Abstract
Dietary supplements are widely marketed and available to health-care consumers who wish to lower their cholesterol levels. As dietary supplements are not considered drugs, there is a lack of regulation in their production and also a deficiency of appropriately designed trials demonstrating their efficacy and safety when used alone or in combination with prescription medications. However, using supplements in place of or in addition to prescribed cholesterol-lowering therapies may be more aligned with the patient’s value system or health-care philosophy. Dietary supplements, such as red yeast rice (RYR), have been shown to have the same mode of action as prescription cholesterol-lowering therapies like 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors. While others, like soluble dietary fiber (SDF), have multiple proposed modes of action. Reasonable data exist to support the use of dietary supplements—in addition to lifestyle modification—as an alternative, or add-on therapy, in those who are unable or unwilling to take conventional lipid-lowering agents.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Dietary supplement
- Lipid-lowering plant extract
- Hypercholesterolemia
- Low-density lipoprotein cholesterol
- Statin intolerance
Introduction
The United States Dietary Supplement and Health Education Act categorizes the use of botanical or natural medicines, including those used for the treatment of hypercholesterolemia, as “dietary supplements” [1]. Patients tend to seek out alternative or complimentary therapies for one of three reasons. First, they may be dissatisfied with conventional or prescription medications that have been ineffective, harmful, too costly, or technologically oriented. Second, the selection of alternative therapies may give the patient a greater sense of autonomy and empowerment with respect to their healthcare decisions. Third, and most commonly, alternative therapies are more compatible with the patient’s beliefs, values, and healthcare philosophy [2].
There is a perception by the general public that botanical products are inherently safe because they are natural and have been used as traditional folk remedies. Little attention is paid to the lack of evidence of their efficacy or safety in well-designed controlled trials. Consumers do not consider that these products may be adulterated with prescription medications or contaminated with harmful substances, as there is a lack of regulation and standardization for composition, biological activity, safety, and reporting of adverse events [3, 4].
Around 20 % of the US population take botanical supplements with the highest consumption in older non-Hispanic white women [5]. The use of supplements in those over 65 years of age is increasing and it is worth noting that almost 30 % of people in this age range also take five or more prescription medications [6, 7]. As less than half of patients disclose the use of supplements to their physician and < 1 % to their pharmacist, there is significant potential for medication interactions [6].
Numerous dietary supplements are taken to lower cholesterol; however, many do not demonstrate efficacy or safety in well-designed clinical trials, and struggle with the limitations listed above. The evidence for the more commonly used supplements is reviewed in this chapter while polyphenols, isoflavones, and plant sterols are reviewed elsewhere.
Red Yeast Rice
Red Yeast Rice (RYR) is a traditional Asian food item that is used to flavor, color, and preserve food (Fig. 23.1). The medicinal value of RYR was first promoted during the Tang dynasty, around AD 800, to aid digestion and circulation [8]. RYR consists mainly of nonglutinous rice, red yeast (Monascus purpureus), and fermentation by-products comprising of polyketides known as monacolins, fatty acids, and trace elements. In 1979, Endo discovered monacolin K, a polyketide that inhibits 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, an early rate-limiting step in cholesterol synthesis [9, 10]. Monacolin K, also known as mevinolin or lovastatin, accounts for around 90 % of the total monacolin fraction in RYR. Monacolin K and its hydroxyl acid form, monacolin KA, are the predominant active ingredients in most commercially available formulations of RYR, but RYR also contains plant sterols, isoflavones, and cis-monounsaturated fatty acids, which are less potent cholesterol-lowering agents [8, 11, 12].
RYR supplements are widely available over the counter and contain unpredictable concentrations of the active constituents. When Gordon et al. analyzed 12 RYR products, there was remarkable variability in the levels of total monacolins (0.31–11.15 mg/capsule), monacolin K (0.10–10.09 mg/capsule), and monacolin KA (0.00–2.30 mg/capsule) [13]. Also, one third of the products contained elevated levels of citrinin, a toxic byproduct of fermentation that is known to be mutagenic and nephrotoxic [14, 15]. The US Food and Drug Administration (FDA) has ruled that RYR is a drug and not a dietary supplement because it contains monacolin K (lovastatin), so manufacturers were told to modify their products so that they do not contain this active component [16, 17]. In spite of this, RYR supplements continue to be sold in the USA with varying but sometimes significant amounts of monacolin K [8].
Multiple clinical trials have evaluated the efficacy of RYR in lowering cholesterol. In one study, 83 subjects with hypercholesterolemia were randomized to 2.4 g/day of RYR (0.4 % monacolins by weight) or placebo. After 12 weeks of treatment, as compared to the placebo, RYR significantly reduced total cholesterol (16.1 %), low-density lipoprotein (LDL) cholesterol (22.4 %), and triglycerides (11.3 %). There was no significant effect on high-density lipoprotein (HDL) cholesterol concentration. The treatment was well tolerated with no hepatic or renal function abnormalities noted in any of the participants. However, there was a single report of musculoskeletal chest pain at week 12 in the treatment group [18].
RYR has demonstrated utility in those who have difficulty tolerating conventional cholesterol-lowering therapy with HMG-CoA reductase inhibitors (statins) due to statin-induced myalgia, myopathy, elevated liver transaminases, and gastrointestinal upset. In a placebo-controlled trial, 62 patients with a history of statin-induced myalgia were randomized to receive 3.6 g/day of RYR (6.48 mg monacolins/day) for 24 weeks. There was no increase in liver transaminases, creatine kinase (CK), or pain scores, but significant reductions in total cholesterol (14.9 %) and LDL-cholesterol (21.3 %) were achieved [19].
Investigators from China randomized 4870 subjects with a history of coronary artery disease (CAD) and dyslipidemia to receive either a placebo or RYR extract containing 12 mg of monacolin K daily for 4.5 years. When compared to placebo, the treatment group had significant reductions in total-cholesterol (10.9 %), LDL-cholesterol (17.6 %), non-HDL cholesterol (16.6 %), and triglycerides (14.6 %), and there was a small but significant increase in HDL-cholesterol of 4.2 %. There was a 45 % relative reduction in coronary events in addition to 30 and 33 % reductions in cardiovascular and total mortality, respectively. Total adverse events and study discontinuation were reported as similar in both groups. There were also minor transient changes in liver transaminase and CK levels of unreported severity or duration in both groups [20].
As monacolins are HMG-CoA reductase inhibitors, it is not surprising that RYR shares the potential adverse effects of prescription statin medications, including myalgia, myopathy, rhabdomyolysis, and elevated liver transaminases [21–24]. Patients should be cautioned not to use RYR preparations in conjunction with statins or medications that affect their metabolism, such as those that utilize the cytochrome P450 3A4 pathway. Allergy to RYR appears to be rare but there is a case report of an anaphylactic reaction in a German butcher who used RYR as an ingredient in sausages [25].
RYR products that contain the active monacolins are effective in lowering total cholesterol, LDL-cholesterol, and triglycerides. The monacolin content varies between brands and even batches of commercially available preparations, which leads to inconsistencies in the dose and efficacy. It may be useful as an alternative lipid-lowering agent in patients who are intolerant to prescription statin therapy, but similar precautions should be taken, as it is an HMG-CoA reductase inhibitor with a similar side effect profile. RYR may also be useful in the secondary prevention of those with established cardiovascular disease but additional research and outcomes data are needed.
Soluble Dietary Fiber (SDF)
The FDA recommends the addition of 3 g/day of β-glucan or 7 g/day of SDF to a low saturated fat, low-cholesterol diet in order to reduce cholesterol and the risk of coronary heart disease (CHD). The Administration issued this recommendation after reviewing 33 clinical studies that evaluated the effect of supplementary dietary fiber on lipid levels [26].
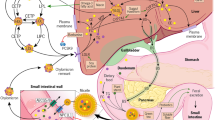
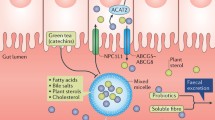
Soluble fiber is thought to lower total and LDL-cholesterol concentrations through several mechanisms. Intestinal absorption of cholesterol is reduced in the presence of soluble fiber, which may be due to viscous soluble fiber forming a physical barrier to absorption, alteration in the emulsification of dietary fat, reduction in the formation of small mixed micelles that are more efficiently absorbed, or a combination [27, 28]. In human studies, soluble fiber increases the fecal excretion of bile acids and cholesterol, resulting in greater bile acid synthesis from circulating cholesterol in the blood [29, 30]. Soluble fiber reduces postprandial glucose absorption and insulin levels, which may reduce cholesterol synthesis, as insulin is a stimulator of HMG-CoA reductase [31–33]. Finally, diets high in fiber can lead to changes in gut microbiota that may have multiple beneficial effects on glucose and lipid metabolism beyond the intestinal lumen [34]. Fermentation of soluble fiber by gut flora produces short-chain fatty acids such as acetate, propionate, and butyrate, which may suppress hepatic cholesterol production [35, 36].
β-glucans are the principal components of the endosperm cell walls in cereals such as oats and barley. They are highly viscous, soluble, nonstarch polysaccharides consisting of linear glucose chains with varying molecular weights depending on the method of extraction [37]. There have been multiple studies and meta-analyses that have evaluated the effect of oat β-glucan on cholesterol concentrations. Brown et al. [38] evaluated data from 25 controlled trials and found that an average of 5 g/day of soluble oat fiber significantly reduced total, HDL, and LDL-cholesterols. There was a significant dose–response effect that for every gram of soluble oat fiber consumed per day, the total cholesterol decreased by 1.4 mg/dL, LDL cholesterol 1.2 mg/dL, and HDL-cholesterol 0.07 mg/dL, but there was no effect on triglyceride concentrations.
A more recent review assessed data from 20 clinical trials, one systematic review and the above meta-analysis. Of the studies reviewed, 70 % reported a significant reduction in circulating cholesterol concentrations and the authors concluded that daily doses of at least 3 g/day of β-glucan result in 5–10 % reductions in total cholesterol and LDL-cholesterol in normocholesterolemic or hypercholesterolemic individuals. The authors found that β-glucan is more effective at lowering LDL-cholesterol when given in a liquid form than when it is delivered in solid form like a muffin [39]. For example, men with moderate hypercholesterolemia experienced a 6 % lowering in both total cholesterol and LDL-cholesterol after drinking oat milk containing 3.8 g of β-glucan per day [40]. Whereas, Kerckhoffs et al. found no significant reduction in cholesterol in either group when they randomized 48 subjects to bread and cookies with wheat fiber or 5.9 g of β-glucan per day. In a subsequent experiment reported in the same paper, when the subjects were given orange juice containing 5 g/day of β-glucan or wheat fiber, the β-glucan group experienced a 7 % reduction in total cholesterol compared with the controls [41]. It is proposed that the cholesterol-lowering efficacy of the baked products may be attenuated because exposure to heat reduces the molecular weight and viscosity of β-glucan polymers [42].
