnormal breath testing. These patients were then randomized to receive neomycin or placebo for 1 week. A follow-up questionnaire revealed that patients in the neomycin group reported a 35% reduction in symptomatology as compared to 11.4% in the placebo group [38]. More recently, Peralta and colleagues assessed 97 patients who met Rome II criteria and found that 56% of these patients had positive lactulose breath tests [39].
Although the exact mechanisms by which altered fecal flora induce disease are poorly understood, it has been shown that fecal short chain fatty acids produced by microbiota, which are critical for maintenance of the colonic epithelium, are significantly reduced in children with diarrhea-predominant IBS [40]. Symptoms especially related to gas production are reduced by an exclusion diet, suggesting an alteration in the activity of hydrogen-consuming bacteria and further emphasizing the importance of fermentation in the pathogenesis of IBS [41]. Lactulose breath testing in IBS subjects does not seem to reflect malabsorption but the pattern of hydrogen excretion is suggestive of bacterial overgrowth [42] and suggests that IBS might be associated with rapid excretion of gaseous products of fermentation [43]. On the other hand, increased bacterial methane production was seen with constipation-predominant IBS [44]. Postprandial serotonin release was also blunted [45], suggesting a possible neurochemical basis for impaired motor function. The discovery that specific changes in gut microbiota contribute to IBS pathophysiology could aid in the development of new therapeutic strategies [46, 47].
The Gut Microbiome in IBS
The gut microbiome is the array of microorganisms that dwell along the human gastrointestinal tract. The human microbiota is estimated to contain as many as 1014 bacterial cells-a number that is 10 times greater than the number of human cells present in our bodies [48–50]. The microbiota colonizes every surface in contact with the external environment but the colon is the most heavily colonized and is estimated to contain over 70% of all the organisms in the human body. The human gut has a large surface area [51] and is rich in nutrients, making it a preferred site for bacterial colonization. The architecture of this population is dynamic and evolves from birth to adulthood. It is influenced by the diet, state of health, external environment and other similar factors. The microbiome is closely associated with many aspects of human health, from nutritional status to immune and stress response. The intricate balance in the make-up of the gut microbial population, as well as the presence or absence of key microbial elements is crucial in ensuring health of the host. Although it is embraced as largely beneficial, it has been postulated that altered bacterial populations or products of bacterial metabolism may contribute to the development of disease in the gastrointestinal tract as well as remote areas of the body. The mechanisms through which microbiota exerts its beneficial or negative influences on the host include the production of signaling pathways and recognition of bacterial proteins by intestinal epithelial and mucosal host immune cells.
Recent data propose a role for the gut microbiota in the development of both central and peripheral neural processes. These interactions, termed the “brain–gut–enteric microbiota axis” [52], as discussed, can be bidirectional, with potential ramifications for disruption of this axis leading to abnormal neurogenic stimulation of the enteric system and the development of disorders such as IBS. The activation of any of the central nervous system and the gut–brain axis has potential in influencing enteric microbiota both directly through interaction between the gut microbiome and the host, and indirectly via changes in their environment [3].
In animal studies, the impact of stress on the composition of the enteric microbiota has become evident [53, 54]. Stress was characterized by transient reductions in the levels of the enteric microbiota in rhesus monkeys. In postnatal, maternal separation-induced stress, reduction in Lactobacilli was associated with the appearance of stress-indicative behaviors.
So is there a quantitative difference in the gut bacteria in IBS? Several studies are beginning to address this question. Osipov and colleagues demonstrate that the concentration of Streptomycetes, Rhodococci, and other members of the Actinomycetales order become dozen folds higher in quantity [55]. In another study by Malinen, a reduction of Lactobacilli, Clostridium coccoides, and Bifidobacterium catenulatem counts were seen in diarrhea-predominant IBS compared with healthy individuals [56]. Si and colleagues noted a reduction of fecal Bifidobacteria and an increase in Enterobacteriaceae, as well as lowered resistance to microbial colonization of the bowel in patients with IBS [57]. Taken together, these studies show encouraging associations between the gut microbiome and IBS.
