Abstract
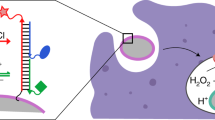
Phagosomal ROS generation is critical for our immune defense against microbial infections. Quantitative assessment of phagosomal ROS production is required to understand the complex relationship between the phagocyte and the microbe, in particular for pathogens that resist phagosomal destruction. ROS detection is difficult due to the transient nature of the reactive species and their multiple interactions with the environment. Direct labeling of phagocytic prey with a ROS-sensitive dye allows to target the dye into the phagosome and to follow the kinetics of phagosomal ROS production on a single phagosome base. Here we describe the basic labeling procedure, the quality assessment, and the imaging technique to achieve this kinetic analysis.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Dupre-Crochet S, Erard M, Nusse O (2013) ROS production in phagocytes: why, when, and where? J Leukoc Biol 94:657–670
Nauseef WM (2008) Biological roles for the NOX family NADPH oxidases. J Biol Chem 283:16961–16965
O’Neill S, Brault J, Stasia MJ, Knaus UG (2015) Genetic disorders coupled to ROS deficiency. Redox Biol 6:135–156
Imlay JA (2008) Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem 77:755–776
Seider K, Heyken A, Lüttich A, Miramón P, Hube B (2010) Interaction of pathogenic yeasts with phagocytes: survival, persistence and escape. Curr Opin Microbiol 13:392–400
Nault L, Bouchab L, Dupré-Crochet S, Nüsse O, Erard M (2016) Environmental effects on ROS-detection - learning from the phagosome. Antioxid Redox Signal 25:564–576
Tlili A, Dupré-Crochet S, Erard M, Nüße O (2011) Kinetic analysis of phagosomal production of reactive oxygen species. Free Radic Biol Med 50:438–447
Wardman P (2007) Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: progress, pitfalls, and prospects. Free Radic Biol Med 43:995–1022
Dewitt S, Laffafian I, Hallett MB (2003) Phagosomal oxidative activity during beta2 integrin (CR3)-mediated phagocytosis by neutrophils is triggered by a non-restricted Ca2+ signal: Ca2+ controls time not space. J Cell Sci 116:2857–2865
Tlili A, Erard M, Faure MC, Baudin X, Piolot T, Dupre-Crochet S, Nusse O (2012) Stable accumulation of p67(phox) at the phagosomal membrane and ROS production within the phagosome. J Leukoc Biol 91:83–95
Song ZM, Bouchab L, Hudik E, Le Bars R, Nüsse O, Dupré-Crochet S (2017) Phosphoinositol 3-phosphate acts as a timer for reactive oxygen species production in the phagosome. J Leukoc Biol 101:1155–1168
Bernardo J, Long HJ, Simons ER (2010) Initial cytoplasmic and phagosomal consequences of human neutrophil exposure to Staphylococcus epidermidis. Cytom Part A 77A:243–252
Russell DG, VanderVen BC, Glennie S, Mwandumba H, Heyderman RS (2009) The macrophage marches on its phagosome: dynamic assays of phagosome function. Nat Rev Immunol 9:594–U84
Vanderven BC, Yates RM, Russell DG (2009) Intraphagosomal measurement of the magnitude and duration of the oxidative burst. Traffic 10:372–378
Kamen LA, Levinsohn J, Cadwallader A, Tridandapani S, Swanson JA (2008) SHIP-A increases early oxidative burst and regulates phagosome maturation in macrophages. J Immunol 180:7497–7505
Chen X, Zhong Z, Xu Z, Chen L, Wang Y (2010) 2′,7′-Dichlorodihydrofluorescein as a fluorescent probe for reactive oxygen species measurement: forty years of application and controversy. Free Radic Res 44:587–604
Kundu K, Knight SF, Willett N, Lee S, Taylor WR, Murthy N (2009) Hydrocyanines: a class of fluorescent sensors that can image reactive oxygen species in cell culture, tissue, and in vivo. Angew Chemie Int Ed Engl 48:299–303
Erard M, Dupre-Crochet S, Nusse O (2018) Biosensors for spatiotemporal detection of reactive oxygen species in cells and tissues. Am J Physiol Integr Comp Physiol 314(5):R667–R683. https://doi.org/10.1152/ajpregu.00140.2017
Schwarzlander M, Dick TP, Meyer AJ, Morgan B (2016) Dissecting redox biology using fluorescent protein sensors. Antioxid Redox Signal 24:680–712
Seider K, Brunke S, Schild L, Jablonowski N, Wilson D, Majer O, Barz D, Haas A, Kuchler K, Schaller M, Hube B (2011) The facultative intracellular pathogen Candida glabrata subverts macrophage cytokine production and phagolysosome maturation. J Immunol 187:3072–3086
Acknowledgments
This work was supported by grants from the FRM (Foundation for Medical Research, DCM20121225747). We wish to thank our past colleagues who have contributed to this method and Elodie Hudik for her excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Dupré-Crochet, S., Erard, M., Nüβe, O. (2019). Kinetic Analysis of Phagosomal ROS Generation. In: Knaus, U., Leto, T. (eds) NADPH Oxidases. Methods in Molecular Biology, vol 1982. Humana, New York, NY. https://doi.org/10.1007/978-1-4939-9424-3_18
Download citation
DOI: https://doi.org/10.1007/978-1-4939-9424-3_18
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-4939-9423-6
Online ISBN: 978-1-4939-9424-3
eBook Packages: Springer Protocols