Psyllium is another popular soluble fiber that is the mucilaginous seed husk of the Plantago ovata plant (Fig. 23.2). The active cholesterol-lowering component of psyllium is believed to be arabinoxylan, a polysaccharide with a xylose backbone and arabinose side chains [43]. As early as 1965, Garvin et al. reported a 9 % reduction in total cholesterol in participants consuming 9.3 g/day of psyllium hydrocolloid for 3 weeks [44]. When Brown et al. [38] analyzed data from 12 trials with a daily dose range of 2–10 g of psyllium, they found that for every gram taken per day, there was a 1.4 mg/dL reduction in total cholesterol, and in the four studies evaluating LDL-cholesterol, a 2.6 mg/dL reduction. The decrease in HDL-cholesterol was trivial (0.15 mg/dL per gram of psyllium) but significant, whereas there was no significant effect on triglycerides.
The meta-analysis that included 21 studies by Wei et al. [45] reported that the use of psyllium (3–20.4 g/day) is associated with a lowering of total cholesterol by 14.5 mg/dL and LDL-cholesterol by 10.8 mg/dL in subjects with mild-to-moderate hypercholesterolemia. Based on their findings, the authors calculated that the consumption of psyllium 5, 10, 15 g/day could result in 5.6, 9.0, and 12.5 % decreases in LDL-cholesterol, respectively. As opposed to β-glucan, the form in which psyllium was consumed, e.g., bulk laxative or enriched food, did not significantly affect the degree of cholesterol lowering. Similar to studies described earlier, the authors noted a significant but minimal reduction in HDL-cholesterol and no effect on triglycerides. These findings are similar to those of Anderson et al. [46] who included data from three unpublished studies in a meta-analysis of eight controlled trials. They found that consuming 10.2 g of psyllium per day, as part of a low-fat diet, reduced total cholesterol by 4 %, LDL cholesterol by 7 %, and the ratio of apolipoprotein B to apolipoprotein A-I by 6 %.
The cholesterol-lowering effects of dietary fiber appear to be additive when used in conjunction with a low-fat diet and statin therapy. In a placebo-controlled study of hypercholesterolemic patients with a mean baseline LDL-cholesterol of 173 mg/dL, 8 weeks of treatment with 10 mg of simvastatin plus 15 g of psyllium husk (Metamucil®) per day reduced LDL-cholesterol levels by 63 mg/dL (36 %), which was the same amount as 20 mg of simvastatin alone [47].
Soluble fiber is generally well tolerated but should be introduced gradually to avoid gastrointestinal upset. Anaphylaxis and allergies to SDF preparations, separate from food intolerances, are rare but have been reported in isolated cases along with hypersensitivity in health-care workers with occupational exposures to psyllium [48–50]. Certain prescription medications, such as oral contraceptives or antidepressants, should be taken at a different time to the SDF supplements as the fiber can interfere with their rate of absorption and the total absorbed dose [51].
SDF works in multiple ways to beneficially reduce total cholesterol and LDL-cholesterol as part of a low-fat diet. Liquid or unheated forms may be more effective because the reduction in molecular weight and viscosity that occurs with cooking may decrease its cholesterol-lowering efficacy. SDF may be useful in patients who are unable to tolerate other cholesterol-lowering agents, as an add-on for those on maximal doses of other agents who are not at goal, or to minimize the dose of statin used and its potential side effects. The two most popular forms of SDF are β-glucan and psyllium. If patients would like to incorporate these fibers into their diet, they should aim for at least 3 g/day of β-glucan and 15 g/day of psyllium to achieve significant cholesterol-lowering.
Nuts
Nuts are a recognized sources of poly- and cis-monounsaturated fatty acids, dietary fiber, vitamins, minerals, and bioactive compounds like phytosterols and polyphenols [52]. In particular, walnuts have a very high ratio of polyunsaturated fatty acids, and almonds are rich in cis-monounsaturated fatty acids [53]. Although the majority of trials have evaluated the cholesterol-lowering effects of walnuts and almonds, other nuts, e.g., pistachios and macadamias, have also been shown to reduce cholesterol levels [53–56]. Recent evidence suggests that consuming > 3 servings of nuts/week as part of a Mediterranean diet is associated with a 30–55 % lower rate of cardiovascular events and mortality. The protective effect of nuts is thought to be due in part to their beneficial effects on lipid metabolism [57, 58].
Zambon et al. carried out a randomized crossover trial with 55 hypercholesterolemic Spanish men and women consuming a Mediterranean diet as the control treatment. In half of the patients, 35 % of the dietary energy from cis-monounsaturated fat was replaced with walnuts (41–56 g/day) for 6 weeks. When compared with the control group, the walnut group experienced a decrease of 4.1 % in total cholesterol of and 5.9 % in LDL cholesterol [59].
A study of almond supplementation in hyperlipidemic subjects found a significant dose–response reduction in cholesterol levels. Groups receiving 37 g/day experienced 3.1 % reduction in total cholesterol and 4.4 % reduction in LDL-cholesterol, while the group that received 73 g/day experienced reductions of 5.6 and 9.4 %, respectively. There was a significant increase in HDL-cholesterol of 4.6 % in the low-dose and 3.8 % in the high-dose almond groups but no significant changes in triglycerides [60].
A recent pooled analysis of 25 trials evaluated the effect of nut consumption on lipid levels in subjects with normal and elevated cholesterol levels. The authors found that the lipid-lowering effect of nuts was dose-related but similar between different varieties of nuts. Cholesterol reduction was greatest in those consuming a Western diet with higher baseline LDL-cholesterol and lower body mass index. An average daily intake of 67 g of nuts corresponded to a reduction in total cholesterol by 10.9 mg/dL (5.1 %) and LDL-cholesterol by 10.2 mg/dL (7.4 %). In those with triglycerides > 150 mg/dL, plasma levels were reduced by 20.6 mg/dL (10.2 %). When triglyceride levels were < 150 mg/dL, nut consumption did not have a significant effect on HDL-cholesterol or on triglyceride levels [61].
Patients should be encouraged to incorporate nuts as part of a balanced calorie-controlled diet. As nuts are calorie-dense, they should be used to displace less healthy foods instead of an additional source of high-fat calories. While the magnitude of cholesterol reduction is small, the numerous nutritional components in nuts (monounsaturated fatty acids, fiber, etc.) may provide additive health benefits to complement other cholesterol-lowering strategies.
Flaxseed
Flax (Linum usitatissimum) is a flowering crop that bears golden-brown seeds (Fig. 23.3). It has been cultivated since 6000 BC and the ancient Greeks and Romans valued the seeds for their laxative effects. Today, flaxseed is consumed as whole seeds, ground (meal or powder), or as an expressed oil [62]. Whole flaxseed is comprised of 41 % fat, 28 % dietary fiber, and 21 % protein. It has a unique fatty acid profile of 73 % polyunsaturated fatty acids, 18 % monounsaturated fatty acids, and 9 % saturated fatty acids. Linoleic acid, an omega-6 fatty acid, makes up approximately 16 % of the total fatty acids , and alpha-linolenic acid (ALA), an ω-3 fatty acid, constitutes around 57 %. Flax is also rich in both soluble and insoluble fiber along with the plant lignan, secoisolariciresinol diglycoside (SDG) [63].
The dietary supplementation of whole flaxseed, flaxseed oil, and lignans has been shown to reduce blood cholesterol in animal studies [64–67]. Flaxseed is thought to lower cholesterol through several mechanisms. First, the SDF component may reduce intestinal cholesterol absorption and promote excretion of bile acids [68]; second, SDG and other lignans have been shown to modulate the activity of 7alpha-hydroxylase and acyl CoA cholesterol transferase [69]; and finally, ALA displaces saturated fats from the diet and may augment LDL-cholesterol catabolism [70].
Many human trials have evaluated the effects of whole flaxseed or its components on cholesterol levels. In a study of 199 postmenopausal Canadian women, Dodin et al. randomized the participants to either 40 g of flaxseed or wheat germ placebo daily for 1 year. At the end of the trial, there was no reduction in total cholesterol or LDL-cholesterol in the flaxseed group compared to the baseline. However, when compared with the placebo group there was a modest but statistically significant reduction in total cholesterol of 7.7 mg/dL and an increase in HDL-cholesterol of 3.1 mg/dL [71].
In contrast, 38 postmenopausal women with hypercholesterolemia were given 38 g of either whole flaxseed or sunflower seeds baked into a muffin every day for 6 weeks. The investigators found that there were significant reductions in total cholesterol in both the flaxseed (6.9 %) and the sunflower seed (5.5 %) groups. Notably, only the flaxseed group saw significant reductions in LDL-cholesterol (14.7 %) and lipoprotein(a) (7.4 %) when compared to the baseline levels [72]. As compared to whole flaxseed, the addition of 30 g/day of ground flaxseed did not lower total cholesterol and LDL-cholesterol more than a low-fat diet in a study of 161 prostate cancer patients [73].
The effect of dietary flaxseed lignan (300 or 600 mg SDG daily) was tested in hypercholesterolemic subjects with a baseline LDL-cholesterol > 140 mg/dL. After 8 weeks of treatment, significant reductions in total cholesterol and LDL-cholesterol were observed in both treatment groups. In the 600 mg/day group, total cholesterol decreased by 22 % and LDL-cholesterol 24 %. Plasma concentrations of lignan metabolites increased in both treatment groups, and these levels were significantly correlated with reductions in cholesterol [74]. By contrast, when the same approach was taken in patients with type 2 diabetes who had LDL-cholesterol > 160 mg/dL, no significant effects on lipid levels were observed with 360 mg/day of SDG after 12 weeks of treatment [75].
Since one of the bioactive components of flaxseed is proposed to be polyunsaturated fatty acids, some studies have evaluated the effects of providing these fatty acids on lipid reduction. In a study by Harper et al., participants received either 3 g per day of ALA or olive oil. At the end of 6 months, the adjusted total cholesterol level in the ALA group was 17 mg/dL higher than in the olive oil group (p = 0.03), but the other lipid fractions as well as the particle sizes were unchanged [76]. Similarly, Paschos et al. compared the effect of 15 mL flaxseed oil (8.1 g ALA) to 15 mL safflower oil (11.2 g linoleic acid) in 35 men with untreated dyslipidemia (total cholesterol > 240 mg/dL), and found no changes in serum lipid concentrations after 12 weeks [77].