Intestinal Inflammation
Considerable attention has been recently directed towards the possibility of microscopic inflammation as a contributor to the development of IBS [58, 59]. Low-grade inflammation found in biopsies of different parts of the intestine in subjects with IBS has fueled this concept [47, 58, 60, 61]. The release of certain inflammatory mediators such as cytokines, interleukins, and histamine, may affect nearby enteric nerves, causing alteration in gut function and sensory perception leading to IBS symptomatology [60, 62]. The study by Chadwick et al. led the way to the new concept of IBS as an inflammatory condition. In their study of 77 IBS subjects, 55% were diarrhea predominant; and none had a confirmed infectious origin for IBS [58]. While 38 subjects had normal histology, 31 demonstrated microscopic inflammation and 8 fulfilled histologic criteria for lymphocytic colitis. Interestingly, even in the group with normal histology, immunohistology demonstrated inflammation with increased intraepithelial lymphocytes as well as an increase in CD3+ and CD25+ cells in the lamina propria. Therefore, all subjects had mucosal immune activation.
Additional studies further support the role of inflammation in IBS. Gonsalkorale and colleagues demonstrated that subjects with IBS have a reduction in interleukin-10 (IL-10), which has an anti-inflammatory effect [63]. Barbara and colleagues’ work demonstrate an increase in colonic mast cell degranulation with direct correlation between the proximity of mast cells in the mucosa and clinical pain severity [62]. Furthermore, Tornblom and colleagues examined full-thickness jejunal biopsies in 10 subjects obtained during laparoscopy [64] and noted low-grade infiltration of lymphocytes in the myenteric plexus in all patients and many had evidence of neuronal degeneration, longitudinal muscle hypertrophy and abnormalities in the number and size of interstitial cells of Cajal. There is also evidence to support an alteration in the ratio between the cytokines IL-10 and IL-12 favoring a Th1 response similar to what is seen in peripheral blood mononuclear cells [65]. Spiller further proposed that the inflammatory changes could represent an immune response to an initial enteric infection in individuals who become susceptible by a relative deficiency of anti-inflammatory cytokines [66].
Although embracing this theory broadens therapeutic options, yet efforts to treat the inflammation in an attempt to improve symptoms have been largely unsuccessful. Subjects with post-infectious IBS randomized to either prednisolone 30 mg daily versus placebo showed no improvement in their symptoms even though T lymphocytes decreased by 22% as compared to 11.5% in the placebo group [67]. Therefore, the clinical significance and application of this important concept of IBS being an inflammatory condition are yet to be defined.
Modulation of Visceral Hypersensitivity
Visceral hypersensitivity is becoming more recognized as a potential contributor to the development of pain in IBS. Studies are now beginning to utilize the concept of the gut microbiome and probiotics to modulate this visceral hypersensitivity. Animal studies using the probiotic Lactobacillus farciminis demonstrate significant attenuation of stress-induced proteins during colorectal distension in rats and suggest a link to the epithelial cell cytoskeletal contraction [25, 26]. A study by Verdu showed that administration of Lactobacillus paracasei attenuates the antibiotic induced visceral hypersensitivity in mice [68]. Perhaps the most interesting link between gut bacteria and visceral sensitivity is highlighted by Rousseaux et al. [69] establishing that oral administration of L. acidophilus induced the expression of mu-opioid and cannabinoid receptors in epithelial cells, and mediated analgesic functions in the intestine in a manner similar to that induced by morphine.
Potential Therapeutic Applications
The modification of the enteric microbiota to treat subjects with IBS and visceral hypersensitivity is attractive due to its ease, relative safety, and current lack of other effective therapeutic alternatives. Such modifications can be achieved by the administration of antibiotics, probiotics, or prebiotics. Clinical studies have produced variable responses to such treatments and vary depending on age, predominant symptoms, and bowel habits. Our current understanding of IBS pathophysiology remains incomplete, and although the complexity of the network of interactions within the enteric microbiota and visceral hypersensitivity is emerging, some studies have provided evidence for a beneficiary role for enhancement or manipulation of the gut bacteria. The use of nonabsorbable antimicrobial therapies such as Rifaximin has shown some promise and probiotics and prebiotics are also emerging as potential therapies. A recent study by Pimentel [70] validated that rifaximin therapy for 2 weeks provided significant relief of IBS symptoms, bloating, abdominal pain, and diarrhea. In another study, 54 patients with positive lactulose breath tests were treated with a 7-day course of rifaximin. Follow-up after 3 weeks revealed that half of their subjects had a subsequent negative lactulose breath test and a statistically significant improvement in symptoms. These results were similar to those found in another recent study by Majewski and associates, in which a 4-week course of rifaximin led to improvement in IBS-related symptoms and a negative breath test in patients who previously had positive tests [71]. Although these results are encouraging, other researchers have failed to confirm these findings and further research is needed in this area [72].