To investigate the effects of flaxseed fiber on plasma lipids, Jenkins et al. performed a randomized crossover study in hyperlipidemic subjects using 50 g of partially defatted flaxseed or wheat germ baked into muffins. After 3 weeks, there were significant reductions in total cholesterol (4.6 %), LDL-cholesterol (7.6 %), apolipoprotein A-I (5.8 %), and apolipoprotein B (5.4 %). In spite of the decrease in apolipoprotein A-I, there was no significant change in HDL-cholesterol [78].
Evaluating the body of evidence, a recent meta-analysis concluded that flaxseed supplementation does lower total and LDL-cholesterol levels without significant effects on HDL-cholesterol or triglycerides. The authors found reductions in total cholesterol when either whole flaxseed (7.3 mg/dL) or flaxseed lignans (10.8 mg/dL) was given. The reduction in LDL-cholesterol was of similar magnitude (6.2 mg/dL) for both whole flaxseed and lignans. Their analysis suggested that flaxseed oil does not have any beneficial effects on cholesterol levels [79].
Overall, flaxseed supplementation is well tolerated with the principle side effect being increased bowel movements [62]. Anaphylaxis from flaxseeds is rare but case reports are present in the literature [80–82].
The data suggest that supplementing the diet with whole, ground, or defatted flaxseed leads to reductions in total cholesterol and LDL-cholesterol. The lack of effect observed from flaxseed oil suggests that the cholesterol-lowering power of flaxseed may be related to its fiber, lignans, or a combination. The cholesterol-lowering effect of isolated flaxseed lignans is dose dependent and more efficacious in patients with higher cholesterol levels. If flaxseeds are added to the diet to reduce cholesterol, patients should strive to consume at least 40 g of whole or ground seeds per day. There are only a few trials that have evaluated the effect of flax lignans, and although a daily dose of 600 mg SDG shows some promise, the evidence is not strong enough to support the use of isolated lignans.
Soy Protein
Soy Protein (SP) is derived from the soybean, Glycine max, in a multistep process that serves to isolate the protein from soybeans by extracting the lipid and fibrous components. This results in an isolated SP concentrate or soy flour that can be further processed into texturized products [83].
There are epidemiological studies that show lower incidences of hypercholesterolemia and ischemic heart disease in Asian countries where greater quantities of soy products are consumed [84, 85]. Interest in the ability of SP to lower cholesterol began when scientists noted that substituting casein (a common milk protein) with SP in atherogenic, but cholesterol-free, diets prevented hypercholesterolemia and atherosclerosis in rabbits [86]. Sirtori et al. then demonstrated that replacing dietary animal protein with SP in hypercholesterolemic patients reduced total and LDL-cholesterol levels, which was subsequently confirmed in a multicenter trial [87, 88]. The evidence is such that the FDA has recommended a daily intake of > 25 g (four servings) of SP as part of a low-fat, low-cholesterol diet to reduce total and LDL-cholesterol levels [89].
Several mechanisms have been proposed for how dietary SP lowers cholesterol. Altered bile acid metabolism and increased gastrointestinal excretion of cholesterol are alleged to be mediated by soy peptides, heat-stable saponins, or trypsin inhibitors that promote cholecystokinin secretion and biliary outflow [89, 90]. However, this has not been supported by clinical studies looking at excretion of fecal neutral steroid or bile acid outputs [91].
It is suggested that phytic acid decreases cholesterol by chelating zinc in the intestine. This results in a higher ratio of copper to zinc, which favors lower cholesterol levels [92, 93]. In vitro studies have demonstrated an increase in LDL-receptor activity that is mediated by storage proteins contained in soy, particularly the 7S globulin [94, 95]. Clinical studies have shown that LDL-receptor activity and LDL-cholesterol degradation by mononuclear cells are increased by SP-enriched diets in patients with hypercholesterolemia [96].
Soy isoflavones are discussed elsewhere in this publication, and the readers are referred to Chap. 22 for further information. Briefly, soy isoflavones are bioactive molecules that are removed from SP preparations during processing with alcohol. Carefully controlled studies designed to determine whether the cholesterol-lowering effect is from the SP or the isoflavones have concluded that the LDL-cholesterol-reducing effect of SP, although modest, is independent of isoflavones [97].
In 1995, Anderson et al. [98] published a meta-analysis of 29 controlled studies, which concluded that SP lowers cholesterol levels proportional to the degree of hypercholesterolemia and not by the quantity consumed, which ranged from 18 to 124 g/day. Total cholesterol levels were reduced by 20 % in those with baseline cholesterol values > 335 mg/dL, 7 % in those 259–333 mg/dL and there was no significant effect in those with total cholesterol levels < 255 mg/dL. However, when they analyzed the data within groups receiving SP, they found a significant dose-related reduction in cholesterol: total cholesterol was reduced by 8.9 mg/dL in the group receiving 25 g SP/day, 17.4 mg/dL in the 50 g SP/day group , and 26.3 mg/dL in the 75 g SP/day group. In this analysis, the type of SP (isolate or textured), diets (usual, low-fat control, etc.), and the age of the subjects did not influence the magnitude or dose dependency of cholesterol reduction.
A more recent meta-analysis by Jenkins et al. [99] analyzed 11 studies with balanced macronutrient profiles and consumption of 20–133 g SP/day. The authors demonstrated that the cholesterol-lowering properties of SP are attributable to both intrinsic and extrinsic factors. They determined that 3.6–6 % of LDL-cholesterol lowering is due to the extrinsic displacement of saturated fats and dietary cholesterol from foods when consuming 13–58 g of SP/day. A further 4.3 % reduction in LDL-cholesterol was attributed to SP’s intrinsic effects.
SP is largely well tolerated but contains at least 16 potential allergens, e.g., soy hydrophobic protein. As a result, soy is on the UN Food and Agriculture Organization’s list of the eight most significant food allergens and is felt to be an under-recognized cause of food-related anaphylaxis [100, 101]. Based on the limited data available, SP products that contain isoflavones do not appear to have a negative impact on health in pregnancy or in hormone-dependent malignancy states such as breast or prostate cancer [102]. However, it would be prudent to exercise vigilance and moderation with intake.
In conclusion, SP modestly lowers total cholesterol and LDL-cholesterol through extrinsic and intrinsic mechanisms when it replaces animal protein from the diet. It is most effective in those with higher baseline cholesterol levels and when more than half of the daily protein requirement (> 25 g/day) is comprised of SP. The beneficial effects of soy on lipids appear to be from the protein component and not isoflavones, which are not recommended for cholesterol lowering because of a lack of evidence.
Garlic
Garlic (Allium sativum) has been widely used in cooking and as a traditional medicine for thousands of years. An ancient Egyptian manuscript, the Codex Ebers, cites it as a treatment for heart disorders, tumors, and numerous other complaints [103]. Garlic is comprised mainly of water (65 %) and its dry weight is made up of fructose-containing carbohydrates, sulfur compounds, fiber, protein, vitamins, minerals, and saponins. The majority of compounds from garlic are water soluble and less than 1 % are oil soluble. As such, it is often difficult to compare studies utilizing different garlic preparations as the content of active compounds will vary depending on whether it is raw whole garlic, garlic powder, garlic oils, or other extracts [104].
In vitro studies using isolated rodent and human hepatocytes have demonstrated that garlic inhibits cholesterol synthesis in a dose-dependent manner without significant toxicity [105–107]. Work by Gebhardt et al. suggests that the reduction in cholesterol synthesis occurs at the level of HMG-CoA reductase, but at higher doses lanosterol and 7-dehydrocholesterol will accumulate, suggesting effects further downstream in the cholesterol synthesis pathway [106]. More recent work proposes that organosulfur compounds in garlic, e.g., cysteine sulfoxides like S-allyl-l-cysteine or allicin, inhibit cholesterol synthesis via 4α-methyl oxidase. Allicin is formed by the action of the heat-sensitive enzyme alliinase on alliin, a sulfur-containing amino acid, when raw garlic is cut or chewed [108]. Water-soluble compounds like S-allyl-l-cysteine can inhibit cholesterol synthesis by up to 60 % but are cytotoxic at higher concentrations [109, 110]. However, it may be that these in vitro cholesterol-lowering effects are mainly due to cytotoxicity.
The German Association of General Practitioners performed the largest multicenter trial to evaluate garlic as a cholesterol-reducing agent by administering a commercially available garlic powder supplement [111]. They randomized 261 patients with type IIa or IIb hyperlipoproteinemia and total cholesterol and/or triglyceride levels > 200 mg/dL to receive either 800 mg of garlic powder (1.3 % alliin content) or placebo daily for 16 weeks. The investigators found a mean decrease in total cholesterol of 12 % (from 266 to 188 mg/dL) and a decrease of 17 % in triglycerides (226–188 mg/dL). The greatest cholesterol lowering was observed in patients with baseline cholesterol levels of 250–300 mg/dL. In this study, 21 % of the treatment group and 9 % of the placebo group complained of a mild garlic smell.
Zhang et al. conducted an 11-week study in which 51 healthy subjects received either 8.2 mg per day of garlic oil (containing allyl sulfides) or placebo. A further 27 volunteers received a garlic powder preparation containing 7.8 mg of allicin per day. There was no significant difference in lipid levels seen in the garlic-treated group as a whole at the end of the interventions with either garlic preparation. However, there was a significant improvement in HDL-cholesterol in women, with an increase of 6.2 mg/dL, and a reduction in total to HDL-cholesterol ratio following the garlic oil treatment specifically [112].
Studies on the use of aged garlic extract (AGE) are very limited. The AGE is made by soaking raw garlic in aqueous ethanol for 20 months at room temperature. The filtered extract is reduced until the final product contains 1.47 g/L of S-allyl-l-cysteine. Macan et al. [113] assessed the safety of using an AGE (Kyolic®) in 52 subjects on oral anticoagulation therapy. Volunteers were randomized to receive 5 mL of AGE twice daily or placebo for 12 weeks. The authors did not comment on the use of lipid-lowering medications in the study population at baseline, but did report that 9 % of subjects in the treatment group and 15 % in the placebo group had a history of hypercholesterolemia. The mean total cholesterol concentration at baseline was 184 mg/dL in both groups and the LDL-cholesterol was 104 mg/dL in the treatment group and 108 mg/dL in the placebo group. Following treatment, there were no significant differences in total cholesterol, LDL-cholesterol, or triglyceride concentrations between groups or within groups. There was, however, a modest but significant increase in mean HDL-cholesterol concentration by 2.9 mg/dL in the group that received AGE.
In contrast to the above study, Lau et al. used the same preparation of AGE and randomized 32 participants with untreated hypercholesterolemia (mean total cholesterol 306 mg/dL) to receive 4 mL of AGE or placebo daily for 6 months. In the AGE group, there was an increase in total cholesterol in almost all subjects for the first 3 months. However, by the end of the study, 11 of 15 subjects achieved > 10 % reduction in total cholesterol. In another experiment, the authors evaluated 14 subjects with total cholesterol levels < 200 mg/dL and found no significant cholesterol-lowering effect after 6 months of the treatment. Finally, when ten participants with baseline cholesterol within 240–380 mg/dL range were treated with AGE, six of ten experienced > 10 % cholesterol lowering at 6 months [114].
A study from India evaluated the effect of eating 10 g of raw garlic after breakfast every day on cholesterol levels in 50 medical students. After 2 months of treatment, there was a significant reduction in total cholesterol of 15.5 % compared to the control group. However, an increase in clotting time and fibrinolytic activity was also seen in these otherwise healthy young volunteers. No comment was made by the authors on how well the therapy was tolerated or if any participants dropped out [115].
A meta-analysis by Reid et al. [116] is the most comprehensive to date, and evaluated 39 primary garlic trials. They concluded that garlic supplements are effective in reducing total cholesterol by 17 mg/dL and LDL-cholesterol by 9 mg/dL in those with cholesterol levels > 200 mg/dL. The magnitude of cholesterol lowering was larger in trials of longer duration and in subjects with higher baseline cholesterol levels. The largest total cholesterol reduction was observed with AGE treatment, while the greatest LDL-cholesterol lowering was seen with garlic powder preparations. There was a small but significant increase in HDL-cholesterol (1.5 mg/dL) but no effect on triglycerides. While generally well tolerated, 60 % of the 39 trials reported side effects. Garlic breath, odor, or taste was most frequently reported in the treatment groups receiving raw garlic or garlic powder. However, gastrointestinal side effects were not more prevalent compared to placebo groups and no abnormalities were observed in hepatic or hematological factors.
The current data suggest that garlic, especially garlic powder, is effective at lowering LDL-cholesterol by 10 % or more in patients with hypercholesterolemia who are supplemented > 3 months. There is also a modest but significant increase in HDL-cholesterol but no change in triglyceride levels. There are no data to demonstrate any benefit of garlic supplements in patients who are already taking conventional lipid-lowering therapy.
Berberine
Berberine is an isoquinoline alkaloid, originally isolated from the rhizomes of the plant Coptis chinensis (Fig. 23.4), which has been used in Asia to treat gastrointestinal infections and diabetes for centuries [117]. The extract has been shown to improve insulin resistance, glucose control, and body weight in several in vitro, animal, and human studies [118–122]. In vitro studies suggest that berberine affects cholesterol metabolism by inhibiting cholesterol synthesis through multiple pathways, including activation of adenosine monophosphate-activated protein kinase, upregulating LDL-receptors through LDL-receptor messenger-ribonucleic acid stabilization in an extracellular signal-regulated kinase (ERK)-dependent manner, increasing transcription of the LDL promoter using the c-Jun N-terminal kinases (JNK) pathway, and reducing proprotein convertase subtilisin/kexin type 9 (PCSK9) mRNA and protein levels [123–126].
In two small studies, obese subjects taking 1.5 g/day of berberine hydrochloride had a nonsignificant 12 % reduction in total cholesterol; while patients with type 2 diabetes had a significant 13 % decrease in total cholesterol after 3 months [127, 128]. More convincingly, a randomized controlled trial of 144 hypercholesterolemic Caucasian subjects showed that consuming 500 mg of berberine twice daily significantly decreased total cholesterol, LDL-cholesterol, and triglycerides by 11.6, 16.4 and 21.2 %, respectively, and increased HDL cholesterol by 9.1 % [129]. When 116 patients with type 2 diabetes and dyslipidemia were randomized to the same dose of berberine (1000 mg/day) for 3 months, there were significant improvements in glucose tolerance and body weight, in addition to significant reductions in total cholesterol (18.1 %), LDL-cholesterol (21.1 %), and triglycerides (35.9 %) [122].
One study evaluated the addition of berberine to conventional statin therapy in a single-center trial. In this study, 63 treatment-naive subjects with hypercholesterolemia were randomized to receive berberine hydrochloride (500 mg twice daily), simvastatin (20 mg once daily), or both for 2 months. There were significant reductions in total cholesterol (9.1, 21.8, 29.1 %), LDL cholesterol (14.3, 23.8, 31.8 %), and triglycerides (11.4, 22.1, 38.9 %) in the berberine, simvastatin, and combination groups, respectively. The cholesterol and triglyceride reductions in the combination group were significantly greater when compared to the simvastatin and berberine monotherapy groups. HDL-cholesterol was not significantly changed in any of the three groups [130]. The addition of berberine to simvastatin therapy appears to be safe and well tolerated. The additional LDL-cholesterol lowering achieved with the combination suggests that berberine could be used with low-dose statin therapy to reduce the dose of statin required as well as potential side effects or toxicities.
Overall, berberine appears to be well tolerated and does not cause elevations in liver transaminases or creatine kinase. The most commonly reported adverse reactions to berberine are gastrointestinal in nature, consisting of self-limiting constipation, flatulence; and in rare instances headache [122, 127, 129, 131]. Berberine appears to be safe for use in patients with chronic liver disease, including chronic hepatitis B, hepatitis C, and alcoholic liver cirrhosis, as evaluated at a single Chinese center. In this study, subjects experienced significant reductions in total cholesterol, LDL-cholesterol, and triglycerides without elevations in liver transaminases or other side effects [132]. In addition to cholesterol-lowering, berberine is known to have antiarrhythmic and vasodilatory effects on the cardiovascular system [133, 134]. There is a case report of a man who was taking berberine for hypercholesterolemia and developed a junctional bradycardia, which reverted to normal sinus rhythm within 10 days of the supplement being discontinued [135].
In conclusion, berberine appears to be a relatively safe agent that moderately reduces total cholesterol, LDL-cholesterol, and triglycerides by around 10–20 %. It may also improve blood glucose control and reduce body weight, which also have beneficial effects on hyperlipidemia. Though data are limited, the available evidence suggests that berberine further lowers cholesterol levels when used in conjunction with statins. Further studies are needed to confirm this effect and the safety of combination therapy before berberine is recommended as a supplemental therapy to conventional lipid-lowering agents or as an alternative in patients with statin intolerance .
Guggul
The Commiphora mukul, also known as the guggul tree, is native to arid parts of the Indian subcontinent. Medicinal use of its gum resin (Fig. 23.5) has been described in ayurvedic texts since 600 BC for the treatment of inflammatory conditions, obesity, and atherosclerosis [136]. The lipid-lowering properties of guggul were first evaluated in the 1960s and commercial preparations have been marketed for this purpose since the late 1980s [137].
The active isomers, E- and Z-guggulsterone [cis- and trans- 4,17(20)-pregnadiene-3,16-dione], are available in ethyl extracts of the resin [138]. One mode of cholesterol-lowering action is thought to be though the inhibition of the farsenoid X receptor (FXR) , which is a nuclear hormone receptor activated by bile acids. For example, FXR-null mice do not exhibit the significant decrease in cholesterol when treated with guggulsterone that is observed in wild-type mice fed with a high-cholesterol diet [139]. There is also evidence that guggulsterone increases hepatic LDL-cholesterol uptake, fecal excretion of sterols and bile acids , LDL-cholesterol catabolism, and inhibits HMG-CoA reductase [140–143].
There are early trials from India that demonstrate significant reductions in LDL-cholesterol with guggulsterone therapy, but many of these suffer from flawed study designs [137, 144, 145]. A randomized, double-blind, placebo-controlled trial with a standardized, commercially available guggul extract (Guggulipid) was performed in 103 adults with primary hypercholesterolemia who were eating Western diets. In this trial, Guggulipid increased mean LDL cholesterol by 4–5 % in both the standard-dose and high-dose treatment groups. Only 18 % of participants treated with Guggulipid experienced a 5 % or greater reduction in LDL cholesterol [146]. Further, Guggulipid caused a hypersensitivity drug rash in 3 % of the standard dose and 15 % of the high-dose recipients. While one study reporter headache in 71% of treated subjects [147], gastrointestinal upset appears to be the most frequently reported side effect [137]. There has been one case of rhabdomyolysis reported in an Italian man who had been taking C. mukul capsules for 2 weeks to treat hypercholesterolemia. He had previously developed elevated serum creatine kinase while on simvastatin therapy, which had normalized before starting C. mukul [148].
It appears that guggulsterones do not reduce serum cholesterol in Western populations consuming a Western diet. Despite plausible biological mechanisms for lowering cholesterol, predominantly from rodent models, rigorous studies of guggulsterone therapy in humans have not been able to replicate the early data from Indian trials. This extract also appears to cause an excess of hypersensitivity skin rashes and gastrointestinal side effects requiring cessation of therapy. Therefore, at this time guggulsterone therapy is not recommended for patients looking for alternative cholesterol-lowering therapies.
Policosanol
Policosanol is a mixture of naturally occurring alcohols extracted from the wax of purified sugar cane (Saccharum officnarum L.). The extract was initially developed in Cuba where it was first approved for use in 1991. The principal components of policosanol are the higher aliphatic primary alcohols octocosanol (CH3–CH2(26)–CH2–OH), triacontanol, and hexacosanol [149].
The mechanism of action of policosanol in humans is unknown. In vitro experiments suggest that policosanol affects cholesterol synthesis at a level upstream of mevalonate formation, enhances LDL-particle uptake and degradation [150]. Animal models suggest that increased clearance of LDL-cholesterol is the primary mode of cholesterol lowering as opposed to reduced cholesterol synthesis [151, 152]. Other experimental models suggest that policosanol prevents lipoprotein peroxidation, has antiplatelet effects, and attenuates the development of atherosclerosis [153–156].
The initial studies on policosanol were performed in Cuba by one consortium. This group reported that doses of 10–20 mg/day reduced total cholesterol by ~ 20 % and LDL-cholesterol up to 31 % in a dose-dependent manner [157–160]. Significant increases in HDL-cholesterol of 24–29 % were also observed in several early studies [160–163].
More robust randomized, placebo-controlled studies have failed to replicate the levels of LDL-cholesterol lowering that were initially reported. These studies have shown no significant lipid reductions in patients of several phenotypes, including primary hypercholesterolemia, heterozygous familial hypercholesterolemia, and combined hyperlipidemia with 8–12 weeks of policosanol treatment, ranging in dose from 10 to 80 mg/day [164, 165].
A comparative study evaluated the lipid-lowering effects of policosanol (20 mg/day) compared to atorvastatin (10 mg/day) for 12 weeks. The authors found that policosanol did not significantly reduce total cholesterol or LDL-cholesterol levels, nor did it provide any additional cholesterol lowering when given in combination with atorvastatin [166].
In spite of promising early reports from Cuban researchers, rigorously conducted trials performed elsewhere have shown that policosanol is ineffective at treating dyslipidemia and should not be recommended to patients.