An important study by O’Mahony et al. [65] showed that Bifidobacterium infantis not only resulted in symptom reduction in IBS, but also correlated with normalization of proinflammatory cytokines, suggesting an immune modulating effect of probiotics. The study by Bazzocchi et al. [73] is the first observation showing a clinical improvement related to changes in the composition of the fecal bacterial flora, fecal biochemistry and colonic motility pattern, all of which was induced by administration of probiotics, in patients with functional diarrhea. In constipation-predominant IBS, Agrawal and colleagues [74] show improvements in abdominal girth and gastrointestinal transit, as well as reduced symptomatology after 4 weeks of Bifidobacerterium lactis consumption.
Pediatric studies addressing the role of probiotics in IBS have recently emerged (Table 5.1). A study published by Bausserman and Michail [75] designed to determine whether oral administration of the probiotic Lactobacillus GG under randomized, placebo-controlled, double-blinded conditions would improve symptoms of IBS in children, showed a lower incidence of perceived abdominal distension but did not alter any of the other parameters. Another double-blinded, randomized controlled trial by Gawronska et al. [76] designed to determine the efficacy of a 4-week therapy with Lactobacillus rhamnosus GG (LGG) in treating functional abdominal pain disorders (FAPD) in children showed a higher incidence of treatment success (i.e., no pain) in children with IBS receiving the probiotic. A more recent study by Guandalini and colleagues [77] suggests a beneficial role for VSL#3 (Sigma-Tau Pharmaceuticals, Inc.) in children with IBS. Finally, reports of amelioration of symptoms of bloating and flatulence in patients with IBS when treated with a poorly absorbed antibiotic, Rifaximin [77, 78], and the poorly absorbed antibiotic Neomycin has been effective in reducing symptoms and decreasing hydrogen and methane production in IBS [38, 79], suggesting major role for intestinal bacteria as a contributor to symptoms of IBS.
Conclusions
Strong evidence suggests that the gut microbiome plays an important role in functional gastrointestinal disorders and interactions between the gut and the nervous system influence intestinal motility and inflammation. Although several reports suggest a disruption in the balance of the enteric microbiota in patients with IBS, considerably more data are needed to establish whether these changes are merely seen due to the dysmotility or indeed there is a causative role for these findings. While most of the studies addressing the role of the enteric bacteria rely on traditional culture techniques to identify the microbiome, studies utilizing molecular technology in identifying the microbiota would prove useful in further investigating the role of these bacteria in gastrointestinal symptomatology and disease. Results from a small number of well-designed, randomized, controlled, clinical trials suggest that, not only does regular intake of certain probiotic bacteria help to treat the symptoms of IBS, but their effects go beyond symptoms and are associated with modulation of biological parameters, such as intestinal transit, abdominal girth and inflammatory markers that in turn influence the gut–brain axis.
Further understanding of the gut microbiota will improve our knowledge of their role in health and disease, and allow for improved future therapeutic and prophylactic modalities. Although significant strides have been made in our journey of deciphering the codes of the gut microbiome, our ability to delve deep into this fascinating organ has been hampered by the complexities of its inhabitants. The introduction of non-culture-based molecular techniques that enable quantitative assessment of the entire enteric microbiota coupled with encouraging results from probiotic research continue to improve our understanding in this area.
References
Vantrappen G, et al. The interdigestive motor complex of normal subjects and patients with bacterial overgrowth of the small intestine. J Clin Invest. 1977;59(6):1158–66.
Wang SH, et al. A research of migrating motor complex in patients with irritable bowel syndrome. Zhonghua Nei Ke Za Zhi. 2009;48(2):106–10.