Artichoke Leaf Extract
The globe artichoke (Cynara scolymus) is a member of the daisy family and is native to the Mediterranean region (Fig. 23.6). Artichoke leaf extract (ALE) has been used medicinally since the Ancient Egyptian times as an aid to digestion and to treat hangovers, jaundice, and snake bites [167]. Since the 1930s, there have been reports suggesting that ALE has favorable effects on cholesterol plaques and lipid metabolism [168]. Up to 4 % of ALE is made of sesquiterpene lactones; up to 2 % consists of phenolic acids such as chlorogenic acid, caffeic acid, and cynarin; and around 1 % of ALE is flavonoids, including luteolin, cynaroside, and scolymoside [167]. Experiments in cell cultures and animal models have demonstrated that ALE decreases cholesterol synthesis through luteolin, which is an intermediate below HMG-CoA reductase in the cholesterogenic pathway, and increases biliary excretion [169–173].
Very few randomized controlled trials have evaluated the effect of ALE on lipoprotein metabolism. Petrowicz et al. [174] published the results, in abstract format, of a randomized controlled trial in 44 subjects with average total cholesterol levels of 204 mg/dL. Although subjects took 640 mg of ALE three times daily, there was no effect on lipid concentrations. However, in a subgroup of 24 participants with baseline total cholesterol levels > 200 mg/dL, there was a reduction in cholesterol that was attributed to ALE, which was dependent on the baseline cholesterol level—the degree of reduction was not disclosed.
A multicenter study by Englisch et al. randomized 143 participants with total cholesterol levels > 280 mg/dL to 1800 mg/day of ALE or placebo for 6 weeks. Compared to baseline values, the ALE group experienced an 18.5 % reduction in total cholesterol and a 22.9 % decrease in LDL-cholesterol with no significant changes in HDL-cholesterol or triglyceride levels [175].
The most recent randomized trial enrolled 131 subjects to receive either 1280 mg/day of a standardized ALE or placebo for 12 weeks. In the treatment group, there was a modest but significant decrease in total cholesterol (4.2 %) but no significant changes in LDL-cholesterol, HDL-cholesterol, or triglycerides [176].
Finally, a study of 17 subjects with familial hypercholesterolemia specifically evaluated the effect of Cynarin, the 1,5-dicaffeyl ester of quinic acid, which is a phenolic acid found in ALE. The intervention failed to produce any significant changes in cholesterol or triglyceride concentrations after 3 months of treatment [177].
None of the studies reported any significant adverse events or laboratory test abnormalities as a result of ALE treatment. However, there are scant reports of transient gastrointestinal effects such as constipation and flatulence [168, 176]. In conclusion, the current evidence, reinforced by a recent Cochrane Database Systematic Review, does not support the use of ALE to lower cholesterol [178].
Conclusions
Healthcare consumers are free to choose from a multitude of readily available and well-promoted dietary supplements. Many patients see these supplements as safer alternatives, which are more aligned with their philosophy on healthcare. However, caution must be exercised as these dietary supplements frequently contain active compounds that are not standardized or regulated, and have the potential to interact with other medications. Patients should be encouraged to discuss the use of all dietary supplements with their physicians and pharmacists to reduce the risk of adverse effects.
There is a distinct role for the use of dietary supplements in patients who are unable or unwilling to take conventional lipid-lowering agents. However, supplements should only be recommended once there is convincing evidence for their safety and efficacy, either as lipid-lowering monotherapies or adjuncts to standard treatments like statins. Reasonable data exist to support the use of several supplements for cholesterol lowering, including RYR, SDF, nuts, flaxseed, SP, garlic, and berberine. In addition to inherent metabolic properties, several of these agents, including SDF, nuts, and soy protein, reduce plasma cholesterol levels simply by displacing lipid-rich or cholesterogenic foods from the diet. Patients should be encouraged to modify their lifestyles and consume a low-fat and low-cholesterol diet as part of any cholesterol-reducing therapy plan, including the use of dietary supplements.
References
Zeisel SH. Regulation of “nutraceuticals”. Science. 1999;285:1853–5.
Astin JA. Why patients use alternative medicine: results of a national study. JAMA. 1998;279:1548–53.
Marcus DM, Grollman AP. Botanical medicines-the need for new regulations. N Engl J Med. 2002;347:2073–6.
Newmaster SG, Grguric M, Shanmughanandhan D, Ramalingam S, Ragupathy S. DNA barcoding detects contamination and substitution in North American herbal products. BMC Med. 2013;11:222.
Bailey RL, Gahche JJ, Lentino CV, Dwyer JT, Engel JS, Thomas PR, Betz JM, Sempos CT, Picciano MF. Dietary supplement use in the United States, 2003–2006. J Nutr. 2011;141:261–6.
Wu C-H, Wang C-C, Kennedy J. Changes in herb and dietary supplement use in the US adult population: a comparison of the 2002 and 2007 National Health Interview Surveys. Clin Ther. 2011;33:1749–58.
Qato DM, Alexander GC, Conti RM, Johnson M, Schumm P, Lindau ST. Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA. 2008;300:2867–78.
Musselman ME, Pettit RS, Derenski KL. A review and update of red yeast rice. J Evid Based Complementary Altern Med. 2012;17:33–9.
Endo A. Monacolin K, a new hypocholesterolemic agent that specifically inhibits 3-hydroxy-3-methylglutaryl coenzyme A reductase. J Antibiot (Tokyo). 1980;33:334–6.
Alberts AW, Chen J, Kuron G, Hunt V, Huff J, Hoffman C, Rothrock J, Lopez M, Joshua H, Harris E, et al. Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc Natl Acad Sci U S A. 1980;77:3957–61.
Ma J, Li Y, Ye Q, Li J, Hua Y, Ju D, Zhang D, Cooper R, Chang M. Constituents of red yeast rice, a traditional Chinese food and medicine. J Agric Food Chem. 2000;48:5220–5.
Journoud M, Jones PJ. Red yeast rice: a new hypolipidemic drug. Life Sci. 2004;74:2675–83.
Gordon RY, Cooperman T, Obermeyer W, Becker DJ. Marked variability of monacolin levels in commercial red yeast rice products: buyer beware! Arch Intern Med. 2010;170:1722–7.
Donmez-Altuntas H, Dumlupinar G, Imamoglu N, Hamurcu Z, Liman BC. Effects of the mycotoxin citrinin on micronucleus formation in a cytokinesis-block genotoxicity assay in cultured human lymphocytes. J Appl Toxicol. 2007;27:337–41.
Kuroda K, Ishii Y, Takasu S, Kijima A, Matsushita K, Watanabe M, Takahashi H, Sugita-Konishi Y, Sakai H, Yanai T, et al. Cell cycle progression, but not genotoxic activity, mainly contributes to citrinin-induced renal carcinogenesis. Toxicology. 2013;311:216–24.
McCarthy M. FDA bans red yeast rice product. Lancet. 1998;351:1637.
Mark DA. All red yeast rice products are not created equal-or legal. Am J Cardiol. 2010;106:448.
Heber D, Yip I, Ashley JM, Elashoff DA, Elashoff RM, Go VL. Cholesterol-lowering effects of a proprietary Chinese red-yeast-rice dietary supplement. Am J Clin Nutr. 1999;69:231–6.
Becker DJ, Gordon RY, Halbert SC, French B, Morris PB, Rader DJ. Red yeast rice for dyslipidemia in statin-intolerant patients: a randomized trial. Ann Intern Med. 2009;150(830–839):W147–W839.
Lu Z, Kou W, Du B, Wu Y, Zhao S, Brusco OA, Morgan JM, Capuzzi DM, Chinese Coronary Secondary Prevention Study G., Li S. Effect of Xuezhikang, an extract from red yeast Chinese rice, on coronary events in a Chinese population with previous myocardial infarction. Am J Cardiol. 2008;101:1689–93.
Mueller PS. Symptomatic myopathy due to red yeast rice. Ann Intern Med. 2006;145:474–5.
Smith DJ, Olive KE. Chinese red rice-induced myopathy. South Med J. 2003;96:1265–7.
Prasad GV, Wong T, Meliton G, Bhaloo S. Rhabdomyolysis due to red yeast rice (Monascus purpureus) in a renal transplant recipient. Transplantation. 2002;74:1200–1.
Grieco A, Miele L, Pompili M, Biolato M, Vecchio FM, Grattagliano I, Gasbarrini G. Acute hepatitis caused by a natural lipid-lowering product: when “alternative” medicine is no “alternative” at all. J Hepatol. 2009;50:1273–7.
Wigger-Alberti W, Bauer A, Hipler UC, Elsner P. Anaphylaxis due to Monascus purpureus-fermented rice (red yeast rice). Allergy. 1999;54:1330–1.
Food and Drug Administartion, HHS. Food labeling: health claims; soluble fiber from certain foods and risk of coronary heart disease. Interim final rule. Fed Regist. 2008;73:9938–47.
Lund E, Gee J, Brown J, Wood P, Johnson I. Effect of oat gum on the physical properties of the gastrointestinal contents and on the uptake of D-galactose and cholesterol by rat small intestine in vitro. Br J Nutr. 1989;62:91–101.
Haikal Z, Play B, Landrier JF, Giraud A, Ghiringhelli O, Lairon D, Jourdheuil-Rahmani D. NPC1L1 and SR-BI are involved in intestinal cholesterol absorption from small-size lipid donors. Lipids. 2008;43:401–8.
Lia A, Hallmans G, Sandberg AS, Sundberg B, Aman P, Andersson H. Oat beta-glucan increases bile acid excretion and a fiber-rich barley fraction increases cholesterol excretion in ileostomy subjects. Am J Clin Nutr. 1995;62:1245–51.
Ellegard L, Andersson H. Oat bran rapidly increases bile acid excretion and bile acid synthesis: an ileostomy study. Eur J Clin Nutr. 2007;61:938–45.
Pastors JG, Blaisdell PW, Balm TK, Asplin CM, Pohl SL. Psyllium fiber reduces rise in postprandial glucose and insulin concentrations in patients with non-insulin-dependent diabetes. Am J Clin Nutr. 1991;53:1431–5.
Lakshmanan MR, Nepokroeff CM, Ness GC, Dugan RE, Porter JW. Stimulation by insulin of rat liver β-hydroxy-β-methylglutaryl coenzyme A reductase and cholesterol-synthesizing activities. Biochem Biophys Res Commun. 1973;50:704–10.
Chandalia M, Garg A, Lutjohann D, von Bergmann K, Grundy SM, Brinkley LJ. Beneficial effects of high dietary fiber intake in patients with type 2 diabetes mellitus. N Engl J Med. 2000;342:1392–8.