Macfarlane S, Dillon JF. Microbial biofilms in the human gastrointestinal tract. J Appl Microbiol. 2007;102(5):1187–96.
Kiliaan AJ, et al. Stress stimulates transepithelial macromolecular uptake in rat jejunum. Am J Physiol. 1998;275(5 Pt 1):G1037–44.
Groot J, et al. Stress-induced decrease of the intestinal barrier function. The role of muscarinic receptor activation. Ann N Y Acad Sci. 2000;915:237–46.
Yates DA, et al. Adaptation of stress-induced mucosal pathophysiology in rat colon involves opioid pathways. Am J Physiol Gastrointest Liver Physiol. 2001;281(1):G124–8.
Soderholm JD, et al. Neonatal maternal separation predisposes adult rats to colonic barrier dysfunction in response to mild stress. Am J Physiol Gastrointest Liver Physiol. 2002;283(6):G1257–63.
Soderholm JD, et al. Chronic stress induces mast cell-dependent bacterial adherence and initiates mucosal inflammation in rat intestine. Gastroenterology. 2002;123(4):1099–108.
Jacob C, et al. Mast cell tryptase controls paracellular permeability of the intestine. Role of protease-activated receptor 2 and beta-arrestins. J Biol Chem. 2005;280(36):31936–48.
Demaude J, et al. Phenotypic changes in colonocytes following acute stress or activation of mast cells in mice: implications for delayed epithelial barrier dysfunction. Gut. 2006;55(5):655–61.
Salzman NH, et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 2010;11(1):76–83.
Alonso C, et al. Maladaptive intestinal epithelial responses to life stress may predispose healthy women to gut mucosal inflammation. Gastroenterology. 2008;135(1):163–172 e1.
Stephens RL, Tache Y. Intracisternal injection of a TRH analogue stimulates gastric luminal serotonin release in rats. Am J Physiol. 1989;256(2 Pt 1):G377–83.
Santos J, et al. Release of mast cell mediators into the jejunum by cold pain stress in humans. Gastroenterology. 1998;114(4):640–8.
Hughes DT, Sperandio V. Inter-kingdom signalling: communication between bacteria and their hosts. Nat Rev Microbiol. 2008;6(2):111–20.
Alverdy J, et al. Gut-derived sepsis occurs when the right pathogen with the right virulence genes meets the right host: evidence for in vivo virulence expression in Pseudomonas aeruginosa. Ann Surg. 2000;232(4):480–9.
Cogan TA, et al. Norepinephrine increases the pathogenic potential of Campylobacter jejuni. Gut. 2007;56(8):1060–5.
Dunlop SP, et al. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology. 2003;125(6):1651–9.
Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453(7195):620–5.
Barbara G, et al. Interactions between commensal bacteria and gut sensorimotor function in health and disease. Am J Gastroenterol. 2005;100(11):2560–8.
Malbert CH. The ileocolonic sphincter. Neurogastroenterol Motil. 2005;17 Suppl 1:41–9.
Dass NB, et al. The relationship between the effects of short-chain fatty acids on intestinal motility in vitro and GPR43 receptor activation. Neurogastroenterol Motil. 2007;19(1):66–74.
Husebye E, et al. Influence of microbial species on small intestinal myoelectric activity and transit in germ-free rats. Am J Physiol Gastrointest Liver Physiol. 2001;280(3):G368–80.
Rhee SH, et al. Pathophysiological role of Toll-like receptor 5 engagement by bacterial flagellin in colonic inflammation. Proc Natl Acad Sci USA. 2005;102(38):13610–5.
Ait-Belgnaoui A, et al. Lactobacillus farciminis treatment attenuates stress-induced overexpression of Fos protein in spinal and supraspinal sites after colorectal distension in rats. Neurogastroenterol Motil. 2009;21(5):567–73. e18–9.
Ait-Belgnaoui A, et al. Lactobacillus farciminis treatment suppresses stress induced visceral hypersensitivity: a possible action through interaction with epithelial cell cytoskeleton contraction. Gut. 2006;55(8):1090–4.
Everhart JE, Renault PF. Irritable bowel syndrome in office-based practice in the United States. Gastroenterology. 1991;100(4):998–1005.