Weickert MO, Pfeiffer AFH. Metabolic effects of dietary fiber consumption and prevention of diabetes. J Nutr. 2008;138:439–42.
Immerstrand T, Andersson KE, Wange C, Rascon A, Hellstrand P, Nyman M, Cui SW, Bergenstahl B, Tragardh C, Oste R. Effects of oat bran, processed to different molecular weights of beta-glucan, on plasma lipids and caecal formation of SCFA in mice. Br J Nutr. 2010;104:364–73.
Hara H, Haga S, Aoyama Y, Kiriyama S. Short-chain fatty acids suppress cholesterol synthesis in rat liver and intestine. J Nutr. 1999;129:942–8.
Brennan CS, Cleary LJ. The potential use of cereal (1→3,1→4)-β-d-glucans as functional food ingredients. J Cereal Sci. 2005;42:1–13.
Brown L, Rosner B, Willett WW, Sacks FM. Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr. 1999;69:30–42.
Othman RA, Moghadasian MH, Jones PJ. Cholesterol-lowering effects of oat β-glucan. Nutr Rev. 2011;69:299–309.
Onning G, Wallmark A, Persson M, Akesson B, Elmstahl S, Oste R. Consumption of oat milk for 5 weeks lowers serum cholesterol and LDL cholesterol in free-living men with moderate hypercholesterolemia. Ann Nutr Metab. 1999;43:301–9.
Kerckhoffs DA, Hornstra G, Mensink RP. Cholesterol-lowering effect of β-glucan from oat bran in mildly hypercholesterolemic subjects may decrease when β-glucan is incorporated into bread and cookies. Am J Clin Nutr. 2003;78:221–7.
Würsch P, Pi-Sunyer FX. The role of viscous soluble fiber in the metabolic control of diabetes: a review with special emphasis on cereals rich in β-glucan. Diabetes Care. 1997;20:1774–80.
Fischer MH, Yu N, Gray GR, Ralph J, Anderson L, Marlett JA. The gel-forming polysaccharide of psyllium husk (Plantago ovata Forsk). Carbohydr Res. 2004;339:2009–17.
Garvin JE, Forman DT, Eiseman WR, Phillips CR. Lowering of human serum cholesterol by an oral hydrophilic colloid. Exp Biol Med. 1965;120:744–6.
Wei ZH, Wang H, Chen XY, Wang BS, Rong ZX, Wang BS, Su BH, Chen HZ. Time- and dose-dependent effect of psyllium on serum lipids in mild-to-moderate hypercholesterolemia: a meta-analysis of controlled clinical trials. Eur J Clin Nutr. 2009;63:821–7.
Anderson JW, Allgood LD, Lawrence A, Altringer LA, Jerdack GR, Hengehold DA, Morel JG. Cholesterol-lowering effects of psyllium intake adjunctive to diet therapy in men and women with hypercholesterolemia: meta-analysis of 8 controlled trials. Am J Clin Nutr. 2000;71:472–9.
Moreyra AE, Wilson AC, Koraym A. Effect of combining psyllium fiber with simvastatin in lowering cholesterol. Arch Intern Med. 2005;165:1161.
Khalili B, Bardana EJ Jr., Yunginger JW. Psyllium-associated anaphylaxis and death: a case report and review of the literature. Ann Allergy Asthma Immunol. 2003;91:579–84.
Vaswani S, Hamilton R, Valentine M, Adkinson N. Psyllium laxative-induced anaphylaxis, asthma, and rhinitis. Allergy. 1996;51:266–8.
Cartier A, Malo JL, Dolovich J. Occupational asthma in nurses handling psyllium. Clin Allergy. 1987;17:1–6.
González Canga A, Fernández Martinez N, Sahagún Prieto A, García Vieitez J, Díez Liébana M, Díez Láiz R, Sierra Vega M. Dietary fiber and its interaction with drugs. Nutr Hosp. 2010;25:535–9.
Kris-Etherton PM, Zhao G, Binkoski AE, Coval SM, Etherton TD. The effects of nuts on coronary heart disease risk. Nutr Rev. 2001;59:103–11.
Abbey M, Noakes M, Belling GB, Nestel PJ. Partial replacement of saturated fatty acids with almonds or walnuts lowers total plasma cholesterol and low-density-lipoprotein cholesterol. Am J Clin Nutr. 1994;59:995–9.
Sabate J, Fraser GE, Burke K, Knutsen SF, Bennett H, Lindsted KD. Effects of walnuts on serum lipid levels and blood pressure in normal men. N Engl J Med. 1993;328:603–7.
Spiller GA, Jenkins DA, Bosello O, Gates JE, Cragen LN, Bruce B. Nuts and plasma lipids: an almond-based diet lowers LDL-C while preserving HDL-C. J Am Coll Nutr. 1998;17:285–90.
Garg ML, Blake RJ, Wills RBH. Macadamia nut consumption lowers plasma total and LDL cholesterol levels in hypercholesterolemic men. J Nutr. 2003;133:1060–3.
Guasch-Ferré M, Bulló M, Martínez-González MÁ, Ros E, Corella D, Estruch R, Fitó M, Arós F, Wärnberg J, Fiol M. Frequency of nut consumption and mortality risk in the PREDIMED nutrition intervention trial. BMC Med. 2013;11:164.
Estruch R, Ros E, Salas-Salvadó J, Covas M-I, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J, et al. Primary prevention of cardiovascular disease with a mediterranean diet. N Engl J Med. 2013;368:1279–90.
Zambon D, Sabate J, Munoz S, Campero B, Casals E, Merlos M, Laguna JC, Ros E. Substituting walnuts for monounsaturated fat improves the serum lipid profile of hypercholesterolemic men and women. A randomized crossover trial. Ann Intern Med. 2000;132:538–46.
Jenkins DJA, Kendall CWC, Marchie A, Parker TL, Connelly PW, Qian W, Haight JS, Faulkner D, Vidgen E, Lapsley KG, et al. Dose response of almonds on coronary heart disease risk factors: blood lipids, oxidized low-density lipoproteins, lipoprotein(a), homocysteine, and pulmonary nitric oxide: a randomized, controlled, crossover trial. Circulation. 2002;106:1327–32.
Sabaté J, Oda K, Ros E. Nut consumption and blood lipid levels: a pooled analysis of 25 intervention trials. Arch Intern Med. 2010;170:821.
Bloedon LT, Szapary PO. Flaxseed and cardiovascular risk. Nutr Rev. 2004;62:18–27.
Morris DH. Essential nutrients and other functional compounds in flaxseed. Nutr Today. 2001;36:159–62.
Lucas EA, Lightfoot SA, Hammond LJ, Devareddy L, Khalil DA, Daggy BP, Smith BJ, Westcott N, Mocanu V, Soung DY. Flaxseed reduces plasma cholesterol and atherosclerotic lesion formation in ovariectomized Golden Syrian hamsters. Atherosclerosis. 2004;173:223–9.
Pellizzon MA, Billheimer JT, Bloedon LT, Szapary PO, Rader DJ. Flaxseed reduces plasma cholesterol levels in hypercholesterolemic mouse models. J Am Coll Nutr. 2007;26:66–75.
Vijaimohan K, Jainu M, Sabitha KE, Subramaniyam S, Anandhan C, Shyamala Devi CS. Beneficial effects of alpha linolenic acid rich flaxseed oil on growth performance and hepatic cholesterol metabolism in high fat diet fed rats. Life Sci. 2006;79:448–54.
Prasad K. Hypocholesterolemic and antiatherosclerotic effect of flax lignan complex isolated from flaxseed. Atherosclerosis. 2005;179:269–75.
Cohn JS, Kamili A, Wat E, Chung RWS, Tandy S. Reduction in intestinal cholesterol absorption by various food components: mechanisms and implications. Atheroscler Suppl. 2010;11:45–8.
Sanghvi A, Divven WF, Seltman H. Proceedings of the symposium on drugs affecting lipid metabolism. Inhibition of rat liver cholesterol 7-alpha hydroxylase and aceyl CoA: cholesterol aceyl transferase activities by entrodiol and enterolactone. 1984. pp. 311–22.
Chan JK, Bruce VM, McDonald BE. Dietary alpha-linolenic acid is as effective as oleic acid and linoleic acid in lowering blood cholesterol in normolipidemic men. Am J Clin Nutr. 1991;53:1230–4.
Dodin S, Lemay A, Jacques H, Légaré F, Forest J-C, Mâsse B. The effects of flaxseed dietary supplement on lipid profile, bone mineral density, and symptoms in menopausal women: a randomized, double-blind, wheat germ placebo-controlled clinical trial. J Clin Endocrinol Metab. 2005;90:1390–7.
Arjmandi BH, Khan DA, Juma S, Drum ML, Venkatesh S, Sohn E, Wei L, Derman R. Whole flaxseed consumption lowers serum LDL-cholesterol and lipoprotein(a) concentrations in postmenopausal women. Nutr Res. 1998;18:1203–14.
Demark-Wahnefried W, Polascik TJ, George SL, Switzer BR, Madden JF, Ruffin MT, Snyder DC, Owzar K, Hars V, Albala DM. Flaxseed supplementation (not dietary fat restriction) reduces prostate cancer proliferation rates in men presurgery. Cancer Epidemiol Biomarkers Prev. 2008;17:3577–87.
Zhang W, Wang X, Liu Y, Tian H, Flickinger B, Empie MW, Sun SZ. Dietary flaxseed lignan extract lowers plasma cholesterol and glucose concentrations in hypercholesterolaemic subjects. Br J Nutr. 2008;99:1301–9.
Pan A, Sun J, Chen Y, Ye X, Li H, Yu Z, Wang Y, Gu W, Zhang X, Chen X. Effects of a flaxseed-derived lignan supplement in type 2 diabetic patients: a randomized, double-blind, cross-over trial. PLoS One. 2007;2:e1148.
Harper CR, Edwards MC, Jacobson TA. Flaxseed oil supplementation does not affect plasma lipoprotein concentration or particle size in human subjects. J Nutr. 2006;136:2844–8.
Paschos GK, Zampelas A, Panagiotakos DB, Katsiougiannis S, Griffin BA, Votteas V, Skopouli FN. Effects of flaxseed oil supplementation on plasma adiponectin levels in dyslipidemic men. Eur J Nutr. 2007;46:315–20.
Jenkins DJ, Kendall CW, Vidgen E, Agarwal S, Rao AV, Rosenberg RS, Diamandis EP, Novokmet R, Mehling CC, Perera T, et al. Health aspects of partially defatted flaxseed, including effects on serum lipids, oxidative measures, and ex vivo androgen and progestin activity: a controlled crossover trial. Am J Clin Nutr. 1999;69:395–402.
Pan A, Yu D, Demark-Wahnefried W, Franco OH, Lin X. Meta-analysis of the effects of flaxseed interventions on blood lipids. Am J Clin Nutr. 2009;90:288–97.
Alonso L, Marcos ML, Blanco JG, Navarro JA, Juste S, del Mar Garcés M, Pérez R, Carretero PJ. Anaphylaxis caused by linseed (flaxseed) intake. J Allergy Clin Immunol. 1996;98:469–70.
Gall H. Food-dependent exercise-induced anaphylaxis to flaxseed. Allergol Int. 2000;49:219–21.
Leóna F, Rodríguez M, Cuevas M. Anaphylaxis to linum. Allergol Immunopathol. 2003;31:47–9.
Endres JG, Soy protein products: characteristics, nutritional aspects and utilization. AOCS press, Champaign, Illinois. 2001.
Nagata C, Takatsuka N, Kurisu Y, Shimizu H. Decreased serum total cholesterol concentration is associated with high intake of soy products in Japanese men and women. J Nutr 1998;128:209–13.
Zhang X, Shu XO, Gao Y-T, Yang G, Li Q, Li H, Jin F, Zheng W. Soy food consumption is associated with lower risk of coronary heart disease in Chinese women. J Nutr. 2003;133:2874–8.
Carroll KK, Kurowska EM. Soy consumption and cholesterol reduction: review of animal and human studies. J Nutr. 1995;125:594S–597S.
Sirtori CR, Agradi E, Conti F, Mantero O, Gatti E. Soybean-protein diet in the treatment of type-II hyperlipoproteinaemia. Lancet. 1977;1:275–7.
Descovich GC, Gaddi A, Mannino G, Cattin L, Senin U, Caruzzo C, Fragiacomo C, Sirtori M, Ceredi C, Benassi MS, et al. Multicentre study of soybean protein diet for outpatient hypercholesterolæmic patients. Lancet. 1980;316:709–12.
Erdman JW, Committee, f.t.A.N. Soy protein and cardiovascular disease: a statement for healthcare professionals from the nutrition committee of the AHA. Circulation. 2000;102:2555–9.
Sidhu G, Oakenfull D. A mechanism for the hypocholesterolaemic activity of saponins. Br J Nutr. 1986;55:643–9.
Fumagalli R, Soleri L, Farina R, Musanti R, Mantero O, Noseda G, Gatti E, Sirtori CR. Fecal cholesterol excretion studies in type II hypercholesterolemic patients treated with the soybean protein diet. Atherosclerosis. 1982;43:341–53.
O’Deli BL, Savage JE. Effect of phytic acid on zinc availability. Exp Biol Med. 1960;103:304–6.
Klevay L. The role of copper and zinc in cholesterol metabolism. In: Draper H, editor. Advances in nutritional research. US: Springer; 1977. pp. 227–52.
Lovati MR, Manzoni C, Gianazza E, Arnoldi A, Kurowska E, Carroll KK, Sirtori CR. Soy protein peptides regulate cholesterol homeostasis in hep G2 cells. J Nutr. 2000;130:2543–9.
Manzoni C, Duranti M, Eberini I, Scharnag H, März W, Castiglioni S, Lovati MR. Subcellular localization of soybean 7S globulin in HepG2 Cells and LDL receptor up-regulation by Its α′ constituent subunit. J Nutr. 2003;133:2149–55.
Lovati M, Manzoni C, Canavesi A, Sirtori M, Vaccarino V, Marchi M, Gaddi G, Sirtori C. Soybean protein diet increases low density lipoprotein receptor activity in mononuclear cells from hypercholesterolemic patients. J Clin Invest. 1987;80:1498.
Sacks FM, Lichtenstein A, Van Horn L, Harris W, Kris-Etherton P, Winston M, Committee, f.t.A.H.A.N. Soy protein, isoflavones, and cardiovascular health: an American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation. 2006;113:1034–44.
Anderson JW, Johnstone BM, Cook-Newell ME. Meta-analysis of the effects of soy protein intake on serum lipids. N Engl J Med. 1995;333:276–82.
Jenkins DJA, Mirrahimi A, Srichaikul K, Berryman CE, Wang L, Carleton A, Abdulnour S, Sievenpiper JL, Kendall CWC, Kris-Etherton PM. Soy protein reduces serum cholesterol by both intrinsic and food displacement mechanisms. J Nutr. 2010;140:2302S–11S.
Cordle CT. Soy protein allergy: incidence and relative severity. J Nutr. 2004;134:1213S–9S.
Foucard T, Malmheden Yman I. A study on severe food reactions in Sweden—is soy protein an underestimated cause of food anaphylaxis? Allergy. 1999;54:261–5.
Munro IC, Harwood M, Hlywka JJ, Stephen AM, Doull J, Flamm WG, Adlercreutz H. Soy isoflavones: a safety review. Nutr Rev. 2003;61:1–33.
Rahman K. Historical perspective on garlic and cardiovascular disease. J Nutr. 2001;131:977S–979S.
Rahman K, Lowe GM. Garlic and cardiovascular disease: a critical review. J Nutr. 2006;136:736S–740S.
Sendl A, Schliack M, Löser R, Stanislaus F, Wagner H. Inhibition of cholesterol synthesis in vitro by extracts and isolated compounds prepared from garlic and wild garlic. Atherosclerosis. 1992;94:79–85.
Gebhardt R. Multiple inhibitory effects of garlic extracts on cholesterol biosynthesis in hepatocytes. Lipids. 1993;28:613–9.
Liu L, Yeh Y-Y. Inhibition of cholesterol biosynthesis by organosulfur compounds derived from garlic. Lipids. 2000;35:197–203.
Tattelman E. Health effects of garlic. Am Fam Physician. 2005;72:103–6.
Yeh YY, Liu L. Cholesterol-lowering effect of garlic extracts and organosulfur compounds: human and animal studies. J Nutr. 2001;131:989S–993S.
Singh DK, Porter TD. Inhibition of sterol 4alpha-methyl oxidase is the principal mechanism by which garlic decreases cholesterol synthesis. J Nutr. 2006;136:759S–764S.
Mader FH. Treatment of hyperlipidaemia with garlic-powder tablets. Evidence from the German Association of General Practitioners’ multicentric placebo-controlled double-blind study. Arzneimittelforschung. 1990;40:1111–6.
Zhang X-H, Lowe D, Giles P, Fell S, Connock MJ, Maslin DJ. Gender may affect the action of garlic oil on plasma cholesterol and glucose levels of normal subjects. J Nutr. 2001;131:1471–8.
Macan H, Uykimpang R, Alconcel M, Takasu J, Razon R, Amagase H, Niihara Y. Aged garlic extract may be safe for patients on warfarin therapy. J Nutr. 2006;136:793–5S.
Lau BHS, Lam F, Wang-Cheng R. Effect of an odor-modified garlic preparation on blood lipids. Nutr Res. 1987;7:139–49.
Gadkari J, Joshi V. Effect of ingestion of raw garlic on serum cholesterol level, clotting time and fibrinolytic activity in normal subjects. J Postgrad Med. 1991;37:128.
Ried K, Toben C, Fakler P. Effect of garlic on serum lipids: an updated meta-analysis. Nutr Rev. 2013;71:282–99.
Tang L-Q, Wei W, Chen L-M, Liu S. Effects of berberine on diabetes induced by alloxan and a high-fat/high-cholesterol diet in rats. J Ethnopharmacol. 2006;108:109–15.
Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, Ye JM, Lee CH, Oh WK, Kim CT, et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55:2256–64.
Ko BS, Choi SB, Park SK, Jang JS, Kim YE, Park S. Insulin sensitizing and insulinotropic action of berberine from Cortidis rhizoma. Biol Pharm Bull. 2005;28:1431–7.
Yin J, Hu R, Chen M, Tang J, Li F, Yang Y, Chen J. Effects of berberine on glucose metabolism in vitro. Metabolism. 2002;51:1439–43.
Yin J, Gao Z, Liu D, Liu Z, Ye J. Berberine improves glucose metabolism through induction of glycolysis. Am J Physiol Endocrinol Metab. 2008;294:E148–E56.
Zhang Y, Li X, Zou D, Liu W, Yang J, Zhu N, Huo L, Wang M, Hong J, Wu P, et al. Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J Clin Endocrinol Metab. 2008;93:2559–65.
Brusq JM, Ancellin N, Grondin P, Guillard R, Martin S, Saintillan Y, Issandou M. Inhibition of lipid synthesis through activation of AMP kinase: an additional mechanism for the hypolipidemic effects of berberine. J Lipid Res. 2006;47:1281–8.
Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C, Wang Y, Wang Z, Si S, Pan H, et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10:1344–51.
Lee S, Lim H-J, Park J-H, Lee K-S, Jang Y, Park H-Y. Berberine-induced LDLR up-regulation involves JNK pathway. Biochem Biophys Res Commun. 2007;362:853–7.
Cameron J, Ranheim T, Kulseth MA, Leren TP, Berge KE. Berberine decreases PCSK9 expression in HepG2 cells. Atherosclerosis. 2008;201:266–73.
Hu Y, Ehli EA, Kittelsrud J, Ronan PJ, Munger K, Downey T, Bohlen K, Callahan L, Munson V, Jahnke M, et al. Lipid-lowering effect of berberine in human subjects and rats. Phytomedicine. 2012;19:861–7.
Yin J, Xing H, Ye J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism. 2008;57:712–7.
Derosa G, D’Angelo A, Bonaventura A, Bianchi L, Romano D, Maffioli P. Effects of berberine on lipid profile in subjects with low cardiovascular risk. Expert Opin Biol Ther. 2013;13:475–82.
Kong WJ, Wei J, Zuo ZY, Wang YM, Song DQ, You XF, Zhao LX, Pan HN, Jiang JD. Combination of simvastatin with berberine improves the lipid-lowering efficacy. Metabolism. 2008;57:1029–37.
Dong H, Wang N, Zhao L, Lu F. Berberine in the treatment of type 2 diabetes mellitus: a systemic review and meta-analysis. Evid Based Complement Alternat Med. 2012;2012:591654.
Zhao W, Xue R, Zhou ZX, Kong WJ, Jiang JD. Reduction of blood lipid by berberine in hyperlipidemic patients with chronic hepatitis or liver cirrhosis. Biomed Pharmacother. 2008;62:730–1.
Lau CW, Yao XQ, Chen ZY, Ko WH, Huang Y. Cardiovascular actions of berberine. Cardiovasc Drug Rev. 2001;19:234–44.
Marin-Neto JA, Maciel BC, Secches AL, Gallo Junior L. Cardiovascular effects of berberine in patients with severe congestive heart failure. Clin Cardiol. 1988;11:253–60.