Di Lorenzo C, et al. Antroduodenal manometry in children and adults with severe non-ulcer dyspepsia. Scand J Gastroenterol. 1994;29(9):799–806.
Fenton TR, Harries JT, Milla PJ. Disordered small intestinal motility: a rational basis for toddlers’ diarrhoea. Gut. 1983;24(10):897–903.
Vassallo MJ, et al. Colonic tone and motility in patients with irritable bowel syndrome. Mayo Clin Proc. 1992;67(8):725–31.
Cann PA, et al. Irritable bowel syndrome: relationship of disorders in the transit of a single solid meal to symptom patterns. Gut. 1983;24(5):405–11.
Manabe N, et al. Lower functional gastrointestinal disorders: evidence of abnormal colonic transit in a 287 patient cohort. Neurogastroenterol Motil. 2010;22(3):293-e82.
Horikawa Y, et al. Gastrointestinal motility in patients with irritable bowel syndrome studied by using radiopaque markers. Scand J Gastroenterol. 1999;34(12):1190–5.
de Boissieu D, et al. Small-bowel bacterial overgrowth in children with chronic diarrhea, abdominal pain, or both. J Pediatr. 1996;128(2):203–7.
Pimentel M, et al. Methane, a gas produced by enteric bacteria, slows intestinal transit and augments small intestinal contractile activity. Am J Physiol Gastrointest Liver Physiol. 2006;290(6):G1089–95.
Pimentel M, et al. Lower frequency of MMC is found in IBS subjects with abnormal lactulose breath test, suggesting bacterial overgrowth. Dig Dis Sci. 2002;47(12):2639–43.
Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol. 2000;95(12):3503–6.
Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome. a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2003;98(2):412–9.
Peralta S, et al. Small intestine bacterial overgrowth and irritable bowel syndrome-related symptoms: experience with Rifaximin. World J Gastroenterol. 2009;15(21):2628–31.
Treem WR, et al. Fecal short-chain fatty acids in patients with diarrhea-predominant irritable bowel syndrome: in vitro studies of carbohydrate fermentation. J Pediatr Gastroenterol Nutr. 1996;23(3):280–6.
King TS, Elia M, Hunter JO. Abnormal colonic fermentation in irritable bowel syndrome. Lancet. 1998;352(9135):1187–9.
Pimentel M, Kong Y, Park S. Breath testing to evaluate lactose intolerance in irritable bowel syndrome correlates with lactulose testing and may not reflect true lactose malabsorption. Am J Gastroenterol. 2003;98(12):2700–4.
Dear KL, Elia M, Hunter JO. Do interventions which reduce colonic bacterial fermentation improve symptoms of irritable bowel syndrome? Dig Dis Sci. 2005;50(4):758–66.
Pimentel M, et al. Methane production during lactulose breath test is associated with gastrointestinal disease presentation. Dig Dis Sci. 2003;48(1):86–92.
Pimentel M, Kong Y, Park S. IBS subjects with methane on lactulose breath test have lower postprandial serotonin levels than subjects with hydrogen. Dig Dis Sci. 2004;49(1):84–7.
Quigley EM. Bacterial flora in irritable bowel syndrome: role in pathophysiology, implications for management. J Dig Dis. 2007;8(1):2–7.
Barbara G, et al. New pathophysiological mechanisms in irritable bowel syndrome. Aliment Pharmacol Ther. 2004;20 Suppl 2:1–9.
Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124(4):837–48.
Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–33.
Whitman WB, Coleman DC, Wiebe WJ. Prokaryotes: the unseen majority. Proc Natl Acad Sci USA. 1998;95(12):6578–83.
Gebbers JO, Laissue JA. Immunologic structures and functions of the gut. Schweiz Arch Tierheilkd. 1989;131(5):221–38.
Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6(5):306–14.
Schaedler RW, Dubos RJ. The fecal flora of various strains of mice. Its bearing on their susceptibility to endotoxin. J Exp Med. 1962;115:1149–60.
Bailey MT, Coe CL. Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Dev Psychobiol. 1999;35(2):146–55.
Osipov GA, et al. [Clinical significance of studies of microorganisms of the intestinal mucosa by culture biochemical methods and mass fragmentography]. Eksp Klin Gastroenterol. 2003;(4):59–67, 115
Malinen E, et al. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am J Gastroenterol. 2005;100(2):373–82.