Cannillo M, Frea S, Fornengo C, Toso E, Mercurio G, Battista S, Gaita F. Berberine behind the thriller of marked symptomatic bradycardia. World J Cardiol. 2013;5:261–4.
Satyavati GV. Gum guggul (Commiphora mukul)-the success story of an ancient insight leading to a modern discovery. Indian J Med Res. 1988;87:327–35.
Ulbricht C, Basch E, Szapary P, Hammerness P, Axentsev S, Boon H, Kroll D, Garraway L, Vora M, Woods J, et al. Guggul for hyperlipidemia: a review by the Natural Standard Research Collaboration. Complement Ther Med. 2005;13:279–90.
Nityanand S, Kapoor NK. Cholesterol lowering activity of the various fractions of the guggal. Indian J Exp Biol. 1973;11:395–6.
Urizar NL, Liverman AB, Dodds DT, Silva FV, Ordentlich P, Yan Y, Gonzalez FJ, Heyman RA, Mangelsdorf DJ, Moore DD. A natural product that lowers cholesterol as an antagonist ligand for FXR. Science. 2002;296:1703–6.
Singh V, Kaul S, Chander R, Kapoor NK. Stimulation of low density lipoprotein receptor activity in liver membrane of guggulsterone treated rats. Pharmacol Res. 1990;22:37–44.
Sheela CG, Augusti KT. Effects of S-allyl cysteine sulfoxide isolated from Allium sativum Linn and gugulipid on some enzymes and fecal excretions of bile acids and sterols in cholesterol fed rats. Indian J Exp Biol. 1995;33:749–51.
Panda S, Kar A. Gugulu (Commiphora mukul) induces triiodothyronine production: possible involvement of lipid peroxidation. Life Sci. 1999;65:PL137–41.
Wu J, Xia C, Meier J, Li S, Hu X, Lala DS. The hypolipidemic natural product guggulsterone acts as an antagonist of the bile acid receptor. Mol Endocrinol. 2002;16:1590–7.
Nityanand S, Srivastava JS, Asthana OP. Clinical trials with gugulipid. A new hypolipidaemic agent. J Assoc Physicians India. 1989;37:323–8.
Kuppurajan K, Rajagopalan SS, Rao TK, Sitaraman R. Effect of guggulu (Commiphora mukul-Engl.) on serum lipids in obese, hypercholesterolemic and hyperlipemic cases. J Assoc Physicians India. 1978;26:367–73.
Szapary PO, Wolfe ML, Bloedon LT, Cucchiara AJ, DerMarderosian AH, Cirigliano MD, Rader DJ. Guggulipid for the treatment of hypercholesterolemia: a randomized controlled trial. JAMA. 2003;290:765–72.
Arora RB, Kapoor V, Gupta SK, Sharma RC. Isolation of a crystalline steroidal compound from Commiphora mukul & its anti-inflammatory activity. Indian J Exp Biol. 1971;9:403–4.
Bianchi A, Cantu P, Firenzuoli F, Mazzanti G, Menniti-Ippolito F, Raschetti R. Rhabdomyolysis caused by Commiphora mukul, a natural lipid-lowering agent. Ann Pharmacother. 2004;38:1222–5.
Gouni-Berthold I, Berthold HK. Policosanol: clinical pharmacology and therapeutic significance of a new lipid-lowering agent. Am Heart J. 2002;143:356–65.
Menendez R, Fernandez SI, Del Rio A, Gonzalez RM, Fraga V, Amor AM, Mas RM. Policosanol inhibits cholesterol biosynthesis and enhances low density lipoprotein processing in cultured human fibroblasts. Biol Res. 1994;27:199–203.
Menendez R, Amor AM, Gonzalez RM, Fraga V, Mas R. Effect of policosanol on the hepatic cholesterol biosynthesis of normocholesterolemic rats. Biol Res. 1996;29:253–7.
Menendez R, Arruzazabala L, Mas R, Del Rio A, Amor AM, Gonzalez RM, Carbajal D, Fraga V, Molina V, Illnait J. Cholesterol-lowering effect of policosanol on rabbits with hypercholesterolaemia induced by a wheat starch-casein diet. Br J Nutr. 1997;77:923–32.
Menendez R, Mas R, Amor AM, Gonzalez RM, Fernandez JC, Rodeiro I, Zayas M, Jimenez S. Effects of policosanol treatment on the susceptibility of low density lipoprotein (LDL) isolated from healthy volunteers to oxidative modification in vitro. Br J Clin Pharmacol. 2000;50:255–62.
Arruzazabala ML, Valdes S, Mas R, Fernandez L, Carbajal D. Effect of policosanol successive dose increases on platelet aggregation in healthy volunteers. Pharmacol Res. 1996;34:181–5.
Batista J, Stüsser R, Penichet M, Uguet E. Doppler-ultrasound pilot study of the effects of long-term policosanol therapy on carotid-vertebral atherosclerosis. Curr Ther Res. 1995;56:906–14.
Stusser R, Batista J, Padron R, Sosa F, Pereztol O. Long-term therapy with policosanol improves treadmill exercise-ECG testing performance of coronary heart disease patients. Int J Clin Pharmacol Ther. 1998;36:469–73.
Pons P, Más R, Illnait J, Fernández L, Rodriguez M, Robaina C, Fernández JC. Efficacy and safety of policosanol in patients with primary hypercholesterolemia. Curr Ther Res. 1992;52:507–13.
Pons P, Rodriguez M, Robaina C, Illnait J, Mas R, Fernandez L, Fernandez JC. Effects of successive dose increases of policosanol on the lipid profile of patients with type II hypercholesterolaemia and tolerability to treatment. Int J Clin Pharmacol Res. 1994;14:27–33.
Canetti M, Moreira M, Mas R, Illnait J, Fernandez L, Fernandez J, Diaz E, Castano G. A two-year study on the efficacy and tolerability of policosanol in patients with type II hyperlipoproteinaemia. Int J Clin Pharmacol Res. 1995;15:159–65.
Hernández F, Illnait J, Más R, Castaño G, Fernández L, González M, Cordovi N, Fernández J. Effect of policosanol on serum lipids and lipoproteins in healthy volunteers. Curr Ther Res. 1992;51:568–75.
Crespo N, Alvarez R, Más R, Illnait J, Fernández L, Fernández JC. Effects of policosanol on patients with non-insulin-dependent diabetes mellitus and hypercholesterolemia: a pilot study. Curr Ther Res. 1997;58:44–51.
Más R, Castaño G, Illnait J, Fernández L, Fernández J, Alemán C, Pontigas V, Lescay M. Effects of policosanol in patients with type II hypercholesterolemia and additional coronary risk factors. Clin Pharmacol Ther. 1999;65:439–47.
Castano G, Mas R, Fernandez L, Fernandez JC, Illnait J, Lopez LE, Alvarez E. Effects of policosanol on postmenopausal women with type II hypercholesterolemia. Gynecol Endocrinol. 2000;14:187–95.
Greyling A, De Witt C, Oosthuizen W, Jerling JC. Effects of a policosanol supplement on serum lipid concentrations in hypercholesterolaemic and heterozygous familial hypercholesterolaemic subjects. Br J Nutr. 2006;95:968–75.
Dulin MF, Hatcher LF, Sasser HC, Barringer TA. Policosanol is ineffective in the treatment of hypercholesterolemia: a randomized controlled trial. Am J Clin Nutr. 2006;84:1543–8.
Cubeddu LX, Cubeddu RJ, Heimowitz T, Restrepo B, Lamas GA, Weinberg GB. Comparative lipid-lowering effects of policosanol and atorvastatin: a randomized, parallel, double-blind, placebo-controlled trial. Am Heart J. 2006;152:982.e981–982.e985.
Joy JF, Haber SL. Clinical uses of artichoke leaf extract. Am J Health Syst Pharm. 2007;64:1904, 1906–9.
Kraft K. Artichoke leaf extract—recent findings reflecting effects on lipid metabolism, liver and gastrointestinal tracts. Phytomedicine. 1997;4:369–78.
Gebhardt R. Inhibition of cholesterol biosynthesis in primary cultured rat hepatocytes by artichoke (Cynara scolymus L.) extracts. J Pharmacol Exp Ther. 1998;286:1122–8.
Gebhardt R. Inhibition of cholesterol biosynthesis in HepG2 cells by artichoke extracts is reinforced by glucosidase pretreatment. Phytother Res. 2002;16:368–72.
Qiang Z, Lee SO, Ye Z, Wu X, Hendrich S. Artichoke extract lowered plasma cholesterol and increased fecal bile acids in Golden Syrian hamsters. Phytother Res. 2012;26:1048–52.
Kirchhoff R, Beckers C, Kirchhoff GM, Trinczek-Gartner H, Petrowicz O, Reimann HJ. Increase in choleresis by means of artichoke extract. Phytomedicine. 1994;1:107–15.
Gebhardt R. Inhibition of cholesterol biosynthesis in primary cultured rat hepatocytes by artichoke (Cynara scolymus L.) extracts. J Pharmacol Exp Ther. 1998;286:1122–8.
Petrowicz O, Gebhardt R, Donner M, Schwandt P, Kraft K. Effects of artichoke leaf extract (ALE) on lipoprotein metabolism in vitro and in vivo. Atherosclerosis. 1997;129:147.
Englisch W, Beckers C, Unkauf M, Ruepp M, Zinserling V. Efficacy of artichoke dry extract in patients with hyperlipoproteinemia. Arzneimittelforschung. 2000;50:260–265.
Bundy R, Walker AF, Middleton RW, Wallis C, Simpson HC. Artichoke leaf extract (Cynara scolymus) reduces plasma cholesterol in otherwise healthy hypercholesterolemic adults: a randomized, double blind placebo controlled trial. Phytomedicine. 2008;15:668–75.
Heckers H, Dittmar K, Schmahl FW, Huth K. Inefficiency of cynarin as therapeutic regimen in familial type ii hyperlipoproteinaemia. Atherosclerosis. 1977;26:249–53.
Wider B, Pittler MH, Thompson-Coon J, Ernst E. Artichoke leaf extract for treating hypercholesterolaemia. Cochrane Database Syst Rev. 2013;3:CD003335.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Humana Press
About this chapter
Cite this chapter
Almandoz, J. (2015). Dietary Supplements for Cholesterol Management. In: Garg, A. (eds) Dyslipidemias. Contemporary Endocrinology. Humana Press, Totowa, NJ. https://doi.org/10.1007/978-1-60761-424-1_23
Download citation
DOI: https://doi.org/10.1007/978-1-60761-424-1_23
Published:
Publisher Name: Humana Press, Totowa, NJ
Print ISBN: 978-1-60761-423-4
Online ISBN: 978-1-60761-424-1
eBook Packages: MedicineMedicine (R0)