Si JM, et al. Intestinal microecology and quality of life in irritable bowel syndrome patients. World J Gastroenterol. 2004;10(12):1802–5.
Chadwick VS, et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology. 2002;122(7):1778–83.
Gwee KA, et al. Increased rectal mucosal expression of interleukin 1beta in recently acquired post-infectious irritable bowel syndrome. Gut. 2003;52(4):523–6.
Weston AP, et al. Terminal ileal mucosal mast cells in irritable bowel syndrome. Dig Dis Sci. 1993;38(9):1590–5.
Faure C, et al. Serotonin signaling is altered in irritable bowel syndrome with diarrhea but not in functional dyspepsia in pediatric age patients. Gastroenterology. 2010;139(1):249–58.
Barbara G, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126(3):693–702.
Cremonini F, et al. Effect of CCK-1 antagonist, dexloxiglumide, in female patients with irritable bowel syndrome: a pharmacodynamic and pharmacogenomic study. Am J Gastroenterol. 2005;100(3):652–63.
Tornblom H, et al. Full-thickness biopsy of the jejunum reveals inflammation and enteric neuropathy in irritable bowel syndrome. Gastroenterology. 2002;123(6):1972–9.
O’Mahony L, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128(3):541–51.
Spiller RC. Role of nerves in enteric infection. Gut. 2002;51(6):759–62.
Dunlop SP, et al. Randomized, double-blind, placebo-controlled trial of prednisolone in post-infectious irritable bowel syndrome. Aliment Pharmacol Ther. 2003;18(1):77–84.
Verdu EF, et al. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55(2):182–90.
Rousseaux C, et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med. 2007;13(1):35–7.
Pimentel M, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364(1):22–32.
Majewski M, McCallum RW. Results of small intestinal bacterial overgrowth testing in irritable bowel syndrome patients: clinical profiles and effects of antibiotic trial. Adv Med Sci. 2007;52:139–42.
Yamini D, Pimentel M. Irritable bowel syndrome and small intestinal bacterial overgrowth. J Clin Gastroenterol. 2010;44(10):672–5.
Bazzocchi G, et al. Intestinal microflora and oral bacteriotherapy in irritable bowel syndrome. Dig Liver Dis. 2002;34 Suppl 2:S48–53.
Agrawal A, et al. Clinical trial: the effects of a fermented milk product containing Bifidobacterium lactis DN-173-010 on abdominal distension and gastrointestinal transit in irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2008;29(1):104–14.
Bausserman M, Michail S. The use of Lactobacillus GG in irritable bowel syndrome in children: a double-blind randomized control trial. J Pediatr. 2005;147(2):197–201.
Gawronska A, et al. A randomized double-blind placebo-controlled trial of Lactobacillus GG for abdominal pain disorders in children. Aliment Pharmacol Ther. 2007;25(2):177–84.
Guandalini S, et al. VSL#3 improves symptoms in children with irritable bowel syndrome: a multicenter, randomized, placebo-controlled, double-blind, crossover study. J Pediatr Gastroenterol Nutr. 2010;51(1):24–30.
Sharara AI, et al. A randomized double-blind placebo-controlled trial of rifaximin in patients with abdominal bloating and flatulence. Am J Gastroenterol. 2006;101(2):326–33.
Frissora CL, Cash BD. Review article: the role of antibiotics vs. conventional pharmacotherapy in treating symptoms of irritable bowel syndrome. Aliment Pharmacol Ther. 2007;25(11):1271–81.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media New York
About this chapter
Cite this chapter
Michail, S., Ng, MW. (2013). Inflammation, Microflora, Motility, and Visceral Sensitivity. In: Faure, C., Di Lorenzo, C., Thapar, N. (eds) Pediatric Neurogastroenterology. Clinical Gastroenterology. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-60761-709-9_5
Download citation
DOI: https://doi.org/10.1007/978-1-60761-709-9_5
Published:
Publisher Name: Humana Press, Totowa, NJ
Print ISBN: 978-1-60761-708-2
Online ISBN: 978-1-60761-709-9
eBook Packages: MedicineMedicine (R0)